Trends in Liver Cirrhosis and Diabetes-Related Mortality Among Adults in the United States: A CDC WONDER Database Analysis (1999–2020)
Abstract
1. Introduction
2. Methods
2.1. Study Setting and Population
2.2. Data Abstraction
2.3. Statistical Analysis
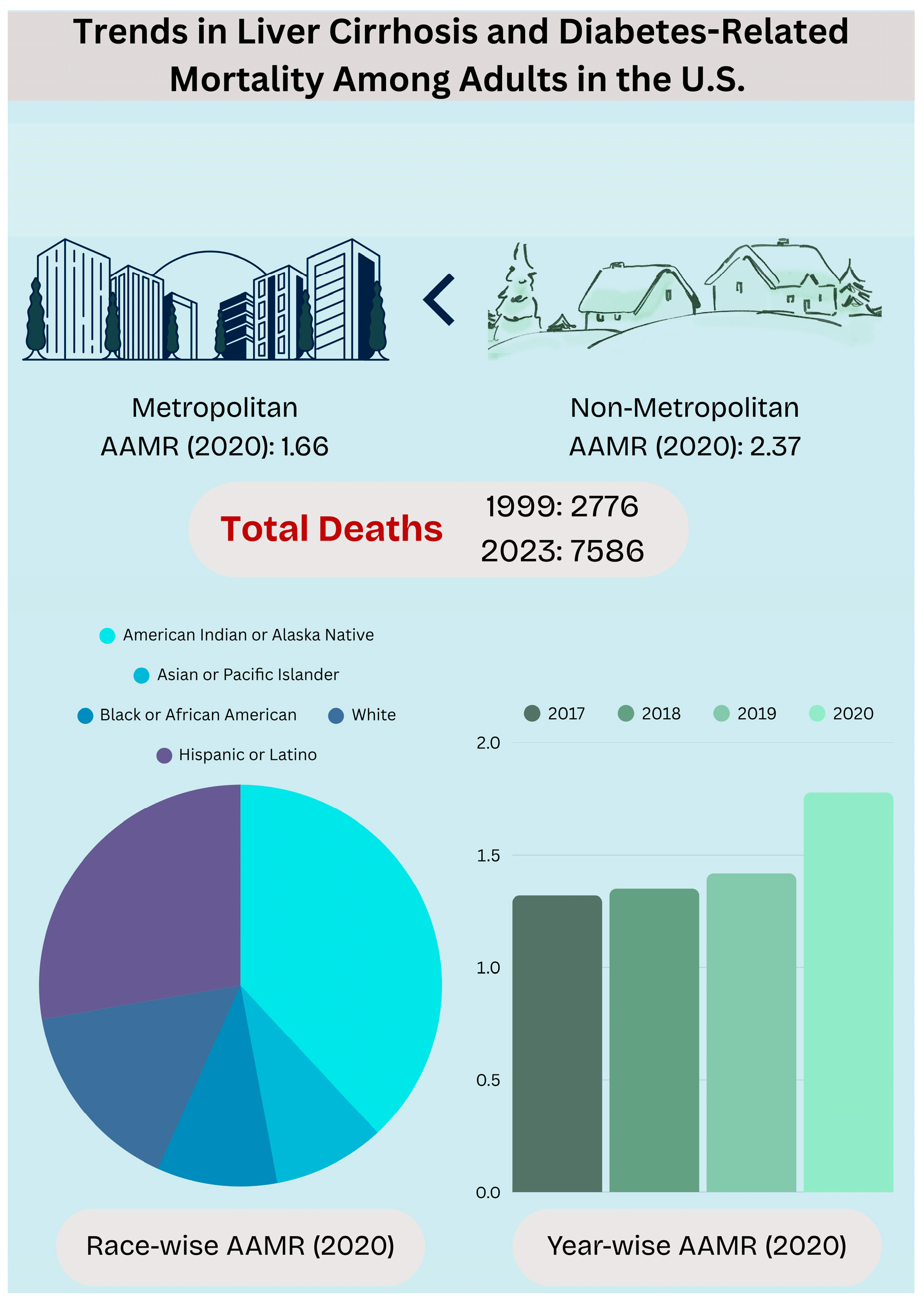
3. Results
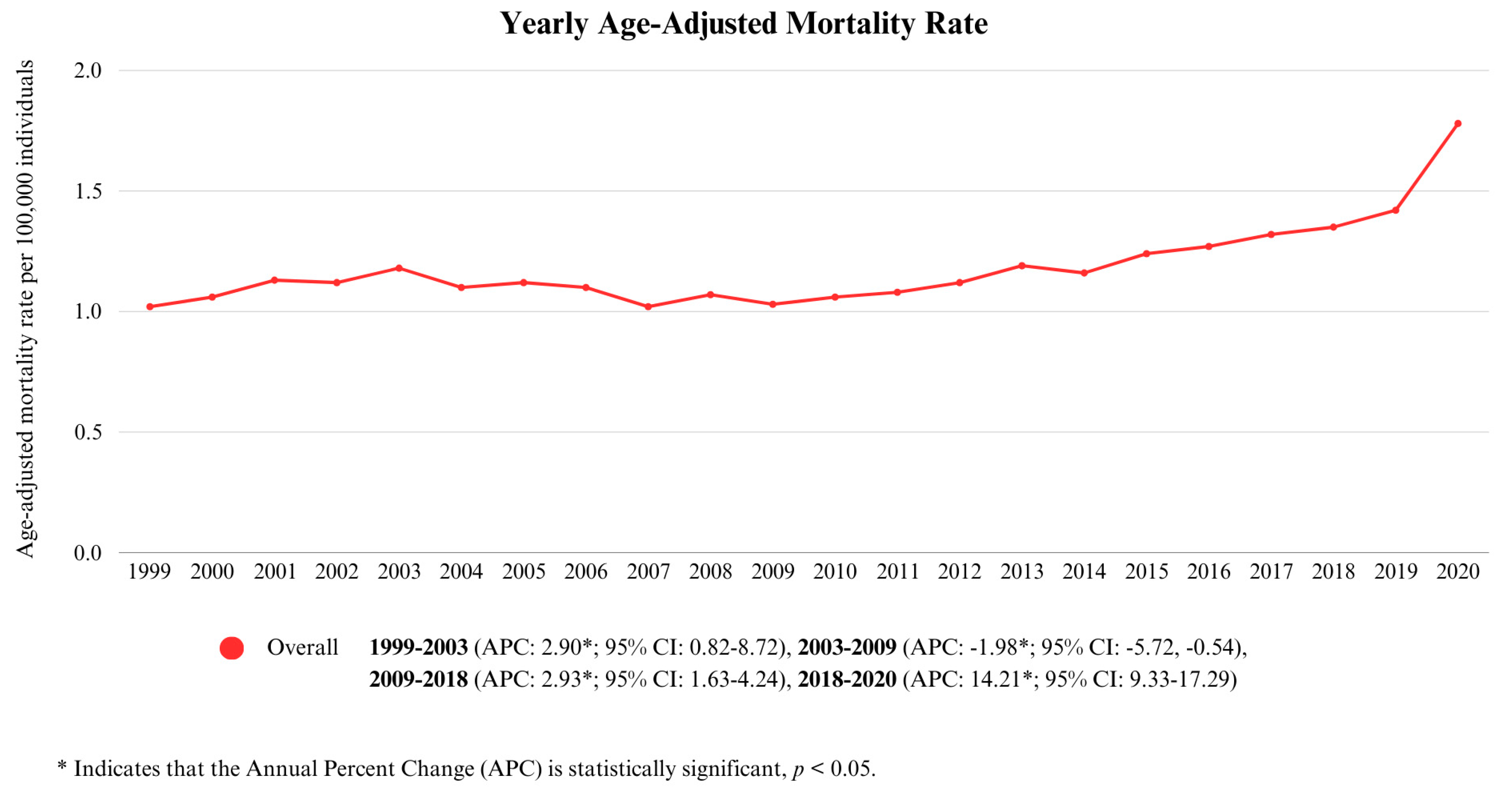
