Threatened Birds in a Changing Mediterranean Wetland: Long-Term Trends and Climate-Driven Threats
Abstract
1. Introduction
2. Materials and Methods
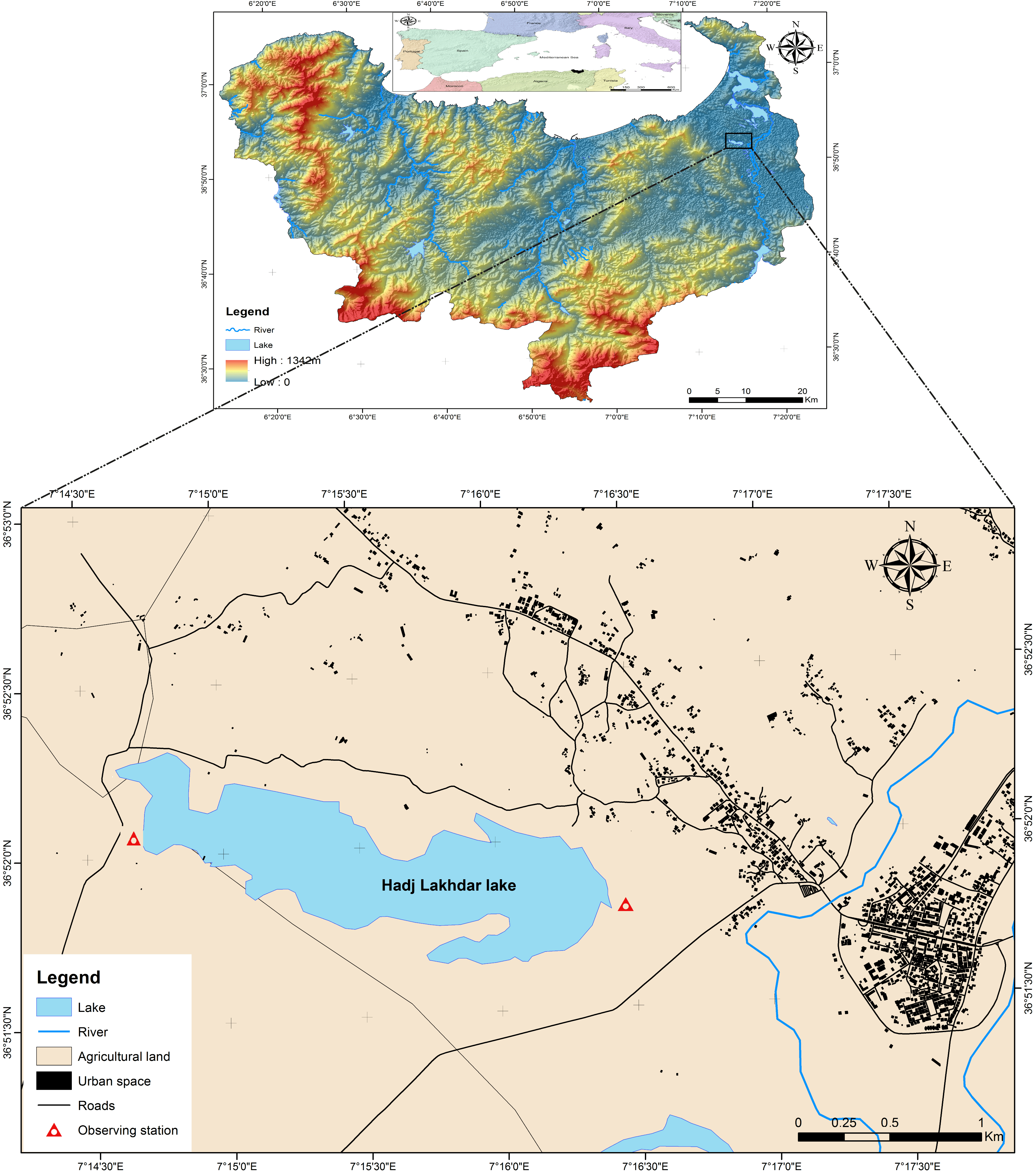
2.1. Study Area
2.2. Bird Counts
2.3. Climatic Variables
Relating Climate Variability and Population Dynamics
2.4. Vegetation Cover
2.5. Statistical Analysis
3. Results
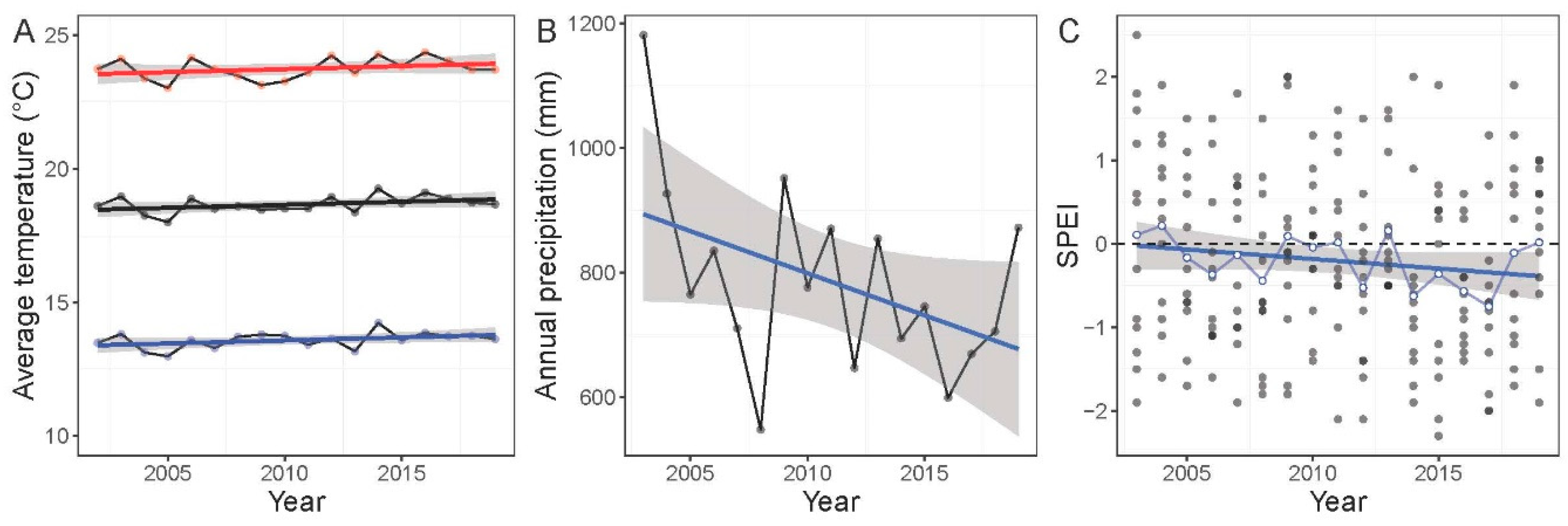
3.1. Environmental Changes
3.1.1. Climate
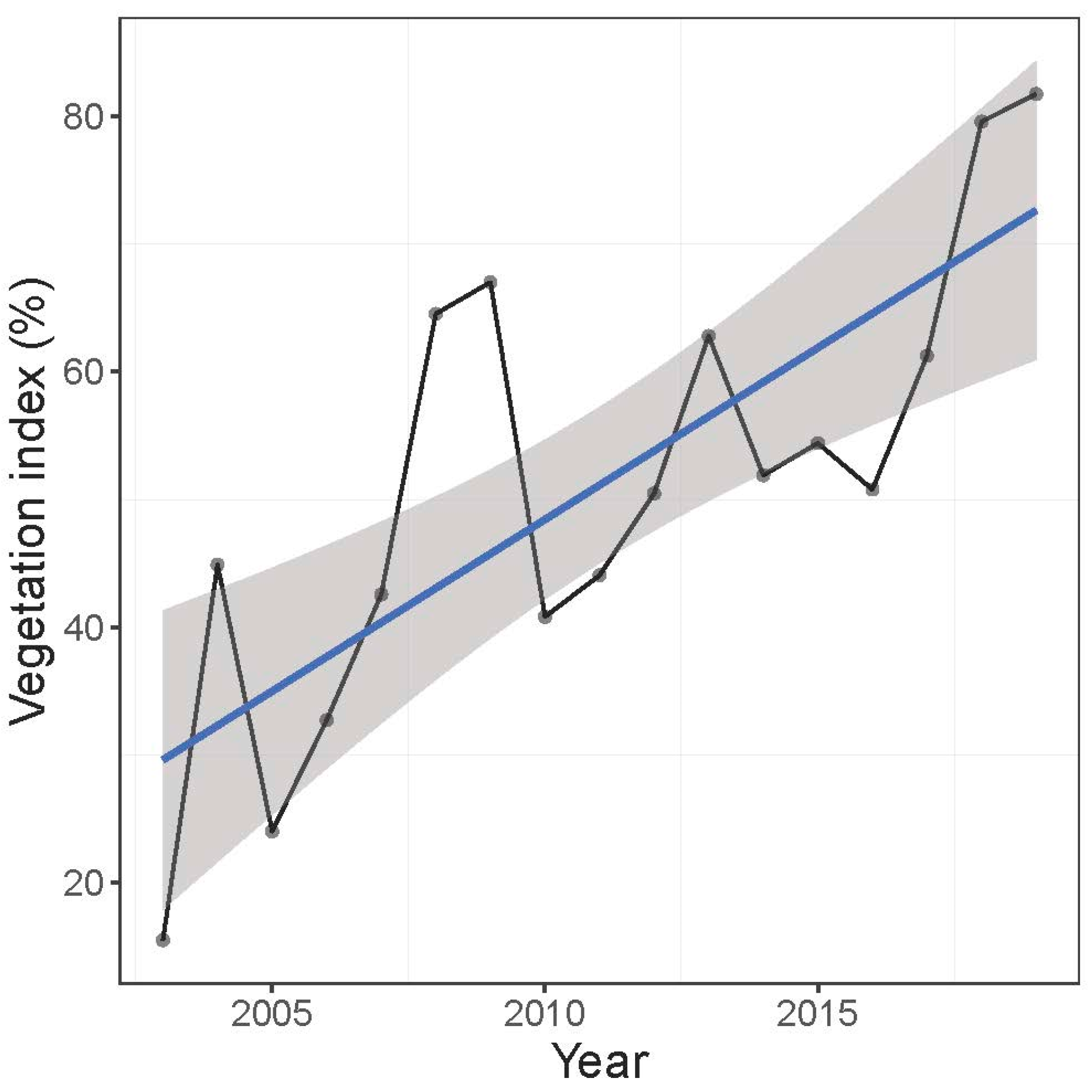
3.1.2. Vegetation Cover
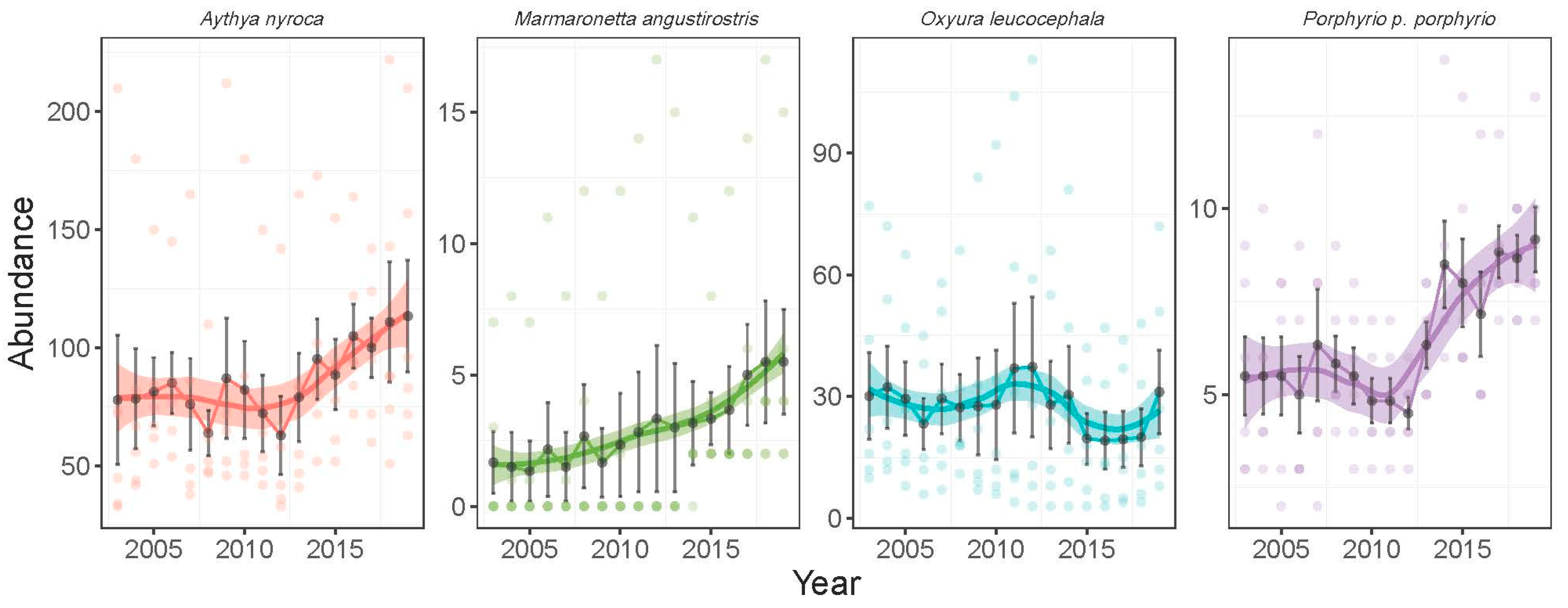
3.2. Temporal Trends of Abundance
3.2.1. Abundance During the Wintering Season
3.2.2. Abundance During the Breeding Season
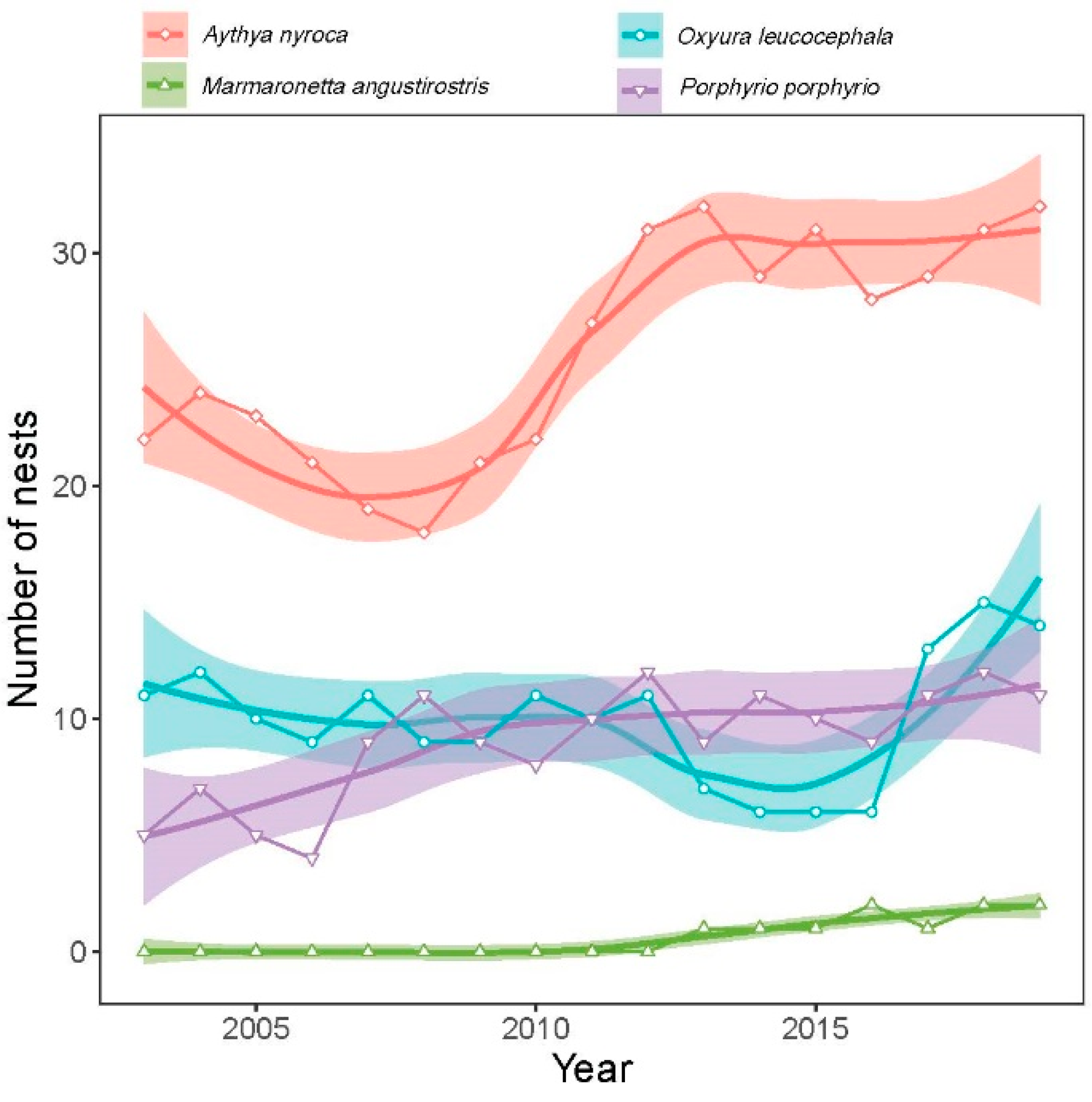
3.2.3. Abundance of Nests
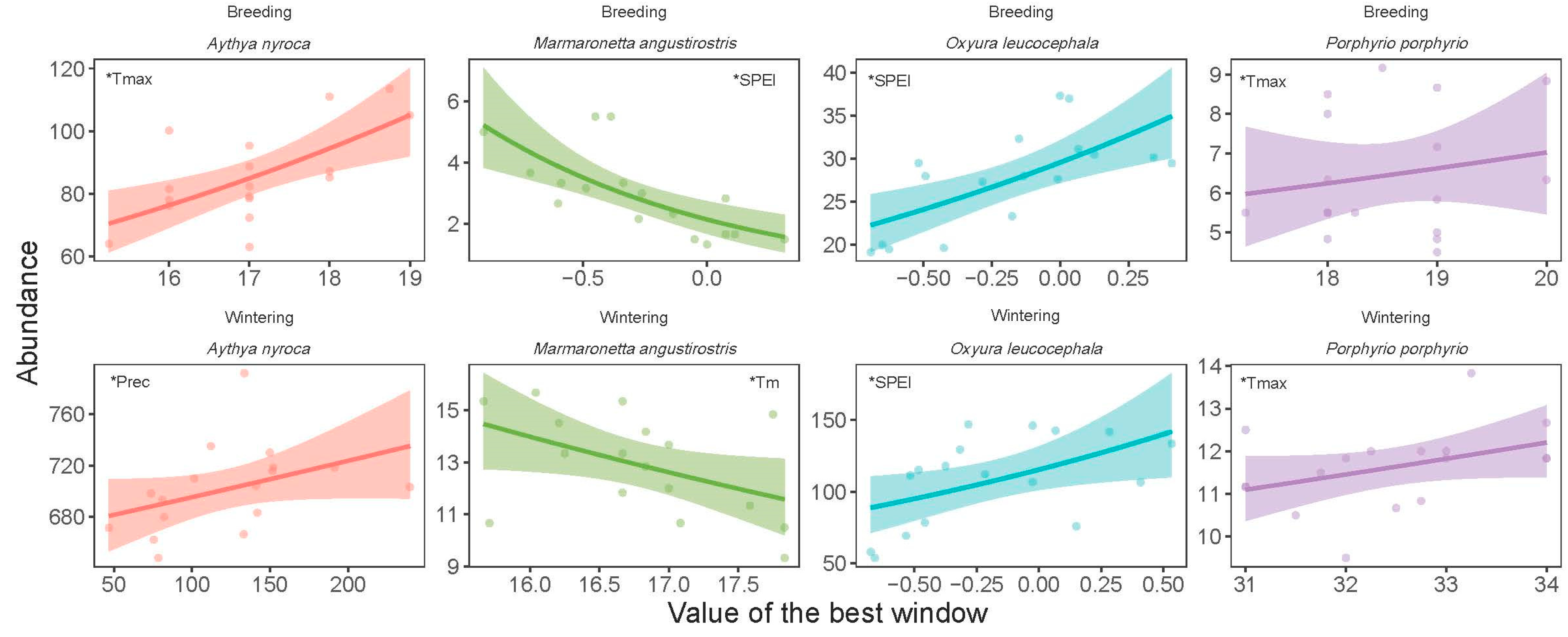
3.3. Correlation of Temporal Trends with Climate Change
4. Discussion
4.1. Vegetation Cover
4.2. Wintering Population
4.3. Breeding Population
4.4. Climate Change Influence
4.5. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davidson, N.C. How much wetland has the world lost? Long-term and recent trends in global wetland area. Mar. Freshw. Res. 2014, 65, 934–941. [Google Scholar] [CrossRef]
- Albert, J.S.; Destouni, G.; Duke-Sylvester, S.M.; Magurran, A.E.; Oberdorff, T.; Reis, R.E.; Winemiller, K.O.; Ripple, W.J. Scientists’ warning to humanity on the freshwater biodiversity crisis. AMBIO 2021, 50, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Gopal, B. Biodiversity in wetlands. Wetl. Handb. 2009, 2, 65–95. [Google Scholar]
- Tickner, D.; Opperman, J.J.; Abell, R.; Acreman, M.; Arthington, A.H.; Bunn, S.E.; Cooke, S.J.; Dalton, J.; Darwall, W.; Edwards, G.; et al. Bending the curve of global freshwater biodiversity loss: An emergency recovery plan. BioScience 2020, 70, 330–342. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Niu, Z.; Chen, Y.; Li, L.; Zhang, H. Global wetlands: Potential distribution, wetland loss, and status. Sci. Total Environ. 2017, 586, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.; Peng, S.; Ciais, P.; Chen, Y. Future impacts of climate change on inland Ramsar wetlands. Nat. Clim. Change 2021, 11, 45–51. [Google Scholar] [CrossRef]
