Non-Celiac Villous Atrophy—A Problem Still Underestimated
Abstract
1. Introduction
2. Pathophysiology of Non-Celiac Villous Atrophy
2.1. Immunological Mechanisms
2.2. Inflammatory Processes
2.3. Genetic and Environmental Factors
3. Primary Causes and Clinical Manifestations
3.1. Autoimmune Conditions
3.1.1. Autoimmune Enteropathy
3.1.2. Associated Autoimmune Disorders
3.2. Immunodeficiency Disorders
3.3. Infectious Etiology
4. Secondary and Iatrogenic Causes
4.1. Drug-Induced Enteropathy
4.2. Demographic and Environmental Factors
- -
- Inducing systemic inflammation;
- -
- Increasing intestinal permeability;
- -
- Disrupting the balance of intestinal microflora;
- -
- Inducing oxidative stress and epithelial cell damage.
4.3. Metabolic Disorders
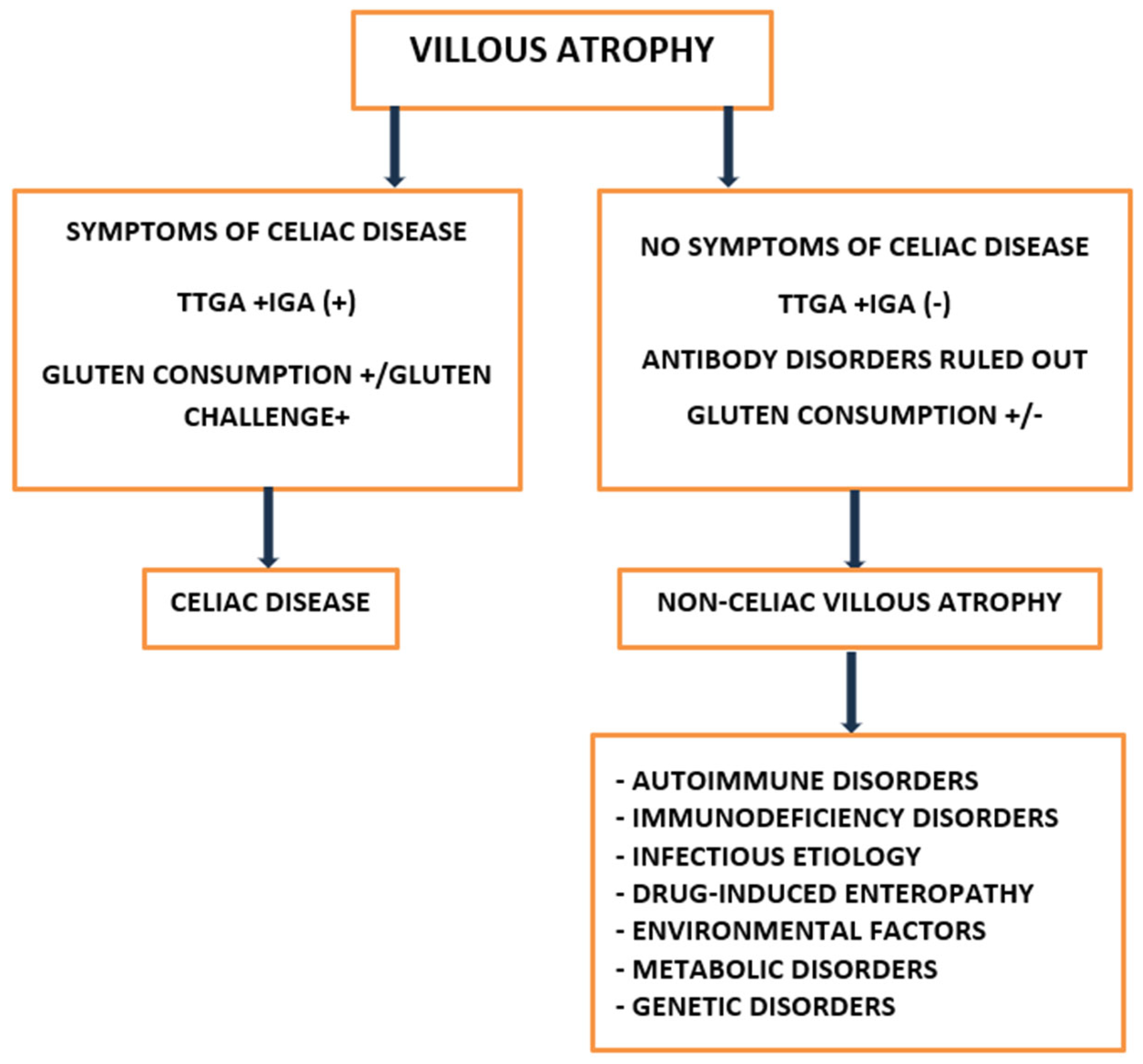
5. Diagnostic Approaches
5.1. Histopathological Assessment
5.2. Serological Testing
5.3. Differential Diagnosis
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AI | Artificial Intelligence |
| AIDS | Acquired Immunodeficiency Syndrome |
| AIE | Autoimmune Enteropathy |
| APS | Autoimmune Polyglandular Syndromes |
| ARBs | Angiotensin Receptor Blockers |
| CD | Celiac Disease |
| CCND2 | Cyclin D2 |
| CTLA4 | Cytotoxic T-Lymphocyte Associated Protein 4 |
| CVID | Common Variable Immunodeficiency |
| CVIDs | Immunodeficiency-related enteropathies |
| DGP-IgG | Deamidated Gliadin Peptide IgG |
| DOAJ | Directory of Open Access Journals |
| DVL2 | Disheveled 2 Protein |
| EMA | Anti-Endomysial Antibodies |
| EATL | Enteropathy-Associated T-cell Lymphoma |
| HIV | Human Immunodeficiency Virus |
| HLA | Human Leukocyte Antigen |
| IFN-γ | Interferon-Gamma |
| Igs | Immunoglobulins |
| IL | Interleukin |
| IL10RA | Interleukin 10 Receptor Subunit Alpha |
| IVA | Idiopathic Villous Atrophy |
| MDPI | Multidisciplinary Digital Publishing Institute |
| MMF | Mycophenolate Mofetil |
| NCVA | Non-Celiac Villous Atrophy |
| NCEs | Non-Celiac Enteropathies |
| NSAIDs | Non-Steroidal Anti-Inflammatory Drugs |
| PCR | Polymerase Chain Reaction |
| ROS | Reactive Oxygen Species |
| SLE | Systemic Lupus Erythematosus |
| SNCD | Seronegative Celiac Disease |
| SNEs | Seronegative Enteropathies |
| SNVA | Seronegative Villous Atrophy |
| SOT | Solid Organ Transplant |
| T1DM | Type 1 Diabetes Mellitus |
| TLA | Three-Letter Acronym |
| TNF-α | Tumor Necrosis Factor-Alpha |
| TPN | Total Parenteral Nutrition |
| tTG-IgA | Tissue Transglutaminase IgA |
| VA | Villous Atrophy |
References
- Schiepatti, A.; Maimaris, S.; Scalvini, D.; Raju, S.A.; Ingham, K.E.; Johnson, C.M.; Rubio-Tapia, A.; Maruggi, C.; Malamut, G.; Lenti, M.V.; et al. Long-term Prognosis of Nonceliac Enteropathies and a Score to Identify Patients with Poor Outcomes: A 30-year Multicenter Longitudinal Study. Am. J. Gastroenterol. 2025. ahead of print. [Google Scholar] [CrossRef]
