How to Pick a Neuroprotective Drug in Stroke Without Losing Your Mind?
Abstract
1. Failures of Neuroprotective Drugs in Cerebral Ischemia
1.1. Human Clinical Trials in Neuroprotection: Methodological Issues and Solutions
1.2. Preclinical Animal Models in Neuroprotection: Methodological Issues and Solutions
1.3. Perceived Headwinds to Achieving Neuroprotection
1.4. Is the Best Neuroprotective Therapy for Translation Being Chosen?
1.5. Proposed Themes Proposed for Picking a Neuroprotective Drug
- (i)
- Re-testing of failed drugs has been advocated for, given that a substantial fraction of patients treated with EVT achieve successful reperfusion, especially for those drugs exhibiting acceptable safety profiles. Re-testing should include drugs that failed (‘back-testing’ [58]) in the era pre-dating IVT or EVT since, in the absence of reperfusion, a drug would in all likelihood not be efficacious; furthermore, this re-testing could take advantage of the increased rigor being adopted in preclinical studies [19,59,60].
- (ii)
- Re-purposing of clinically approved drugs minimizes intellectual property concerns and improving drug accessibility in this manner to investigators should in principle allow broader participation in the field. Various organizations have made small libraries of drugs available to investigators, which are usually quite diverse, FDA-approved and off-patent [61,62], although this approach does not seem to enjoy widespread adoption.
- (iii)
- The inability of the one-target one-drug approach to result in a clinical success has been a strong motivator for seeking alternative or additional solutions. However, single-target drug development is still encouraged, particularly against novel targets or against conventional targets in novel ways although, even then, single targets that act as a hub for multiple pathways seem preferred [14].
- (iv)
- Endogenous neuroprotective mechanisms such as preconditioning and postconditioning have attracted considerable study [63], particularly if coupled with acute neuroprotective properties. Advances have been made such as in the field of neutraceuticals, in which the therapy acts as both a preconditioner and can exert acute effects against stroke [64,65].
- (v)
- Pleiotropic drugs that target more than one neurotoxic signaling pathway have attracted considerable interest, using the rationale that cerebral ischemia activates numerous parallel and serial pathways [7,66,67], so a single drug with multimodal modes of action against the signaling activated by cerebral ischemia should be pursued [1,68]. In fact, the most recent STAIR XI in 2021 advocated for interventions exerting multiple mechanisms of action [14], again citing the continued failure of one-target/one-drug clinical trials [1]. As examples cited, hypothermia or antioxidants target various toxic cellular signaling pathways activated by cerebral ischemia. Some drugs have been designed to incorporate two active ingredients [69,70]. Off-target effects of multi-modal drugs may be subject to historical biases against ‘dirty’ drugs, partly due to concerns of increased toxicity. What is missing, though, in discussions about pleiotropism is whether the most critical signaling pathways activated by ischemia are being targeted, and in an effective enough manner. Instead, it is often implicitly assumed that targeting several components in the signaling cascade will result in synergistic or additive neuroprotection.
- (vi)
- Combination therapy to target more than one neurotoxic signaling pathway can improve efficacy. A systematic review and meta-analysis from over 11,000 studies found that infarct size was reduced by 20% and 38% in focal cerebral ischemia by single and double therapy, respectively, which were adjusted to 14 and 28% when corrected for publication bias, in focal cerebral ischemia [71]. Limitations to implementing combination therapy include logistical issues (industrial, academic and regulatory). However, in principle, combination therapy allows different components of the neurotoxic signaling cascade to be targeted, at the optimal doses required to achieve efficacy; optimized combination therapy may allow synergistic neuroprotection, potentially decreasing the individual doses required, thereby reducing adverse effects. Strategies for picking the individual constituents vary considerably, raising the question of whether the selection process has been optimal.
- (vii)
- It is important to evaluate the veracity of each of these concepts. But, the process of examination must not stop at the individual level. Given the overall relatively low levels of efficacy achieved in animal models of cerebral ischemia, and the considerable headwinds, it is necessary to go beyond demonstrating proof of concept to demonstrating superiority of concept. Out of all of these concepts—re-testing, re-purposing, single-target hubs, endogenous signaling, pleiotropism and combination therapy—is there one (or more) which offers the best chance of success? To aid in this decision, it is crucial to have a strategy to allow a more informed choice to be made about which concept offers the greatest potential for translational success.
2. A New ‘Neuroprotective Framework’ to Prioritize Therapeutic Selection
2.1. The Cells: Neurons
2.2. The Biological Model: In Vitro
2.3. The Test Insult: Oxygen–Glucose Deprivation (OGD)
2.4. The Strategy: Utilize the OGD Continuum
- (i)
- Sub-lethal preconditioning: At the shortest end of the OGD continuum, a ‘mild’ OGD insult which is sufficient to stress neurons, yet not severe enough to exert any neurotoxicity, is a classical form of preconditioning neurons to withstand a subsequent otherwise lethal insult caused by OGD or other excitotoxicity-based insults [81]. However, how preconditioning actually protects neurons—typically defined as the end effector—and the robustness of this neuroprotection should be defined, particularly relative to the other concepts introduced above: essentially, this represents a comparison between endogenous neuroprotection offered by preconditioning versus the exogenous based concepts cited above (re-testing, re-purposing, single-target hubs, pleiotropism and combination therapy).
- (ii)
- Lethal: Increasing the duration of OGD sufficient to cause ≤100% neurotoxicity represents a classical approach and typically comprises most studies. This level of neurotoxicity probably models a penumbral-like insult, representing tissue at risk most amenable to neuroprotection. Adjusting various other features can modulate the harshness of the insult. A more dense, almost confluent density of neurons or 3D neurospheres will succumb more easily to OGD than a lower density, due to higher cellular release of glutamate. Other variations include altering the ion composition of the extracellular media to more closely mimic the ischemic milieu [82,83,84], or altering the degree of oxygen deprivation. The majority of drugs that have been tested in neuron cell culture models of OGD fit within this category: the goal is usually to evaluate mechanism of action (MOA) and to demonstrate proof-of-concept of neuroprotection as a basis for translation to testing in animal models of cerebral ischemia.
- (iii)
- Supra-lethal: Increasing the duration of OGD further results in an insult capable of killing neurons many times over. This type of insult has usually been performed much less frequently, since most drugs lose their neuroprotective ability under these circumstances. Instead of avoiding this type of insult, it should be embraced as a method to eliminate most neuroprotective modalities. Those therapies that are still neuroprotective against supra-lethal OGD (‘last man standing’) should be considered from an efficacy standpoint as the strongest candidates for translation to in vivo models of cerebral ischemia. As outlined extensively below, such approaches offer good correspondence with results obtained from other in vitro and in vivo systems employing increasingly harsh ischemia models. We propose that such an approach can form a bulwark in prioritizing a neuroprotective therapy amongst many other options.
3. The Neuronal Biological Target in the OGD Continuum
3.1. Lethal OGD: Excitotoxicity as the Neuronal Target
3.2. Sub-Lethal OGD: Preconditioning Is Anti-Excitotoxic
3.3. Supra-Lethal OGD: Continued Relevance of Excitotoxicity
- (i)
- Neurotoxicity caused by increasingly lethal OGD durations can be averted with more potent NMDAR antagonists. For instance, the more highly potent MK-801 is more neuroprotective compared to other competitive, uncompetitive and glycine-site NMDAR antagonists in neuron cultures and slice preparations exposed to OGD [106,107,108,109,110,111,112].
- (ii)
- The concentration of the NMDAR antagonist must increase with progressive OGD duration [85,113,114,115,116]. In the case of MK-801, elevating its concentration—up to a remarkably consistent maximally effective concentration of ~10 uM—allows successively longer durations of supra-lethal OGD to be withstood [85,117,118,119,120]. The Kd for MK-801 may rise from 37 nM to < 500 nM in depolarized neurons [121], but why concentrations well above IC50′s of different classes of NMDAR antagonists are necessary to maximize neuroprotection is not fully understood. It has been suggested that NMDARs on cultured hippocampal neurons contain two populations with very different inhibition properties by MK-801, with a high (IC50 = 105 nM) and a low (IC50 = ~55 µM) affinity site [122]. Interestingly, adding another NMDAR antagonist with MK-801 improved neuroprotection [107,121,123], although this was not a universal finding [119].
- (iii)
- Neuroprotection by even maximal amounts of MK-801 fails with further lengthening of the supra-lethal OGD duration [116,117,120,124,125,126]. This failure also extends to in vivo studies [127,128] and for other methods of blocking the NMDAR in vitro [121,129]. Neuroprotection with the maximal MK-801 dose also fails if the degree of oxygen deprivation is severe, even if glucose is present [130] or with longer term evaluation after a severe insult [113].
- (iv)
- Antagonists of different receptors and ion channels must be combined to maintain neuroprotection at even harsher supra-lethal OGD. The most common combinations of antagonists are NMDAR and AMPAR with L-type voltage-gated Ca2+ channels, with the most common cocktail composed of MK-801 (10 µM), CNQX (10 µM) and nimodipine (2–10 µM) [111,114,116,117,119,121,124,131,132,133,134,135,136]. Blocking AMPAR’s may help prevent removal of the voltage-dependent block of NMDAR [111], enhancing neuroprotection.
- (v)
- (vi)
- Neuroprotection can be achieved under extreme supra-lethal OGD conditions, but laboratories sharply diverge in the nature of the inhibitors used to supplement the anti-excitotoxic cocktails. Augmenting this MK-801/CNQX/nifedipine cocktail with TRPM7 or TRPM2 channel inhibitors protected neurons from supra-lethal OGD [117]. However, we [85] could not reproduce the finding that augmenting the cocktail with the TRPM7 inhibitor Gd3+ was neuroprotective [117]. The method of activation of TRPM7 in particular was attributed to the formation of ONOO− from the reaction of O2− and NO, which were blocked by the superoxide dismutase inhibitor MnTBAP and an nNOS inhibitor, respectively [117]. We also could not reproduce this finding with the nNOS inhibitor. As for MnTBAP, the mechanism of action is not as a superoxide dismutase [140]; instead, in a series of studies, we identified an off-target ability of MnTBAP (and other metalloporphyrins) to potently block Ca2+ influx through NMDAR’s, as well as from other sources, which was the basis for neuroprotection [90,91,92,93]. To determine if O2− was truly involved, we augmented the MK-801/CNQX/nifedipine with various antioxidants, but this was ineffective too. Other studies report no role for TRPM7 in slices either [136].
- (vii)
- Instead, we found that augmenting, manipulating and then changing the base MK-801/CNQX/nifedipine cocktail provided neuroprotection against successively longer durations of supra-lethal OGD. First, augmenting this cocktail with agents known to block Ca2+ influx (MnTBAP, ZnTBAP, verapamil and Ni2+) was neuroprotective. This suggested that the base cocktail MK-801/CNQX/nifedipine failed to sufficiently prevent Ca2+ influx (and likely accounts for why Aarts et al. [117] observed neuroprotection with the MnTBAP addition). Second, even longer supra-lethal OGD durations necessitated increasing the CNQX concentration and then exchanging CNQX with the more potent NBQX (less potent AMPAR antagonists were ineffective), at maximal concentrations. Third, more severe supra-lethal OGD necessitated exchanging MK-801 with a glycine-site NMDAR antagonist at maximal concentration, with the rank order of neuroprotection correlating with the rank order of potency of the glycine-site NMDAR antagonists. Figure 1 schematically summarizes the rationale used to identify the most neuroprotective cocktail ever identified in vitro [85].
3.4. Excitotoxicity Dominates the OGD Continuum
4. Role of Excitotoxicity in Other Model Systems
4.1. Excitotoxicity in Brain Slices and In Vivo
4.2. Implications of Energy Deprivation on Anti-Excitotoxic Approaches
4.3. Proposed Refinements to Excitotoxicity
- (i)
- (ii)
- A refinement of the source specificity hypothesis is to block neurotoxic signaling downstream of NMDAR activation, while still allowing normal basal synaptic function to be undisturbed [178]. Targeting death signaling cascades may extend the temporal window of activation, whereas NMDAR antagonists will have a much shorter temporal window. This has been the basis for development of PSD-95 peptides [179] and other peptides.