- Anand, V.; Oinam, B. Future land use land cover prediction with special emphasis on urbanization and wetlands. Remote Sens. Lett. 2020, 11, 225–234. [Google Scholar] [CrossRef]
- Gregory, R.D.; Van Strien, A. Wild Bird Indicators: Using Composite Population Trends of Birds as Measures of Environmental Health. Ornithol. Sci. 2010, 9, 3–22. [Google Scholar] [CrossRef]
- Green, A.J.; Elmberg, J. Ecosystem services provided by waterbirds. Biol. Rev. 2014, 89, 105–122. [Google Scholar] [CrossRef]
- Lees, A.C.; Haskell, L.; Allinson, T.; Bezeng, S.B.; Burfield, I.J.; Renjifo, L.M.; Rosenberg, K.V.; Viswanathan, A.; Butchart, S.H. State of the World’s Birds. Annu. Rev. Environ. Resour. 2022, 47, 231–260. [Google Scholar] [CrossRef]
- Verniest, F.; Le Viol, I.; Julliard, R.; Dami, L.; Guelmami, A.; Suet, M.; Abdou, W.; Azafzaf, H.; Bendjedda, N.; Bino, T.; et al. Anticipating the effects of climate warming and natural habitat conversion on waterbird communities to address protection gaps. Biol. Conserv. 2023, 279, 109939. [Google Scholar] [CrossRef]
- Mokany, K.; Ware, C.; Harwood, T.D.; Schmidt, R.K.; Ferrier, S. Habitat-based biodiversity assessment for ecosystem accounting in the Murray–Darling Basin. Conserv. Biol. 2022, 36, e13915. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ouyang, Z. Focusing on rapid urbanization areas can control the rapid loss of migratory water bird habitats in China. Glob. Ecol. Conserv. 2019, 20, e00801. [Google Scholar] [CrossRef]
- Söderquist, P.; Gunnarsson, G.; Elmberg, J.; Dessborn, L. Survival of wild and farmed-released mallards: The Swedish example. Eur. J. Wildl. Res. 2021, 67, 19. [Google Scholar] [CrossRef]
- Pérez-García, J.M.; Sebastián-González, E.; Rodríguez-Caro, R.; Sanz-Aguilar, A.; Botella, F. Blind shots: Non-natural mortality counteracts conservation efforts of a threatened waterbird. Anim. Conserv. 2023, 27, 293–307. [Google Scholar] [CrossRef]