- DeGaetani, M.; Tennyson, C.A.; Lebwohl, B.; Lewis, S.K.; Abu Daya, H.; Arguelles-Grande, C.; Bhagat, G.; Green, P.H. Villous atrophy and negative celiac serology: A diagnostic and therapeutic dilemma. Am. J. Gastroenterol. 2013, 108, 647–653. [Google Scholar] [CrossRef]
- Jansson-Knodell, C.L.; Rubio-Tapia, A. Villous Atrophy: Flat Mucosa, Raised Questions. Dig. Dis. Sci. 2024, 69, 655–656. [Google Scholar] [CrossRef] [PubMed]
- Schiepatti, A.; Sanders, D.S.; Zuffada, M.; Luinetti, O.; Iraqi, A.; Biagi, F. Overview in the clinical management of patients with seronegative villous atrophy. Eur. J. Gastroenterol. Hepatol. 2019, 31, 409–417. [Google Scholar] [CrossRef]
- Schiepatti, A.; Cincotta, M.; Biagi, F.; Sanders, D.S. Enteropathies with Villous Atrophy but Negative Coeliac Serology in Adults: Current Issues. BMJ Open Gastroenterol. 2021, 8, e000630. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.H.; Li, H. Olmesartan and Drug-Induced Enteropathy. P T 2014, 39, 47–50. [Google Scholar] [PubMed]
- Gómez-Escudero, O. Drug-Related Enteropathy. In Benign Anorectal Disorders—An Update; IntechOpen: London, UK, 2022; ISBN 978-1-80355-706-9. [Google Scholar]
- Osama, M.A.; Dhawan, S.; Rao, S.; Arora, A.K. Common Variable Immunodeficiency Enteropathy and Its Unpredictable Biopsy Findings: Not Everything Is Black and White. Middle East J. Dig. Dis. 2022, 14, 478–482. [Google Scholar] [CrossRef]
- Jansson-Knodell, C.L.; Hujoel, I.A.; Rubio-Tapia, A.; Murray, J.A. Not All That Flattens Villi Is Celiac Disease: A Review of Enteropathies. Mayo Clin. Proc. 2018, 93, 509–517. [Google Scholar] [CrossRef]
- Mi, K.; Cao, S.; Adams, D. Non-celiac Enteropathies. Curr. Gastroenterol. Rep. 2025, 27, 27. [Google Scholar] [CrossRef]
- Schiepatti, A.; Maimaris, S.; Raju, S.A.; Green, O.L.; Mantica, G.; Therrien, A.; Flores-Marin, D.; Linden, J.; Fernández-Bañares, F.; Esteve, M.; et al. Persistent villous atrophy predicts development of complications and mortality in adult patients with coeliac disease: A multicentre longitudinal cohort study and development of a score to identify high-risk patients. Gut 2023, 72, 2095–2102. [Google Scholar] [CrossRef]
- van Wanrooij, R.L.J.; Neefjes-Borst, E.A.; Bontkes, H.J.; Schreurs, M.W.J.; Langerak, A.W.; Mulder, C.J.J.; Bouma, G. Adult-Onset Autoimmune Enteropathy in an European Tertiary Referral Center. Clin. Transl. Gastroenterol. 2021, 12, e00387. [Google Scholar] [CrossRef]
- Dennis, M.; Amelie Therrien, A. Non Celiac Enteropathy: Damage to the Small Intestine Not Caused by Celiac Disease 2021. Available online: https://www.bidmc.org/-/media/files/beth-israel-org/centers-and-departments/digestive-disease-center/celiac-center/celiacnow/non-celiac-enteropathy.pdf (accessed on 29 May 2025).
