Long COVID Mechanisms, Microvascular Effects, and Evaluation Based on Incidence
Abstract
1. Introduction
2. Long COVID and Microcirculation
3. Proposed Long COVID Pathophysiological Mechanisms
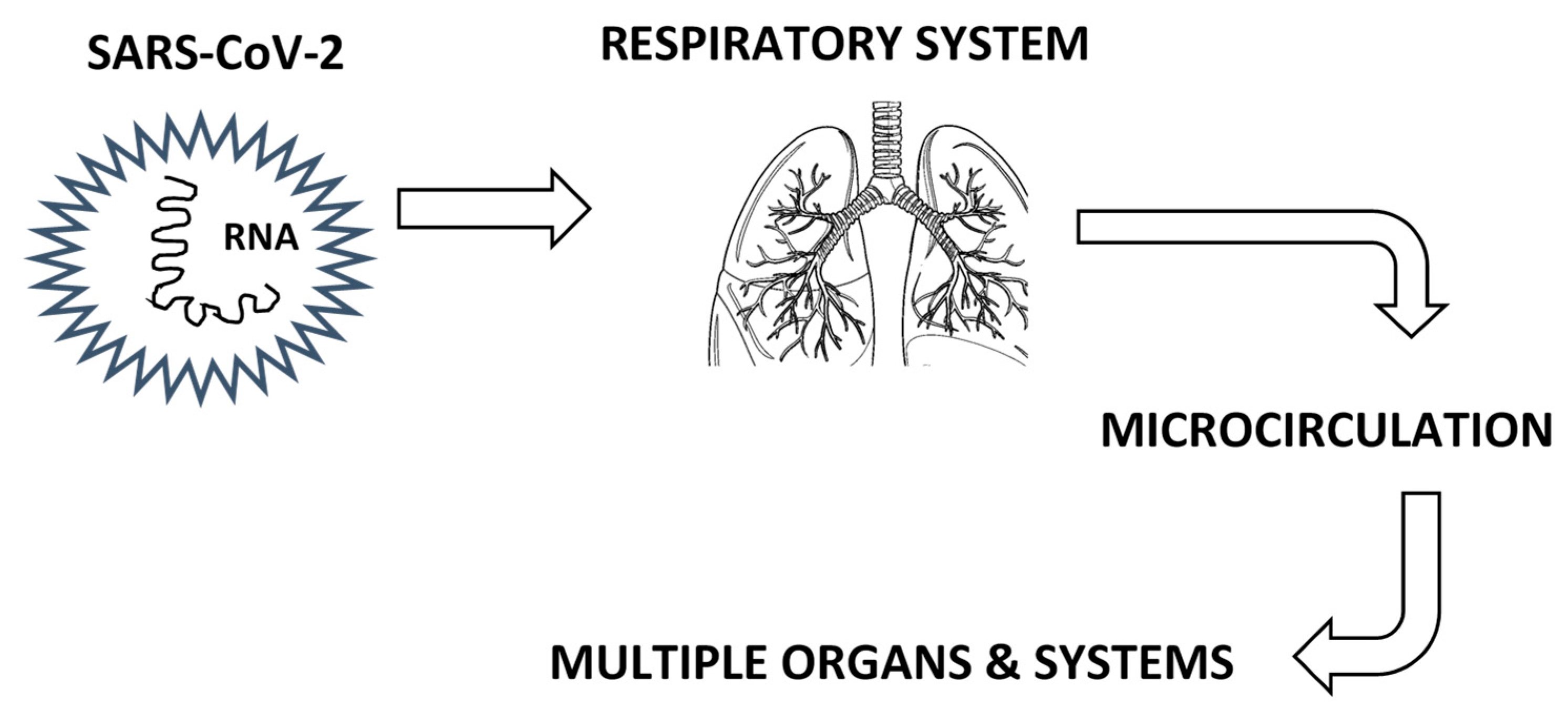
3.1. Respiratory System
3.2. Immune System
3.3. Viral Persistence
3.4. Nervous System
3.5. Gastrointestinal System
3.6. Cardiovascular System and Microcirculation
3.6.1. In Vitro Studies and Blood Coagulation
3.6.2. The Endothelium
3.6.3. Microcirculation
3.6.4. Heart and Large Vessels
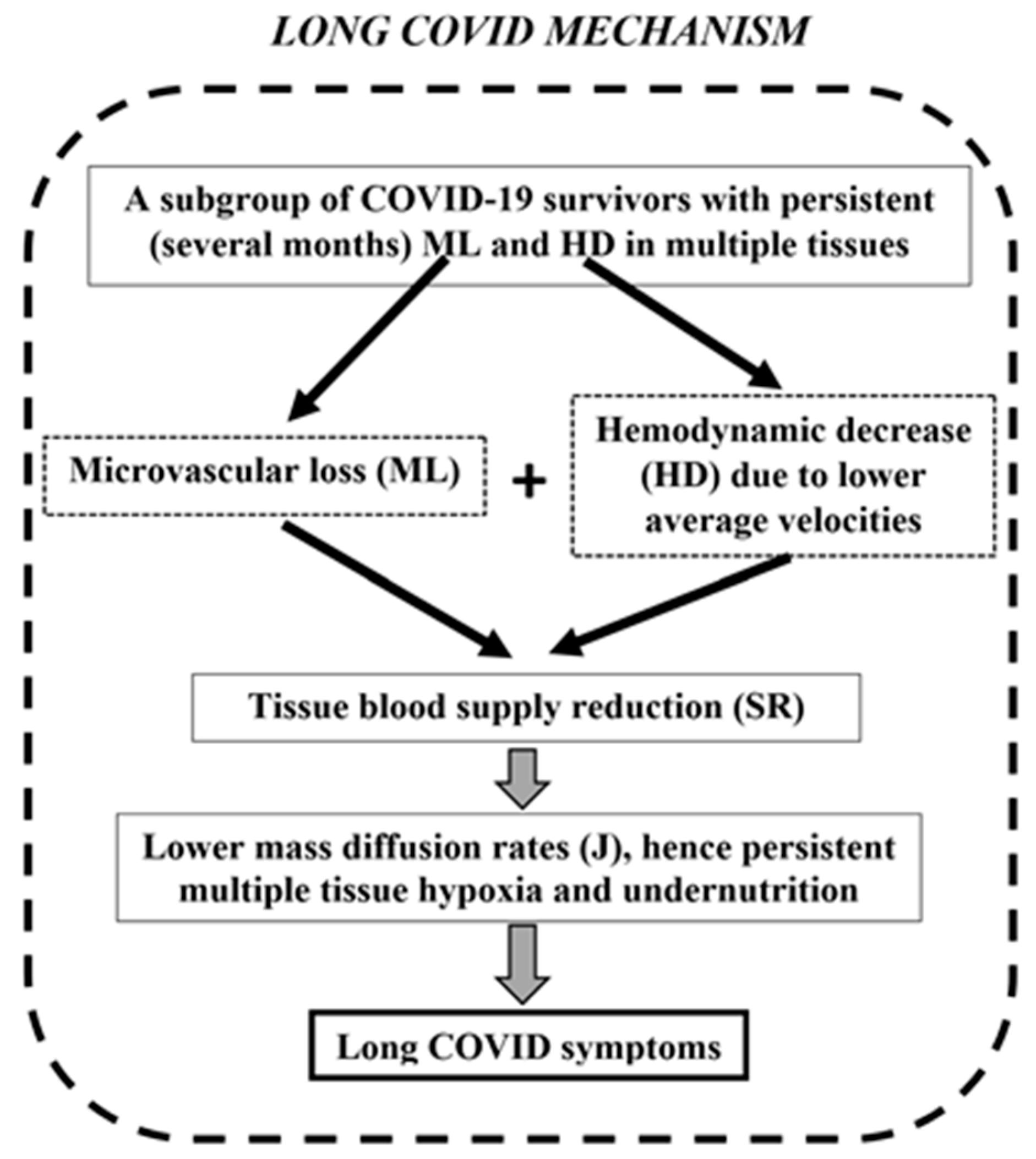
4. SR Mechanism Evaluation Based on Long COVID Symptom Incidence
5. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- WHO. COVID-19 Epidemiological Update, Edition 175; World Health Organization: Geneva, Switzerland, 2025. [Google Scholar]
- Aldous, C.; Gkioulekas, E.; Oldfield, P. Controversies in the Pandemic; Varon, J., Marik, P.E., Rendell, M., Iglesias, J., de Souza, C., Prabhudesai, P., Eds.; Jaypee Brothers Medical Publishers: New Delhi, India, 2024; ISBN 978-93-5696-730-4. [Google Scholar]
- Machado, N.R.; Dias, K.T.; Cortes, B.F.S.; Rodrigues, S.F. Effect of Coronaviruses on Blood Vessel Permeability: Potential Therapeutic Targets. Ther. Adv. Respir. Dis. 2023, 17, 17534666231162252. [Google Scholar] [CrossRef]
- Zlojutro, B.; Jandric, M.; Momcicevic, D.; Dragic, S.; Kovacevic, T.; Djajic, V.; Stojiljkovic, M.P.; Skrbic, R.; Djuric, D.M.; Kovacevic, P. Dynamic Changes in Coagulation, Hematological and Biochemical Parameters as Predictors of Mortality in Critically Ill COVID–19 Patients: A Prospective Observational Study. Clin. Hemorheol. Microcirc. 2023, 83, 137–148. [Google Scholar] [CrossRef]
- Karahan, S.; Aydin, K.; Cetinkaya, A.; Sirakaya, H.A. Nailfold Videocapillaroscopy in Patients with COVID-19-Associated Pneumonia in Intensive Care Units. J. Coll. Physicians Surg. Pak. 2022, 32, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Malgaj Vrečko, M.; Aleš-Rigler, A.; Borštnar, Š.; Večerić-Haler, Ž. Coronavirus Disease 2019-Associated Thrombotic Microangiopathy: A Single-Center Experience. Int. J. Mol. Sci. 2024, 25, 12475. [Google Scholar] [CrossRef] [PubMed]
- Rosei, C.A.; Gaggero, A.; Famà, F.; Malerba, P.; Chiarini, G.; Nardin, M.; Brami, V.; Rossini, C.; Coschignano, M.A.; Porteri, E.; et al. Skin Capillary Alterations in Patients with Acute SarsCoV2 Infection. J. Hypertens. 2022, 40, 2385–2393. [Google Scholar] [CrossRef] [PubMed]
- Jung, F.; Connes, P. Morphology and Function of Red Blood Cells in COVID-19 Patients: Current Overview 2023. Life 2024, 14, 460. [Google Scholar] [CrossRef]
- Kelliher, S.; Weiss, L.; Cullivan, S.; O’Rourke, E.; Murphy, C.A.; Toolan, S.; Lennon, Á.; Szklanna, P.B.; Comer, S.P.; Macleod, H.; et al. Non-severe COVID-19 Is Associated with Endothelial Damage and Hypercoagulability Despite Pharmacological Thromboprophylaxis. J. Thromb. Haemost. 2022, 20, 1008–1014. [Google Scholar] [CrossRef]
- Østergaard, L. SARS CoV-2 Related Microvascular Damage and Symptoms during and after COVID-19: Consequences of Capillary Transit-time Changes, Tissue Hypoxia and Inflammation. Physiol. Rep. 2021, 9, e14726. [Google Scholar] [CrossRef]
- Russu, E.; Arbănaşi, E.-M.; Șchiopu, A. Special Issue “COVID-19 Coagulopathy: Advances on Pathophysiology and Therapies”. Int. J. Mol. Sci. 2024, 25, 3548. [Google Scholar] [CrossRef]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major Findings, Mechanisms and Recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef]
