Investigating the Polystyrene (PS) Biodegradation Potential of Phanerochaete chrysosporium Strain NA3: A Newly Isolated Soil Fungus
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Polystyrene Film
2.3. Isolation of Fungal Strain with Polystyrene-Degrading Potential
2.4. Extraction of DNA from Fungal Mycelia
2.5. Amplification, Purification, and Sequencing of rDNA of the Isolated Fungal Strain
2.6. Biodegradation Kinetics of Polystyrene Using Phanerochaete Chrysosporium Strain NA3
2.6.1. Inoculum Preparation
2.6.2. Carbon Dioxide (CO2) Evolution Test (Sturm Test)
2.6.3. Biodegradation of Polystyrene (PS) Films with Newly Isolated Fungal Strain NA3
2.6.4. Evaluation of the Effect of Thermal and UV Pretreatment on the Biodegradation of Polystyrene (PS) Films
2.6.5. Evaluating Bioaugmentation of Fungal Strain NA3 to Enhance Soil Biodegradation of Polystyrene (PS)
2.6.6. Biodegradation of Polystyrene–Starch Blend Film by Fungal Strain NA3
2.7. Analysis of Biodegradation
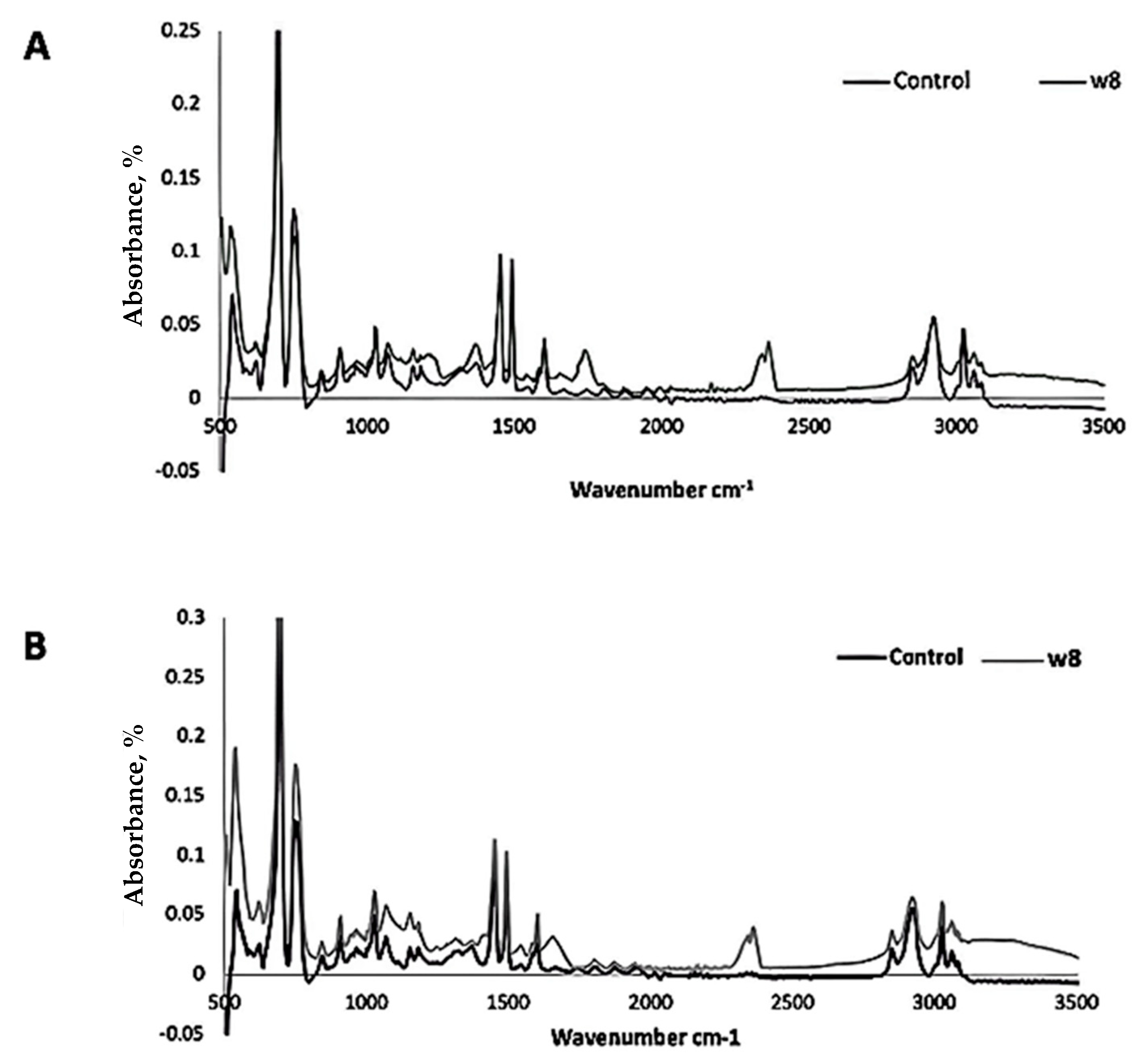
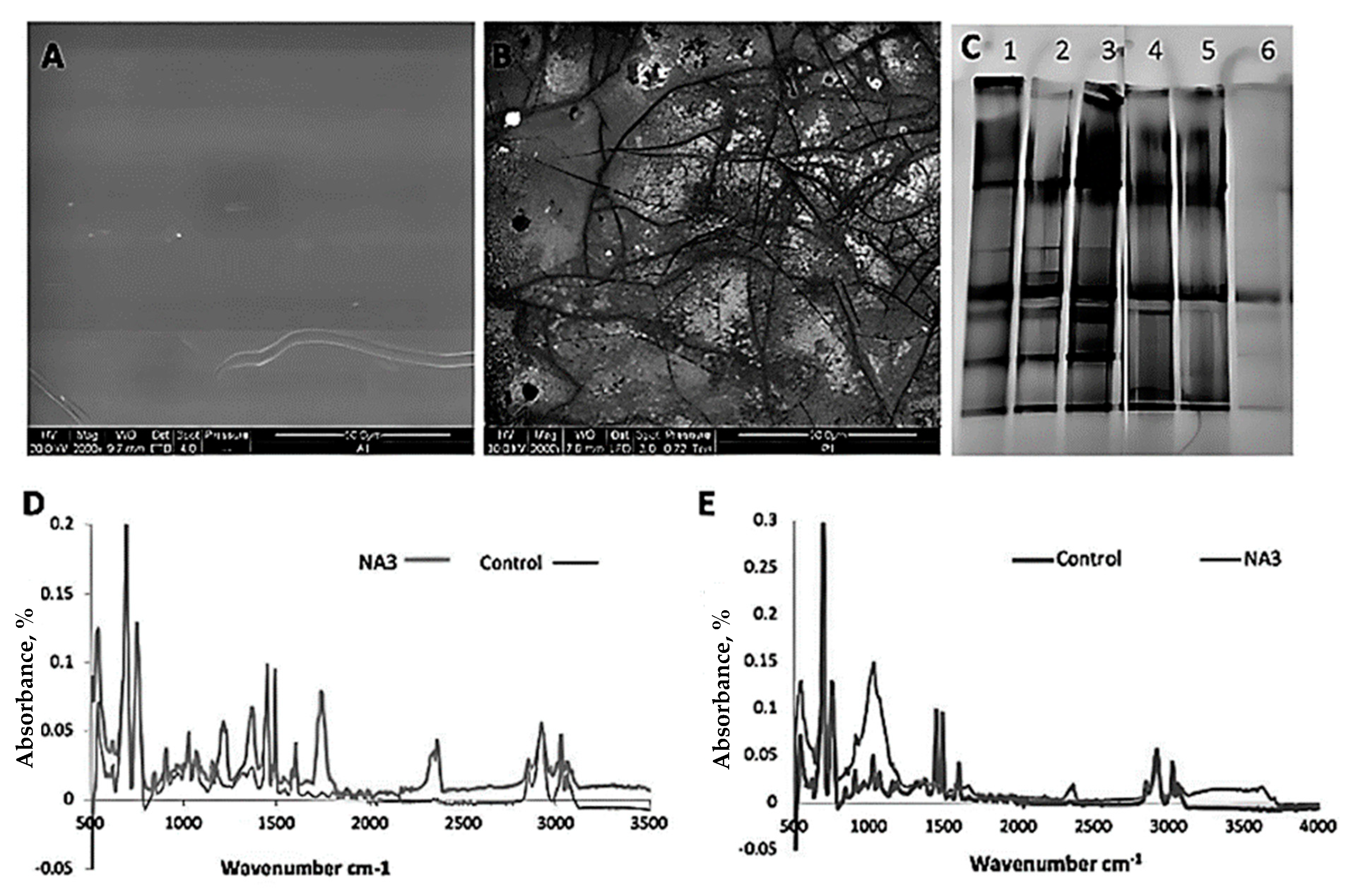
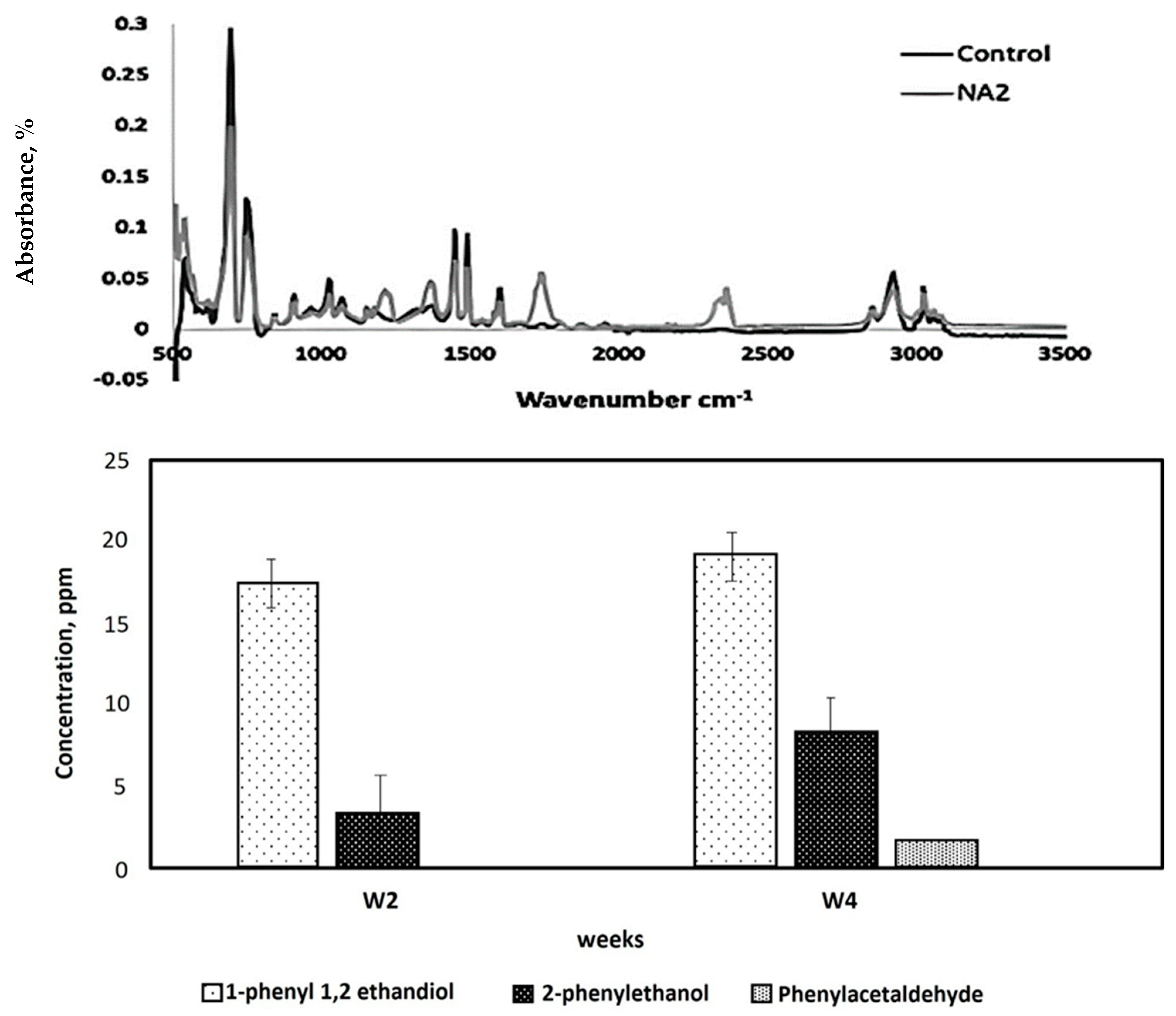
2.7.1. Fourier Transform Infrared Spectroscopy (FTIR)
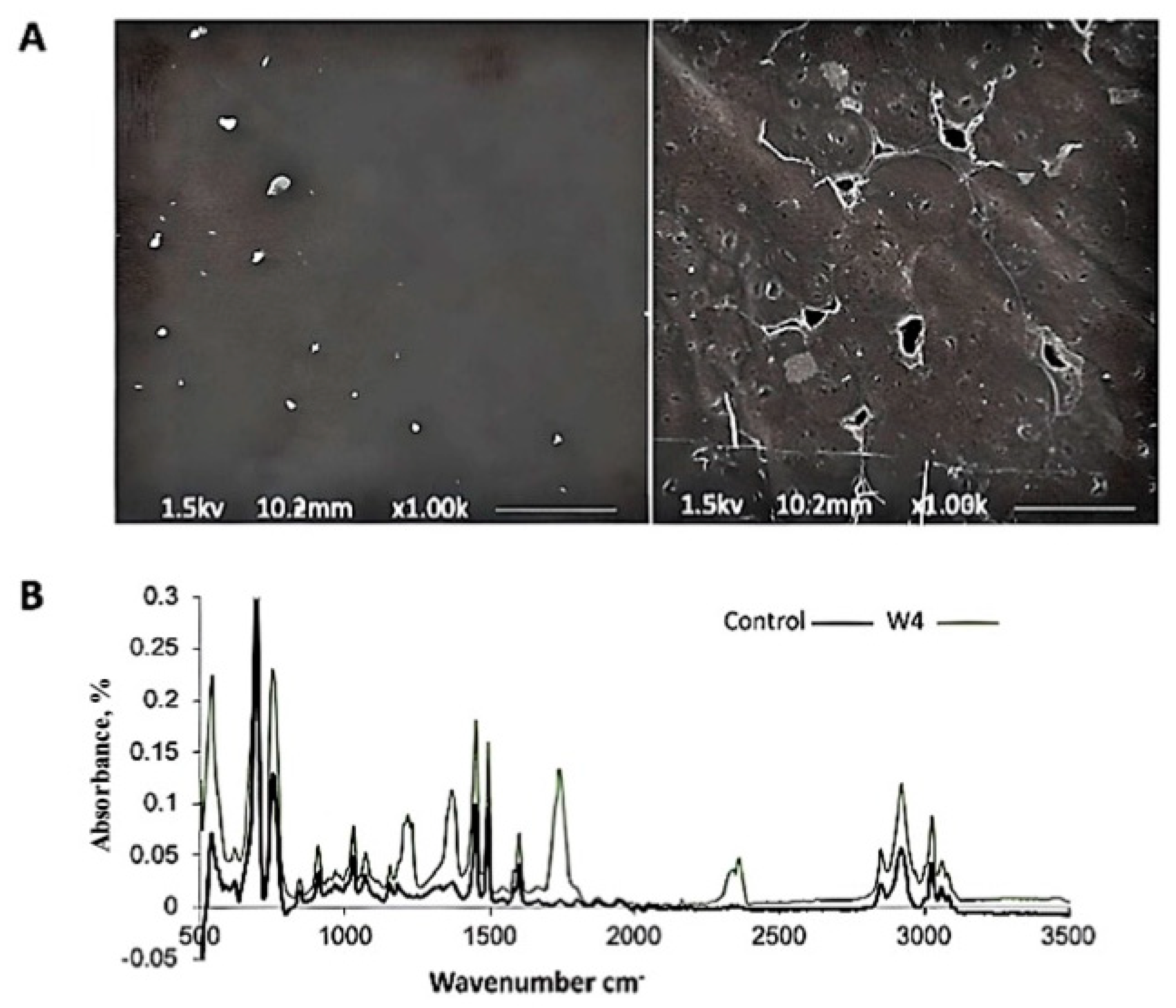
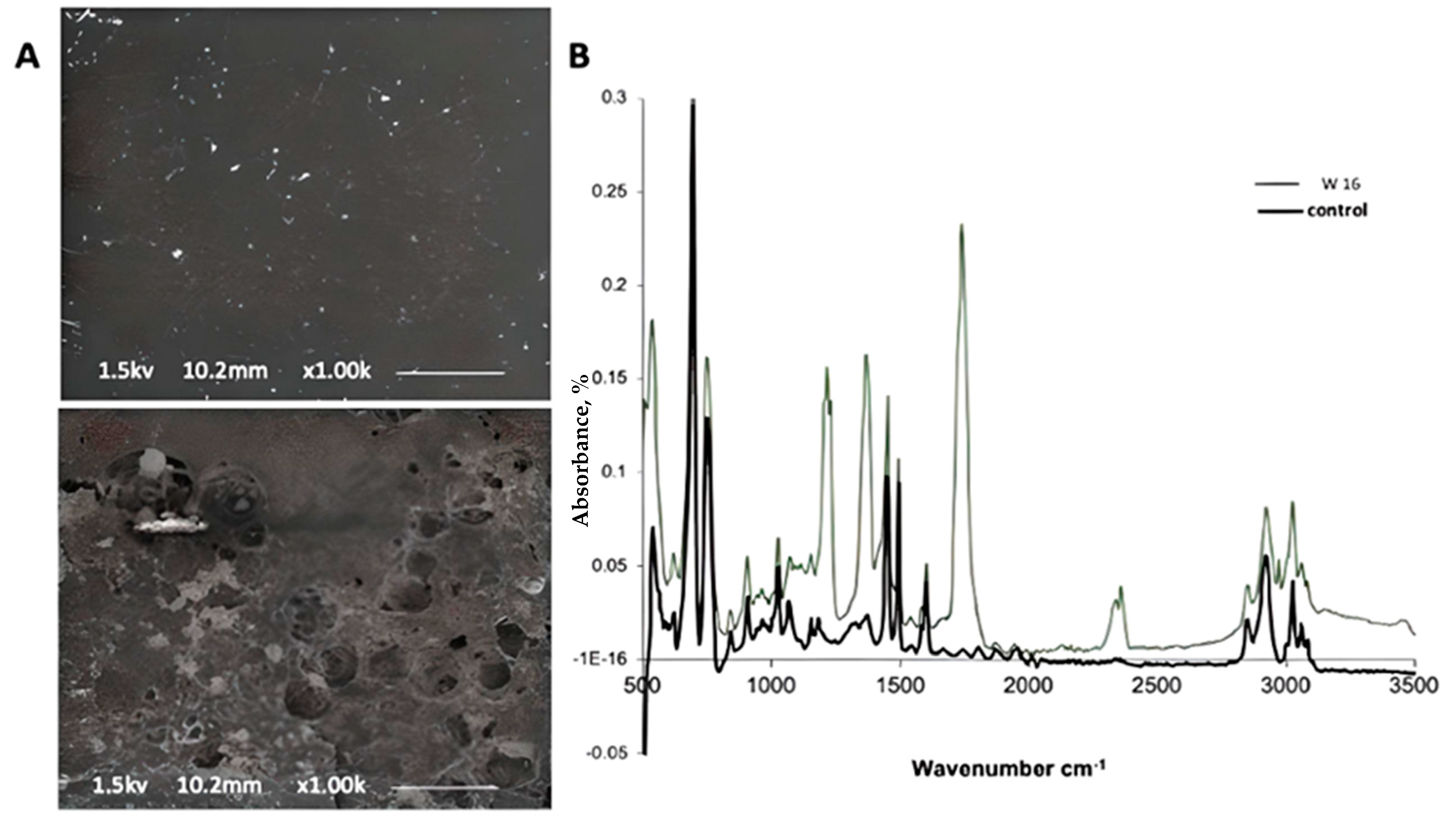
2.7.2. Environmental Scanning Electron Microscopy (ESEM)
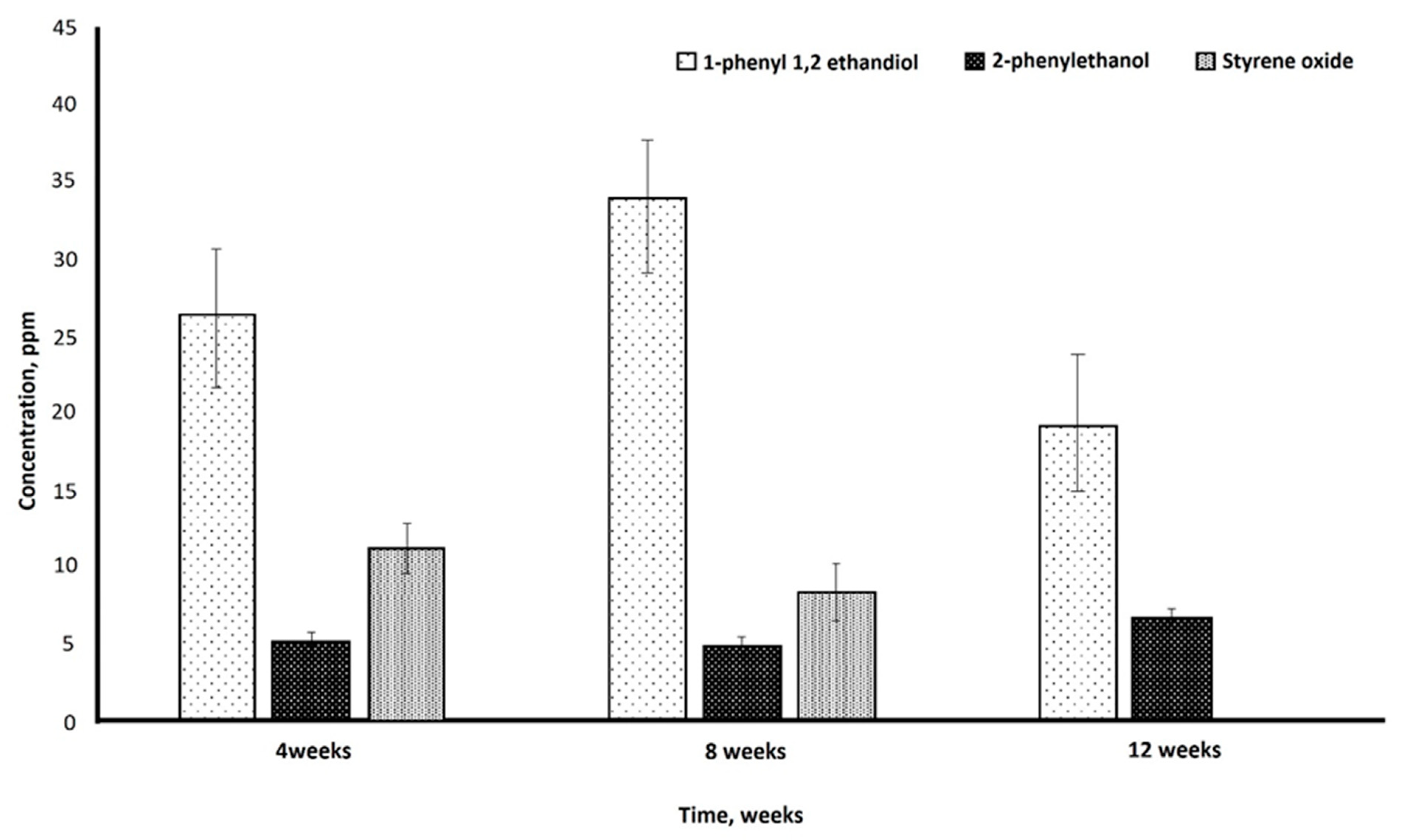
2.7.3. Chromatographic Techniques for the Analysis of Biodegradation
Gel Permeation Chromatography (GPC)
High-Pressure Liquid Chromatography (HPLC)
2.8. Statistical Analysis
3. Results
3.1. Isolation of Fungal Strain with Polystyrene-Degrading Potential
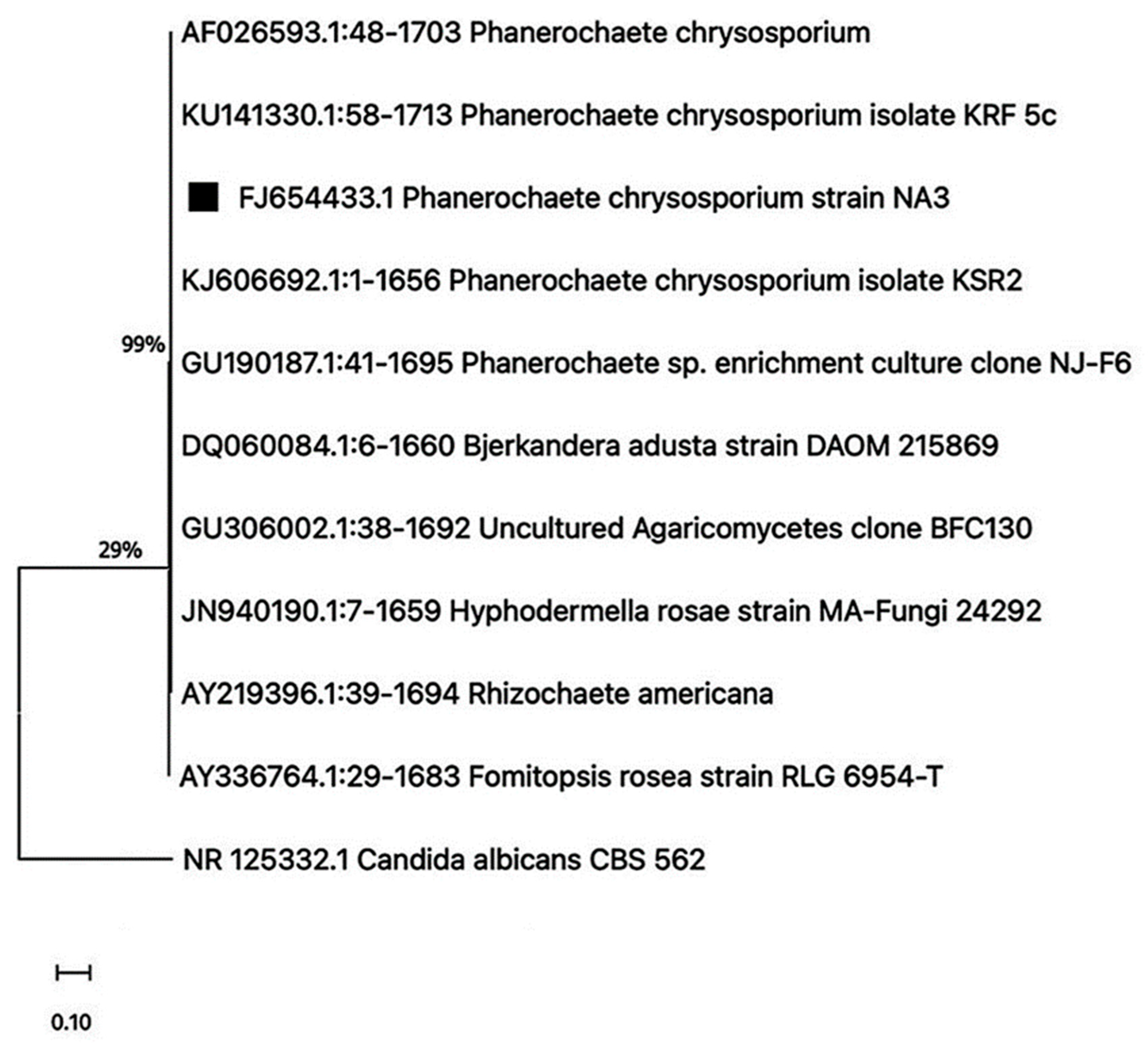
3.2. Molecular Identification of Fungal Strain
3.3. Biodegradation Kinetics of Polystyrene Using Phanerochaete Chrysosporium Strain NA3
3.3.1. Carbon Dioxide (CO2) Evolution Test
3.3.2. Biodegradation of Polystyrene (PS) Films with Newly Isolated Fungal Strain NA3 Under Shake Flask Conditions
3.4. Evaluation of the Effect of Thermal and UV Pretreatment on Biodegradation of Polystyrene (PS) Film
3.5. Biodegradation of PS in Soil; Evaluating Bio-Augmentation of Phanerochaete Chrysosporium Strain NA3
3.6. Biodegradation of Polystyrene Starch Blend
4. Discussion
4.1. Isolation, Identification, and Phylogenetic Analysis of PS-Degrading Fungi
4.2. Assessing Biodegradation and Mineralization of Polystyrene (PS) Through Carbon Dioxide (CO2) Evolution Test
4.3. Assessing the Biodegradation Potential of Polystyrene (PS) Films Using a Novel Fungal Strain NA3 Under Shake Flask Conditions
4.4. Evaluation of the Effect of Thermal and UV Pretreatment on Biodegradation of Polystyrene (PS) Film
4.5. Biodegradation of PS in Soil; Evaluating Bio-Augmentation of Phanerochaete Chrysosporium Strain NA3
4.6. Biodegradation of Polystyrene Starch Blend