- Lázaro de Lucas, C.; Tesouro Rodríguez, L.; Magallares García, L.N.; Martínez-Ojinaga Nodal, E.; Ramos Boluda, E. Diarrea grave por enteropatía autoinmune: Tratamiento y evolución [Severe diarrhoea due to autoimmune enteropathy: Treatment and outcomes]. An. de Pediatr. 2018, 88, 350–351. [Google Scholar] [CrossRef] [PubMed]
- Krigel, A.; Turner, K.O.; Makharia, G.K.; Green, P.H.; Genta, R.M.; Lebwohl, B. Ethnic Variations in Duodenal Villous Atrophy Consistent with Celiac Disease in the United States. Clin. Gastroenterol. Hepatol. 2016, 14, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Karanam, P.K.; Al-Hamadani, M.; Go, R.S. Enteropathy-associated T-cell lymphoma in the US: Higher incidence and poorer survival among Asians. Br. J. Haematol. 2016, 172, 990–992. [Google Scholar] [CrossRef] [PubMed]
- Leonard, M.M.; Lebwohl, B.; Rubio-Tapia, A.; Biagi, F. AGA Clinical Practice Update on the Evaluation and Management of Seronegative Enteropathies: Expert Review. Gastroenterology 2021, 160, 437–444. [Google Scholar] [CrossRef]
- Moral Moral, P.; Cabañero-Navalon, D.; Garcia-Bustos, V.; Núñez Beltrán, M.; Salavert, M. Norovirus Infection as a Model of Chronic or Recurrent Infection in Common Variable Immunodeficiency. Rev. Esp. Quimioter. 2022, 35 (Suppl. S3), 63–66. [Google Scholar] [CrossRef]
- Pensieri, M.V.; Pulvirenti, F.; Schiepatti, A.; Maimaris, S.; Lattanzio, S.; Quinti, I.; Klersy, C.; Corazza, G.R.; Biagi, F. The High Mortality of Patients with Common Variable Immunodeficiency and Small Bowel Villous Atrophy. Scand. J. Gastroenterol. 2019, 54, 164–168. [Google Scholar] [CrossRef]
- Chakraborty, H.; De, B. Drug-Induced Gastrointestinal Disorders: A Comprehensive Review. Acta Sci. Gastrointest. Disord. 2024, 7, 3. [Google Scholar]
- Yoosuf, S.; Makharia, G.K. Evolving Therapy for Celiac Disease. Front. Pediatr. 2019, 7, 193. [Google Scholar] [CrossRef]
- Abbasi, A.; Bazzaz, S.; Ibrahim, S.A.; Hekmatdoost, A.; Hosseini, H.; Sabahi, S.; Sheykhsaran, E.; Rahbar Saadat, Y.; Asghari Ozma, M.; Lahouty, M. A Critical Review on the Gluten-Induced Enteropathy/Celiac Disease: Gluten-Targeted Dietary and Non-Dietary Therapeutic Approaches. Food Rev. Int. 2024, 40, 883–923. [Google Scholar] [CrossRef]
- Agarwal, S.; Mayer, L. Gastrointestinal Manifestations in Primary Immune Disorders. Inflamm. Bowel Dis. 2010, 16, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Elli, L.; Branchi, F.; Sidhu, R.; Guandalini, S.; Assiri, A.; Rinawi, F.; Shamir, R.; Das, P.; Makharia, G.K. Small Bowel Villous Atrophy: Celiac Disease and Beyond. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Aziz, I.; Peerally, M.F.; Barnes, J.-H.; Kandasamy, V.; Whiteley, J.C.; Partridge, D.; Vergani, P.; Cross, S.S.; Green, P.H.; Sanders, D.S. The Clinical and Phenotypical Assessment of Seronegative Villous Atrophy; a Prospective UK Centre Experience Evaluating 200 Adult Cases over a 15-Year Period (2000–2015). Gut 2017, 66, 1563–1572. [Google Scholar] [CrossRef]
- Hvas, C.L.; Jensen, M.D.; Reimer, M.C.; Riis, L.B.; Johannes, J.; Skovbjerg, H.; Teisner, A.; Wildt, S. Celiac Disease: Diagnosis and Treatment. Available online: https://www.mayoclinic.org/diseases-conditions/celiac-disease/diagnosis-treatment/drc-20352225 (accessed on 29 May 2025).