- Taquet, M.; Dercon, Q.; Luciano, S.; Geddes, J.R.; Husain, M.; Harrison, P.J. Incidence, Co-Occurrence, and Evolution of Long-COVID Features: A 6-Month Retrospective Cohort Study of 273,618 Survivors of COVID-19. PLoS Med. 2021, 18, e1003773. [Google Scholar] [CrossRef] [PubMed]
- Van Der Knaap, N.; Klinkhammer, S.; Postma, A.A.; Visser-Meily, J.M.A.; Horn, J.; Van Heugten, C.M.; Voorter, P.H.M.; Van Der Thiel, M.M.; Drenthen, G.S.; Backes, W.H.; et al. Post-COVID Microvascular Dysfunction in Hospitalized COVID-19 Survivors Is Associated with Acute Disease Severity and Persistent Cognitive Complaints. J. Neurol. Sci. 2025, 472, 123464. [Google Scholar] [CrossRef]
- Ariza, M.; Delas, B.; Rodriguez, B.; De Frutos, B.; Cano, N.; Segura, B.; Barrué, C.; Bejar, J.; Asaad, M.; Cortés, C.U.; et al. Retinal Microvasculature Changes Linked to Executive Function Impairment after COVID-19. J. Clin. Med. 2024, 13, 5671. [Google Scholar] [CrossRef] [PubMed]
- Moka, S.; Koutsiaris, A.G.; Garas, A.; Messinis, I.; Tachmitzi, S.V.; Giannoukas, A.; Tsironi, E.E. Blood Flow Velocity Comparison in the Eye Capillaries and Postcapillary Venules between Normal Pregnant and Non-Pregnant Women. Microvasc. Res. 2020, 127, 103926. [Google Scholar] [CrossRef]
- Karakasis, P.; Nasoufidou, A.; Sagris, M.; Fragakis, N.; Tsioufis, K. Vascular Alterations Following COVID-19 Infection: A Comprehensive Literature Review. Life 2024, 14, 545. [Google Scholar] [CrossRef]
- Koutsiaris, A.G. Hemodynamics in the Microcirculation. Ann. Biomed. Eng. 2016, 44, 1321–1322. [Google Scholar] [CrossRef]
- Koutsiaris, A.G. Meta-Analysis of Conjunctival Microvascular Hemorheology Metrics. Microvasc. Res. 2022, 142, 104369. [Google Scholar] [CrossRef] [PubMed]
- Koutsiaris, A.G.; Batis, V.; Liakopoulou, G.; Tachmitzi, S.V.; Detorakis, E.T.; Tsironi, E.E. Optical Coherence Tomography Angiography (OCTA) of the Eye: A Review on Basic Principles, Advantages, Disadvantages and Device Specifications. Clin. Hemorheol. Microcirc. 2023, 83, 247–271. [Google Scholar] [CrossRef]
- Fang, D.-Q.; Yang, D.-W.; Mai, X.-T.; Cheung, C.Y.; Chen, H.-Y. Repeatability, Interocular Correlation and Agreement of Optic Nerve Head Vessel Density in Healthy Eyes: A Swept-Source Optical Coherence Tomographic Angiography Study. Int. J. Ophthalmol. 2024, 17, 896–903. [Google Scholar] [CrossRef]
- Ribeiro Reis, A.P.; Ioannidou, E.; Wagner, S.K.; Struyven, R.; Sun, Z.; Foster, P.; Khawaja, A.P.; Petzold, A.; Sivaprasad, S.; Pontikos, N.; et al. Retinal Morphology across the Menstrual Cycle: Insights from the UK Biobank. Npj Womens Health 2024, 2, 38. [Google Scholar] [CrossRef]
- Bayraktar, M.F.; Toprak, G.; Alkan, Y. The Relationship between Choroidal Vascular Index and Non-Invasive Ultrasonographic Atherosclerosis Predictors. Photodiagnosis Photodyn. Ther. 2024, 46, 104046. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-M.; Kang, M.; Wang, J.-Y.; Xu, S.-H.; Chen, C.; Wei, H.; Ling, Q.; He, L.-Q.; Zou, J.; Wang, Y.-X.; et al. Microvascular Alterations of the Ocular Surface and Retina in Connective Tissue Disease-Related Interstitial Lung Disease. Int. J. Ophthalmol. 2024, 17, 1869–1879. [Google Scholar] [CrossRef] [PubMed]
- Mwale, P.; Zheng, H.; Zheng, Y.; Jiang, B.; Li, Y.; Wang, Y.; Li, F.; Chen, X.; Ke, M. Detecting Pre and Early Retinal Changes in Patients with Type 2 Diabetes Mellitus Using Optical Coherence Tomography Angiography. Open J. Ophthalmol. 2024, 14, 369–384. [Google Scholar] [CrossRef]