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Poddar, A.K.; Patel, S.S.; Patel, H.D. Synthesis, characterization and applications of conductive polymers: A brief review. Polym. Adv. Technol. 2021, 32, 4616–4641. [Google Scholar] [CrossRef]
- Stevens, E.S. Green Plastics: An Introduction to the New Science of Biodegradable Plastics; Princeton University Press: Princeton, NJ, USA, 2001. [Google Scholar] [CrossRef]
- Okan, M.; Aydin, H.M.; Barsbay, M. Current approaches to waste polymer utilization and minimization: A review. J. Chem. Tech. Biotechnol. 2019, 94, 8–21. [Google Scholar] [CrossRef]
- Block, C.; Van Caneghem, J.; Van Brecht, A.; Wauters, G.; Vandecasteele, C. Incineration of hazardous waste: A sustainable process? Waste Biomass Valori. 2015, 6, 137–145. [Google Scholar] [CrossRef]
- Ngugi, H.N.; Kaluli, J.W.; Abiero-Gariy, Z. Use of expanded polystyrene technology and materials recycling for building construction in Kenya. Am. J. Eng. Technol. Manag. 2017, 2, 64–71. [Google Scholar] [CrossRef]
- Nugnes, R.; Lavorgna, M.; Orlo, E.; Russo, C.; Isidori, M. Toxic impact of polystyrene microplastic particles in freshwater organisms. Chemosphere 2022, 299, 134373. [Google Scholar] [CrossRef]
- Farrelly, T.A.; Shaw, I.C. Polystyrene as hazardous household waste. In Household Hazardous Waste Management; Mmereki, D., Ed.; Intechopen: London, UK, 2017; p. 45. [Google Scholar] [CrossRef]
- Zhang, Y.; Pedersen, J.N.; Eser, B.E.; Guo, Z. Biodegradation of polyethylene and polystyrene: From microbial deterioration to enzyme discovery. Biotechnol. Adv. 2022, 60, 107991. [Google Scholar] [CrossRef]
- Satti, S.; Shah, A. Polyester-based biodegradable plastics: An approach towards sustainable development. Lett. Appl. Microbiol. 2020, 70, 413–430. [Google Scholar] [CrossRef]
- Arunrattiyakorn, P.; Ponprateep, S.; Kaennonsang, N.; Charapok, Y.; Punphuet, Y.; Krajangsang, S.; Tangteerawatana, P.; Limtrakul, A. Biodegradation of polystyrene by three bacterial strains isolated from the gut of Superworms (Zophobas atratus larvae). J. Appl. Microbiol. 2022, 132, 2823–2831. [Google Scholar] [CrossRef]
- Sun, J.; Prabhu, A.; Aroney, S.; Rinke, C. Insights into plastic biodegradation: Community composition and functional capabilities of the superworm (Zophobas morio) microbiome in styrofoam feeding trials. bioRxiv 2022, 8, 000842. [Google Scholar]
- Kim, H.W.; Jo, J.H.; Kim, Y.B.; Le, T.K.; Cho, C.W.; Yun, C.H.; Chi, W.S.; Yeom, S.J. Biodegradation of polystyrene by bacteria from the soil in common environments. J. Hazard. Mater. 2021, 416, 126239. [Google Scholar] [CrossRef]
- Naima, A.; Safia, A.; Bashir, A.; Geoffery, R. Isolation and identification of polystyrene biodegrading bacteria from soil. Afr. J. Microbiol. Res. 2010, 4, 1537–1541. [Google Scholar]
- Yang, S.; Brandon, A.M.; Xing, D.; Yang, J.; Pang, J.; Criddle, C.; Ren, N.; Wu, W. Progresses in polystyrene biodegradation and prospects for solutions to plastic waste pollution. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Phuket, Thailand, 10–12 January 2018; IOP Publishing Ltd.: Bristol, UK, 2018; p. 012005. [Google Scholar] [CrossRef]