- Kleijn, D.; Cherkaoui, I.; Goedhart, P.W.; van der Hout, J.; Lammertsma, D. Waterbirds increase more rapidly in Ramsar-designated wetlands than in unprotected wetlands. J. Appl. Ecol. 2014, 51, 289–298. [Google Scholar] [CrossRef]
- Salimi, S.; Almuktar, S.A.; Scholz, M. Impact of climate change on wetland ecosystems: A critical review of experimental wetlands. J. Environ. Manag. 2021, 286, 112160. [Google Scholar] [CrossRef]
- Amano, T.; Székely, T.; Wauchope, H.S.; Sandel, B.; Nagy, S.; Mundkur, T.; Langendoen, T.; Blanco, D.; Michel, N.L.; Sutherland, W.J. Responses of global waterbird populations to climate change vary with latitude. Nat. Clim. Change 2020, 10, 959–964. [Google Scholar] [CrossRef]
- Proença, V.; Martin, L.J.; Pereira, H.M.; Fernandez, M.; McRae, L.; Belnap, J.; Böhm, M.; Brummitt, N.; García-Moreno, J.; Gregory, R.D.; et al. Global biodiversity monitoring: From data sources to Essential Biodiversity Variables. Biol. Conserv. 2017, 213, 256–263. [Google Scholar] [CrossRef]
- Gaget, E.; Le Viol, I.; Pavón-Jordán, D.; Cazalis, V.; Kerbiriou, C.; Jiguet, F.; Popoff, N.; Dami, L.; Mondain-Monval, J.; du Rau, P.D.; et al. Assessing the effectiveness of the Ramsar Convention in preserving wintering waterbirds in the Mediterranean. Biol. Conserv. 2020, 243, 108485. [Google Scholar] [CrossRef]
- Khelifa, R.; Mahdjoub, H.; Samways, M.J. Combined climatic and anthropogenic stress threaten resilience of important wetland sites in an arid region. Sci. Total Environ. 2022, 806, 150806. [Google Scholar] [CrossRef] [PubMed]
- BirdLife International. Country Profile: Algeria. Available online: http://datazone.birdlife.org/country/algeria (accessed on 30 November 2023).
- BirdLife International. Species Factsheet: Oxyura Leucocephala. Available online: https://datazone.birdlife.org/species/factsheet/white-headed-duck-oxyura-leucocephala (accessed on 7 June 2024).