- (iii)
- Extrasynaptic NMDARs promote death—and therefore should be preferentially blocked—while synaptic NMDAR activation promotes survival, and therefore should remain undisturbed [180,181]. Indeed, our preconditioning protocol of 4-AP with bicuculline to hyper-activate synaptic NMDARs ranked highest in providing efficacy against early supra-lethal OGD [101]. However, neuroprotection provided by peptides inhibiting synaptic PSD-95/nNOS signaling suggest that synaptic NMDARs can also be excitotoxic, a concept that has been confirmed [87]. This theory is predicated upon the assumption that activation of synaptic and extrasynaptic NMDARs cause equal loading of Ca2+ (emphasizing the location and not the Ca2+ loading), but this dogma has been strongly challenged [182]. Another key requirement is that targeting extrasynaptic NMDARs requires choosing weaker NMDAR antagonists at lower concentrations [183] (at odds with strong cocktail requirements). Memantine has attracted considerable attention due to preferentially blocking extrasynaptic over synaptic NMDAR currents [184].
- (iv)
- The rationale behind Pathologically Activated Therapeutics (PATs) is to inhibit receptors that are excessively activated under pathological conditions, while having minimal effect on the target’s normal physiological activity. The uncompetitive open-channel NMDAR antagonist memantine has been proposed to be a PAT, since relatively lower concentrations of memantine block the effects of higher concentrations of NMDA due to more channels being open, while its fast off-rate allows normal transmission [183]. However, memantine is less potent than MK-801 in NMDAR-mediated current and neurotoxicity [185], and is therefore far less neuroprotective against supra-lethal OGD, even at maximal doses [85]. Since memantine will be repelled from the NMDAR during the membrane depolarization that accompanies ischemia, NitroMemantine was developed, which possesses an additional function of targeting an NO-generating group to the redox modulatory sites of NMDARs, outperforming memantine against cerebral ischemia [70]. A variation of PATs are ‘context-dependent’ NMDAR inhibitors which are more potent at acidic pH (such as exists in ischemic tissue) [186,187], one of which successfully completed a phase 1 clinical trial [188].
- (v)
- Subunit-specific antagonists have been proposed: NR2B-containing NMDARs may be more toxic, possibly due to a more of an extrasynaptic location, than NR2A subunits located in synaptic pro-survival locations [189], but these subunits are located in both locations [190]. However, NR2B-containing subunits can mediate pro-survival signaling on their own [191] and, conversely, blocking NR2A-subunits can augment neuroprotection [182].
- (vi)
- The Ca2+ overload hypothesis suggests that the amount of Ca2+ loading within neurons is the most important determinant of neurotoxicity, particularly with NMDAR-mediated entry [86,192,193], although see reference [173]. However, a concern with implementation of therapies preventing Ca2+ overload is that glutamate receptor activation (synaptic) is required for neuron survival, so blocking these receptors may harm naïve neurons (such as in non-infarcted regions), or prevent recruitment of endogenous recovery mechanisms in the injured region [54,152,194,195,196]. A dual issue has been suggested in which blocking only NMDARs may be insufficient so Ca2+ overload is not prevented in the core or near-penumbra, or can even be harmful by causing Ca2+ starvation and apoptosis further from the core and later time intervals (reviewed in [152]).
- (vii)
- Additional or alternative target besides excitotoxicity have been suggested: between 1993 and 2001, 28 anti-excitotoxic approaches all failed in clinical trials so, perhaps inevitably (despite all of the methodological issues), given the intense focus on blocking excitotoxicity in clinical trials, many investigators have since come to doubt this hypothesis or, at the very least, suggested refinements [142].
4.4. Are Proposed Refinements to Excitotoxicity Relevant to Supra-Lethal OGD?
5. The Path Forward
5.1. Achieving Neuroprotection with Re-Canalization
5.2. A New Chapter: Timing of Delivery an Essential a Trade-Off
5.3. Determining the Therapeutic Index In Vitro
6. How Do Past and Current Prospects Fit Within the Proposed Neuroprotective Framework?
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Paul, S.; Candelario-Jalil, E. Emerging neuroprotective strategies for the treatment of ischemic stroke: An overview of clinical and preclinical studies. Exp. Neurol. 2021, 335, 113518. [Google Scholar] [CrossRef] [PubMed]
- Feuerstein, G.Z.; Chavez, J. Translational medicine for stroke drug discovery: The pharmaceutical industry perspective. Stroke 2009, 40, S121–S125. [Google Scholar] [CrossRef] [PubMed]
- Jonas, S.; Ayigari, V.; Viera, D.; Waterman, P. Neuroprotection against cerebral ischemia. A review of animal studies and correlation with human trial results. Ann. N. Y. Acad. Sci. 1999, 890, 2–3. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, M.D. Neuroprotection for ischemic stroke: Past, present and future. Neuropharmacology 2008, 55, 363–389. [Google Scholar] [CrossRef]
- Zaleska, M.M.; Mercado, M.L.; Chavez, J.; Feuerstein, G.Z.; Pangalos, M.N.; Wood, A. The development of stroke therapeutics: Promising mechanisms and translational challenges. Neuropharmacology 2009, 56, 329–341. [Google Scholar] [CrossRef]
- Grotta, J. Neuroprotection is unlikely to be effective in humans using current trial designs. Stroke 2002, 33, 306–307. [Google Scholar] [CrossRef]
- Fisher, M.; Savitz, S.I. Pharmacological brain cytoprotection in acute ischaemic stroke-renewed hope in the reperfusion era. Nat. Rev. Neurol. 2022, 18, 193–202. [Google Scholar] [CrossRef]
- Muir, K.W.; Teal, P.A. Why have neuro-protectants failed?: Lessons learned from stroke trials. J. Neurol. 2005, 252, 1011–1020. [Google Scholar] [CrossRef]
- Tymianski, M. Novel approaches to neuroprotection trials in acute ischemic stroke. Stroke 2013, 44, 2942–2950. [Google Scholar] [CrossRef]
- Hartley, D.M.; Choi, D.W. Delayed rescue of N-methyl-D-aspartate receptor-mediated neuronal injury in cortical culture. J. Pharmacol. Exp. Ther. 1989, 250, 752–758. [Google Scholar] [CrossRef]
- Gerasimov, V.D.; Artemenko, D.P.; Krishtal, O.A. Therapeutic time window for the neuroprotective action of MK-801 after decapitation ischemia: Hippocampal slice data. Brain Res. 2004, 1017, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Margaill, I.; Parmentier, S.; Callebert, J.; Allix, M.; Boulu, R.G.; Plotkine, M. Short therapeutic window for MK-801 in transient focal cerebral ischemia in normotensive rats. J. Cereb. Blood Flow Metab. 1996, 16, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Rod, M.R.; Auer, R.N. Pre- and post-ischemic administration of dizocilpine (MK-801) reduces cerebral necrosis in the rat. Can. J. Neurol. Sci. 1989, 16, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Lyden, P.; Buchan, A.; Boltze, J.; Fisher, M. Top Priorities for Cerebroprotective Studies-A Paradigm Shift: Report from STAIR XI. Stroke 2021, 52, 3063–3071. [Google Scholar] [CrossRef]
- Dammavalam, V.; Lin, S.; Nessa, S.; Daksla, N.; Stefanowski, K.; Costa, A.; Bergese, S. Neuroprotection during Thrombectomy for Acute Ischemic Stroke: A Review of Future Therapies. Int. J. Mol. Sci. 2024, 25, 891. [Google Scholar] [CrossRef]
- Nam, H.S.; Kim, B.M. Advance of Thrombolysis and Thrombectomy in Acute Ischemic Stroke. J. Clin. Med. 2023, 12, 720. [Google Scholar] [CrossRef]
- Goyal, M.; Rinkel, L.A.; Ospel, J.M. A Review on Adjunctive Therapies for Endovascular Treatment in Acute Ischemic Stroke. J. Neuroendovasc Ther. 2023, 17, 263–271. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, B.J.; Han, M.K.; Park, T.H.; Lee, K.B.; Lee, B.C.; Yu, K.H.; Oh, M.S.; Cha, J.K.; Kim, D.H.; et al. Futile reperfusion and predicted therapeutic benefits after successful endovascular treatment according to initial stroke severity. BMC Neurol. 2019, 19, 11. [Google Scholar] [CrossRef]
- Shi, L.; Rocha, M.; Leak, R.K.; Zhao, J.; Bhatia, T.N.; Mu, H.; Wei, Z.; Yu, F.; Weiner, S.L.; Ma, F.; et al. A new era for stroke therapy: Integrating neurovascular protection with optimal reperfusion. J. Cereb. Blood Flow Metab. 2018, 38, 2073–2091. [Google Scholar] [CrossRef]
- Grotta, J. Why do all drugs work in animals but none in stroke patients? 2. Neuroprotective therapy. J. Intern. Med. 1995, 237, 89–94. [Google Scholar] [CrossRef]
- Lo, E.H. Experimental models, neurovascular mechanisms and translational issues in stroke research. Br. J. Pharmacol. 2008, 153, S396–S405. [Google Scholar] [CrossRef] [PubMed]
- Lo, E.H.; Moskowitz, M.A.; Jacobs, T.P. Exciting, radical, suicidal: How brain cells die after stroke. Stroke 2005, 36, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Sena, E.; van der Worp, H.B.; Howells, D.; Macleod, M. How can we improve the pre-clinical development of drugs for stroke? Trends Neurosci. 2007, 30, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Savitz, S.I. A critical appraisal of the NXY-059 neuroprotection studies for acute stroke: A need for more rigorous testing of neuroprotective agents in animal models of stroke. Exp. Neurol. 2007, 205, 20–25. [Google Scholar] [CrossRef]