- Ugueri, U.; Atuanya, E.; Zainab, U. Biodegradability of polystyrene plastics by bacterial isolates from plastic composted waste soil and molecular characterization of plastic degrading bacterial isolates. Afr. J. Microbiol. Res. 2022, 16, 247–257. [Google Scholar]
- Ming, Z.; Feng, S.; Yilihamu, A.; Ma, Q.; Yang, S.; Yang, S.T. Toxicity of pristine and chemically functionalized fullerenes to white rot fungus Phanerochaete chrysosporium. Nanomaterials 2018, 8, 120. [Google Scholar] [CrossRef]
- Glenn, J.K.; Gold, M.H. Reprint of: Purification and Characterization of an Extracellular Mn (ll)-Dependent Peroxidase from the Lignin-Degrading Basidiomycete, Phanerochaete chrysosporium. Arch. Biochem. Biophys. 2022, 726, 109251. [Google Scholar] [CrossRef]
- Feng, J.; Hwang, R.; Chang, K.; Hwang, S.; Strelkov, S.; Gossen, B.; Zhou, Q. An inexpensive method for extraction of genomic DNA from fungal mycelia. Can. J. Plant Pathol. 2010, 32, 396–401. [Google Scholar] [CrossRef]
- Gouy, M.; Guindon, S.; Gascuel, O. SeaView Version 4: A Multiplatform Graphical User Interface for Sequence Alignment and Phylogenetic Tree Building. Mol. Biol. Evol. 2009, 27, 221–224. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar]
- Felsenstein, J. Confidence Limits on Phylogenies: An Approach Using the Bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Osman, R.B.; van der Veen, A.J.; Huiberts, D.; Wismeijer, D.; Alharbi, N. 3D-printing zirconia implants; a dream or a reality? An in-vitro study evaluating the dimensional accuracy, surface topography and mechanical properties of printed zirconia implant and discs. J. Mech. Behav Biomed. Mater. 2017, 75, 521–528. [Google Scholar] [CrossRef]
- Yasin, N.M.; Akkermans, S.; Van Impe, J.F. Enhancing the biodegradation of (bio) plastic through pretreatments: A critical review. Waste Manage. 2022, 150, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kundungal, H.; Gangarapu, M.; Sarangapani, S.; Patchaiyappan, A.; Devipriya, S.P. Role of pretreatment and evidence for the enhanced biodegradation and mineralization of low-density polyethylene films by greater waxworm. Environ. Technol. 2021, 42, 717–730. [Google Scholar] [CrossRef]
- Sakai, Y.; Isokawa, M.; Masuda, T.; Yoshioka, H.; Hayatsu, M.; Hayano, K. Usefulness of soil p-nitrophenyl acetate esterase activity as a tool to monitor biodegradation of polybutylene succinate (PBS) in cultivated soil. Polym. J. 2002, 34, 767–774. [Google Scholar] [CrossRef]
- Sang, B.I.; Hori, K.; Tanji, Y.; Unno, H. Fungal contribution to in situ biodegradation of poly (3-hydroxybutyrate-co-3-hydroxyvalerate) film in soil. Appl. Microbiol. Biotechnol. 2002, 58, 241–247. [Google Scholar] [CrossRef]