- Giménez, M.; Botella, F.; Pérez-García, J.M. Cerceta Pardilla Marmaronetta angustirostris. In Libro Rojo de las Aves de España; López-Jiménez, N., Ed.; SEO/BirdLife: Madrid, Spain, 2021; pp. 178–184. [Google Scholar]
- Salvador, A.; Amat, J.A.; Green, A.J. Marbled Duck (Marmaronetta angustirostris), version 2.0. In Birds of the World; Kirwan, G.M., Keeney, B.K., Eds.; Cornell Lab of Ornithology: Ithaca, NY, USA, 2023. [Google Scholar] [CrossRef]
- BirdLife International. Species Factsheet: Ferruginous Duck Aythya Nyroca, 2019. Available online: https://datazone.birdlife.org/species/factsheet/ferruginous-duck-aythya-nyroca (accessed on 5 April 2025).
- Mouslim, B.; Eddine, M.S.; Rassim, K.; Zihad, B.; Moussa, H. Aspects of the breeding ecology of the Purple Swamphen Porphyrio porphyrio in the wetland complex of Guerbes-Sanhadja, north-east Algeria. Ostrich 2014, 85, 185–191. [Google Scholar] [CrossRef]
- Cramp, S.; Simmons, K. (Eds.) The Birds of the Western Palearctic; OUP: Oxford, UK, 1977; Volume 1. [Google Scholar]
- Anstey, S. The Status and Conservation of the White-Headed Duck Oxyura Leucocephala.; International Waterfowl and Wetlands Research Bureau: Slimbridge, UK, 1989. [Google Scholar]
- Green, A.J.; Hilton, G.M.; Hughes, B.; Fox, A.D.; Yarar, M. The Ecology and Behavior of the White-Headed Duck Oxyura Leucocephala at Burdur Gölü, Turkey; Wildfowl and Wetlands Trust: Slimbridge, UK, 1993. [Google Scholar]
- Petkov, N. The Ferruginous Duck Aythya nyroca as a potential indicator species for tracking ecological changes at the Srebarna Lake managed reserve (NE Bulgaria). Acrocephalus 2006, 27, 37–43. [Google Scholar]
- Samraoui, F.; Nedjah, R.; Alfarhan, A.H.; Samraoui, B. An overview of the Rallidae of Algeria with particular reference to the breeding ecology of the Purple Swamp-Hen Porphyrio porphyrio. Wetl. Ecol. Manag. 2015, 23, 505–517. [Google Scholar] [CrossRef]
- Fiche Descriptive Ramsar. Algérie: Complexe de Zones Humides de la Plaine de Guerbes-Sanhadja. Available online: https://rsis.ramsar.org/ris/1056 (accessed on 12 June 2019).