- Macleod, M.R.; O’Collins, T.; Howells, D.W.; Donnan, G.A. Pooling of animal experimental data reveals influence of study design and publication bias. Stroke 2004, 35, 1203–1208. [Google Scholar] [CrossRef]
- Dirnagl, U. Bench to bedside: The quest for quality in experimental stroke research. J. Cereb. Blood Flow Metab. 2006, 26, 1465–1478. [Google Scholar] [CrossRef] [PubMed]
- Lyden, P.D. Cerebroprotection for Acute Ischemic Stroke: Looking Ahead. Stroke 2021, 52, 3033–3044. [Google Scholar] [CrossRef]
- Ioannidis, J.P. Why most published research findings are false. PLoS Med. 2005, 2, e124. [Google Scholar] [CrossRef]
- Schmidt-Pogoda, A.; Bonberg, N.; Koecke, M.H.M.; Strecker, J.K.; Wellmann, J.; Bruckmann, N.M.; Beuker, C.; Schäbitz, W.R.; Meuth, S.G.; Wiendl, H.; et al. Why Most Acute Stroke Studies Are Positive in Animals but Not in Patients: A Systematic Comparison of Preclinical, Early Phase, and Phase 3 Clinical Trials of Neuroprotective Agents. Ann. Neurol. 2020, 87, 40–51. [Google Scholar] [CrossRef]
- Minnerup, J.; Wersching, H.; Schilling, M.; Schabitz, W.R. Analysis of early phase and subsequent phase III stroke studies of neuroprotectants: Outcomes and predictors for success. Exp. Transl. Stroke Med. 2014, 6, 2–6. [Google Scholar] [CrossRef]
- Sena, E.S.; van der Worp, H.B.; Bath, P.M.; Howells, D.W.; Macleod, M.R. Publication bias in reports of animal stroke studies leads to major overstatement of efficacy. PLoS Biol. 2010, 8, e1000344. [Google Scholar] [CrossRef] [PubMed]
- Bath, P.M.; Macleod, M.R.; Green, A.R. Emulating multicentre clinical stroke trials: A new paradigm for studying novel interventions in experimental models of stroke. Int. J. Stroke 2009, 4, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Kent, T.A.; Mandava, P. Embracing Biological and Methodological Variance in a New Approach to Pre-Clinical Stroke Testing. Transl. Stroke Res. 2016, 7, 274–283. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dirnagl, U. Thomas Willis Lecture: Is Translational Stroke Research Broken, and if So, How Can We Fix It? Stroke 2016, 47, 2148–2153. [Google Scholar] [CrossRef]
- Dirnagl, U.; Fisher, M. International, multicenter randomized preclinical trials in translational stroke research: It’s time to act. J. Cereb. Blood Flow Metab. 2012, 32, 933–935. [Google Scholar] [CrossRef]
- Bosetti, F.; Koenig, J.I.; Ayata, C.; Back, S.A.; Becker, K.; Broderick, J.P.; Carmichael, S.T.; Cho, S.; Cipolla, M.J.; Corbett, D.; et al. Translational Stroke Research: Vision and Opportunities. Stroke 2017, 48, 2632–2637. [Google Scholar] [CrossRef]
- Mergenthaler, P.; Meisel, A. Do stroke models model stroke? Dis. Model. Mech. 2012, 5, 718–725. [Google Scholar] [CrossRef]
- Gelderblom, M.; Koch, S.; Strecker, J.K.; Jørgensen, C.; Garcia-Bonilla, L.; Ludewig, P.; Schädlich, I.S.; Piepke, M.; Degenhardt, K.; Bernreuther, C.; et al. A preclinical randomized controlled multi-centre trial of anti-interleukin-17A treatment for acute ischaemic stroke. Brain Commun. 2023, 5, fcad090. [Google Scholar] [CrossRef]
- Balduini, W.; Carloni, S.; Cimino, M. Preclinical randomized controlled multicenter trials (pRCT) in stroke research: A new and valid approach to improve translation? Ann. Transl. Med. 2016, 4, 549. [Google Scholar] [CrossRef]
- Llovera, G.; Hofmann, K.; Roth, S.; Salas-Pérdomo, A.; Ferrer-Ferrer, M.; Perego, C.; Zanier, E.R.; Mamrak, U.; Rex, A.; Party, H.; et al. Results of a preclinical randomized controlled multicenter trial (pRCT): Anti-CD49d treatment for acute brain ischemia. Sci. Transl. Med. 2015, 7, 299ra121. [Google Scholar] [CrossRef]
- Llovera, G.; Liesz, A. The next step in translational research: Lessons learned from the first preclinical randomized controlled trial. J. Neurochem. 2016, 139, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Maysami, S.; Wong, R.; Pradillo, J.M.; Denes, A.; Dhungana, H.; Malm, T.; Koistinaho, J.; Orset, C.; Rahman, M.; Rubio, M.; et al. A cross-laboratory preclinical study on the effectiveness of interleukin-1 receptor antagonist in stroke. J. Cereb. Blood Flow Metab. 2016, 36, 596–605. [Google Scholar] [CrossRef]
- Lyden, P.D.; Bosetti, F.; Diniz, M.A.; Rogatko, A.; Koenig, J.I.; Lamb, J.; Nagarkatti, K.A.; Cabeen, R.P.; Hess, D.C.; Kamat, P.K.; et al. The Stroke Preclinical Assessment Network: Rationale, Design, Feasibility, and Stage 1 Results. Stroke 2022, 53, 1802–1812. [Google Scholar] [CrossRef]
- Lyden, P.D.; Diniz, M.A.; Bosetti, F.; Lamb, J.; Nagarkatti, K.A.; Rogatko, A.; Kim, S.; Cabeen, R.P.; Koenig, J.I.; Akhter, K.; et al. A multi-laboratory preclinical trial in rodents to assess treatment candidates for acute ischemic stroke. Sci. Transl. Med. 2023, 15, eadg8656. [Google Scholar] [CrossRef] [PubMed]
- Nutt, D.J. The full cost and burden of disorders of the brain in Europe exposed for the first time. Eur. Neuropsychopharmacol. 2011, 21, 715–717. [Google Scholar] [CrossRef]
- Wegener, G.; Rujescu, D. The current development of CNS drug research. Int. J. Neuropsychopharmacol. 2013, 16, 1687–1693. [Google Scholar] [CrossRef]
- Fisher, M.; Feuerstein, G.; Howells, D.W.; Hurn, P.D.; Kent, T.A.; Savitz, S.I.; Lo, E.H. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke 2009, 40, 2244–2250. [Google Scholar] [CrossRef] [PubMed]
- Lyden, P.; Lapchak, P. Sisyphus and translational stroke research. Sci. Transl. Med. 2012, 4, 156ps120. [Google Scholar] [CrossRef]
- Kidwell, C.S.; Liebeskind, D.S.; Starkman, S.; Saver, J.L. Trends in acute ischemic stroke trials through the 20th century. Stroke 2001, 32, 1349–1359. [Google Scholar] [CrossRef]
- Choi, D.W.; Armitage, R.; Brady, L.S.; Coetzee, T.; Fisher, W.; Hyman, S.; Pande, A.; Paul, S.; Potter, W.; Roin, B.; et al. Medicines for the mind: Policy-based “pull” incentives for creating breakthrough CNS drugs. Neuron 2014, 84, 554–563. [Google Scholar] [CrossRef]
- DiMasi, J.A.; Grabowski, H.G.; Hansen, R.W. Innovation in the pharmaceutical industry: New estimates of R&D costs. J. Health Econ. 2016, 47, 20–33. [Google Scholar] [CrossRef] [PubMed]
- Lapchak, P.A.; Zhang, J.H. The High Cost of Stroke and Stroke Cytoprotection Research. Transl. Stroke Res. 2017, 8, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Moskowitz, M.A. Brain protection: Maybe yes, maybe no. Stroke 2010, 41, S85–S86. [Google Scholar] [CrossRef]
- Hoyte, L.; Barber, P.A.; Buchan, A.M.; Hill, M.D. The rise and fall of NMDA antagonists for ischemic stroke. Curr. Mol. Med. 2004, 4, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Green, A.R. Why do neuroprotective drugs that are so promising in animals fail in the clinic? An industry perspective. Clin. Exp. Pharmacol. Physiol. 2002, 29, 1030–1034. [Google Scholar] [CrossRef]
- O’Collins, V.E.; Macleod, M.R.; Donnan, G.A.; Horky, L.L.; van der Worp, B.H.; Howells, D.W. 1,026 experimental treatments in acute stroke. Ann. Neurol. 2006, 59, 467–477. [Google Scholar] [CrossRef]
- Jonas, S.; Aiyagari, V.; Vieira, D.; Figueroa, M. The failure of neuronal protective agents versus the success of thrombolysis in the treatment of ischemic stroke. The predictive value of animal models. Ann. N. Y. Acad. Sci. 2001, 939, 257–267. [Google Scholar] [CrossRef]
- Antonic, A.; Sena, E.S.; Donnan, G.A.; Howells, D.W. Human in vitro models of ischaemic stroke: A test bed for translation. Transl. Stroke Res. 2012, 3, 306–309. [Google Scholar] [CrossRef]
- Neuhaus, A.A.; Couch, Y.; Hadley, G.; Buchan, A.M. Neuroprotection in stroke: The importance of collaboration and reproducibility. Brain 2017, 140, 2079–2092. [Google Scholar] [CrossRef]
- Garber, K. Stroke treatment—Light at the end of the tunnel? Nat. Biotechnol. 2007, 25, 838–840. [Google Scholar] [CrossRef]
- Hill, J.W.; Thompson, J.F.; Carter, M.B.; Edwards, B.S.; Sklar, L.A.; Rosenberg, G.A. Identification of isoxsuprine hydrochloride as a neuroprotectant in ischemic stroke through cell-based high-throughput screening. PLoS ONE 2014, 9, e96761. [Google Scholar] [CrossRef] [PubMed]
- Rothstein, J.D.; Patel, S.; Regan, M.R.; Haenggeli, C.; Huang, Y.H.; Bergles, D.E.; Jin, L.; Dykes, H.M.; Vidensky, S.; Chung, D.S.; et al. Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature 2005, 433, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Endres, M.; Engelhardt, B.; Koistinaho, J.; Lindvall, O.; Meairs, S.; Mohr, J.P.; Planas, A.; Rothwell, N.; Schwaninger, M.; Schwab, M.E.; et al. Improving outcome after stroke: Overcoming the translational roadblock. Cerebrovasc. Dis. 2008, 25, 268–278. [Google Scholar] [CrossRef]
- Tauskela, J.S.; Bourourou, M.; Blondeau, N. Tackling issues in the path toward clinical translation in brain conditioning: Potential offered by nutraceuticals. Brain Circ. 2017, 3, 78–86. [Google Scholar] [CrossRef]
- Blondeau, N. The nutraceutical potential of omega-3 alpha-linolenic acid in reducing the consequences of stroke. Biochimie 2016, 120, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Lapchak, P.A. Emerging Therapies: Pleiotropic Multi-target Drugs to Treat Stroke Victims. Transl. Stroke Res. 2011, 2, 129–135. [Google Scholar] [CrossRef]
- Moskowitz, M.A.; Lo, E.H.; Iadecola, C. The science of stroke: Mechanisms in search of treatments. Neuron 2010, 67, 181–198. [Google Scholar] [CrossRef]
- Savitz, S.I.; Fisher, M. Future of neuroprotection for acute stroke: In the aftermath of the SAINT trials. Ann. Neurol. 2007, 61, 396–402. [Google Scholar] [CrossRef]