- Yamamoto-Tamura, K.; Hiradate, S.; Watanabe, T.; Koitabashi, M.; Sameshima-Yamashita, Y.; Yarimizu, T.; Kitamoto, H. Contribution of soil esterase to biodegradation of aliphatic polyester agricultural mulch film in cultivated soils. AMB Express 2015, 5, 10. [Google Scholar] [CrossRef]
- Muyzer, G.; De Waal, E.C.; Uitterlinden, A. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl. Environ. Microbiol. 1993, 59, 695–700. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, J.; Wu, W.M.; Zhao, J.; Song, Y.; Gao, L.; Yang, R.; Jiang, L. Biodegradation and mineralization of polystyrene by plastic-eating mealworms: Part 1. Chemical and physical characterization and isotopic tests. Environ. Sci. Technol. 2015, 49, 12080–12086. [Google Scholar] [CrossRef]
- Mohan, A.J.; Sekhar, V.C.; Bhaskar, T.; Nampoothiri, K.M. Microbial assisted high impact polystyrene (HIPS) degradation. Bioresour. Technol. 2016, 213, 204–207. [Google Scholar] [CrossRef]
- Sekhar, V.C.; Nampoothiri, K.M.; Mohan, A.J.; Nair, N.R.; Bhaskar, T.; Pandey, A. Microbial degradation of high impact polystyrene (HIPS), an e-plastic with decabromodiphenyl oxide and antimony trioxide. J. Hazard. Mater. 2016, 318, 347–354. [Google Scholar] [CrossRef]
- Subramani, M.; Sepperumal, U. FTIR analysis of bacterial mediated chemical changes in polystyrene foam. Ann. Biol. Res. 2016, 7, 55–61. [Google Scholar]
- Savoldelli, J.; Tomback, D.; Savoldelli, H. Breaking down polystyrene through the application of a two-step thermal degradation and bacterial method to produce usable byproducts. Waste Manag. 2017, 60, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Kolvenbach, B.; Corvini, N.; Wang, S.; Tavanaie, N.; Wang, L.; Ma, Y.; Scheu, S.; Corvini, P.F.X.; Ji, R. Mineralisation of 14C-labelled polystyrene plastics by Penicillium variabile after ozonation pre-treatment. New Biotechnol. 2017, 38, 101–105. [Google Scholar] [CrossRef]
- Brandon, A.M.; Gao, S.H.; Tian, R.; Ning, D.; Yang, S.S.; Zhou, J.; Wu, W.M.; Criddle, C.S. Biodegradation of polyethylene and plastic mixtures in mealworms (larvae of Tenebrio molitor) and effects on the gut microbiome. Environ. Sci. Technol. 2018, 52, 6526–6533. [Google Scholar] [CrossRef]
- Chauhan, D.; Agrawal, G.; Deshmukh, S.; Roy, S.S.; Priyadarshini, R. Biofilm formation by Exiguobacterium sp. DR11 and DR14 alter polystyrene surface properties and initiate biodegradation. RSC Adv. 2018, 8, 37590–37599. [Google Scholar] [CrossRef]
- Yang, S.S.; Brandon, A.M.; Flanagan, J.C.A.; Yang, J.; Ning, D.; Cai, S.Y.; Fan, H.Q.; Wang, Z.Y.; Ren, J.; Benbow, E. Biodegradation of polystyrene wastes in yellow mealworms (larvae of Tenebrio molitor Linnaeus): Factors affecting biodegradation rates and the ability of polystyrene-fed larvae to complete their life cycle. Chemosphere 2018, 191, 979–989. [Google Scholar] [CrossRef]