- Sasahara, Y.; Uchida, T.; Suzuki, T.; Abukawa, D. Primary Immunodeficiencies Associated with Early-Onset Inflammatory Bowel Disease in Southeast and East Asia. Front. Immunol. 2021, 12, 786538. [Google Scholar] [CrossRef] [PubMed]
- Caliskan, M.; Dogan, G.; Orenay-Boyacioglu, S. Relationship between Villous Atrophy and Wnt Pathway Gene Expressions in Pediatric Celiac Patients. Rev. Assoc. Med. Bras. 2023, 69, e20221496. [Google Scholar] [CrossRef]
- Christodoulidis, G.; Agko, S.E.; Kouliou, M.N.; Koumarelas, K.E.; Zacharoulis, D. Advances and Challenges in Diagnosing and Managing Adult Autoimmune Enteropathy. World J. Gastroenterol. 2025, 31, 99118. [Google Scholar] [CrossRef]
- Téllez Villajos, L.; Crespo Pérez, L.; Cano Ruiz, A. Atrofia Vellositaria Sin Enfermedad Celíaca: ¿un Nuevo Síndrome o Más Confusión? Med. Clínica 2015, 144, 121–125. [Google Scholar] [CrossRef]
- Montalto, M.; D’Onofrio, F.; Santoro, L.; Gallo, A.; Gasbarrini, A.; Gasbarrini, G. Autoimmune Enteropathy in Children and Adults. Scand. J. Gastroenterol. 2009, 44, 1029–1036. [Google Scholar] [CrossRef]
- Owens, S.R.; Greenson, J.K. The Pathology of Malabsorption: Current Concepts. Histopathology 2007, 50, 64–82. [Google Scholar] [CrossRef]
- Kluger, N.; Jokinen, M.; Krohn, K.; Ranki, A. Gastrointestinal Manifestations in APECED Syndrome. J. Clin. Gastroenterol. 2013, 47, 112–120. [Google Scholar] [CrossRef]
- Agarwal, S.; Mayer, L. Pathogenesis and Treatment of Gastrointestinal Disease in Antibody Deficiency Syndromes. J. Allergy Clin. Immunol. 2009, 124, 658–664. [Google Scholar] [CrossRef]
- Kotler, D.P. Human Immunodeficiency Virus-Related Wasting: Malabsorption Syndromes. Semin. Oncol. 1998, 25, 70–75. [Google Scholar] [PubMed]
- Weclawiak, H.; Ould-Mohamed, A.; Bournet, B.; Guilbeau-Frugier, C.; Fortenfant, F.; Muscari, F.; Sallusto, F.; Dambrin, C.; Esposito, L.; Guitard, J.; et al. Duodenal Villous Atrophy: A Cause of Chronic Diarrhea After Solid-Organ Transplantation. Am. J. Transplant. 2011, 11, 575–582. [Google Scholar] [CrossRef]
- Syndrome of Intractable Diarrhoea with Persistent Villous Atrophy in Early Childhood: A Clinicopathological Survey of 47 Cases-Goulet-1998-Journal of Pediatric Gastroenterology and Nutrition-Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/10.1002/j.1536-4801.1998.tb00744.x (accessed on 11 March 2025).
- Marietta, E.V.; Cartee, A.; Rishi, A.; Murray, J.A. Drug-Induced Enteropathy. Dig. Dis. 2015, 33, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Eusébio, M.; Caldeira, P.; Antunes, A.G.; Ramos, A.; Velasco, F.; Cadillá, J.; Guerreiro, H. Olmesartan-Induced Enteropathy: An Unusual Cause of Villous Atrophy. GE Port. J. Gastroenterol. 2016, 23, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Malinowski, M.; Martus, P.; Lock, J.F.; Neuhaus, P.; Stockmann, M. Systemic Influence of Immunosuppressive Drugs on Small and Large Bowel Transport and Barrier Function. Transpl. Int. 2011, 24, 184–193. [Google Scholar] [CrossRef]
- Sjölund, K.; Persson, J.; Bergman, L. Can Villous Atrophy Be Induced by Chronic Alcohol Consumption? J. Intern. Med. 1989, 226, 133–135. [Google Scholar] [CrossRef]
- Maccioni, L.; Fu, Y.; Horsmans, Y.; Leclercq, I.; Stärkel, P.; Kunos, G.; Gao, B. Alcohol-Associated Bowel Disease: New Insights into Pathogenesis. eGastroenterology 2023, 1, e100013. [Google Scholar] [CrossRef]
- Yoseph, B.P.; Breed, E.; Overgaard, C.E.; Ward, C.J.; Liang, Z.; Wagener, M.E.; Lexcen, D.R.; Lusczek, E.R.; Beilman, G.J.; Burd, E.M.; et al. Correction: Chronic Alcohol Ingestion Increases Mortality and Organ Injury in a Murine Model of Septic Peritonitis. PLoS ONE 2020, 15, e0239568. [Google Scholar] [CrossRef]
- Ferretti, G.; Bacchetti, T.; Masciangelo, S.; Saturni, L. Celiac Disease, Inflammation and Oxidative Damage: A Nutrigenetic Approach. Nutrients 2012, 4, 243–257. [Google Scholar] [CrossRef]
- Uchida, H.; Nakajima, Y.; Ohtake, K.; Ito, J.; Morita, M.; Kamimura, A.; Kobayashi, J. Protective Effects of Oral Glutathione on Fasting-Induced Intestinal Atrophy through Oxidative Stress. World J. Gastroenterol. 2017, 23, 6650–6664. [Google Scholar] [CrossRef] [PubMed]