- Racioppo, P.; Alhasany, A.; Pham, N.V.; Wang, Z.; Corradetti, G.; Mikaelian, G.; Paulus, Y.M.; Sadda, S.R.; Hu, Z. Automated Foveal Avascular Zone Segmentation in Optical Coherence Tomography Angiography Across Multiple Eye Diseases Using Knowledge Distillation. Bioengineering 2025, 12, 334. [Google Scholar] [CrossRef]
- Vagiakis, I.; Bakirtzis, C.; Andravizou, A.; Pirounides, D. Unlocking the Potential of Vessel Density and the Foveal Avascular Zone in Optical Coherence Tomography Angiography as Biomarkers in Alzheimer’s Disease. Healthcare 2024, 12, 1589. [Google Scholar] [CrossRef]
- Azar, G.; Abdelmassih, Y.; Bonnin, S.; Guindolet, D.; Vasseur, V.; Behar Cohen, F.; Salmon, D.; Mauget-Faÿsse, M. Endothelial Glycocalyx Anomalies and Ocular Manifestations in Patients with Post-Acute COVID-19. J. Clin. Med. 2024, 13, 7272. [Google Scholar] [CrossRef]
- Song, X.; Yu, Y.; Zhou, H.; Zhang, Y.; Mao, Y.; Wang, H.; Cao, X.; Zhu, X.; Li, Z.; Li, L.; et al. Acute Macular Neuroretinopathy Associated with COVID-19 Pandemic: A Real-World Observation Study. Asia-Pac. J. Ophthalmol. 2024, 13, 100103. [Google Scholar] [CrossRef]
- Bilbao-Malavé, V.; González-Zamora, J.; Saenz De Viteri, M.; De La Puente, M.; Gándara, E.; Casablanca-Piñera, A.; Boquera-Ventosa, C.; Zarranz-Ventura, J.; Landecho, M.F.; García-Layana, A. Persistent Retinal Microvascular Impairment in COVID-19 Bilateral Pneumonia at 6-Months Follow-Up Assessed by Optical Coherence Tomography Angiography. Biomedicines 2021, 9, 502. [Google Scholar] [CrossRef]
- Cennamo, G.; Reibaldi, M.; Montorio, D.; D’Andrea, L.; Fallico, M.; Triassi, M. Optical Coherence Tomography Angiography Features in Post-COVID-19 Pneumonia Patients: A Pilot Study. Am. J. Ophthalmol. 2021, 227, 182–190. [Google Scholar] [CrossRef]
- Dipu, T.; Goel, R.; Arora, R.; Thakar, M.; Gautam, A.; Shah, S.; Gupta, Y.; Chhabra, M.; Kumar, S.; Singh, K.; et al. Ocular Sequelae in Severe COVID-19 Recovered Patients of Second Wave. Indian J. Ophthalmol. 2022, 70, 1780–1786. [Google Scholar] [CrossRef]
- El-Haddad, N.S.E.-D.M.; Abd El-Wahed, E.; Abd El-Wahab, A.; Shalaby, S.; Farag, M.M.A.; Mohammed, N.S.; Shawky, S. The Effect of Post-Coronavirus Disease 2019 Infection on the Retinal Microvasculature. J. Curr. Ophthalmol. 2023, 35, 50–55. [Google Scholar] [CrossRef] [PubMed]
- González-Zamora, J.; Bilbao-Malavé, V.; Gándara, E.; Casablanca-Piñera, A.; Boquera-Ventosa, C.; Landecho, M.F.; Zarranz-Ventura, J.; García-Layana, A. Retinal Microvascular Impairment in COVID-19 Bilateral Pneumonia Assessed by Optical Coherence Tomography Angiography. Biomedicines 2021, 9, 247. [Google Scholar] [CrossRef]
- Kal, M.; Winiarczyk, M.; Cieśla, E.; Płatkowska-Adamska, B.; Walczyk, A.; Biskup, M.; Pabjan, P.; Głuszek, S.; Odrobina, D.; Mackiewicz, J.; et al. Retinal Microvascular Changes in COVID-19 Bilateral Pneumonia Based on Optical Coherence Tomography Angiography. J. Clin. Med. 2022, 11, 3621. [Google Scholar] [CrossRef] [PubMed]
- Kalaw, F.G.P.; Warter, A.; Cavichini, M.; Knight, D.; Li, A.; Deussen, D.; Galang, C.; Heinke, A.; Mendoza, V.; Borooah, S.; et al. Retinal Tissue and Microvasculature Loss in COVID-19 Infection. Sci. Rep. 2023, 13, 5100. [Google Scholar] [CrossRef] [PubMed]
- Koutsiaris, A.G.; Riri, K.; Boutlas, S.; Panagiotou, T.N.; Kotoula, M.; Daniil, Z.; Tsironi, E.E. COVID-19 Hemodynamic and Thrombotic Effect on the Eye Microcirculation After Hospitalization: A Quantitative Case-Control Study. Clin. Hemorheol. Microcirc. 2022, 82, 379–390. [Google Scholar] [CrossRef]
- Ozturk, M.; Kumova Guler, D.; Oskan, E.E.; Onder, F. Long-Term Effects of COVID-19 on Optic Disc and Retinal Microvasculature Assessed by Optical Coherence Tomography Angiography. Diagnostics 2025, 15, 114. [Google Scholar] [CrossRef]