- Bouchaala, L.; Elafri, A.; Charchar, N.; Boukhemza, M.; Houhamdi, M. Wintering behaviour and spatial ecology of Eurasian Wigeon Anas penelope in a coastal Mediterranean wetland complex (Guerbes-Sanhadja) of northeastern Algeria. Avian Biol. Res. 2017, 10, 84–91. [Google Scholar] [CrossRef]
- Metallaoui, S.; Houhamdi, M. Données préliminaires sur l’avifaune aquatique de la Garaet Hadj-Tahar (Skikda, nord-est algérien). Bull. Afr. Bird Club 2008, 15, 71–76. [Google Scholar] [CrossRef]
- Bibby, C.J.; Jones, M.; Marsden, S. Bird Surveys; Expedition Advisory Centre: London, UK, 1998. [Google Scholar]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Vicente-Serrano; Sergio, M.; National Center for Atmospheric Research Staff (Eds.) The Climate Data Guide: Standardized Precipitation Evapotranspiration Index (SPEI). Available online: https://climatedataguide.ucar.edu/climate-data/standardized-precipitation-evapotranspiration-index-spei (accessed on 30 September 2015).
- Ushey, K. RcppRoll: Efficient Rolling/Windowed Operations. R Package Version 0.3.0. Available online: https://CRAN.R-project.org/package=RcppRoll (accessed on 25 October 2018).
- Haest, B.; Hüppop, O.; Bairlein, F. The influence of weather on avian spring migration phenology: What, where and when? Glob. Change Biol. 2018, 24, 5769–5788. [Google Scholar] [CrossRef]
- Zhou, J.; Zhou, L.; Xu, W. Diversity of wintering waterbirds enhanced by restoring aquatic vegetation at Shengjin Lake, China. Sci. Total Environ. 2020, 737, 140190. [Google Scholar] [CrossRef]
- Lantz, S.M.; Gawlik, D.E.; Cook, M.I. The effects of water depth and emergent vegetation on foraging success and habitat selection of wading birds in the Everglades. Waterbirds 2011, 34, 439–447. [Google Scholar] [CrossRef]
- Alam, A.; Bhat, M.S.; Maheen, M. Using Landsat satellite data for assessing the land use and land cover change in Kashmir valley. GeoJournal 2020, 85, 1529–1543. [Google Scholar] [CrossRef]
- QGIS Development Team. QGIS Geographic Information System. Open Source Geospatial Foundation Project. Available online: http://qgis.org (accessed on 3 February 2024).
- Ashok, A.; Rani, H.P.; Jayakumar, K. Monitoring of dynamic wetland changes using NDVI and NDWI based landsat imagery. Remote Sens. Appl. Soc. Environ. 2021, 23, 100547. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- Kleiber, C.; Zeileis, A. Applied Econometrics with R; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- John, F.; Weisberg, S. An R Companion to Applied Regression; Sage Publications: Thousand Oaks, CA, USA, 2018. [Google Scholar]
- Boussaha, A.; Bezzalla, A.; Zebsa, R.; Amari, H.; Houhamdi, M.; Chenchouni, H. Monitoring and assessment of spatial and seasonal variability in water quality at Lake of Birds (Algeria) using physicochemical parameters and bacterial quality indicators. Environ. Nanotechnol. Monit. Manag. 2024, 22, 100955. [Google Scholar] [CrossRef]
- Loucif, K.; Neffar, S.; Menasria, T.; Maazi, M.C.; Houhamdi, M.; Chenchouni, H. Physico-chemical and bacteriological quality assessment of surface water at Lake Tonga in Algeria. Environ. Nanotechnol. Monit. Manag. 2020, 13, 100284. [Google Scholar] [CrossRef]
- Chen, C.; Park, T.; Wang, X.; Piao, S.; Xu, B.; Chaturvedi, R.K.; Fuchs, R.; Brovkin, V.; Ciais, P.; Fensholt, R.; et al. China and India lead in greening of the world through land-use management. Nat. Sustain. 2019, 2, 122–129. [Google Scholar] [CrossRef]
- Zhu, Z.; Piao, S.; Myneni, R.B.; Huang, M.; Zeng, Z.; Canadell, J.G.; Ciais, P.; Sitch, S.; Friedlingstein, P.; Arneth, A.; et al. Greening of the Earth and its drivers. Nat. Clim. Change 2016, 6, 791–795. [Google Scholar] [CrossRef]
- Zhao, L.; Dai, A.; Dong, B. Changes in global vegetation activity and its driving factors during 1982–2013. Agric. For. Meteorol. 2018, 249, 198–209. [Google Scholar] [CrossRef]
- Eid, A.N.M.; Olatubara, C.; Ewemoje, T.; Farouk, H.; El-Hennawy, M.T. Coastal wetland vegetation features and digital Change Detection Mapping based on remotely sensed imagery: El-Burullus Lake, Egypt. Int. Soil Water Conserv. Res. 2020, 8, 66–79. [Google Scholar]
- Zhang, M.; Lin, H.; Long, X.; Cai, Y. Analyzing the spatiotemporal pattern and driving factors of wetland vegetation changes using 2000-2019 time-series Landsat data. Sci. Total Environ. 2021, 780, 146615. [Google Scholar] [CrossRef]
- Mu, S.; Li, B.; Yao, J.; Yang, G.; Wan, R.; Xu, X. Monitoring the spatio-temporal dynamics of the wetland vegetation in Poyang Lake by Landsat and MODIS observations. Sci. Total Environ. 2020, 725, 138096. [Google Scholar] [CrossRef]
- Magnússon, R.Í.; Limpens, J.; Kleijn, D.; van Huissteden, K.; Maximov, T.C.; Lobry, S.; Heijmans, M.M. Shrub decline and expansion of wetland vegetation revealed by very high resolution land cover change detection in the Siberian lowland tundra. Sci. Total Environ. 2021, 782, 146877. [Google Scholar] [CrossRef]
- Lind, L.; Eckstein, R.L.; Relyea, R.A. Direct and indirect effects of climate change on distribution and community composition of macrophytes in lentic systems. Biol. Rev. 2022, 97, 1677–1690. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Piao, S.; Ciais, P.; Myneni, R.B.; Chen, A.; Chevallier, F.; Dolman, A.J.; Janssens, I.A.; Peñuelas, J.; Zhang, G.; et al. Asymmetric effects of daytime and night-time warming on Northern Hemisphere vegetation. Nature 2013, 501, 88–92. [Google Scholar] [CrossRef]
- Wooliver, R.; Vtipilthorpe, E.; Wiegmann, A.M.; Sheth, S.N. A viewpoint on ecological and evolutionary study of plant thermal performance curves in a warming world. AoB Plants 2022, 14, plac016. [Google Scholar] [CrossRef]