- Isaacson, R.E. Enteric Bacterial Pathogens, Villus Atrophy and Microbial Growth. Veter-Q. 1998, 20, 68–72. [Google Scholar] [CrossRef][Green Version]
- Costa, C.; Bartilotti Matos, F.; Carvalho Sá, D.; Neves Maia, J. Tropical Sprue: A Rare Cause of Malabsorption Syndrome. Cureus 2024, 16, e53748. [Google Scholar] [CrossRef] [PubMed]
- Brar, H.S.; Aloysius, M.M.; Shah, N.J. Tropical Sprue. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Ayob, N.; Muhammad Nawawi, K.N.; Mohamad Nor, M.H.; Raja Ali, R.A.; Ahmad, H.F.; Oon, S.F.; Mohd Mokhtar, N. The Effects of Probiotics on Small Intestinal Microbiota Composition, Inflammatory Cytokines and Intestinal Permeability in Patients with Non-Alcoholic Fatty Liver Disease. Biomedicines 2023, 11, 640. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.Y.; Groer, M.; Dutra, S.V.O.; Sarkar, A.; McSkimming, D.I. Gut Microbiota and Immune System Interactions. Microorganisms 2020, 8, 1587. [Google Scholar] [CrossRef]
- Felli, C.; Baldassarre, A.; Masotti, A. Intestinal and Circulating MicroRNAs in Coeliac Disease. Int. J. Mol. Sci. 2017, 18, 1907. [Google Scholar] [CrossRef]
- Szaflarska-Popławska, A. The Role of the Gluten-Free Diet in the Management of Seronegative Enteropathy. Nutrients 2021, 13, 4027. [Google Scholar] [CrossRef]
- Sinha, P.; Saini, V.; Varshney, N.; Pandey, R.K.; Jha, H.C. The Infiltration of Microplastics in Human Systems: Gastrointestinal Accumulation and Pathogenic Impacts. Heliyon 2025, 11, e42606. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (Contam). Presence of Microplastics and Nanoplastics in Food, with Particular Focus on Seafood. EFSA J. 2016, 14, e04501. [Google Scholar] [CrossRef]
- Jin, Y.; Lu, L.; Tu, W.; Luo, T.; Fu, Z. Impacts of Polystyrene Microplastic on the Gut Barrier, Microbiota and Metabolism of Mice. Sci. Total Environ. 2019, 649, 308–317. [Google Scholar] [CrossRef]
- Qiao, R.; Deng, Y.; Zhang, S.; Wolosker, M.B.; Zhu, Q.; Ren, H.; Zhang, Y. Accumulation of Different Shapes of Microplastics Initiates Intestinal Injury and Gut Microbiota Dysbiosis in the Gut of Zebrafish. Chemosphere 2019, 236, 124334. [Google Scholar] [CrossRef] [PubMed]
- Sofield, C.E.; Anderton, R.S.; Gorecki, A.M. Mind over Microplastics: Exploring Microplastic-Induced Gut Disruption and Gut-Brain-Axis Consequences. Curr. Issues Mol. Biol. 2024, 46, 4186–4202. [Google Scholar] [CrossRef] [PubMed]
- Kou, Y.; Ye, S.; Du, W.; Lu, Z.; Yang, K.; Zhan, L.; Huang, Y.; Qin, L.; Yang, Y. Long-term exposure to air pollution and gastrointestinal disease: Findings from a nationwide cohort study in China. BMC Public Health 2025, 25, 1011. [Google Scholar] [CrossRef]
- Bhardwaj, G.; Riadi, Y.; Afzal, M.; Bansal, P.; Kaur, H.; Deorari, M.; Tonk, R.K.; Almalki, W.H.; Kazmi, I.; Alzarea, S.I.; et al. The hidden threat: Environmental toxins and their effects on gut microbiota. Pathol. Res. Pract. 2024, 255, 155173. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, P.; Shang, X.; Lu, Y.; Li, Y. Exposure of Lead on Intestinal Structural Integrity and the Diversity of Gut Microbiota of Common Carp. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 239, 108877. [Google Scholar] [CrossRef]
- Shi, X.; Xu, W.; Che, X.; Cui, J.; Shang, X.; Teng, X.; Jia, Z. Effect of Arsenic Stress on the Intestinal Structural Integrity and Intestinal Flora Abundance of Cyprinus Carpio. Front. Microbiol. 2023, 14, 1179397. [Google Scholar] [CrossRef]
- Kabir, M.A.; Rabbane, G.; Hernandez, M.R.; Shaikh, M.A.A.; Moniruzzaman, M.; Chang, X. Impaired Intestinal Immunity and Microbial Diversity in Common Carp Exposed to Cadmium. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2024, 276, 109800. [Google Scholar] [CrossRef]
- Nehzomi, Z.S.; Shirani, K. The Gut Microbiota: A Key Player in Cadmium Toxicity—Implications for Disease, Interventions, and Combined Toxicant Exposures. J. Trace Elem. Med. Biol. 2025, 88, 127570. [Google Scholar] [CrossRef]
- Mesnage, R.; Defarge, N.; Spiroux de Vendômois, J.; Séralini, G.E. Potential Toxic Effects of Glyphosate and Its Commercial Formulations below Regulatory Limits. Food Chem. Toxicol. 2015, 84, 133–153. [Google Scholar] [CrossRef]