- Kazantzis, D.; Machairoudia, G.; Theodossiadis, G.; Theodossiadis, P.; Chatziralli, I. Retinal Microvascular Changes in Patients Recovered from COVID-19 Compared to Healthy Controls: A Meta-Analysis. Photodiagnosis Photodyn. Ther. 2023, 42, 103556. [Google Scholar] [CrossRef]
- Saloň, A.; De Boever, P.; Goswami, N. Microvascular Changes During Viral Infections: A Systematic Review of Studies Using Retinal Vessel Diameter Assessments. Biomedicines 2024, 12, 1488. [Google Scholar] [CrossRef]
- Koutsiaris, A.G.; Riri, K.; Boutlas, S.; Daniil, Z.; Tsironi, E.E. A Normative Blood Velocity Model in the Exchange Microvessels for Discriminating Health from Disease: Healthy Controls versus COVID-19 Cases. Clin. Hemorheol. Microcirc. 2023, 84, 215–226. [Google Scholar] [CrossRef]
- Çakmak, F.; Demirbuga, A.; Demirkol, D.; Gümüş, S.; Torun, S.H.; Kayaalp, G.K.; Ömeroglu, R.E.; Somer, A.; Uysalol, M.; Yıldız, R.; et al. Nailfold Capillaroscopy: A Sensitive Method for Evaluating Microvascular Involvement in Children with SARS-CoV-2 Infection. Microvasc. Res. 2021, 138, 104196. [Google Scholar] [CrossRef]
- Zharkikh, E.V.; Loktionova, Y.I.; Fedorovich, A.A.; Gorshkov, A.Y.; Dunaev, A.V. Assessment of Blood Microcirculation Changes after COVID-19 Using Wearable Laser Doppler Flowmetry. Diagnostics 2023, 13, 920. [Google Scholar] [CrossRef]
- Karstarli Bakay, O.S.; Cetin, N.; Bakay, U.; Cinar, G.; Goksin, S. A Window into the Vascular Endothelium in COVID-19: Nails. Dermatol. Pract. Concept. 2025, 15, 4927. [Google Scholar] [CrossRef]
- Sulli, A.; Gotelli, E.; Bica, P.F.; Schiavetti, I.; Pizzorni, C.; Aloè, T.; Grosso, M.; Barisione, E.; Paolino, S.; Smith, V.; et al. Detailed Videocapillaroscopic Microvascular Changes Detectable in Adult COVID-19 Survivors. Microvasc. Res. 2022, 142, 104361. [Google Scholar] [CrossRef]
- Osiaevi, I.; Schulze, A.; Evers, G.; Harmening, K.; Vink, H.; Kümpers, P.; Mohr, M.; Rovas, A. Persistent Capillary Rarefication in Long COVID Syndrome. Angiogenesis 2023, 26, 53–61. [Google Scholar] [CrossRef]
- Cho, J.L.; Villacreses, R.; Nagpal, P.; Guo, J.; Pezzulo, A.A.; Thurman, A.L.; Hamzeh, N.Y.; Blount, R.J.; Fortis, S.; Hoffman, E.A.; et al. Quantitative Chest CT Assessment of Small Airways Disease in Post-Acute SARS-CoV-2 Infection. Radiology 2022, 304, 185–192. [Google Scholar] [CrossRef]
- Littlefield, K.M.; Watson, R.O.; Schneider, J.M.; Neff, C.P.; Yamada, E.; Zhang, M.; Campbell, T.B.; Falta, M.T.; Jolley, S.E.; Fontenot, A.P.; et al. SARS-CoV-2-Specific T Cells Associate with Inflammation and Reduced Lung Function in Pulmonary Post-Acute Sequalae of SARS-CoV-2. PLoS Pathog. 2022, 18, e1010359. [Google Scholar] [CrossRef]
- Dal Negro, R.W.; Turco, P.; Povero, M. mRNA Vaccines Protect from the Lung Microvasculature Injury and the Capillary Blood Volume Loss Occurring in SARS-CoV-2 Paucisymptomatic Infections. Multidiscip. Respir. Med. 2024, 19, 2. [Google Scholar] [CrossRef]
- Untersmayr, E.; Venter, C.; Smith, P.; Rohrhofer, J.; Ndwandwe, C.; Schwarze, J.; Shannon, E.; Sokolowska, M.; Sadlier, C.; O’Mahony, L. Immune Mechanisms Underpinning Long COVID: Collegium Internationale Allergologicum Update 2024. Int. Arch. Allergy Immunol. 2024, 185, 489–502. [Google Scholar] [CrossRef]
- Stein, S.R.; Ramelli, S.C.; Grazioli, A.; Chung, J.-Y.; Singh, M.; Yinda, C.K.; Winkler, C.W.; Sun, J.; Dickey, J.M.; Ylaya, K.; et al. SARS-CoV-2 Infection and Persistence in the Human Body and Brain at Autopsy. Nature 2022, 612, 758–763. [Google Scholar] [CrossRef]