- Yang, S.S.; Wu, W.M.; Brandon, A.M.; Fan, H.Q.; Receveur, J.P.; Li, Y.; Wang, Z.-Y.; Fan, R.; McClellan, R.L.; Gao, S.H. Ubiquity of polystyrene digestion and biodegradation within yellow mealworms, larvae of Tenebrio molitor Linnaeus (Coleoptera: Tenebrionidae). Chemosphere 2018, 212, 262–271. [Google Scholar] [CrossRef]
- Syranidou, E.; Karkanorachaki, K.; Amorotti, F.; Avgeropoulos, A.; Kolvenbach, B.; Zhou, N.-Y.; Fava, F.; Corvini, P.F.X.; Kalogerakis, N. Biodegradation of mixture of plastic films by tailored marine consortia. J. Hazard. Mater. 2019, 375, 33–42. [Google Scholar] [CrossRef]
- Matyja, K.; Rybak, J.; Hanus-Lorenz, B.; Wróbel, M.; Rutkowski, R. Effects of polystyrene diet on Tenebrio molitor larval growth, development and survival: Dynamic Energy Budget (DEB) model analysis. Environ. Pollut. 2020, 264, 114740. [Google Scholar] [CrossRef]
- Peng, B.Y.; Li, Y.; Fan, R.; Chen, Z.; Chen, J.; Brandon, A.M.; Criddle, C.S.; Zhang, Y.; Wu, W.M. Biodegradation of low-density polyethylene and polystyrene in superworms, larvae of Zophobas atratus (Coleoptera: Tenebrionidae): Broad and limited extent depolymerization. Environ. Pollut. 2020, 266, 115206. [Google Scholar] [CrossRef]
- Wang, S.; Shi, W.; Huang, Z.; Zhou, N.; Xie, Y.; Tang, Y.; Hu, F.; Liu, G.; Zheng, H. Complete digestion/biodegradation of polystyrene microplastics by greater wax moth (Galleria mellonella) larvae: Direct in vivo evidence, gut microbiota independence, and potential metabolic pathways. J. Hazard. Mater. 2022, 423, 127213. [Google Scholar] [CrossRef]
- Philippe, A.; Salaun, M.; Quemener, M.; Noël, C.; Tallec, K.; Lacroix, C.; Coton, E.; Burgaud, G. Colonization and biodegradation potential of fungal communities on immersed polystyrene vs. biodegradable plastics: A time series study in a marina environment. J. Fungi 2024, 10, 428. [Google Scholar] [CrossRef]
- Temporiti, M.E.E.; Nicola, L.; Nielsen, E.; Tosi, S. Fungal enzymes involved in plastics biodegradation. Microorganisms 2022, 10, 1180. [Google Scholar] [CrossRef]
- Lü, H.; Wei, J.L.; Tang, G.X.; Chen, Y.S.; Huang, Y.H.; Hu, R.; Mo, C.H.; Zhao, H.M.; Xiang, L.; Li, Y.W.; et al. Microbial consortium degrading of organic pollutants: Source, degradation efficiency, pathway, mechanism and application. J. Clean. Prod. 2024, 451, 141913. [Google Scholar] [CrossRef]
- Jaiswal, S.; Sharma, B.; Shukla, P. Integrated approaches in microbial degradation of plastics. Environ. Technol. Innov. 2020, 17, 100567. [Google Scholar] [CrossRef]
- Zambrano, M.C.; Pawlak, J.J.; Venditti, R.A. Effects of chemical and morphological structure on biodegradability of fibers, fabrics, and other polymeric materials. BioResources 2020, 15, 9786. [Google Scholar] [CrossRef]
- Mor, R.; Sivan, A. Biofilm formation and partial biodegradation of polystyrene by the actinomycete Rhodococcus ruber. Biodegradation 2008, 19, 851–858. [Google Scholar] [CrossRef]