- Saleh, S.M.; Elghareeb, T.A.; Atia, M.M.; Ahmed, M.A.I. Impact of Glyphosate-Roundup® in the Ileal Structure of Male and Female Rats: A Morphological and Immunohistochemical Study. Microsc. Microanal. 2021, 27, 1547–1563. [Google Scholar] [CrossRef]
- Davidson, G.P.; Cutz, E.; Hamilton, J.R.; Gall, D.G. Familial Enteropathy: A Syndrome of Protracted Diarrhea from Birth, Failure to Thrive, and Hypoplastic Villus Atrophy. Gastroenterology 1978, 75, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Christl, S.U.; Scheppach, W. Metabolic Consequences of Total Colectomy. Scand. J. Gastroenterol. 1997, 32, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Calzadilla, N.; Comiskey, S.M.; Dudeja, P.K.; Saksena, S.; Gill, R.K.; Alrefai, W.A. Bile Acids as Inflammatory Mediators and Modulators of Intestinal Permeability. Front. Immunol. 2022, 13, 1021924. [Google Scholar] [CrossRef]
- Pavlidis, P.; Powell, N.; Vincent, R.P.; Ehrlich, D.; Bjarnason, I.; Hayee, B. Systematic Review: Bile Acids and Intestinal Inflammation-Luminal Aggressors or Regulators of Mucosal Defence? Aliment. Pharmacol. Ther. 2015, 42, 802–817. [Google Scholar] [CrossRef]
- Westergaard, H. Tropical Sprue. Curr. Treat. Options Gastroenterol. 2004, 7, 7–11. [Google Scholar] [CrossRef]
- Ge, Y.; Zadeh, M.; Mohamadzadeh, M. Vitamin B12 Regulates the Transcriptional, Metabolic, and Epigenetic Programing in Human Ileal Epithelial Cells. Nutrients 2022, 14, 2825. [Google Scholar] [CrossRef]
- Qudsiya, Z.; De Jesus, O. Subacute Combined Degeneration of the Spinal Cord(Archived). In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Malinowski, M.; Martus, P.; Neuhaus, P.; Stockmann, M. The Influence of Commonly Used Immunosuppressive Drugs on the Small Bowel Functions—A Comparative Experimental Study. Ann. Transplant. 2009, 14, 38–44. [Google Scholar] [PubMed]
- Tapia, O.; Villaseca, M.; Sierralta, A.; Roa, J.C. Duodenal Villous Atrophy Associated with Mycophenolate Mofetil: Report of One Case. Rev. Med. Chil. 2010, 138, 590–594. [Google Scholar]
- Schiepatti, A.; Premoli, A.; Maimaris, S.; Rizzo, M.; Marples, M.; Villani, L.; Scott, N.; Sottotetti, F.; Sanders, D.S.; Biagi, F.; et al. Small Bowel Villous Atrophy Due to Immune-Checkpoint Inhibitors: Report of Two Cases and Literature Review. PLoS ONE 2022, 11, 2022-6-3. [Google Scholar] [CrossRef]
- Louis-Auguste, J.; Greenwald, S.; Simuyandi, M.; Soko, R.; Banda, R.; Kelly, P. High Dose Multiple Micronutrient Supplementation Improves Villous Morphology in Environmental Enteropathy without HIV Enteropathy: Results from a Double-Blind Randomised Placebo Controlled Trial in Zambian Adults. BMC Gastroenterol. 2014, 14, 15. [Google Scholar] [CrossRef]
- Jinga, M.; Balaban, D.V.; Peride, I.; Niculae, A.; DuŢescu, I.M.; Vasilescu, F.; Mäki, M.; Popp, A.M. Crypt Hyperplastic Enteropathy in Distal Duodenum in Helicobacter Pylori Infection—Report of Two Cases without Evidence of Celiac Disease. Rom. J. Morphol. Embryol. Rev. Roum. Morphol. Embryol. 2017, 58, 685–688. [Google Scholar]
- Savidge, T.C.; Shmakov, A.N.; Walker-Smith, J.A.; Phillips, A.D. Epithelial Cell Proliferation in Childhood Enteropathies. Gut 1996, 39, 185–193. [Google Scholar] [CrossRef][Green Version]
- Smyrk, T.C. Practical Approach to the Flattened Duodenal Biopsy. Surg. Pathol. Clin. 2017, 10, 823–839. [Google Scholar] [CrossRef] [PubMed]
- Villanacci, V. The Histological Classification of Biopsy in Celiac Disease: Time for a Change? Dig. Liver Dis. 2015, 47, 2–3. [Google Scholar] [CrossRef]
- Sali, R.; Ehsan, L.; Kowsari, K.; Khan, M.; Moskaluk, C.; Syed, S.; Brown, D. CeliacNet: Celiac Disease Severity Diagnosis on Duodenal Histopathological Images Using Deep Residual Networks. In Proceedings of the IEEE International Conference on Bioinformatics and Biomedicine, San Diego, CA, USA, 21 November 2019; Volume 2019. [Google Scholar]
- Corazza, G.R.; Villanacci, V.; Zambelli, C.; Milione, M.; Luinetti, O.; Vindigni, C.; Chioda, C.; Albarello, L.; Bartolini, D.; Donato, F. Comparison of the Interobserver Reproducibility with Different Histologic Criteria Used in Celiac Disease. Clin. Gastroenterol. Hepatol. 2007, 5, 838–843. [Google Scholar] [CrossRef]