- Kankaala, P.; Ojala, A.; Tulonen, T.; Haapamäki, J.; Arvola, L. Response of littoral vegetation on climate warming in the boreal zone; an experimental simulation. Aquat. Ecol. 2000, 34, 433–444. [Google Scholar] [CrossRef]
- Xiong, Y.; Mo, S.; Wu, H.; Qu, X.; Liu, Y.; Zhou, L. Influence of human activities and climate change on wetland landscape pattern—A review. Sci. Total Environ. 2023, 879, 163112. [Google Scholar] [CrossRef]
- Amat, J.A.; Sanchez, A. Biología y Ecología de la Malvasïa (Oxyura leucocephala) en Andalucía. Doñana Acta Vertebr. 1982, 9, 251–320. [Google Scholar]
- Chettibi, F.; Khelifa, R.; Aberkane, M.; Bouslama, Z.; Houhamdi, M. Diurnal activity budget and breeding ecology of the White-headed Duck Oxyura leucocephala at Lake Tonga (North-east Algeria). Zool. Ecol. 2013, 23, 183–190. [Google Scholar] [CrossRef]
- Loyn, R.H.; Rogers, D.I.; Swindley, R.J.; Menkhorst, P.W.; Stamation, K.; Haynes, S.; Graham, H.; Hepworth, G.; Steele, W.K. Waterfowl populations decline with nutrient reduction and increase with nutrient restoration: 20 years of adaptive management at a Ramsar-listed wastewater treatment plant. Hydrobiologia 2023, 850, 4127–4147. [Google Scholar] [CrossRef]
- Sánchez, M.I.; Green, A.J.; Dolz, J.C. The diets of the White-headed Duck Oxyura leucocephala, Ruddy Duck O. jamaicensis and their hybrids from Spain. Bird Study 2000, 47, 275–284. [Google Scholar] [CrossRef]
- Boudraa, W.; Bouslama, Z.; Houhamdi, M. Waterbird inventory and monitoring surveys of Boussedra Marsh (Annaba, northeast Algeria). Bull. De La Société Zool. De France 2014, 139, 281–295. [Google Scholar]
- Aissaoui, R.; Tahar, A.; Saheb, M.; Guergueb, L.; Houhamdi, M. Diurnal behaviour of Ferruginous Duck Aythya nyroca wintering at the El-Kala wetlands (northeast Algeria). Bull. De L’institut Sci. Rabat Sect. Sci. De La Vie 2011, 33, 67–75. [Google Scholar]
- Ouassou, A.; Dakki, M.; Lahrouz, S.; El Agbani, M.A.; Qninba, A. Status and Trends of the Ferruginous Duck’s (Aythya nyroca) Wintering Population in Morocco: Analysis of 35 Years of Winter Census Data (1983-2017). Int. J. Zool. 2018, 2018, 5767194. [Google Scholar] [CrossRef]
- Mouslim, B.; Seyf-Eddine, M.; Zihad, B.; Moussa, H. Biodiversity and Phenology of the Rallidae and the Anatidae in Garaet Hadj Tahar (Norteast of Algeria). Ann. Biol. Res. 2013, 4, 249–253. [Google Scholar]
- Draidi, K.; Djemadi, I.; Bakhouche, B.; Narsis, S.; Bouslama, Z.; Moussouni, A.; Tiar, G. A multi-year survey on aquatic avifauna consolidates the eligibility of a small significant peri-urban wetland in northeast Algeria (Boussedra marsh) to be included on the Important Bird Areas network. Wetl. Ecol. Manag. 2023, 31, 629–648. [Google Scholar] [CrossRef]
- Bouzegag, A.; Saheb, M.; Bensaci, E.; Nouidjem, Y.; Houhamdi, M. Ecologie de la Sarcelle Marbrée Marmaronetta angustirostris (Ménétries, 1832) dans l’éco-complexe de zones humides de la vallée de l’oued Righ (Sahar Algérien). Bull. De L’institut Sci. Rabat Sect. Sci. De La Vie 2013, 35, 141–149. [Google Scholar]
- Nouidjem, Y.; Mimeche, F.; Bensaci, E.; Merouani, S.; Arar, A.; Saheb, M. Check list of waterbirds at Wadi Djedi in Ziban Oasis-Algeria. Arx. De Miscel·Lània Zoològica 2019, 17, 34–43. [Google Scholar] [CrossRef]
- Ouassou, A.; Dakki, M.; El Agbani, M.A.; Qninba, A.; El Hamoumi, R.H. Distribution and Numbers of Three Globally Threatened Waterbird Species Wintering in Morocco: The Common Pochard, Marbled Teal, and White-Headed Duck. Int. J. Zool. 2021, 2021, 8846203. [Google Scholar] [CrossRef]
- Austin, G.E.; Rehfisch, M.M. Shifting nonbreeding distributions of migratory fauna in relation to climatic change. Glob. Change Biol. 2005, 11, 31–38. [Google Scholar] [CrossRef]
- Sánchez-Lafuente, A.M.; Rey, P.; Valera, F.; Muñoz-Cobo, J. Past and current distribution of the purple swamphen Porphyrio porphyrio L. in the Iberian Peninsula. Biol. Conserv. 1992, 61, 23–30. [Google Scholar] [CrossRef]
- Cherkaoui, S.I.; Magri, N.; Hanane, S. Factors predicting Ramsar site occupancy by threatened waterfowl: The case of the Marbled Teal Marmaronetta angustirostris and Ferruginous Duck Aythya nyroca in Morocco. Ardeola 2016, 63, 295–309. [Google Scholar] [CrossRef]
- Green, A.J. Habitat selection by the Marbled Teal Marmaronetta angustirostris, Ferruginous Duck Aythya nyroca and other ducks in the Göksu Delta, Turkey, in summer. Rev. D’ecologie Terre Et Vie 1998, 53, 225–243. [Google Scholar] [CrossRef]
- Lazli, A.; Boumezbeur, A.; Moali-Grine, N.; Moali, A. Évolution de la population nicheuse de l’Érismature à tête blanche Oxyura leucocephala sur le lac Tonga (Algérie). Rev. D’ecologie Terre Et Vie 2011, 66, 173–181. [Google Scholar] [CrossRef]
- Amine, H.M.; Rabah, Z.; Zinette, B.; Abdeldjalil, Y.; Mouslim, B.; Sadek, A.; Mohammed, D.; Menouar, S.; Moussa, H. Abundance and diurnal time activity budget of the threatened species white-headed ducks (Anatidae: Oxyura leucocephala) in an unprotected area, Boussedra Marsh, Northeast Algeria. Ekológia 2021, 40, 384–391. [Google Scholar] [CrossRef]
- Petkov, N. Ferruginous duck Aythya nyroca breeding population development and habitat selection at Durankulak Lake, Bulgaria. Acrocephalus 2003, 24, 87–96. [Google Scholar]
- Loucif, K.; Maazi, M.C.; Houhamdi, M.; Chenchouni, H. Nest site selection and breeding ecology of the Ferruginous Duck (Aythya nyroca) in Algeria. Glob. Ecol. Conserv. 2021, 26, e01524. [Google Scholar] [CrossRef]
- Pacheco, C.; McGregor, P.K. Conservation of the purple gallinule (Porphyrio porphyrio L.) in Portugal: Causes of decline, recovery and expansion. Biol. Conserv. 2004, 119, 115–120. [Google Scholar] [CrossRef]