- Gáspár, Z.; Szabó, B.G.; Ceglédi, A.; Lakatos, B. Human Herpesvirus Reactivation and Its Potential Role in the Pathogenesis of Post-Acute Sequelae of SARS-CoV-2 Infection. GeroScience 2025, 47, 167–187. [Google Scholar] [CrossRef]
- Kempuraj, D.; Aenlle, K.K.; Cohen, J.; Mathew, A.; Isler, D.; Pangeni, R.P.; Nathanson, L.; Theoharides, T.C.; Klimas, N.G. COVID-19 and Long COVID: Disruption of the Neurovascular Unit, Blood-Brain Barrier, and Tight Junctions. Neuroscientist 2024, 30, 421–439. [Google Scholar] [CrossRef]
- Greene, C.; Connolly, R.; Brennan, D.; Laffan, A.; O’Keeffe, E.; Zaporojan, L.; O’Callaghan, J.; Thomson, B.; Connolly, E.; Argue, R.; et al. Blood–Brain Barrier Disruption and Sustained Systemic Inflammation in Individuals with Long COVID-Associated Cognitive Impairment. Nat. Neurosci. 2024, 27, 421–432. [Google Scholar] [CrossRef]
- Qiao, H.; Deng, X.; Qiu, L.; Qu, Y.; Chiu, Y.; Chen, F.; Xia, S.; Muenzel, C.; Ge, T.; Zhang, Z.; et al. SARS-CoV-2 Induces Blood-brain Barrier and Choroid Plexus Barrier Impairments and Vascular Inflammation in Mice. J. Med. Virol. 2024, 96, e29671. [Google Scholar] [CrossRef]
- Reiken, S.; Sittenfeld, L.; Dridi, H.; Liu, Y.; Liu, X.; Marks, A.R. Alzheimer’s-like Signaling in Brains of COVID-19 Patients. Alzheimers Dement. 2022, 18, 955–965. [Google Scholar] [CrossRef]
- Charnley, M.; Islam, S.; Bindra, G.K.; Engwirda, J.; Ratcliffe, J.; Zhou, J.; Mezzenga, R.; Hulett, M.D.; Han, K.; Berryman, J.T.; et al. Neurotoxic Amyloidogenic Peptides in the Proteome of SARS-CoV2: Potential Implications for Neurological Symptoms in COVID-19. Nat. Commun. 2022, 13, 3387. [Google Scholar] [CrossRef]
- Peluso, M.J.; Deeks, S.G.; Mustapic, M.; Kapogiannis, D.; Henrich, T.J.; Lu, S.; Goldberg, S.A.; Hoh, R.; Chen, J.Y.; Martinez, E.O.; et al. SARS-CoV-2 and Mitochondrial Proteins in Neural-Derived Exosomes of COVID-19. Ann. Neurol. 2022, 91, 772–781. [Google Scholar] [CrossRef]
- Díaz-Resendiz, K.J.G.; Benitez-Trinidad, A.B.; Covantes-Rosales, C.E.; Toledo-Ibarra, G.A.; Ortiz-Lazareno, P.C.; Girón-Pérez, D.A.; Bueno-Durán, A.Y.; Pérez-Díaz, D.A.; Barcelos-García, R.G.; Girón-Pérez, M.I. Loss of Mitochondrial Membrane Potential (Δ Ψ m) in Leucocytes as Post-COVID-19 Sequelae. J. Leukoc. Biol. 2022, 112, 23–29. [Google Scholar] [CrossRef]
- Zhang, B.-Z.; Chu, H.; Han, S.; Shuai, H.; Deng, J.; Hu, Y.; Gong, H.; Lee, A.C.-Y.; Zou, Z.; Yau, T.; et al. SARS-CoV-2 Infects Human Neural Progenitor Cells and Brain Organoids. Cell Res. 2020, 30, 928–931. [Google Scholar] [CrossRef]
- Oaklander, A.L.; Mills, A.J.; Kelley, M.; Toran, L.S.; Smith, B.; Dalakas, M.C.; Nath, A. Peripheral Neuropathy Evaluations of Patients With Prolonged Long COVID. Neurol. Neuroimmunol. Neuroinflamm. 2022, 9, e1146. [Google Scholar] [CrossRef]
- Larsen, N.W.; Stiles, L.E.; Shaik, R.; Schneider, L.; Muppidi, S.; Tsui, C.T.; Geng, L.N.; Bonilla, H.; Miglis, M.G. Characterization of Autonomic Symptom Burden in Long COVID: A Global Survey of 2,314 Adults. Front. Neurol. 2022, 13, 1012668. [Google Scholar] [CrossRef]
- Campen, C.L.M.C.V.; Visser, F.C. Long-Haul COVID Patients: Prevalence of POTS Are Reduced but Cerebral Blood Flow Abnormalities Remain Abnormal with Longer Disease Duration. Healthcare 2022, 10, 2105. [Google Scholar] [CrossRef]
- Tavee, J. Current Concepts in Long COVID-19 Brain Fog and Postural Orthostatic Tachycardia Syndrome. Ann. Allergy. Asthma. Immunol. 2024, 133, 522–530. [Google Scholar] [CrossRef]