- Xu, J.; Wang, A.; Meng, X.; Yalkun, G.; Xu, A.; Gao, Z.; Chen, H.; Ji, Y.; Xu, J.; Geng, D.; et al. Edaravone Dexborneol Versus Edaravone Alone for the Treatment of Acute Ischemic Stroke: A Phase III, Randomized, Double-Blind, Comparative Trial. Stroke 2021, 52, 772–780. [Google Scholar] [CrossRef]
- Takahashi, H.; Xia, P.; Cui, J.; Talantova, M.; Bodhinathan, K.; Li, W.; Saleem, S.; Holland, E.A.; Tong, G.; Piña-Crespo, J.; et al. Pharmacologically targeted NMDA receptor antagonism by NitroMemantine for cerebrovascular disease. Sci. Rep. 2015, 5, 14781. [Google Scholar] [CrossRef]
- O’Collins, V.E.; Macleod, M.R.; Donnan, G.A.; Howells, D.W. Evaluation of combination therapy in animal models of cerebral ischemia. J. Cereb. Blood Flow Metab. 2012, 32, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Meairs, S.; Wahlgren, N.; Dirnagl, U.; Lindvall, O.; Rothwell, P.; Baron, J.C.; Hossmann, K.; Engelhardt, B.; Ferro, J.; McCulloch, J.; et al. Stroke research priorities for the next decade—A representative view of the European scientific community. Cerebrovasc. Dis. 2006, 22, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Lapchak, P.A. Translational Stroke Research Opportunities and a Strategy to Develop Effective Cytoprotection. Transl. Stroke Res. 2017, 8, 318–321. [Google Scholar] [CrossRef]
- Lyden, P.D.; Lamb, J.; Kothari, S.; Toossi, S.; Boitano, P.; Rajput, P.S. Differential effects of hypothermia on neurovascular unit determine protective or toxic results: Toward optimized therapeutic hypothermia. J. Cereb. Blood Flow Metab. 2019, 39, 1693–1709. [Google Scholar] [CrossRef]
- Rajput, P.; Brookshier, A.; Kothari, S.; Eckstein, L.; Chang, H.; Liska, S.; Lamb, J.; Sances, S.; Lyden, P. Differential Vulnerability and Response to Injury among Brain Cell Types Comprising the Neurovascular Unit. J. Neurosci. 2024, 44, e1093222024. [Google Scholar] [CrossRef]
- Goldberg, M.P.; Choi, D.W. Combined oxygen and glucose deprivation in cortical cell culture: Calcium-dependent and calcium-independent mechanisms of neuronal injury. J. Neurosci. 1993, 13, 3510–3524. [Google Scholar] [CrossRef]
- Inoue, M.; Tanida, T.; Kondo, T.; Takenaka, S.; Nakajima, T. Oxygen-glucose deprivation-induced glial cell reactivity in the rat primary neuron-glia co-culture. J. Vet. Med. Sci. 2023, 85, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Brookshier, A.; Lyden, P. Differential vulnerability among cell types in the neurovascular unit: Description and mechanisms. J. Cereb. Blood Flow Metab. 2025, 45, 3–12. [Google Scholar] [CrossRef]
- Medvedeva, Y.V.; Ji, S.G.; Yin, H.Z.; Weiss, J.H. Differential Vulnerability of CA1 versus CA3 Pyramidal Neurons After Ischemia: Possible Relationship to Sources of Zn2+ Accumulation and Its Entry into and Prolonged Effects on Mitochondria. J. Neurosci. 2017, 37, 726–737. [Google Scholar] [CrossRef]
- Meloni, B.P.; Majda, B.T.; Knuckey, N.W. Establishment of neuronal in vitro models of ischemia in 96-well microtiter strip-plates that result in acute, progressive and delayed neuronal death. Neuroscience 2001, 108, 17–26. [Google Scholar] [CrossRef]
- Tauskela, J.S.; Comas, T.; Hewitt, K.; Monette, R.; Paris, J.; Hogan, M.; Morley, P. Cross-tolerance to otherwise lethal N-methyl-D-aspartate and oxygen-glucose deprivation in preconditioned cortical cultures. Neuroscience 2001, 107, 571–584. [Google Scholar] [CrossRef] [PubMed]
- Cronberg, T.; Rytter, A.; Asztely, F.; Soder, A.; Wieloch, T. Glucose but not lactate in combination with acidosis aggravates ischemic neuronal death in vitro. Stroke 2004, 35, 753–757. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Sun, X.; Gu, X.; Wang, J.; Haddad, G.G. Cell death in an ischemic infarct rim model. J. Neurochem. 2007, 103, 1644–1653. [Google Scholar] [CrossRef]
- Rytter, A.; Cronberg, T.; Asztely, F.; Nemali, S.; Wieloch, T. Mouse hippocampal organotypic tissue cultures exposed to in vitro “ischemia” show selective and delayed CA1 damage that is aggravated by glucose. J. Cereb. Blood Flow Metab. 2003, 23, 23–33. [Google Scholar] [CrossRef]
- Tauskela, J.S.; Brunette, E.; Aylsworth, A.; Zhao, X. Neuroprotection against supra-lethal ‘stroke in a dish’ insults by an anti-excitotoxic receptor antagonist cocktail. Neurochem. Int. 2022, 158, 105381. [Google Scholar] [CrossRef]
- Szydlowska, K.; Tymianski, M. Calcium, ischemia and excitotoxicity. Cell Calcium 2010, 47, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.W. Excitotoxicity: Still Hammering the Ischemic Brain in 2020. Front. Neurosci. 2020, 14, 579953. [Google Scholar] [CrossRef]
- Tauskela, J.S.; Aylsworth, A.; Hewitt, M.; Brunette, E.; Blondeau, N. Failure and rescue of preconditioning-induced neuroprotection in severe stroke-like insults. Neuropharmacology 2016, 105, 533–542. [Google Scholar] [CrossRef]
- Tauskela, J.S.; Mealing, G.; Comas, T.; Brunette, E.; Monette, R.; Small, D.L.; Morley, P. Protection of cortical neurons against oxygen-glucose deprivation and N-methyl-D-aspartate by DIDS and SITS. Eur. J. Pharmacol. 2003, 464, 17–25. [Google Scholar] [CrossRef]
- Tauskela, J.S.; Brunette, E. Neuroprotection against staurosporine by metalloporphyrins independent of antioxidant capability. Neurosci. Lett. 2009, 466, 41–46. [Google Scholar] [CrossRef]
- Tauskela, J.S.; Brunette, E.; Hewitt, M.; Mealing, G.; Morley, P. Competing approaches to excitotoxic neuroprotection by inert and catalytic antioxidant porphyrins. Neurosci. Lett. 2006, 401, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Tauskela, J.S.; Brunette, E.; Kiedrowski, L.; Lortie, K.; Hewitt, M.; Morley, P. Unconventional neuroprotection against Ca2+ -dependent insults by metalloporphyrin catalytic antioxidants. J. Neurochem. 2006, 98, 1324–1342. [Google Scholar] [CrossRef] [PubMed]
- Tauskela, J.S.; Brunette, E.; O’Reilly, N.; Mealing, G.; Comas, T.; Gendron, T.F.; Monette, R.; Morley, P. An alternative Ca2+-dependent mechanism of neuroprotection by the metalloporphyrin class of superoxide dismutase mimetics. FASEB J. 2005, 19, 1734–1736. [Google Scholar] [CrossRef] [PubMed]
- Salinska, E.; Sobczuk, A.; Lazarewicz, J.W. Dantrolene antagonizes the glycineB site of the NMDA receptor. Neurosci. Lett. 2008, 432, 137–140. [Google Scholar] [CrossRef]
- Chepkova, A.N.; Sergeeva, O.A.; Haas, H.L. Carbenoxolone impairs LTP and blocks NMDA receptors in murine hippocampus. Neuropharmacology 2008, 55, 139–147. [Google Scholar] [CrossRef]
- Tauskela, J.S.; Chakravarthy, B.R.; Murray, C.L.; Wang, Y.; Comas, T.; Hogan, M.; Hakim, A.; Morley, P. Evidence from cultured rat cortical neurons of differences in the mechanism of ischemic preconditioning of brain and heart. Brain Res. 1999, 827, 143–151. [Google Scholar] [CrossRef]
- Tauskela, J.S.; Brunette, E.; Monette, R.; Comas, T.; Morley, P. Preconditioning of cortical neurons by oxygen-glucose deprivation: Tolerance induction through abbreviated neurotoxic signaling. Am. J. Physiol. Cell Physiol. 2003, 285, C899–C911. [Google Scholar] [CrossRef]
- Grabb, M.C.; Lobner, D.; Turetsky, D.M.; Choi, D.W. Preconditioned resistance to oxygen-glucose deprivation-induced cortical neuronal death: Alterations in vesicular GABA and glutamate release. Neuroscience 2002, 115, 173–183. [Google Scholar] [CrossRef]
- Kosugi, T.; Kawahara, K.; Yamada, T.; Nakajima, T.; Tanaka, M. Functional significance of the preconditioning-induced down-regulation of glutamate transporter GLT-1 in neuron/astrocyte co-cultures. Neurochem. Res. 2005, 30, 1109–1116. [Google Scholar] [CrossRef]
- Yamada, T.; Kawahara, K.; Kosugi, T.; Tanaka, M. Nitric oxide produced during sublethal ischemia is crucial for the preconditioning-induced down-regulation of glutamate transporter GLT-1 in neuron/astrocyte co-cultures. Neurochem. Res. 2006, 31, 49–56. [Google Scholar] [CrossRef]
- Tauskela, J.S.; Kuebler, E.S.; Thivierge, J.P.; Aylsworth, A.; Hewitt, M.; Zhao, X.; Mielke, J.G.; Martina, M. Resilience of network activity in preconditioned neurons exposed to ‘stroke-in-a-dish’ insults. Neurochem. Int. 2021, 146, 105035. [Google Scholar] [CrossRef] [PubMed]
- Tauskela, J.S.; Fang, H.; Hewitt, M.; Brunette, E.; Ahuja, T.; Thivierge, J.P.; Comas, T.; Mealing, G.A. Elevated synaptic activity preconditions neurons against an in vitro model of ischemia. J. Biol. Chem. 2008, 283, 34667–34676. [Google Scholar] [CrossRef] [PubMed]
- Tauskela, J.S.; Aylsworth, A.; Hewitt, M.; Brunette, E.; Mealing, G.A. Preconditioning induces tolerance by suppressing glutamate release in neuron culture ischemia models. J. Neurochem. 2012, 122, 470–481. [Google Scholar] [CrossRef] [PubMed]
- Abe, H.; Nowak, T.S., Jr. Induced hippocampal neuron protection in an optimized gerbil ischemia model: Insult thresholds for tolerance induction and altered gene expression defined by ischemic depolarization. J. Cereb. Blood Flow Metab. 2004, 24, 84–97. [Google Scholar] [CrossRef]
- Ueda, M.; Nowak, T.S., Jr. Protective preconditioning by transient global ischemia in the rat: Components of delayed injury progression and lasting protection distinguished by comparisons of depolarization thresholds for cell loss at long survival times. J. Cereb. Blood Flow Metab. 2005, 25, 949–958. [Google Scholar] [CrossRef]
- Frankiewicz, T.; Pilc, A.; Parsons, C.G. Differential effects of NMDA-receptor antagonists on long-term potentiation and hypoxic/hypoglycaemic excitotoxicity in hippocampal slices. Neuropharmacology 2000, 39, 631–642. [Google Scholar] [CrossRef]