- Satti, S.M.; Shah, Z.; Luqman, A.; Hasan, F.; Osman, M.; Shah, A.A. Biodegradation of Poly (3-hydroxybutyrate) and Poly (3-hydroxybutyrate-co-3-hydroxyvalerate) by newly isolated Penicillium oxalicum SS2 in soil microcosms and partial characterization of extracellular depolymerase. Curr. Microbiol. 2020, 77, 1622–1636. [Google Scholar] [CrossRef]
- Shah, A.A.; Hasan, F.; Hameed, A.; Ahmed, S. Biological degradation of plastics: A comprehensive review. Biotechnol. Adv. 2008, 26, 246–265. [Google Scholar] [CrossRef]
- Wang, S.Y.; Jiao, H. Scavenging capacity of berry crops on superoxide radicals, hydrogen peroxide, hydroxyl radicals, and singlet oxygen. J. Agric. Food Chem. 2000, 48, 5677–5684. [Google Scholar] [CrossRef]
- Osman, M.; Satti, S.M.; Luqman, A.; Hasan, F.; Shah, Z.; Shah, A.A. Degradation of polyester polyurethane by Aspergillus sp. strain S45 isolated from soil. J. Polym. Environ. 2018, 26, 301–310. [Google Scholar] [CrossRef]
- Mayekar, P.C.; Castro-Aguirre, E.; Auras, R.; Selke, S.; Narayan, R. Effect of nano-clay and surfactant on the biodegradation of poly (lactic acid) films. Polymers 2020, 12, 311. [Google Scholar] [CrossRef] [PubMed]
- Castro-Aguirre, E.; Auras, R.; Selke, S.; Rubino, M.; Marsh, T. Impact of nanoclays on the biodegradation of poly (lactic acid) nanocomposites. Polymers 2018, 10, 202. [Google Scholar] [CrossRef] [PubMed]
- Satti, S.M.; Shah, A.A.; Auras, R.; Marsh, T.L. Isolation and characterization of bacteria capable of degrading poly (lactic acid) at ambient temperature. Polym. Degrad. Stab. 2017, 144, 392–400. [Google Scholar] [CrossRef]
- Arvanitoyannis, I.; Biliaderis, C.G. Physical properties of polyol-plasticized edible blends made of methyl cellulose and soluble starch. Carbohydr. Polym. 1999, 38, 47–58. [Google Scholar] [CrossRef]
- Scott, G.; Wiles, D.M. Programmed-life plastics from polyolefins: A new look at sustainability. Biomacromolecules 2001, 2, 615–622. [Google Scholar] [CrossRef]
- Gu, J.G.; Gu, J.D. Methods currently used in testing microbiological degradation and deterioration of a wide range of polymeric materials with various degree of degradability: A review. J. Polym. Environ. 2005, 13, 65–74. [Google Scholar] [CrossRef]
- Kyrikou, I.; Briassoulis, D. Biodegradation of agricultural plastic films: A critical review. J. Polym. Environ. 2007, 15, 125–150. [Google Scholar] [CrossRef]
- Chaudhary, A.K.; Chaitanya, K.; Vijayakumar, R. Synergistic effect of UV and chemical treatment on biological degradation of Polystyrene by Cephalosporium strain NCIM 1251. Arch. Microbiol. 2021, 203, 2183–2191. [Google Scholar] [CrossRef]
- Karlberg, J.; Albertsson-Wikland, K. Growth in full-term small-for-gestational-age infants: From birth to final height. Pediatr. Res. 1995, 38, 733–739. [Google Scholar] [CrossRef]