- Fedorowski, A.; Fanciulli, A.; Raj, S.R.; Sheldon, R.; Shibao, C.A.; Sutton, R. Cardiovascular Autonomic Dysfunction in Post-COVID-19 Syndrome: A Major Health-Care Burden. Nat. Rev. Cardiol. 2024, 21, 379–395. [Google Scholar] [CrossRef]
- Yeoh, Y.K.; Zuo, T.; Lui, G.C.-Y.; Zhang, F.; Liu, Q.; Li, A.Y.; Chung, A.C.; Cheung, C.P.; Tso, E.Y.; Fung, K.S.; et al. Gut Microbiota Composition Reflects Disease Severity and Dysfunctional Immune Responses in Patients with COVID-19. Gut 2021, 70, 698–706. [Google Scholar] [CrossRef]
- Liu, Q.; Mak, J.W.Y.; Su, Q.; Yeoh, Y.K.; Lui, G.C.-Y.; Ng, S.S.S.; Zhang, F.; Li, A.Y.L.; Lu, W.; Hui, D.S.-C.; et al. Gut Microbiota Dynamics in a Prospective Cohort of Patients with Post-Acute COVID-19 Syndrome. Gut 2022, 71, 544–552. [Google Scholar] [CrossRef]
- König, R.S.; Albrich, W.C.; Kahlert, C.R.; Bahr, L.S.; Löber, U.; Vernazza, P.; Scheibenbogen, C.; Forslund, S.K. The Gut Microbiome in Myalgic Encephalomyelitis (ME)/Chronic Fatigue Syndrome (CFS). Front. Immunol. 2022, 12, 628741. [Google Scholar] [CrossRef]
- Pretorius, E.; Venter, C.; Laubscher, G.J.; Kotze, M.J.; Oladejo, S.O.; Watson, L.R.; Rajaratnam, K.; Watson, B.W.; Kell, D.B. Prevalence of Symptoms, Comorbidities, Fibrin Amyloid Microclots and Platelet Pathology in Individuals with Long COVID/Post-Acute Sequelae of COVID-19 (PASC). Cardiovasc. Diabetol. 2022, 21, 148. [Google Scholar] [CrossRef]
- Bellone, S.; Siegel, E.R.; Scheim, D.E.; Santin, A.D. Increased von Willebrand and Factor VIII Plasma Levels in Gynecologic Patients with Post-Acute-COVID-Sequela (PASC)/Long COVID. Gynecol. Oncol. Rep. 2024, 51, 101324. [Google Scholar] [CrossRef]
- Popazu, C.; Romila, A.; Petrea, M.; Grosu, R.M.; Lescai, A.-M.; Vlad, A.L.; Oprea, V.D.; Baltă, A.A.Ș. Overview of Inflammatory and Coagulation Markers in Elderly Patients with COVID-19: Retrospective Analysis of Laboratory Results. Life 2025, 15, 370. [Google Scholar] [CrossRef]
- Baldassarro, V.A.; Alastra, G.; Cescatti, M.; Quadalti, C.; Lorenzini, L.; Giardino, L.; Calzà, L. SARS-CoV-2-Related Peptides Induce Endothelial-to-Mesenchymal Transition in Endothelial Capillary Cells Derived from Different Body Districts: Focus on Membrane (M) Protein. Cell Tissue Res. 2024, 397, 241–262. [Google Scholar] [CrossRef]
- Gultom, M.; Lin, L.; Brandt, C.B.; Milusev, A.; Despont, A.; Shaw, J.; Döring, Y.; Luo, Y.; Rieben, R. Sustained Vascular Inflammatory Effects of SARS-CoV-2 Spike Protein on Human Endothelial Cells. Inflammation 2024, 1–17. [Google Scholar] [CrossRef]
- Šuligoj, T.; Coombes, N.S.; Booth, C.; Savva, G.M.; Bewley, K.R.; Funnell, S.G.P.; Juge, N. Modelling SARS-CoV-2 Infection in a Human Alveolus Microphysiological System. Access Microbiol. 2024, 6, 000814-v3. [Google Scholar] [CrossRef]
- Romanowska-Kocejko, M.; Braczko, A.; Jędrzejewska, A.; Żarczyńska-Buchowiecka, M.; Kocejko, T.; Kutryb-Zając, B.; Hellmann, M. Follow-up Assessment of the Microvascular Function in Patients with Long COVID. Microvasc. Res. 2025, 157, 104748. [Google Scholar] [CrossRef]
- Valencia, I.; Lumpuy-Castillo, J.; Magalhaes, G.; Sánchez-Ferrer, C.F.; Lorenzo, Ó.; Peiró, C. Mechanisms of Endothelial Activation, Hypercoagulation and Thrombosis in COVID-19: A Link with Diabetes Mellitus. Cardiovasc. Diabetol. 2024, 23, 75. [Google Scholar] [CrossRef]
- Ståhlberg, M.; Fischer, K.; Tahhan, M.; Zhao, A.; Fedorowski, A.; Runold, M.; Nygren-Bonnier, M.; Björnson, M.; Lund, L.H.; Bruchfeld, J.; et al. Post-Acute COVID-19 Syndrome: Prevalence of Peripheral Microvascular Endothelial Dysfunction and Associations with NT-ProBNP Dynamics. Am. J. Med. 2024, 138, 1019–1028. [Google Scholar] [CrossRef]