- Sánchez-Lafuente, A.M.; Valera, F.; Godino, A.; Muela, F. Natural and human-mediated factors in the recovery and subsequent expansion of the Purple swamphen Porphyrio porphyrio L. (Rallidae) in the Iberian Peninsula. Biodivers. Conserv. 2001, 10, 851–867. [Google Scholar] [CrossRef]
- Pranty, B. Population growth, spread, and persistence of Purple Swamphens (Porphyrio porphyrio) in Florida. Fla. Field Nat. 2012, 40, 1–40. [Google Scholar]
- Anderson, A.M.; Friis, C.; Gratto-Trevor, C.L.; Harris, C.M.; Love, O.P.; Morrison, R.G.; Prosser, S.W.; Nol, E.; Smith, P.A. Drought at a coastal wetland affects refuelling and migration strategies of shorebirds. Oecologia 2021, 197, 661–674. [Google Scholar] [CrossRef]
- Çolak, M.A.; Öztaş, B.; Özgencil, İ.K.; Soyluer, M.; Korkmaz, M.; Ramírez-García, A.; Metin, M.; Yılmaz, G.; Ertuğrul, S.; Tavşanoğlu, Ü.N.; et al. Increased Water Abstraction and Climate Change Have Substantial Effect on Morphometry, Salinity, and Biotic Communities in Lakes: Examples from the Semi-Arid Burdur Basin (Turkey). Water 2022, 14, 1241. [Google Scholar] [CrossRef]
- Causarano, F.; Battisti, C.; Sorace, A. Effect of winter water stress on the breeding bird assemblage of a remnant wetland in Central Italy. Rev. D’ecologie Terre Et Vie 2009, 64, 61–72. [Google Scholar] [CrossRef]
- Petrie, M.J.; Fleskes, J.P.; Wolder, M.A.; Isola, C.R.; Yarris, G.S.; Skalos, D.A. Potential effects of drought on carrying capacity for wintering waterfowl in the Central Valley of California. J. Fish Wildl. Manag. 2016, 7, 408–422. [Google Scholar] [CrossRef]
- Chambers, L.E.; Loyn, R.H. The influence of climate variability on numbers of three waterbird species in Western Port, Victoria, 1973–2002. Int. J. Biometeorol. 2006, 50, 292–304. [Google Scholar] [CrossRef]
- Kahara, S.N.; Skalos, D.; Madurapperuma, B.; Hernandez, K. Habitat quality and drought effects on breeding mallard and other waterfowl populations in California, USA. J. Wildl. Manag. 2022, 86, e22133. [Google Scholar] [CrossRef]
- Jitariu, V.; Dorosencu, A.; Ichim, P.; Ion, C. Severe Drought Monitoring by Remote Sensing Methods and Its Impact on Wetlands Birds Assemblages in Nuntași and Tuzla Lakes (Danube Delta Biosphere Reserve). Land 2022, 11, 672. [Google Scholar] [CrossRef]
- González-Gajardo, A.; Sepúlveda, P.V.; Schlatter, R. Waterbird assemblages and habitat characteristics in wetlands: Influence of temporal variability on species-habitat relationships. Waterbirds 2009, 32, 225–233. [Google Scholar] [CrossRef]
- Bouali, N.; Baaloudj, A.; Touarfia, M.; Houhamdi, I.; Maazi, M.C.; Houhamdi, M. Ecology, phenology and wintering behavior of Anatidae in the wetlands of Souk–Ahras (north–eastern Algeria). Arx. De Miscel. Lània Zoològica 2021, 19, 135–149. [Google Scholar] [CrossRef]
- Petkov, N. Habitat characteristics assessment of the wetlands with breeding Ferruginous Duck Aythya nyroca and Pochard A. ferina in Bulgaria. Acrocephalus 2011, 32, 127–134. [Google Scholar] [CrossRef][Green Version]
- Daneshvar, F.; Nejadhashemi, A.P.; Herman, M.R.; Abouali, M. Response of benthic macroinvertebrate communities to climate change. Ecohydrol. Hydrobiol. 2017, 17, 63–72. [Google Scholar] [CrossRef]
- Daneshvar, F.; Nejadhashemi, A.P.; Herman, M.R.; Abouali, M. Impacts of environmental factors on over-wintering aquatic bird communities in yamzho yumco lake, china. Sustainability 2023, 16, 254. [Google Scholar] [CrossRef]
- Pavón-Jordán, D.; Santangeli, A.; Lehikoinen, A. Effects of flyway-wide weather conditions and breeding habitat on the breeding abundance of migratory boreal waterbirds. J. Avian Biol. 2017, 48, 988–996. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, W.; Zeng, Q.; Tian, H.; Jia, Y.; Lei, G.; Wen, L. Lake productivity and waterbird functional diversity across geographic and environmental gradients in temperate china. Ecol. Evol. 2020, 10, 11237–11250. [Google Scholar] [CrossRef]
- Li, X.; Zeng, Q.; Lei, G.; Sun, G. Effects of meteorological factors on waterbird functional diversity and community composition in liaohe estuary, china. Int. J. Environ. Res. Public Health 2022, 19, 5392. [Google Scholar] [CrossRef]
- Gaget, E.; Johnston, A.; Pavón-Jordán, D.; Lehikoinen, A.S.; Sandercock, B.K.; Soultan, A.; Božič, L.; Clausen, P.; Devos, K.; Domsa, C.; et al. Protected area characteristics that help waterbirds respond to climate warming. Conserv. Biol. 2022, 36, e13877. [Google Scholar] [CrossRef]
- Gaget, E.; Pavón-Jordán, D.; Johnston, A.; Lehikoinen, A.; Hochachka, W.M.; Sandercock, B.K.; Soultan, A.; Azafzaf, H.; Bendjedda, N.; Bino, T.; et al. Benefits of protected areas for nonbreeding waterbirds adjusting their distributions under climate warming. Conserv. Biol. 2021, 35, 834–845. [Google Scholar] [CrossRef]
- Mukherjee, A.; Pal, S.; Adhurya, S.; Mukhopadhyay, S.K. Guanotrophy: Waterbirds Pay for Using Resources at Their Wintering Habitats. Integr. Conserv. 2024, 3, 382–397. [Google Scholar] [CrossRef]
- Rush, S.A.; Verkoeyen, S.; Dobbie, T.; Dobbyn, S.; Hebert, C.E.; Gagnon, J.; Fisk, A.T. Influence of increasing populations of Double-crested Cormorants on soil nutrient characteristics of nesting islands in western Lake Erie. J. Great Lakes Res. 2011, 37, 305–309. [Google Scholar] [CrossRef]
- Batanero, G.L.; León-Palmero, E.; Li, L.; Green, A.J.; Rendón-Martos, M.; Suttle, C.A.; Reche, I. Flamingos and drought as drivers of nutrients and microbial dynamics in a saline lake. Sci. Rep. 2017, 7, 12173. [Google Scholar] [CrossRef]
- Li, C.; Zhang, Y.; Zha, D.; Yang, S.; Huang, Z.Y.X.; de Boer, W.F. Assembly processes of waterbird communities across subsidence wetlands in China: A functional and phylogenetic approach. Divers. Distrib. 2019, 25, 1118–1129. [Google Scholar] [CrossRef]
- Roberts, A.J.; Conover, M.R. Nutrient Recycling by Eared Grebes in the Great Salt Lake. West. N. Am. Nat. 2016, 76, 281–286. [Google Scholar] [CrossRef]