- Vos, T.; Abajobir, A.A.; Abate, K.H.; Abbafati, C.; Abbas, K.M.; Abd-Allah, F.; Abdulkader, R.S.; Abdulle, A.M.; Abebo, T.A.; Abera, S.F. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1211–1259. [Google Scholar] [CrossRef]
- Lee, C.M.; Shleifer, A.; Thaler, R.H. Investor sentiment and the closed-end fund puzzle. J. Financ. 1991, 46, 75–109. [Google Scholar]
- Karthik, M.; Usha, S.; Venkateswaran, K.; Panchal, H.; Suresh, M.; Priya, V.; Hinduja, K. Evaluation of electromagnetic intrusion in brushless DC motor drive for electric vehicle applications with manifestation of mitigating the electromagnetic interference. Int. J. Ambient. Energy 2020. [Google Scholar] [CrossRef]
- Abbas-Abadi, M.S. The effect of process and structural parameters on the stability, thermo-mechanical and thermal degradation of polymers with hydrocarbon skeleton containing PE, PP, PS, PVC, NR, PBR and SBR. J. Therm. Anal. Calorim. 2021, 143, 2867–2882. [Google Scholar] [CrossRef]
- Aly, M.M.; Jaar, T.A.M.; Bokhari, F.M. Poly-β-hydroxybutyrate degradation by Aspergillus fumigates isolated from soil samples collected from Jeddah, Saudi arabia. J. Pharm. Biol. Sci. 2017, 12, 53–61. [Google Scholar] [CrossRef]
- De Oliveira, T.A.; Barbosa, R.; Mesquita, A.B.; Ferreira, J.H.; De Carvalho, L.H.; Alves, T.S. Fungal degradation of reprocessed PP/PBAT/thermoplastic starch blends. J. Mater. Res. Technol. 2020, 9, 2338–2349. [Google Scholar] [CrossRef]
- Prenafeta-Boldu, F.X.; Guivernau, M.; Gallastegui, G.; Viñas, M.; de Hoog, G.S.; Elias, A. Fungal/bacterial interactions during the biodegradation of TEX hydrocarbons (toluene, ethylbenzene and p-xylene) in gas biofilters operated under xerophilic conditions. FEMS Microbiol. Ecol. 2012, 80, 722–734. [Google Scholar] [CrossRef]
- Agarwal, S.; Qian, W.; Tan, R. Household Finance: A Functional Approach, 1st ed.; Palgrave Macmillan: Singapore, 2020; p. 352. [Google Scholar] [CrossRef]
- Rebouh, N.Y.; Khugaev, C.V.; Utkina, A.O.; Isaev, K.V.; Mohamed, E.S.; Kucher, D.E. Contribution of Eco-Friendly Agricultural Practices in Improving and Stabilizing Wheat Crop Yield: A Review. Agronomy 2023, 13, 2400. [Google Scholar] [CrossRef]
- Hajji-Hedfi, L.; Rhouma, A.; Hajlaoui, H.; Hajlaoui, F.; Rebouh, N.Y. Understanding the influence of applying two culture filtrates to control gray mold disease (Botrytis cinerea) in tomato. Agronomy 2023, 13, 1774. [Google Scholar] [CrossRef]
- Lee, J.W.; Choi, S.P.; Thiruvenkatachari, R.; Shim, W.G.; Moon, H. Submerged microfiltration membrane coupled with alum coagulation/powdered activated carbon adsorption for complete decolorization of reactive dyes. Water Res. 2006, 40, 435–444. [Google Scholar] [CrossRef]
| Fungal Isolate | CO2 Produced in Test (g/L) | CO2 Produced in Control (g/L) | CO2 Evolved Due to Biodegradation (g/L) |
|---|---|---|---|
| Phanerochaete chrysosporium | 19.81 ± 0.94 | 10.16 ± 0.63 | 9.65 ± 0.35 |