- Aird, W.C. Phenotypic Heterogeneity of the Endothelium: I. Structure, Function, and Mechanisms. Circ. Res. 2007, 100, 158–173. [Google Scholar] [CrossRef]
- Koutsiaris, A.G. Wall Shear Stress in the Human Eye Microcirculation in Vivo, Segmental Heterogeneity and Performance of in Vitro Cerebrovascular Models. Clin. Hemorheol. Microcirc. 2016, 63, 15–33. [Google Scholar] [CrossRef]
- Koutsiaris, A.G. A Velocity Profile Equation for Blood Flow in Small Arterioles and Venules of Small Mammals in Vivo and an Evaluation Based on Literature Data. Clin. Hemorheol. Microcirc. 2009, 43, 321–334. [Google Scholar] [CrossRef]
- Mierke, C.T. Mechanosensory Entities and Functionality of Endothelial Cells. Front. Cell Dev. Biol. 2024, 12, 1446452. [Google Scholar] [CrossRef]
- Krüger-Genge, A.; Blocki, A.; Franke, R.-P.; Jung, F. Vascular Endothelial Cell Biology: An Update. Int. J. Mol. Sci. 2019, 20, 4411. [Google Scholar] [CrossRef]
- Boegehold, M.A. Heterogeneity of Endothelial Function within the Circulation. Curr. Opin. Nephrol. Hypertens. 1998, 7, 71–78. [Google Scholar] [CrossRef]
- Cutolo, M.; Sulli, A.; Smith, V.; Gotelli, E. Emerging Nailfold Capillaroscopic Patterns in COVID-19: From Acute Patients to Survivors. Reumatismo 2023, 74, 139–143. [Google Scholar] [CrossRef]
- Koutsiaris, A.G. A Blood Supply Pathophysiological Microcirculatory Mechanism for Long COVID. Life 2024, 14, 1076. [Google Scholar] [CrossRef]
- Koutsiaris, A.G. The Velocity-Diffusion Equation in the Exchange Microvessels. Clin. Hemorheol. Microcirc. 2023, 84, 83–88. [Google Scholar] [CrossRef]
- Liu, P.; Ernst, T.; Liang, H.; Jiang, D.; Cunningham, E.; Ryan, M.; Lu, H.; Kottilil, S.; Chang, L. Elevated Cerebral Oxygen Extraction in Patients with Post-COVID Conditions. NeuroImmune Pharmacol. Ther. 2024, 3, 169–174. [Google Scholar] [CrossRef]
- Romanowska-Kocejko, M.; Jędrzejewska, A.; Braczko, A.; Stawarska, K.; Król, O.; Frańczak, M.; Harasim, G.; Smoleński, R.T.; Hellmann, M.; Kutryb-Zając, B. Red Blood Cell Adenylate Energetics Is Related to Endothelial and Microvascular Function in Long COVID. Biomedicines 2024, 12, 554. [Google Scholar] [CrossRef]
- Jamieson, A.; Al Saikhan, L.; Alghamdi, L.; Hamill Howes, L.; Purcell, H.; Hillman, T.; Heightman, M.; Treibel, T.; Orini, M.; Bell, R.; et al. Mechanisms Underlying Exercise Intolerance in Long COVID: An Accumulation of Multisystem Dysfunction. Physiol. Rep. 2024, 12, e15940. [Google Scholar] [CrossRef]
- Lafetá, M.L.; Souza, V.C.; Menezes, T.C.F.; Verrastro, C.G.Y.; Mancuso, F.J.; Albuquerque, A.L.P.; Tanni, S.E.; Izbicki, M.; Carlstron, J.P.; Nery, L.E.; et al. Exercise Intolerance in Post-Coronavirus Disease 2019 Survivors after Hospitalisation. ERJ Open Res. 2023, 9, 00538–02022. [Google Scholar] [CrossRef]
- Russell, S.L.; Okwose, N.C.; Rahman, M.; Lee, B.J.; McGregor, G.; Raleigh, S.M.; Sandhu, H.; Roden, L.C.; Banerjee, P.; Jakovljevic, D.G. The Effect of COVID-19 on Cardiovascular Function and Exercise Tolerance in Healthy Middle-Age and Older Individuals. Scand. Cardiovasc. J. 2025, 59, 2468339. [Google Scholar] [CrossRef]
- Kell, D.B.; Pretorius, E. The Potential Role of Ischaemia–Reperfusion Injury in Chronic, Relapsing Diseases Such as Rheumatoid Arthritis, Long COVID, and ME/CFS: Evidence, Mechanisms, and Therapeutic Implications. Biochem. J. 2022, 479, 1653–1708. [Google Scholar] [CrossRef]