- Almeida, B.A.; Sebastián-González, E.; dos Anjos, L.; Green, A.J. Comparing the diversity and composition of waterbird functional traits between natural, restored, and artificial wetlands. Freshw. Biol. 2020, 65, 2196–2210. [Google Scholar] [CrossRef]
- Soriano-Redondo, A.; Inger, R.; Sherley, R.B.; Rees, E.C.; Abadi, F.; McElwaine, G.; Colhoun, K.; Einarsson, O.; Thorstensen, S.; Newth, J.; et al. Demographic rates reveal the benefits of protected areas in a long-lived migratory bird. Proc. Natl. Acad. Sci. USA 2023, 120, e2212035120. [Google Scholar] [CrossRef]
- Dowd, W.W.; King, F.A.; Denny, M.W. Thermal variation, thermal extremes and the physiological performance of individuals. J. Exp. Biol. 2015, 218, 1956–1967. [Google Scholar] [CrossRef]
- Brunner, L.; Voigt, A. Pitfalls in diagnosing temperature extremes. Nat. Commun. 2024, 15, 2087. [Google Scholar] [CrossRef]
- Cohen, J.M.; Fink, D.; Zuckerberg, B. Extreme winter weather disrupts bird occurrence and abundance patterns at geographic scales. Ecography 2021, 44, 1143–1155. [Google Scholar] [CrossRef]
- Olin, A.B.; Dück, L.; Berglund, P.A.; Karlsson, E.; Bohm, M.; Olsson, O.; Hentati-Sundberg, J. Breeding failures and reduced nest attendance in response to heat stress in a high-latitude seabird. Mar. Ecol. Prog. Ser. 2023, 737, 147–160. [Google Scholar] [CrossRef]
- McKinney, R.R.A.; McWilliams, S.T.R. A new model to estimate daily energy expenditure for wintering waterfowl. Wilson Bull. 2005, 117, 44–55. [Google Scholar] [CrossRef]
- Londe, D.W.; Elmore, R.D.; Davis, C.A.; Fuhlendorf, S.D.; Hovick, T.J.; Luttbeg, B.; Rutledge, J. Fine-scale habitat selection limits trade-offs between foraging and temperature in a grassland bird. Behav. Ecol. 2021, 32, 625–637. [Google Scholar] [CrossRef]
- Zuckerberg, B.; Bonter, D.N.; Hochachka, W.M.; Koenig, W.D.; DeGaetano, A.T.; Dickinson, J.L. Climatic constraints on wintering bird distributions are modified by urbanization and weather. J. Anim. Ecol. 2011, 80, 403–413. [Google Scholar] [CrossRef]

| Season | Species | Corr.Coef | p | Variable | Window Duration (Month) | Window Location |
|---|---|---|---|---|---|---|
| Wintering | Aythya nyroca | 0.580 | 0.015 | Prec | 2 | 10 |
| Marmaronetta angustirostris | −0.530 | 0.029 | Tmean | 3 | 12 | |
| Oxyura leucocephala | 0.517 | 0.036 | SPEI | 12 | 4 | |
| Porphyrio p. porphyrio | 0.599 | 0.011 | Tmax | 2 | 8 | |
| Breeding | Aythya nyroca | 0.609 | 0.009 | Tmax | 1 | 12 |
| Marmaronetta angustirostris | −0.747 | 0.001 | SPEI | 8 | 7 | |
| Oxyura leucocephala | 0.719 | 0.001 | SPEI | 12 | 3 | |
| Porphyrio p. porphyrio | 0.584 | 0.014 | Tmax | 12 | 1 |
| Species | Season | Term | Estimate | LCI | UCI | Std. Error | Statistic | p |
|---|---|---|---|---|---|---|---|---|
| Aythya nyroca | Wintering | Intercept | 6.505 | 6.440 | 6.560 | 0.031 | 211.859 | <0.0001 |
| Climate [P] | 0.0001 | −0.0001 | 0.001 | 0.0001 | 1.713 | 0.107 | ||
| Breeding | Intercept | 2.812 | 1.710 | 3.910 | 0.561 | 5.016 | 0.0002 | |
| Climate [Tmax] | 0.088 | 0.020 | 0.156 | 0.035 | 2.532 | 0.024 | ||
| Vegetation | 0.003 | −0.001 | 0.007 | 0.002 | 1.288 | 0.218 | ||
| Oxyura leucocephala | Wintering | Intercept | 4.748 | 4.610 | 4.880 | 0.067 | 70.855 | <0.0001 |
| Climate [SPEI] | 0.387 | 0.053 | 0.718 | 0.170 | 2.282 | 0.037 | ||
| Breeding | Intercept | 3.323 | 3.070 | 3.570 | 0.128 | 25.981 | <0.0001 | |
| Climate [SPEI] | 0.446 | 0.170 | 0.723 | 0.141 | 3.164 | 0.007 | ||
| Vegetation | 0.001 | −0.004 | 0.006 | 0.003 | 0.539 | 0.598 | ||
| Porphyrio porphyrio | Wintering | Intercept | 0.564 | −1.020 | 2.160 | 0.812 | 0.695 | 0.498 |
| Climate [Tmax] | 0.058 | 0.009 | 0.107 | 0.025 | 2.329 | 0.034 | ||
| Breeding | Intercept | −3.428 | −8.660 | 1.790 | 2.665 | −1.286 | 0.219 | |
| Climate [Tmax] | 0.207 | −0.015 | 0.430 | 0.114 | 1.824 | 0.090 | ||
| Vegetation | 0.007 | 0.002 | 0.013 | 0.003 | 2.611 | 0.021 | ||
| Marmaronetta angustirostris | Wintering | Intercept | 4.488 | 2.940 | 6.030 | 0.789 | 5.691 | 0.0001 |
| Climate [Tm] | −0.105 | −0.196 | −0.013 | 0.047 | −2.246 | 0.041 | ||
| Vegetation | −0.003 | −0.007 | 0.000 | 0.002 | −1.918 | 0.076 | ||
| Breeding | Intercept | 0.070 | −0.330 | 0.455 | 0.200 | 0.349 | 0.732 | |
| Climate [SPEI] | −0.683 | −1.050 | −0.315 | 0.188 | −3.632 | 0.003 | ||
| Vegetation | 0.015 | 0.007 | 0.022 | 0.004 | 4.016 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouregbi, I.; Bensakhri, Z.; Zebsa, R.; Zouaimia, A.; Bensouilah, S.; Bouteraa, O.; Khelifa, R.; Ouakid, M.L.; Mahdjoub, H.; Houhamdi, M. Threatened Birds in a Changing Mediterranean Wetland: Long-Term Trends and Climate-Driven Threats. Life 2025, 15, 892. https://doi.org/10.3390/life15060892
Bouregbi I, Bensakhri Z, Zebsa R, Zouaimia A, Bensouilah S, Bouteraa O, Khelifa R, Ouakid ML, Mahdjoub H, Houhamdi M. Threatened Birds in a Changing Mediterranean Wetland: Long-Term Trends and Climate-Driven Threats. Life. 2025; 15(6):892. https://doi.org/10.3390/life15060892
Chicago/Turabian StyleBouregbi, Imane, Zinette Bensakhri, Rabah Zebsa, Abdelheq Zouaimia, Soufyane Bensouilah, Oualid Bouteraa, Rassim Khelifa, Mohamed Laid Ouakid, Hayat Mahdjoub, and Moussa Houhamdi. 2025. "Threatened Birds in a Changing Mediterranean Wetland: Long-Term Trends and Climate-Driven Threats" Life 15, no. 6: 892. https://doi.org/10.3390/life15060892
APA StyleBouregbi, I., Bensakhri, Z., Zebsa, R., Zouaimia, A., Bensouilah, S., Bouteraa, O., Khelifa, R., Ouakid, M. L., Mahdjoub, H., & Houhamdi, M. (2025). Threatened Birds in a Changing Mediterranean Wetland: Long-Term Trends and Climate-Driven Threats. Life, 15(6), 892. https://doi.org/10.3390/life15060892