- Singhi, A.D.; Goyal, A.; Davison, J.M.; Regueiro, M.D.; Roche, R.L.; Ranganathan, S. Pediatric Autoimmune Enteropathy: An Entity Frequently Associated with Immunodeficiency Disorders. Mod. Pathol. 2014, 27, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Leong, R.W.L.; Lawrance, I.C.; Chow, D.K.L.; To, K.-F.; Lau, J.Y.; Wu, J.; Leung, W.K.; Chan, F.K.L.; Sung, J.J.Y. Association of Intestinal Granulomas with Smoking, Phenotype, and Serology in Chinese Patients with Crohn’s Disease. Am. J. Gastroenterol. 2006, 101, 1024–1029. [Google Scholar] [CrossRef]
- Kurppa, K.; Ashorn, M.; Iltanen, S.; Koskinen, L.L.E.; Saavalainen, P.; Koskinen, O.; Mäki, M.; Kaukinen, K. Celiac Disease without Villous Atrophy in Children: A Prospective Study. J. Pediatr. 2010, 157, 373–380.e1. [Google Scholar] [CrossRef]
- Miyazaki, H.; Hoshi, N.; Kohashi, M.; Tokunaga, E.; Ku, Y.; Takenaka, H.; Ooi, M.; Yamamoto, N.; Uemura, S.; Nishimura, N.; et al. A Case of Autoimmune Enteropathy with CTLA4 Haploinsufficiency. Intest. Res. 2022, 20, 144–149. [Google Scholar] [CrossRef]
- Yazdani, R.; Moazzami, B.; Madani, S.P.; Behniafard, N.; Azizi, G.; Aflatoonian, M.; Abolhassani, H.; Aghamohammadi, A. Candidiasis Associated with Very Early Onset Inflammatory Bowel Disease: First IL10RB Deficient Case from the National Iranian Registry and Review of the Literature. Clin. Immunol. 2019, 205, 35–42. [Google Scholar] [CrossRef]
- Stern, M. Comparative Evaluation of Serologic Tests for Celiac Disease: A European Initiative toward Standardization. J. Pediatr. Gastroenterol. Nutr. 2000, 31, 513–519. [Google Scholar] [PubMed]
- Chong, A.; Kashani, A.; Ansstas, M.; Jamil, L.; Guindi, M. Seronegative Autoimmune Enteropathy with Duodenal Sparing and Colonic Clues in an Adult Female. Clin. J. Gastroenterol. 2021, 14, 546–550. [Google Scholar] [CrossRef]
- McCabe, P.; Alli-Akintade, L.; Stondell, J. Seronegative Adult Autoimmune Enteropathy in a Male Traveler. ACG Case Rep. J. 2017, 4, e19. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Jarzabek-Chorzelska, M.; Sulej, J.; Karnewska, K.; Farrell, T.; Jablonska, S. Celiac Disease and Immunoglobulin a Deficiency: How Effective Are the Serological Methods of Diagnosis? Clin. Diagn. Lab. Immunol. 2002, 9, 1295–1300. [Google Scholar] [CrossRef] [PubMed]
- Giorgio, F.; Principi, M.; Losurdo, G.; Piscitelli, D.; Iannone, A.; Barone, M.; Amoruso, A.; Ierardi, E.; Leo, A.D. Seronegative Celiac Disease and Immunoglobulin Deficiency: Where to Look in the Submerged Iceberg? Nutrients 2015, 7, 7486–7504. [Google Scholar] [CrossRef]
- Olfe, L.; von Hardenberg, S.; Hofmann, W.; Auber, B.; Baumann, U.; Beier, R.; Adriawan, I.R.; Atschekzei, F.; Witte, T.; Sogkas, G. CTLA-4 Insufficiency Due to a Novel CTLA-4 Deletion, Identified through Copy Number Variation Analysis. Int. Arch. Allergy Immunol. 2022, 184, 76–84. [Google Scholar] [CrossRef]
- Guillem, M.M.; Comas, M.E. How to Approach Screening for Celiac Disease in 2008. Gastroenterol. Hepatol. 2008, 31, 454–458. [Google Scholar]
- Lee, M.; Betman, S.; Iuga, A.; Yang, H.-M.; Fleming, J.; Green, P.H.R.; Lebwohl, B.; Lagana, S.M. An Association between Crypt Apoptotic Bodies and Mucosal Flattening in Celiac Disease Patients Exposed to Dietary Gluten. Diagn. Pathol. 2019, 14, 98. [Google Scholar] [CrossRef]
- Mårild, K.; Lebwohl, B.; Green, P.H.R.; Murray, J.A.; Ludvigsson, J.F. Blockers of Angiotensin Other Than Olmesartan in Patients with Villous Atrophy: A Nationwide Case-Control Study. Mayo Clin. Proc. 2015, 90, 730–737. [Google Scholar] [CrossRef]

| Cause | Etiology | Type | Clinical Manifestation | Histological Findings |
|---|---|---|---|---|
| Primary Causes | Autoimmune Conditions and Disorders | Autoimmune Enteropathy (AIE) | Persistent diarrhea Malabsorption Weight loss | Villous atrophy Minimal intraepithelial lymphocytosis Apoptotic bodies in crypts Absence of cup cells or Paneth cells |
| Type 1 Diabetes Mellitus (T1DM) | Malabsorption Glycemic instability Suboptimal diabetes management poor glycemic control | Lack of specific histological findings | ||
| Autoimmune Thyroiditis (Hashimoto’s Thyroiditis; Grave’s Disease) | Malabsorption Osteoporosis Anemia Dysregulated systemic metabolism and hyper/hypothyreosis symptoms | Lack of specific histological findings | ||