- Grigg, J.J.; Anderson, E.G. Competitive and noncompetitive N-methyl-D-aspartate antagonists modify hypoxia-induced membrane potential changes and protect rat hippocampal slices from functional failure: A quantitative comparison. J. Pharmacol. Exp. Ther. 1990, 253, 130–135. [Google Scholar] [CrossRef]
- Priestley, T.; Horne, A.L.; McKernan, R.M.; Kemp, J.A. The effect of NMDA receptor glycine site antagonists on hypoxia-induced neurodegeneration of rat cortical cell cultures. Brain Res. 1990, 531, 183–188. [Google Scholar] [CrossRef]
- Pringle, A.K.; Iannotti, F.; Wilde, G.J.; Chad, J.E.; Seeley, P.J.; Sundstrom, L.E. Neuroprotection by both NMDA and non-NMDA receptor antagonists in in vitro ischemia. Brain Res. 1997, 755, 36–46. [Google Scholar] [CrossRef]
- Schurr, A.; Payne, R.S.; Rigor, B.M. Protection by MK-801 against hypoxia-, excitotoxin-, and depolarization-induced neuronal damage in vitro. Neurochem. Int. 1995, 26, 519–525. [Google Scholar] [CrossRef]
- Virgili, M.; Contestabile, A.; Barnabei, O. Simultaneous blockade of non-NMDA ionotropic receptors and NMDA receptor-associated ionophore partially protects hippocampal slices from protein synthesis impairment due to simulated ischemia. Hippocampus 1995, 5, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Pringle, A.K.; Self, J.; Eshak, M.; Iannotti, F. Reducing conditions significantly attenuate the neuroprotective efficacy of competitive, but not other NMDA receptor antagonists in vitro. Eur. J. Neurosci. 2000, 12, 3833–3842. [Google Scholar] [CrossRef] [PubMed]
- Ahlgren, H.; Henjum, K.; Ottersen, O.P.; Rundén-Pran, E. Validation of organotypical hippocampal slice cultures as an ex vivo model of brain ischemia: Different roles of NMDA receptors in cell death signalling after exposure to NMDA or oxygen and glucose deprivation. Cell Tissue Res. 2011, 345, 329–341. [Google Scholar] [CrossRef]
- Arias, R.L.; Tasse, J.R.; Bowlby, M.R. Neuroprotective interaction effects of NMDA and AMPA receptor antagonists in an in vitro model of cerebral ischemia. Brain Res. 1999, 816, 299–308. [Google Scholar] [CrossRef]
- Schurr, A. Neuroprotection against ischemic/hypoxic brain damage: Blockers of ionotropic glutamate receptor and voltage sensitive calcium channels. Curr. Drug Targets 2004, 5, 603–618. [Google Scholar] [CrossRef]
- Strasser, U.; Fischer, G. Protection from neuronal damage induced by combined oxygen and glucose deprivation in organotypic hippocampal cultures by glutamate receptor antagonists. Brain Res. 1995, 687, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Aarts, M.; Iihara, K.; Wei, W.L.; Xiong, Z.G.; Arundine, M.; Cerwinski, W.; MacDonald, J.F.; Tymianski, M. A key role for TRPM7 channels in anoxic neuronal death. Cell 2003, 115, 863–877. [Google Scholar] [CrossRef]
- Buddle, M.; Eberhardt, E.; Ciminello, L.H.; Levin, T.; Wing, R.; DiPasquale, K.; Raley-Susman, K.M. Microtubule-associated protein 2 (MAP2) associates with the NMDA receptor and is spatially redistributed within rat hippocampal neurons after oxygen-glucose deprivation. Brain Res. 2003, 978, 38–50. [Google Scholar] [CrossRef]
- Kaku, D.A.; Goldberg, M.P.; Choi, D.W. Antagonism of non-NMDA receptors augments the neuroprotective effect of NMDA receptor blockade in cortical cultures subjected to prolonged deprivation of oxygen and glucose. Brain Res. 1991, 554, 344–347. [Google Scholar] [CrossRef]
- Katsuki, H.; Nonaka, M.; Shirakawa, H.; Kume, T.; Akaike, A. Endogenous D-serine is involved in induction of neuronal death by N-methyl-D-aspartate and simulated ischemia in rat cerebrocortical slices. J. Pharmacol. Exp. Ther. 2004, 311, 836–844. [Google Scholar] [CrossRef]
- Kaku, D.A.; Giffard, R.G.; Choi, D.W. Neuroprotective effects of glutamate antagonists and extracellular acidity. Science 1993, 260, 1516–1518. [Google Scholar] [CrossRef] [PubMed]
- Vandame, D.; Desmadryl, G.; Becerril Ortega, J.; Teigell, M.; Crouzin, N.; Buisson, A.; Privat, A.; Hirbec, H. Comparison of the pharmacological properties of GK11 and MK801, two NMDA receptor antagonists: Towards an explanation for the lack of intrinsic neurotoxicity of GK11. J. Neurochem. 2007, 103, 1682–1696. [Google Scholar] [CrossRef] [PubMed]
- McQuate, A.; Barria, A. Rapid exchange of synaptic and extrasynaptic NMDA receptors in hippocampal CA1 neurons. J. Neurophysiol. 2020, 123, 1004–1014. [Google Scholar] [CrossRef]
- Möckel, V.; Fischer, G. Vulnerability to excitotoxic stimuli of cultured rat hippocampal neurons containing the calcium-binding proteins calretinin and calbindin D28K. Brain Res. 1994, 648, 109–120. [Google Scholar] [CrossRef]
- Vahabzadeh, G.; Rahbar-Roshandel, N.; Ebrahimi, S.A.; Mahmoudian, M. Neuroprotective effect of noscapine on cerebral oxygen-glucose deprivation injury. Pharmacol. Rep. 2015, 67, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Vornov, J.J.; Thomas, A.G.; Jo, D. Protective effects of extracellular acidosis and blockade of sodium/hydrogen ion exchange during recovery from metabolic inhibition in neuronal tissue culture. J. Neurochem. 1996, 67, 2379–2389. [Google Scholar] [CrossRef]
- Buchan, A.; Li, H.; Pulsinelli, W.A. The N-methyl-D-aspartate antagonist, MK-801, fails to protect against neuronal damage caused by transient, severe forebrain ischemia in adult rats. J. Neurosci. 1991, 11, 1049–1056. [Google Scholar] [CrossRef]
- Nellgård, B.; Gustafson, I.; Wieloch, T. Lack of protection by the N-methyl-D-aspartate receptor blocker dizocilpine (MK-801) after transient severe cerebral ischemia in the rat. Anesthesiology 1991, 75, 279–287. [Google Scholar] [CrossRef]
- Obeidat, A.S.; Jarvis, C.R.; Andrew, R.D. Glutamate does not mediate acute neuronal damage after spreading depression induced by O2/glucose deprivation in the hippocampal slice. J. Cereb. Blood Flow Metab. 2000, 20, 412–422. [Google Scholar] [CrossRef]
- Newell, D.W.; Barth, A.; Papermaster, V.; Malouf, A.T. Glutamate and non-glutamate receptor mediated toxicity caused by oxygen and glucose deprivation in organotypic hippocampal cultures. J. Neurosci. 1995, 15, 7702–7711. [Google Scholar] [CrossRef]
- Gwag, B.J.; Lobner, D.; Koh, J.Y.; Wie, M.B.; Choi, D.W. Blockade of glutamate receptors unmasks neuronal apoptosis after oxygen-glucose deprivation in vitro. Neuroscience 1995, 68, 615–619. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M.; Sawada, K.; Miyagawa, T.; Kuwada, M.; Katayama, K.; Nishizawa, Y. Role of glutamate receptors and voltage-dependent calcium and sodium channels in the extracellular glutamate/aspartate accumulation and subsequent neuronal injury induced by oxygen/glucose deprivation in cultured hippocampal neurons. J. Pharmacol. Exp. Ther. 1998, 285, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Lobner, D.; Lipton, P. Intracellular calcium levels and calcium fluxes in the CA1 region of the rat hippocampal slice during in vitro ischemia: Relationship to electrophysiological cell damage. J. Neurosci. 1993, 13, 4861–4871. [Google Scholar] [CrossRef] [PubMed]
- Mosinger, J.L.; Price, M.T.; Bai, H.Y.; Xiao, H.; Wozniak, D.F.; Olney, J.W. Blockade of both NMDA and non-NMDA receptors is required for optimal protection against ischemic neuronal degeneration in the in vivo adult mammalian retina. Exp. Neurol. 1991, 113, 10–17. [Google Scholar] [CrossRef]
- Noh, J.H.; Gwag, B.J.; Chung, J.M. Underlying mechanism for NMDA receptor antagonism by the anti-inflammatory drug, sulfasalazine, in mouse cortical neurons. Neuropharmacology 2006, 50, 1–15. [Google Scholar] [CrossRef]
- Revah, O.; Lasser-Katz, E.; Fleidervish, I.A.; Gutnick, M.J. The earliest neuronal responses to hypoxia in the neocortical circuit are glutamate-dependent. Neurobiol. Dis. 2016, 95, 158–167. [Google Scholar] [CrossRef]
- Seo, S.Y.; Kim, E.Y.; Kim, H.; Gwag, B.J. Neuroprotective effect of high glucose against NMDA, free radical, and oxygen-glucose deprivation through enhanced mitochondrial potentials. J. Neurosci. 1999, 19, 8849–8855. [Google Scholar] [CrossRef]
- Stork, C.J.; Li, Y.V. Rising zinc: A significant cause of ischemic neuronal death in the CA1 region of rat hippocampus. J. Cereb. Blood Flow Metab. 2009, 29, 1399–1408. [Google Scholar] [CrossRef]
- Lynch, J.J.; Yu, S.P.; Canzoniero, L.M.; Sensi, S.L.; Choi, D.W. Sodium channel blockers reduce oxygen-glucose deprivation-induced cortical neuronal injury when combined with glutamate receptor antagonists. J. Pharmacol. Exp. Ther. 1995, 273, 554–560. [Google Scholar] [CrossRef]
- Reboucas, J.S.; Spasojevic, I.; Batinic-Haberle, I. Pure manganese(III) 5,10,15,20-tetrakis(4-benzoic acid)porphyrin (MnTBAP) is not a superoxide dismutase mimic in aqueous systems: A case of structure-activity relationship as a watchdog mechanism in experimental therapeutics and biology. J. Biol. Inorg. Chem. 2008, 13, 289–302. [Google Scholar] [CrossRef]
- Andrew, R.D.; Hartings, J.A.; Ayata, C.; Brennan, K.C.; Dawson-Scully, K.D.; Farkas, E.; Herreras, O.; Kirov, S.A.; Müller, M.; Ollen-Bittle, N.; et al. The Critical Role of Spreading Depolarizations in Early Brain Injury: Consensus and Contention. Neurocrit Care 2022, 37, 83–101. [Google Scholar] [CrossRef] [PubMed]
- Andrew, R.D.; Farkas, E.; Hartings, J.A.; Brennan, K.C.; Herreras, O.; Müller, M.; Kirov, S.A.; Ayata, C.; Ollen-Bittle, N.; Reiffurth, C.; et al. Questioning Glutamate Excitotoxicity in Acute Brain Damage: The Importance of Spreading Depolarization. Neurocrit Care 2022, 37, 11–30. [Google Scholar] [CrossRef] [PubMed]