- Wirth, K.J.; Löhn, M. Microvascular Capillary and Precapillary Cardiovascular Disturbances Strongly Interact to Severely Affect Tissue Perfusion and Mitochondrial Function in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Evolving from the Post COVID-19 Syndrome. Medicina 2024, 60, 194. [Google Scholar] [CrossRef]
- Panagiotides, N.G.; Poledniczek, M.; Andreas, M.; Hülsmann, M.; Kocher, A.A.; Kopp, C.W.; Piechota-Polanczyk, A.; Weidenhammer, A.; Pavo, N.; Wadowski, P.P. Myocardial Oedema as a Consequence of Viral Infection and Persistence—A Narrative Review with Focus on COVID-19 and Post COVID Sequelae. Viruses 2024, 16, 121. [Google Scholar] [CrossRef]
- Theresa, C.; Katebe, B.; Shibao, C.A.; Kirabo, A. Arterial Stiffness in Adults with Long COVID in sub-Saharan Africa. Physiol. Rep. 2024, 12, e70029. [Google Scholar] [CrossRef]
- Goldstein, R.E.; Hulten, E.A.; Arnold, T.B.; Thomas, V.M.; Heroy, A.; Walker, E.N.; Fox, K.; Lee, H.; Libbus, J.; Markos, B.; et al. Exercise Stress Echocardiography Shows Impaired Left Ventricular Function after Hospitalization with COVID-19 without Overt Myocarditis: A Pilot Study. Physiol. Rep. 2024, 12, e70138. [Google Scholar] [CrossRef]
- Passos, C.R.; Moreira, A.A.; Reis, R.F.; Dos Santos, R.W.; Lobosco, M.; Rocha, B.M. A Coupled Model of the Cardiovascular and Immune Systems to Analyze the Effects of COVID-19 Infection. BioTech 2025, 14, 19. [Google Scholar] [CrossRef]
| Physiological System | Long COVID Symptom | Incidence (%) |
|---|---|---|
| Respiratory | Abnormal breathing | 19 |
| Chest/throat pain | 13 |
| Metric | Average (%) | Tissue | N |
|---|---|---|---|
| HD | 37 | Conjunctiva/Skin/Brain | 72 |
| ML | 16 | Retina/Choroid/Sublingual | 562 |
| SR | 47 | Multiple Tissues | 634 |
| Physiological System | Long COVID Symptom | Incidence (%) |
|---|---|---|
| Nervous | Anxiety/Depression | 23 |
| Headache | 9 | |
| Cognitive | 8 |
| Physiological System | Long COVID Symptom | Incidence (%) |
|---|---|---|
| Nervous/Muscular/ Microvascular | Fatigue/Malaise (ME/CFS) | 13 |
| Myalgia (ME/CFS) | 3 |
| Long COVID Symptom | Incidence (%) | Normalized Incidence (%) |
|---|---|---|
| Anxiety/Depression | 23 | 20 |
| Abnormal breathing | 19 | 16 |
| Abdominal symptoms | 16 | 14 |
| Fatigue/Malaise | 13 | 11 |
| Chest/Throat pain | 13 | 11 |
| Other pain | 12 | 10 |
| Headache | 9 | 8 |
| Cognitive | 8 | 7 |
| Myalgia | 3 | 3 |
| TOTAL | 116 | 100 |
| Physiological System | Long COVID Symptom | Normalized Incidence (%) |
|---|---|---|
| Respiratory (Table 1) | Abnormal breathing | 16 |
| Chest/Throat pain | 11 | |
| Nervous (Table 3) | Anxiety/Depression | 20 |
| Headache | 8 | |
| Cognitive | 7 | |
| Nervous/Muscular/ Microvascular (Table 4) | Fatigue/Malaise (ME/CFS) | 11 |
| Myalgia (ME/CFS) | 3 | |
| TOTAL | 76 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koutsiaris, A.G.; Karakousis, K. Long COVID Mechanisms, Microvascular Effects, and Evaluation Based on Incidence. Life 2025, 15, 887. https://doi.org/10.3390/life15060887
Koutsiaris AG, Karakousis K. Long COVID Mechanisms, Microvascular Effects, and Evaluation Based on Incidence. Life. 2025; 15(6):887. https://doi.org/10.3390/life15060887
Chicago/Turabian StyleKoutsiaris, Aristotle G., and Kostas Karakousis. 2025. "Long COVID Mechanisms, Microvascular Effects, and Evaluation Based on Incidence" Life 15, no. 6: 887. https://doi.org/10.3390/life15060887
APA StyleKoutsiaris, A. G., & Karakousis, K. (2025). Long COVID Mechanisms, Microvascular Effects, and Evaluation Based on Incidence. Life, 15(6), 887. https://doi.org/10.3390/life15060887