| Systemic Lupus Erythematosus (SLE) | Diarrhea Malabsorption Mimicry to other villous atrophy causes Serological markers associated with SLE (anti-dsDNA; anti-SM antibodies) | Lack of specific histological findings | ||
| Autoimmune Polyglandular Syndromes (APS) | Adrenal insufficiency, T1DM, autoimmune thyroiditis in APS type II Nutrient malabsorption | Lack of specific histological findings | ||
| Immunodeficiency Disorders | Common Variable Immunodeficiency Disease (CVID) | Diarrhea Weight loss Malabsorption syndrome Insufficient immunoglobulin production Recurrent infections | Lack of specific histological findings | |
| HIV/AIDS | Malabsorption Osteomalacia Anemia Hypocholesterolemia HIV/AIDS-associated infections | Enterocyte injury Partial villous atrophy Crypt hyperplasia | ||
| Infectious Etiology | Giardia lamblia | Malabsorption Diarrhea Weight loss Nutritional deficiencies | Mucosal inflammation Disruption of tight junctions in epithelial cells Crypt hypertrophy with associated atrophic changes | |
| Cryptosporidium | Very severe malabsorption | Epithelial cell death Flattening of the villi | ||
| Noroviruses | Treatment-resistant diarrhea Impaired nutrient absorption Prolonged malnutrition | Permanent villous damage | ||
| Mycobacterium tuberculosis | Impaired absorption | Granulomatous inflammation Villous destruction | ||
| Tropheryma whipplei (Whipple’s disease) | Malabsorption | Lamina propria infiltration Macrophage accumulation Flattening of the villi | ||
| Tropical Splenomegaly Syndrome (TSS) | Bacterial dysbiosis Nutritional deficiencies Chronic diarrhea Weight loss Malabsorption Splenomegaly | Villous atrophy | ||
| Secondary and Iatrogenic Causes | Drug-Induced Enteropathy | Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) | Lack of specific clinical findings | Chronic inflammation Mucosal damage Villous atrophy |
| Angiotensin Receptor Blockers (ex. Olmesartan) | Severe diarrhea Weight loss Dehydration Organ failure | Villous atrophy Lymphocytic infiltration Shortened intestinal villi Overlapping with celiac disease | ||
| Mycophenolate Mofetil (MMF) | Malabsorption Abnormal glucose absorption and chloride loss | Villous atrophy | ||
| Environmental Factors | Alcohol | Impairment of nutrient absorption Diarrhea Weight loss | Reversible villous atrophy | |
| Bacterial Toxins (ex. tropical sprue) | Bacterial dysbiosis Nutrient malabsorption Diarrhea | Villous atrophy | ||
| Dietary toxins/food additives/environmental pollutants (heavy metals, pesticides) | Lack of specific clinical findings | Villous atrophy Microbiota disruption Low-grade inflammation | ||
| Metabolic Disorders | Congenital Enteropathy | Decreased glucose and sodium absorption Severe malnutrition Dehydration | Hypoplastic villous atrophy Absence of a brush border Absence of inflammation | |
| Genetic Disorders | Hereditary alpha-tryptasemia | Chronic abdominal pain Diarrhea/constipation, Gastroesophageal reflux, Bloating Irritable bowel syndrome-like symptoms Postural orthostatic tachycardia syndrome (POTS) | Villous atrophy Increased number of mast cells |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Napiórkowska-Baran, K.; Treichel, P.; Wawrzeńczyk, A.; Alska, E.; Zacniewski, R.; Szota, M.; Przybyszewska, J.; Zoń, A.; Bartuzi, Z. Non-Celiac Villous Atrophy—A Problem Still Underestimated. Life 2025, 15, 1098. https://doi.org/10.3390/life15071098
Napiórkowska-Baran K, Treichel P, Wawrzeńczyk A, Alska E, Zacniewski R, Szota M, Przybyszewska J, Zoń A, Bartuzi Z. Non-Celiac Villous Atrophy—A Problem Still Underestimated. Life. 2025; 15(7):1098. https://doi.org/10.3390/life15071098
Chicago/Turabian StyleNapiórkowska-Baran, Katarzyna, Paweł Treichel, Adam Wawrzeńczyk, Ewa Alska, Robert Zacniewski, Maciej Szota, Justyna Przybyszewska, Amanda Zoń, and Zbigniew Bartuzi. 2025. "Non-Celiac Villous Atrophy—A Problem Still Underestimated" Life 15, no. 7: 1098. https://doi.org/10.3390/life15071098
APA StyleNapiórkowska-Baran, K., Treichel, P., Wawrzeńczyk, A., Alska, E., Zacniewski, R., Szota, M., Przybyszewska, J., Zoń, A., & Bartuzi, Z. (2025). Non-Celiac Villous Atrophy—A Problem Still Underestimated. Life, 15(7), 1098. https://doi.org/10.3390/life15071098