- Heit, B.S.; Chu, A.; Sane, A.; Featherstone, D.E.; Park, T.J.; Larson, J. Tonic extracellular glutamate and ischaemia: Glutamate antiporter system x(c) (-) regulates anoxic depolarization in hippocampus. J. Physiol. 2023, 601, 607–629. [Google Scholar] [CrossRef] [PubMed]
- Heit, B.S.; Dykas, P.; Chu, A.; Sane, A.; Larson, J. Synaptic and Network Contributions to Anoxic Depolarization in Mouse Hippocampal Slices. Neuroscience 2021, 461, 102–117. [Google Scholar] [CrossRef]
- Madry, C.; Haglerød, C.; Attwell, D. The role of pannexin hemichannels in the anoxic depolarization of hippocampal pyramidal cells. Brain 2010, 133, 3755–3763. [Google Scholar] [CrossRef]
- Bickler, P.E.; Hansen, B.M. Causes of calcium accumulation in rat cortical brain slices during hypoxia and ischemia: Role of ion channels and membrane damage. Brain Res. 1994, 665, 269–276. [Google Scholar] [CrossRef]
- Martínez-Sánchez, M.; Striggow, F.; Schröder, U.H.; Kahlert, S.; Reymann, K.G.; Reiser, G. Na+ and Ca2+ homeostasis pathways, cell death and protection after oxygen-glucose-deprivation in organotypic hippocampal slice cultures. Neuroscience 2004, 128, 729–740. [Google Scholar] [CrossRef]
- Kubo, T.; Yokoi, T.; Hagiwara, Y.; Fukumori, R.; Goshima, Y.; Misu, Y. Characteristics of protective effects of NMDA antagonist and calcium channel antagonist on ischemic calcium accumulation in rat hippocampal CA1 region. Brain Res. Bull. 2001, 54, 413–419. [Google Scholar] [CrossRef]
- LoPachin, R.M.; Gaughan, C.L.; Lehning, E.J.; Weber, M.L.; Taylor, C.P. Effects of ion channel blockade on the distribution of Na, K, Ca and other elements in oxygen-glucose deprived CA1 hippocampal neurons. Neuroscience 2001, 103, 971–983. [Google Scholar] [CrossRef]
- Zhang, Y.; Lipton, P. Cytosolic Ca2+ changes during in vitro ischemia in rat hippocampal slices: Major roles for glutamate and Na+-dependent Ca2+ release from mitochondria. J. Neurosci. 1999, 19, 3307–3315. [Google Scholar] [CrossRef]
- Soria, F.N.; Pérez-Samartín, A.; Martin, A.; Gona, K.B.; Llop, J.; Szczupak, B.; Chara, J.C.; Matute, C.; Domercq, M. Extrasynaptic glutamate release through cystine/glutamate antiporter contributes to ischemic damage. J. Clin. Investig. 2014, 124, 3645–3655. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Zipfel, G.J.; Choi, D.W. The changing landscape of ischaemic brain injury mechanisms. Nature 1999, 399, A7–A14. [Google Scholar] [CrossRef] [PubMed]
- Nelson, A.N.; Calhoun, M.S.; Thomas, A.M.; Tavares, J.L.; Ferretti, D.M.; Dillon, G.M.; Mandelblat-Cerf, Y. Temporal Progression of Excitotoxic Calcium Following Distal Middle Cerebral Artery Occlusion in Freely Moving Mice. Front. Cell Neurosci. 2020, 14, 566789. [Google Scholar] [CrossRef]
- Wang, E.Y.; Lai, T.W. Drug-Induced Hyperglycemia as a Potential Contributor to Translational Failure of Uncompetitive NMDA Receptor Antagonists. Eneuro 2021, 8. [Google Scholar] [CrossRef]
- Liu, C.W.; Liao, K.H.; Tseng, H.; Wu, C.M.; Chen, H.Y.; Lai, T.W. Hypothermia but not NMDA receptor antagonism protects against stroke induced by distal middle cerebral arterial occlusion in mice. PLoS ONE 2020, 15, e0229499. [Google Scholar] [CrossRef]
- Murphy, T.H.; Li, P.; Betts, K.; Liu, R. Two-photon imaging of stroke onset in vivo reveals that NMDA-receptor independent ischemic depolarization is the major cause of rapid reversible damage to dendrites and spines. J. Neurosci. 2008, 28, 1756–1772. [Google Scholar] [CrossRef] [PubMed]
- Maier, C.M.; Sun, G.H.; Kunis, D.M.; Giffard, R.G.; Steinberg, G.K. Neuroprotection by the N-methyl-D-aspartate receptor antagonist CGP 40116: In vivo and in vitro studies. J. Neurochem. 1995, 65, 652–659. [Google Scholar] [CrossRef]
- Pérez-Pinzón, M.A.; Maier, C.M.; Yoon, E.J.; Sun, G.H.; Giffard, R.G.; Steinberg, G.K. Correlation of CGS 19755 neuroprotection against in vitro excitotoxicity and focal cerebral ischemia. J. Cereb. Blood Flow Metab. 1995, 15, 865–876. [Google Scholar] [CrossRef]
- Ohtani, K.; Tanaka, H.; Yoneda, Y.; Yasuda, H.; Ito, A.; Nagata, R.; Nakamura, M. In vitro and in vivo antagonistic activities of SM-31900 for the NMDA receptor glycine-binding site. Brain Res. 2002, 944, 165–173. [Google Scholar] [CrossRef]
- Novelli, A.; Reilly, J.A.; Lysko, P.G.; Henneberry, R.C. Glutamate becomes neurotoxic via the N-methyl-D-aspartate receptor when intracellular energy levels are reduced. Brain Res. 1988, 451, 205–212. [Google Scholar] [CrossRef]
- Henneberry, R.C. The role of neuronal energy in the neurotoxicity of excitatory amino acids. Neurobiol. Aging 1989, 10, 611–613. [Google Scholar] [CrossRef] [PubMed]
- Thorn, T.L.; He, Y.; Jackman, N.A.; Lobner, D.; Hewett, J.A.; Hewett, S.J. A Cytotoxic, Co-operative Interaction Between Energy Deprivation and Glutamate Release From System xc- Mediates Aglycemic Neuronal Cell Death. ASN Neuro 2015, 7, 1759091415614301. [Google Scholar] [CrossRef] [PubMed]
- Frandsen, A.; Drejer, J.; Schousboe, A. Direct evidence that excitotoxicity in cultured neurons is mediated via N-methyl-D-aspartate (NMDA) as well as non-NMDA receptors. J. Neurochem. 1989, 53, 297–299. [Google Scholar] [CrossRef]
- Munns, S.E.; Meloni, B.P.; Knuckey, N.W.; Arthur, P.G. Primary cortical neuronal cultures reduce cellular energy utilization during anoxic energy deprivation. J. Neurochem. 2003, 87, 764–772. [Google Scholar] [CrossRef]
- Kass, I.S.; Lipton, P. Protection of hippocampal slices from young rats against anoxic transmission damage is due to better maintenance of ATP. J. Physiol. 1989, 413, 1–11. [Google Scholar] [CrossRef]
- Kass, I.S.; Chambers, G.; Cottrell, J.E. The N-methyl-D-aspartate antagonists aminophosphonovaleric acid and MK-801 reduce anoxic damage to dentate granule and CA1 pyramidal cells in the rat hippocampal slice. Exp. Neurol. 1989, 103, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Ziebarth, T.; Pape, N.; Nelson, J.S.E.; van Alphen, F.I.M.; Kalia, M.; Meijer, H.G.E.; Rose, C.R.; Reiner, A. Atypical plume-like events contribute to glutamate accumulation in metabolic stress conditions. iScience 2025, 28, 112256. [Google Scholar] [CrossRef]
- Besancon, E.; Guo, S.; Lok, J.; Tymianski, M.; Lo, E.H. Beyond NMDA and AMPA glutamate receptors: Emerging mechanisms for ionic imbalance and cell death in stroke. Trends Pharmacol. Sci. 2008, 29, 268–275. [Google Scholar] [CrossRef]
- Medvedeva, Y.V.; Lin, B.; Shuttleworth, C.W.; Weiss, J.H. Intracellular Zn2+ accumulation contributes to synaptic failure, mitochondrial depolarization, and cell death in an acute slice oxygen-glucose deprivation model of ischemia. J. Neurosci. 2009, 29, 1105–1114. [Google Scholar] [CrossRef]
- Pivovarova, N.B.; Stanika, R.I.; Kazanina, G.; Villanueva, I.; Andrews, S.B. The interactive roles of zinc and calcium in mitochondrial dysfunction and neurodegeneration. J. Neurochem. 2014, 128, 592–602. [Google Scholar] [CrossRef]
- Deshpande, L.S.; Limbrick, D.D., Jr.; Sombati, S.; DeLorenzo, R.J. Activation of a novel injury-induced calcium-permeable channel that plays a key role in causing extended neuronal depolarization and initiating neuronal death in excitotoxic neuronal injury. J. Pharmacol. Exp. Ther. 2007, 322, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Foster, A.C.; Kemp, J.A. Glutamate- and GABA-based CNS therapeutics. Curr. Opin. Pharmacol. 2006, 6, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Gardoni, F.; Di Luca, M. New targets for pharmacological intervention in the glutamatergic synapse. Eur. J. Pharmacol. 2006, 545, 2–10. [Google Scholar] [CrossRef]
- Sattler, R.; Xiong, Z.; Lu, W.Y.; Hafner, M.; MacDonald, J.F.; Tymianski, M. Specific coupling of NMDA receptor activation to nitric oxide neurotoxicity by PSD-95 protein. Science 1999, 284, 1845–1848. [Google Scholar] [CrossRef]
- Sattler, R.; Charlton, M.P.; Hafner, M.; Tymianski, M. Distinct influx pathways, not calcium load, determine neuronal vulnerability to calcium neurotoxicity. J. Neurochem. 1998, 71, 2349–2364. [Google Scholar] [CrossRef]
- Tymianski, M.; Charlton, M.P.; Carlen, P.L.; Tator, C.H. Source specificity of early calcium neurotoxicity in cultured embryonic spinal neurons. J. Neurosci. 1993, 13, 2085–2104. [Google Scholar] [CrossRef]
- Stanika, R.I.; Villanueva, I.; Kazanina, G.; Andrews, S.B.; Pivovarova, N.B. Comparative impact of voltage-gated calcium channels and NMDA receptors on mitochondria-mediated neuronal injury. J. Neurosci. 2012, 32, 6642–6650. [Google Scholar] [CrossRef]
- Lai, T.W.; Shyu, W.C.; Wang, Y.T. Stroke intervention pathways: NMDA receptors and beyond. Trends Mol. Med. 2011, 17, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Aarts, M.; Liu, Y.; Liu, L.; Besshoh, S.; Arundine, M.; Gurd, J.W.; Wang, Y.T.; Salter, M.W.; Tymianski, M. Treatment of ischemic brain damage by perturbing NMDA receptor- PSD-95 protein interactions. Science 2002, 298, 846–850. [Google Scholar] [CrossRef]
- Hardingham, G.E.; Bading, H. Synaptic versus extrasynaptic NMDA receptor signalling: Implications for neurodegenerative disorders. Nat. Rev. Neurosci. 2010, 11, 682–696. [Google Scholar] [CrossRef]