| Treatment | Weight Average Molecular Weight Mw (Daltons) | Number Average Molecular Weight Mn (Daltons) | Polydispersity (Mw/Mn) |
|---|---|---|---|
| Control | 198,066 ± 10.20 | 107,553 ± 10.61 | 1.842 ± 0.12 |
| Phanerochaete chrysosporium NA3 | 158,169 ± 7.65 | 71,668 ± 9.82 | 2.248 ± 0.14 |
| Heat-Pretreated Polystyrene | |||
| Samples | Weight Average Molecular Weight | Number Average Molecular Weight | Polydispersity |
| Mw (Daltons) | Mn (Daltons) | (Mw/Mn) | |
| With no fungus (control) | 232,142 ± 9.61 | 73,191 ± 10.19 | 2.013 ± 0.09 |
| With P. chrysosporium NA3 | 218,921 ± 7.53 | 63,909 ± 4.66 | 3.426 ± 0.04 |
| UV-Pretreated Polystyrene | |||
| Samples | Weight Average Molecular Weight | Number Average Molecular Weight | Polydispersity |
| Mw (Daltons) | Mn (Daltons) | (Mw/Mn) | |
| With no fungus (control) | 226,780 ± 11.25 | 80,186 ± 10.69 | 2.828 ± 0.02 |
| With P. chrysosporium NA3 | 203,818 ± 8.38 | 60,118 ± 4.08 | 3.39 ± 0.05 |
| Unsterilized Soil Burial | |||
| Treatment | Weight Average Molecular Weight | Number Average Molecular Weight | Polydispersity |
| Mw (Daltons) | Mn (Daltons) | (Mw/Mn) | |
| Unsterilized soil uninoculated control | 186,069 ± 10.11 | 77,107 ± 5.93 | 2.413 ± 0.07 |
| P. chrysosporium NA3 | 243,086 ± 8.76 | 72,318 ± 7.32 | 3.361 ± 0.08 |
| Sterilized Soil Burial | |||
| Treatment | Weight Average Molecular Weight | Number Average Molecular Weight | Polydispersity |
| Mw (Daltons) | Mn (Daltons) | (Mw/Mn) | |
| Sterilized soil uninoculated control | 193,829 ± 5.64 | 94,958 ± 8.55 | 2.041 ± 0.08 |
| P. chrysosporium NA3 | 189,834 ± 9.77 | 39,147 ± 6.71 | 4.849 ± 0.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shereen, M.A.; Satti, S.M.; Abbasi, A.; Atiq, N.; Yousafi, Q.; Ahmed, S.; Parveen, K.; Rebouh, N.Y. Investigating the Polystyrene (PS) Biodegradation Potential of Phanerochaete chrysosporium Strain NA3: A Newly Isolated Soil Fungus. Life 2025, 15, 869. https://doi.org/10.3390/life15060869
Shereen MA, Satti SM, Abbasi A, Atiq N, Yousafi Q, Ahmed S, Parveen K, Rebouh NY. Investigating the Polystyrene (PS) Biodegradation Potential of Phanerochaete chrysosporium Strain NA3: A Newly Isolated Soil Fungus. Life. 2025; 15(6):869. https://doi.org/10.3390/life15060869
Chicago/Turabian StyleShereen, Muhammad Adnan, Sadia Mehmood Satti, Asim Abbasi, Naima Atiq, Qudsia Yousafi, Safia Ahmed, Kousar Parveen, and Nazih Y. Rebouh. 2025. "Investigating the Polystyrene (PS) Biodegradation Potential of Phanerochaete chrysosporium Strain NA3: A Newly Isolated Soil Fungus" Life 15, no. 6: 869. https://doi.org/10.3390/life15060869
APA StyleShereen, M. A., Satti, S. M., Abbasi, A., Atiq, N., Yousafi, Q., Ahmed, S., Parveen, K., & Rebouh, N. Y. (2025). Investigating the Polystyrene (PS) Biodegradation Potential of Phanerochaete chrysosporium Strain NA3: A Newly Isolated Soil Fungus. Life, 15(6), 869. https://doi.org/10.3390/life15060869