- Hardingham, G.E.; Fukunaga, Y.; Bading, H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat. Neurosci. 2002, 5, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Stanika, R.I.; Pivovarova, N.B.; Brantner, C.A.; Watts, C.A.; Winters, C.A.; Andrews, S.B. Coupling diverse routes of calcium entry to mitochondrial dysfunction and glutamate excitotoxicity. Proc. Natl. Acad. Sci. USA 2009, 106, 9854–9859. [Google Scholar] [CrossRef] [PubMed]
- Lipton, S.A. Pathologically activated therapeutics for neuroprotection. Nat. Rev. Neurosci. 2007, 8, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Xia, P.; Chen, H.S.; Zhang, D.; Lipton, S.A. Memantine preferentially blocks extrasynaptic over synaptic NMDA receptor currents in hippocampal autapses. J. Neurosci. 2010, 30, 11246–11250. [Google Scholar] [CrossRef]
- Volbracht, C.; van Beek, J.; Zhu, C.; Blomgren, K.; Leist, M. Neuroprotective properties of memantine in different in vitro and in vivo models of excitotoxicity. Eur. J. Neurosci. 2006, 23, 2611–2622. [Google Scholar] [CrossRef]
- Myers, S.J.; Ruppa, K.P.; Wilson, L.J.; Tahirovic, Y.A.; Lyuboslavsky, P.; Menaldino, D.S.; Dentmon, Z.W.; Koszalka, G.W.; Zaczek, R.; Dingledine, R.J.; et al. A Glutamate N-Methyl-d-Aspartate (NMDA) Receptor Subunit 2B-Selective Inhibitor of NMDA Receptor Function with Enhanced Potency at Acidic pH and Oral Bioavailability for Clinical Use. J. Pharmacol. Exp. Ther. 2021, 379, 41–52. [Google Scholar] [CrossRef]
- Yuan, H.; Myers, S.J.; Wells, G.; Nicholson, K.L.; Swanger, S.A.; Lyuboslavsky, P.; Tahirovic, Y.A.; Menaldino, D.S.; Ganesh, T.; Wilson, L.J.; et al. Context-dependent GluN2B-selective inhibitors of NMDA receptor function are neuroprotective with minimal side effects. Neuron 2015, 85, 1305–1318. [Google Scholar] [CrossRef]
- Zaczek, R.; Traynelis, S.F.; Dingledine, R.; Koszalka, G.W.; Laskowitz, D.T. Phase 1 Clinical Results for NP10679, a pH-sensitive GluN2B-selective N-methyl-d-aspartate Receptor Inhibitor. Clin. Pharmacol. Drug Dev. 2023, 12, 706–717. [Google Scholar] [CrossRef]
- Liu, Y.; Wong, T.P.; Aarts, M.; Rooyakkers, A.; Liu, L.; Lai, T.W.; Wu, D.C.; Lu, J.; Tymianski, M.; Craig, A.M.; et al. NMDA receptor subunits have differential roles in mediating excitotoxic neuronal death both in vitro and in vivo. J. Neurosci. 2007, 27, 2846–2857. [Google Scholar] [CrossRef]
- Thomas, C.G.; Miller, A.J.; Westbrook, G.L. Synaptic and extrasynaptic NMDA receptor NR2 subunits in cultured hippocampal neurons. J. Neurophysiol. 2006, 95, 1727–1734. [Google Scholar] [CrossRef]
- Martel, M.A.; Wyllie, D.J.; Hardingham, G.E. In developing hippocampal neurons, NR2B-containing N-methyl-d-aspartate receptors (NMDARs) can mediate signaling to neuronal survival and synaptic potentiation, as well as neuronal death. Neuroscience 2008, 158, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Hartley, D.M.; Kurth, M.C.; Bjerkness, L.; Weiss, J.H.; Choi, D.W. Glutamate receptor-induced 45Ca2+ accumulation in cortical cell culture correlates with subsequent neuronal degeneration. J. Neurosci. 1993, 13, 1993–2000. [Google Scholar] [CrossRef]
- Cheng, C.; Fass, D.M.; Reynolds, I.J. Emergence of excitotoxicity in cultured forebrain neurons coincides with larger glutamate-stimulated Ca2+(i increases and NMDA receptor mRNA levels. Brain Res. 1999, 849, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Hardingham, G.E.; Bading, H. The Yin and Yang of NMDA receptor signalling. Trends Neurosci. 2003, 26, 81–89. [Google Scholar] [CrossRef]
- Hetman, M.; Kharebava, G. Survival signaling pathways activated by NMDA receptors. Curr. Top. Med. Chem. 2006, 6, 787–799. [Google Scholar] [CrossRef] [PubMed]
- Ikonomidou, C.; Turski, L. Why did NMDA receptor antagonists fail clinical trials for stroke and traumatic brain injury? Lancet Neurol. 2002, 1, 383–386. [Google Scholar] [CrossRef]
- Stanika, R.I.; Winters, C.A.; Pivovarova, N.B.; Andrews, S.B. Differential NMDA receptor-dependent calcium loading and mitochondrial dysfunction in CA1 vs. CA3 hippocampal neurons. Neurobiol. Dis. 2010, 37, 403–411. [Google Scholar] [CrossRef]
- Hyrc, K.; Handran, S.D.; Rothman, S.M.; Goldberg, M.P. Ionized intracellular calcium concentration predicts excitotoxic neuronal death: Observations with low-affinity fluorescent calcium indicators. J. Neurosci. 1997, 17, 6669–6677. [Google Scholar] [CrossRef]
- Stout, A.K.; Reynolds, I.J. High-affinity calcium indicators underestimate increases in intracellular calcium concentrations associated with excitotoxic glutamate stimulations. Neuroscience 1999, 89, 91–100. [Google Scholar] [CrossRef]
- Seo, S.Y.; Yun, B.S.; Ryoo, I.J.; Choi, J.S.; Joo, C.K.; Chang, S.Y.; Chung, J.M.; Oh, S.; Gwag, B.J.; Yoo, I.D. Complestatin is a noncompetitive peptide antagonist of N-methyl-D-aspartate and alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid/kainate receptors: Secure blockade of ischemic neuronal death. J. Pharmacol. Exp. Ther. 2001, 299, 377–384. [Google Scholar] [CrossRef]
- Griauzde, J.; Ravindra, V.M.; Chaudhary, N.; Gemmete, J.J.; Pandey, A.S. Neuroprotection for ischemic stroke in the endovascular era: A brief report on the future of intra-arterial therapy. J. Clin. Neurosci. 2019, 69, 289–291. [Google Scholar] [CrossRef] [PubMed]
- Vos, E.M.; Geraedts, V.J.; van der Lugt, A.; Dippel, D.W.J.; Wermer, M.J.H.; Hofmeijer, J.; van Es, A.; Roos, Y.; Peeters-Scholte, C.; van den Wijngaard, I.R. Systematic Review-Combining Neuroprotection With Reperfusion in Acute Ischemic Stroke. Front. Neurol. 2022, 13, 840892. [Google Scholar] [CrossRef]
- Hill, M.D.; Goyal, M.; Menon, B.K.; Nogueira, R.G.; McTaggart, R.A.; Demchuk, A.M.; Poppe, A.Y.; Buck, B.H.; Field, T.S.; Dowlatshahi, D.; et al. Efficacy and safety of nerinetide for the treatment of acute ischaemic stroke (ESCAPE-NA1): A multicentre, double-blind, randomised controlled trial. Lancet 2020, 395, 878–887. [Google Scholar] [CrossRef] [PubMed]
- Goyal, M.; Menon, B.K.; Ospel, J.; Almekhlafi, M.; Zerna, C.; Nogueira, R.; McTaggart, R.; Demchuk, A.M.; Poppe, A.Y.; Buck, B.; et al. Factors Influencing Nerinetide Effect on Clinical Outcome in Patients Without Alteplase Treatment in the ESCAPE-NA1 Trial. J. Stroke 2025, 27, 95–101. [Google Scholar] [CrossRef]
- Martel, M.A.; Soriano, F.X.; Baxter, P.; Rickman, C.; Duncan, R.; Wyllie, D.J.; Hardingham, G.E. Inhibiting pro-death NMDA receptor signaling dependent on the NR2 PDZ ligand may not affect synaptic function or synaptic NMDA receptor signaling to gene expression. Channels 2009, 3, 12–15. [Google Scholar] [CrossRef]
- Bratane, B.T.; Cui, H.; Cook, D.J.; Bouley, J.; Tymianski, M.; Fisher, M. Neuroprotection by freezing ischemic penumbra evolution without cerebral blood flow augmentation with a postsynaptic density-95 protein inhibitor. Stroke 2011, 42, 3265–3270. [Google Scholar] [CrossRef]
- Henninger, N.; Fisher, M. Extending the Time Window for Endovascular and Pharmacological Reperfusion. Transl. Stroke Res. 2016, 7, 284–293. [Google Scholar] [CrossRef]
- Savitz, S.I.; Baron, J.C.; Yenari, M.A.; Sanossian, N.; Fisher, M. Reconsidering Neuroprotection in the Reperfusion Era. Stroke 2017, 48, 3413–3419. [Google Scholar] [CrossRef] [PubMed]
- Aksenov, D.P.; Doubovikov, E.D. Diffusion constraints in neuroprotection: Implications for clinical trials. Front. Pharmacol. 2025, 16, 1542431. [Google Scholar] [CrossRef]
- Saver, J.L.; Starkman, S.; Eckstein, M.; Stratton, S.J.; Pratt, F.D.; Hamilton, S.; Conwit, R.; Liebeskind, D.S.; Sung, G.; Kramer, I.; et al. Prehospital use of magnesium sulfate as neuroprotection in acute stroke. N. Engl. J. Med. 2015, 372, 528–536. [Google Scholar] [CrossRef]
- Muir, K.W. Magnesium for neuroprotection in ischaemic stroke: Rationale for use and evidence of effectiveness. CNS Drugs 2001, 15, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Nowak, L.; Bregestovski, P.; Ascher, P.; Herbet, A.; Prochiantz, A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature 1984, 307, 462–465. [Google Scholar] [CrossRef] [PubMed]
- Hallak, M. Effect of parenteral magnesium sulfate administration on excitatory amino acid receptors in the rat brain. Magnes. Res. 1998, 11, 117–131. [Google Scholar] [PubMed]
- Clerc, P.; Young, C.A.; Bordt, E.A.; Grigore, A.M.; Fiskum, G.; Polster, B.M. Magnesium sulfate protects against the bioenergetic consequences of chronic glutamate receptor stimulation. PLoS ONE 2013, 8, e79982. [Google Scholar] [CrossRef]
- Deshpande, L.S.; Lou, J.K.; Mian, A.; Blair, R.E.; Sombati, S.; Attkisson, E.; DeLorenzo, R.J. Time course and mechanism of hippocampal neuronal death in an in vitro model of status epilepticus: Role of NMDA receptor activation and NMDA dependent calcium entry. Eur. J. Pharmacol. 2008, 583, 73–83. [Google Scholar] [CrossRef]
- Meloni, B.P.; Zhu, H.; Knuckey, N.W. Is magnesium neuroprotective following global and focal cerebral ischaemia? A review of published studies. Magnes. Res. 2006, 19, 123–137. [Google Scholar] [PubMed]
- Meloni, B.P.; Cross, J.L.; Brookes, L.M.; Clark, V.W.; Campbell, K.; Knuckey, N.W. FAST-Mag protocol with or without mild hypothermia (35 degrees C) does not improve outcome after permanent MCAO in rats. Magnes. Res. 2013, 26, 67–73. [Google Scholar]
- Zhu, H.; Meloni, B.P.; Moore, S.R.; Majda, B.T.; Knuckey, N.W. Intravenous administration of magnesium is only neuroprotective following transient global ischemia when present with post-ischemic mild hypothermia. Brain Res. 2004, 1014, 53–60. [Google Scholar] [CrossRef]
- Rogalewski, A.; Schneider, A.; Ringelstein, E.B.; Schabitz, W.R. Toward a multimodal neuroprotective treatment of stroke. Stroke 2006, 37, 1129–1136. [Google Scholar]
- Milani, D.; Knuckey, N.W.; Anderton, R.S.; Cross, J.L.; Meloni, B.P. The R18 Polyarginine Peptide Is More Effective Than the TAT-NR2B9c (NA-1) Peptide When Administered 60 Minutes after Permanent Middle Cerebral Artery Occlusion in the Rat. Stroke Res. Treat. 2016, 2016, 2372710. [Google Scholar] [CrossRef]
- Meloni, B.P.; Milani, D.; Edwards, A.B.; Anderton, R.S.; O’Hare Doig, R.L.; Fitzgerald, M.; Palmer, T.N.; Knuckey, N.W. Neuroprotective peptides fused to arginine-rich cell penetrating peptides: Neuroprotective mechanism likely mediated by peptide endocytic properties. Pharmacol. Ther. 2015, 153, 36–54. [Google Scholar] [CrossRef] [PubMed]
- Muir, K.W.; Lees, K.R. Clinical experience with excitatory amino acid antagonist drugs. Stroke 1995, 26, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Dawson, D.A.; Wadsworth, G.; Palmer, A.M. A comparative assessment of the efficacy and side-effect liability of neuroprotective compounds in experimental stroke. Brain Res. 2001, 892, 344–350. [Google Scholar] [CrossRef]
- Link, T.W.; Santillan, A.; Patsalides, A. Intra-arterial neuroprotective therapy as an adjunct to endovascular intervention in acute ischemic stroke: A review of the literature and future directions. Interv. Neuroradiol. 2020, 26, 405–415. [Google Scholar] [CrossRef]
- Maniskas, M.E.; Roberts, J.M.; Aron, I.; Fraser, J.F.; Bix, G.J. Stroke neuroprotection revisited: Intra-arterial verapamil is profoundly neuroprotective in experimental acute ischemic stroke. J. Cereb. Blood Flow Metab. 2016, 36, 721–730. [Google Scholar] [CrossRef]
- Maniskas, M.E.; Roberts, J.M.; Gorman, A.; Bix, G.J.; Fraser, J.F. Intra-arterial combination therapy for experimental acute ischemic stroke. Clin. Transl. Sci. 2022, 15, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Olson, E.S.; Whitney, M.A.; Friedman, B.; Aguilera, T.A.; Crisp, J.L.; Baik, F.M.; Jiang, T.; Baird, S.M.; Tsimikas, S.; Tsien, R.Y.; et al. In vivo fluorescence imaging of atherosclerotic plaques with activatable cell-penetrating peptides targeting thrombin activity. Integr Biol 2012, 4, 595–605. [Google Scholar] [CrossRef]
- Fraser, J.F.; Maniskas, M.; Trout, A.; Lukins, D.; Parker, L.; Stafford, W.L.; Alhajeri, A.; Roberts, J.; Bix, G.J. Intra-arterial verapamil post-thrombectomy is feasible, safe, and neuroprotective in stroke. J. Cereb. Blood Flow Metab. 2017, 37, 3531–3543. [Google Scholar] [CrossRef]
- Van Winkle, J.A.; Chen, B.; Lei, I.F.; Pereira, B.; Rajput, P.S.; Lyden, P.D. Concurrent middle cerebral artery occlusion and intra-arterial drug infusion via ipsilateral common carotid artery catheter in the rat. J. Neurosci. Methods 2013, 213, 63–69. [Google Scholar] [CrossRef]
- le Feber, J.; Erkamp, N.; van Putten, M.; Hofmeijer, J. Loss and recovery of functional connectivity in cultured cortical networks exposed to hypoxia. J. Neurophysiol. 2017, 118, 394–403. [Google Scholar] [CrossRef]
- le Feber, J.; Tzafi Pavlidou, S.; Erkamp, N.; van Putten, M.J.; Hofmeijer, J. Progression of Neuronal Damage in an In Vitro Model of the Ischemic Penumbra. PLoS ONE 2016, 11, e0147231. [Google Scholar] [CrossRef] [PubMed]
- Santiago, J.; Kreutzer, J.; Bossink, E.; Kallio, P.; le Feber, J. Oxygen gradient generator to improve in vitro modeling of ischemic stroke. Front. Neurosci. 2023, 17, 1110083. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, A.F.; Gross, G.W.; Weiss, D.G.; Schroeder, O.H.; Gramowski, A.; Shafer, T.J. Microelectrode arrays: A physiologically based neurotoxicity testing platform for the 21st century. Neurotoxicology 2010, 31, 331–350. [Google Scholar] [CrossRef] [PubMed]
- O’Collins, V.E.; Macleod, M.R.; Cox, S.F.; Van, R.L.; Aleksoska, E.; Donnan, G.A.; Howells, D.W. Preclinical drug evaluation for combination therapy in acute stroke using systematic review, meta-analysis, and subsequent experimental testing. J. Cereb. Blood Flow Metab. 2011, 31, 962–975. [Google Scholar] [CrossRef]
- Culmsee, C.; Junker, V.; Kremers, W.; Thal, S.; Plesnila, N.; Krieglstein, J. Combination therapy in ischemic stroke: Synergistic neuroprotective effects of memantine and clenbuterol. Stroke 2004, 35, 1197–1202. [Google Scholar] [CrossRef]
- Jung, K.H.; Chu, K.; Lee, S.T.; Park, H.K.; Kim, J.H.; Kang, K.M.; Kim, M.; Lee, S.K.; Roh, J.K. Augmentation of nitrite therapy in cerebral ischemia by NMDA receptor inhibition. Biochem. Biophys. Res. Commun. 2009, 378, 507–512. [Google Scholar] [CrossRef]
- Lyden, P.; Lonzo, L.; Nunez, S. Combination chemotherapy extends the therapeutic window to 60 minutes after stroke. J. Neurotrauma 1995, 12, 223–230. [Google Scholar] [CrossRef]
- Lyden, P.D.; Jackson-Friedman, C.; Shin, C.; Hassid, S. Synergistic combinatorial stroke therapy: A quantal bioassay of a GABA agonist and a glutamate antagonist. Exp. Neurol. 2000, 163, 477–489. [Google Scholar] [CrossRef]
- Lyden, P.D.; Lonzo, L. Combination therapy protects ischemic brain in rats. A glutamate antagonist plus a gamma-aminobutyric acid agonist. Stroke 1994, 25, 189–196. [Google Scholar] [CrossRef]
- Auer, R.N. Combination therapy with U74006F (tirilazad mesylate), MK-801, insulin and diazepam in transient forebrain ischaemia. Neurol. Res. 1995, 17, 132–136. [Google Scholar] [CrossRef]
- Dirnagl, U.; Macleod, M.R. Stroke research at a road block: The streets from adversity should be paved with meta-analysis and good laboratory practice. Br. J. Pharmacol. 2009, 157, 1154–1156. [Google Scholar] [CrossRef] [PubMed]
- Bath, P.M.; Gray, L.J.; Bath, A.J.; Buchan, A.; Miyata, T.; Green, A.R. Effects of NXY-059 in experimental stroke: An individual animal meta-analysis. Br. J. Pharmacol. 2009, 157, 1157–1171. [Google Scholar] [CrossRef] [PubMed]
- Macleod, M.R.; van der Worp, H.B.; Sena, E.S.; Howells, D.W.; Dirnagl, U.; Donnan, G.A. Evidence for the efficacy of NXY-059 in experimental focal cerebral ischaemia is confounded by study quality. Stroke 2008, 39, 2824–2829. [Google Scholar] [CrossRef] [PubMed]
- Dehouck, M.P.; Cecchelli, R.; Richard Green, A.; Renftel, M.; Lundquist, S. In vitro blood-brain barrier permeability and cerebral endothelial cell uptake of the neuroprotective nitrone compound NXY-059 in normoxic, hypoxic and ischemic conditions. Brain Res. 2002, 955, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, S.; Tsuchidate, R.; Smith, M.L.; Maples, K.R.; Siesjö, B.K. Neuroprotective effects of a novel nitrone, NXY-059, after transient focal cerebral ischemia in the rat. J. Cereb. Blood Flow Metab. 1999, 19, 778–787. [Google Scholar] [CrossRef]
- Fisher, M.; Lees, K.; Papadakis, M.; Buchan, A.M. NXY-059: Brain or vessel protection. Stroke 2006, 37, 2189–2190. [Google Scholar] [CrossRef]
- Antonic, A.; Dottori, M.; Macleod, M.R.; Donnan, G.A.; Howells, D.W. NXY-059, a Failed Stroke Neuroprotectant, Offers No Protection to Stem Cell-Derived Human Neurons. J. Stroke Cerebrovasc. Dis. 2018, 27, 2158–2165. [Google Scholar] [CrossRef]
- Wang, J.K.; Portbury, S.; Thomas, M.B.; Barney, S.; Ricca, D.J.; Morris, D.L.; Warner, D.S.; Lo, D.C. Cardiac glycosides provide neuroprotection against ischemic stroke: Discovery by a brain slice-based compound screening platform. Proc. Natl. Acad. Sci. USA 2006, 103, 10461–10466. [Google Scholar] [CrossRef]
- Ginsberg, M.D. Life after cerovive: A personal perspective on ischemic neuroprotection in the post-NXY-059 era. Stroke 2007, 38, 1967–1972. [Google Scholar] [CrossRef]
- Martin, R.H.; Yeatts, S.D.; Hill, M.D.; Moy, C.S.; Ginsberg, M.D.; Palesch, Y.Y. ALIAS (Albumin in Acute Ischemic Stroke) Trials: Analysis of the Combined Data From Parts 1 and 2. Stroke 2016, 47, 2355–2359. [Google Scholar] [CrossRef]
- Yu, Z.F.; Bruce-Keller, A.J.; Goodman, Y.; Mattson, M.P. Uric acid protects neurons against excitotoxic and metabolic insults in cell culture, and against focal ischemic brain injury in vivo. J. Neurosci. Res. 1998, 53, 613–625. [Google Scholar] [CrossRef]
- Chamorro, Á.; Dirnagl, U.; Urra, X.; Planas, A.M. Neuroprotection in acute stroke: Targeting excitotoxicity, oxidative and nitrosative stress, and inflammation. Lancet Neurol. 2016, 15, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Gwag, B.J.; Lee, Y.A.; Ko, S.Y.; Lee, M.J.; Im, D.S.; Yun, B.S.; Lim, H.R.; Park, S.M.; Byun, H.Y.; Son, S.J.; et al. Marked prevention of ischemic brain injury by Neu2000, an NMDA antagonist and antioxidant derived from aspirin and sulfasalazine. J. Cereb. Blood Flow Metab. 2007, 27, 1142–1151. [Google Scholar] [CrossRef] [PubMed]
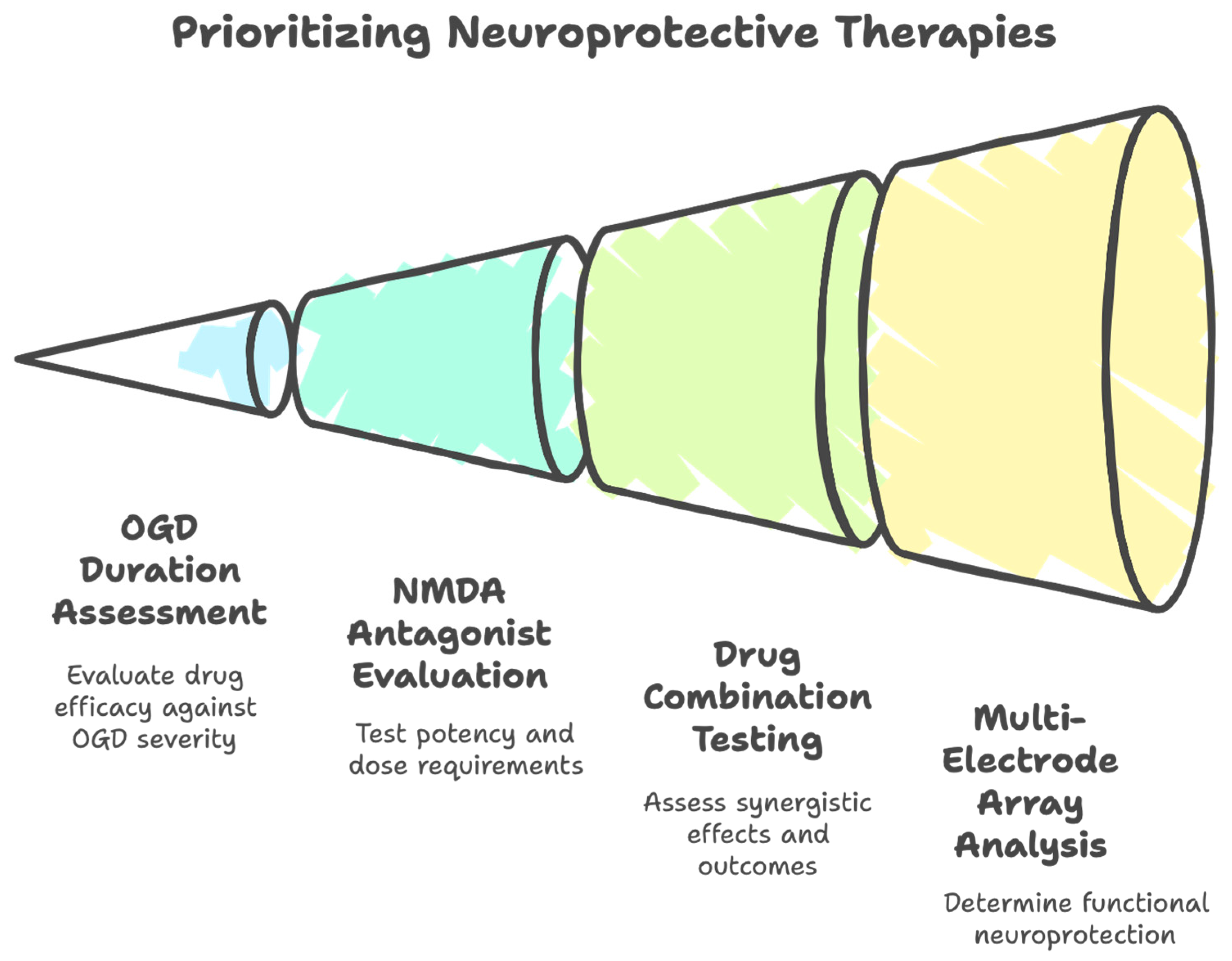
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tauskela, J.S.; Blondeau, N. How to Pick a Neuroprotective Drug in Stroke Without Losing Your Mind? Life 2025, 15, 883. https://doi.org/10.3390/life15060883
Tauskela JS, Blondeau N. How to Pick a Neuroprotective Drug in Stroke Without Losing Your Mind? Life. 2025; 15(6):883. https://doi.org/10.3390/life15060883
Chicago/Turabian StyleTauskela, Joseph S., and Nicolas Blondeau. 2025. "How to Pick a Neuroprotective Drug in Stroke Without Losing Your Mind?" Life 15, no. 6: 883. https://doi.org/10.3390/life15060883
APA StyleTauskela, J. S., & Blondeau, N. (2025). How to Pick a Neuroprotective Drug in Stroke Without Losing Your Mind? Life, 15(6), 883. https://doi.org/10.3390/life15060883