3.1. Annual Trends for Cirrhosis and Diabetes Mellitus-Related Mortality
3.2. Cirrhosis and Diabetes Mellitus-Related Mortality Stratified by Demographics
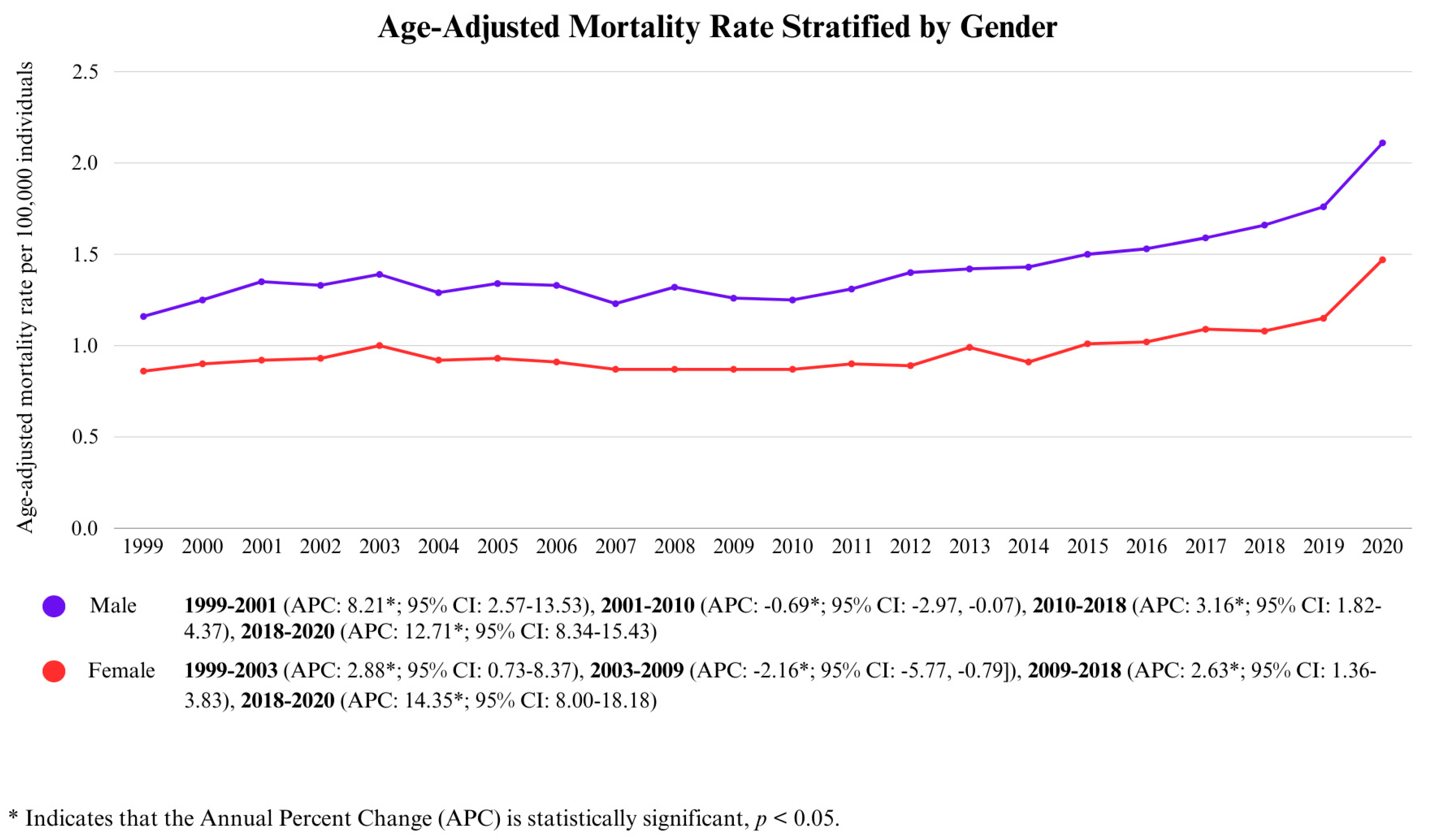
3.2.1. Gender-Wise Analysis
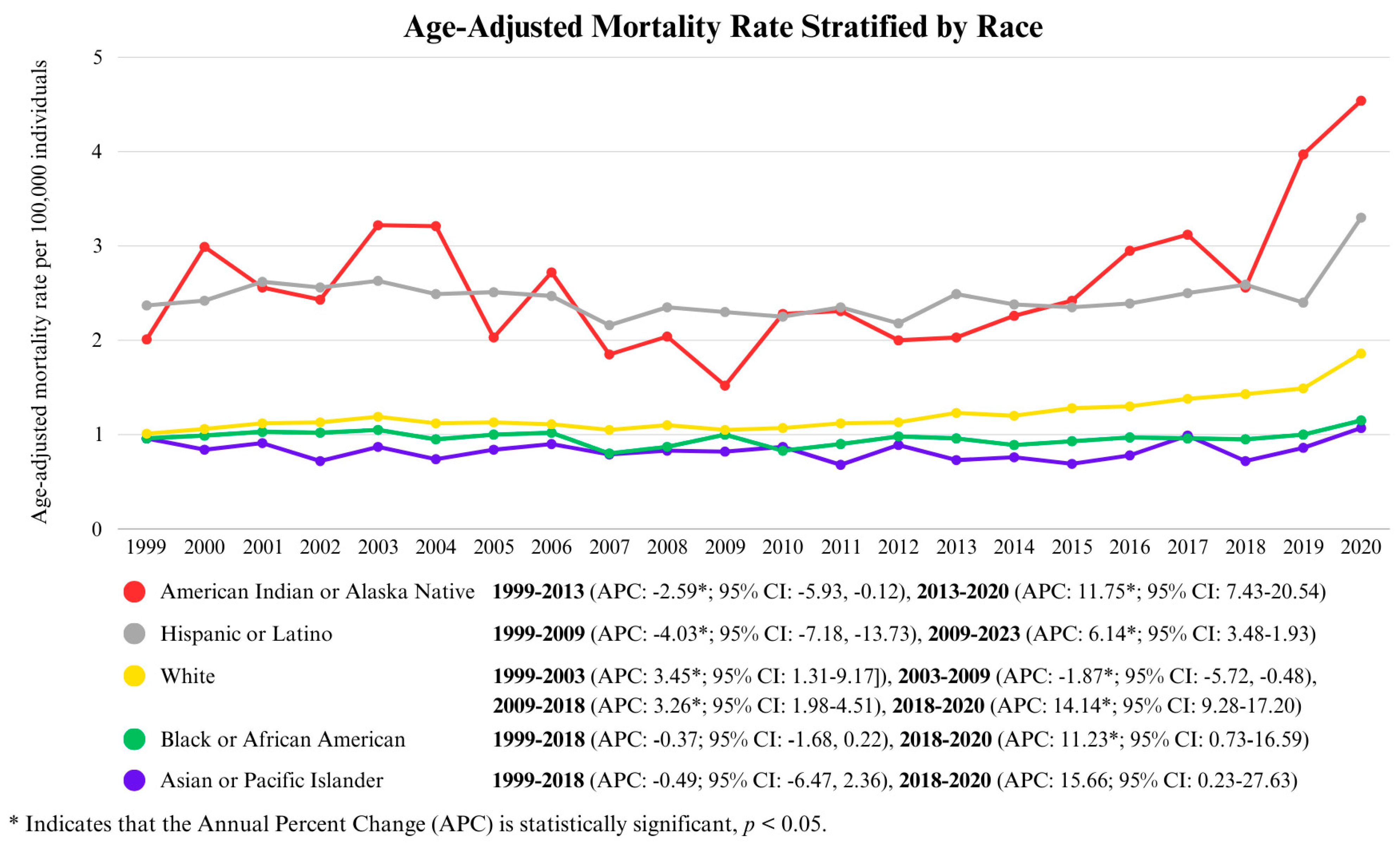
3.2.2. Race-Wise Analysis
3.2.3. Age-Wise Analysis
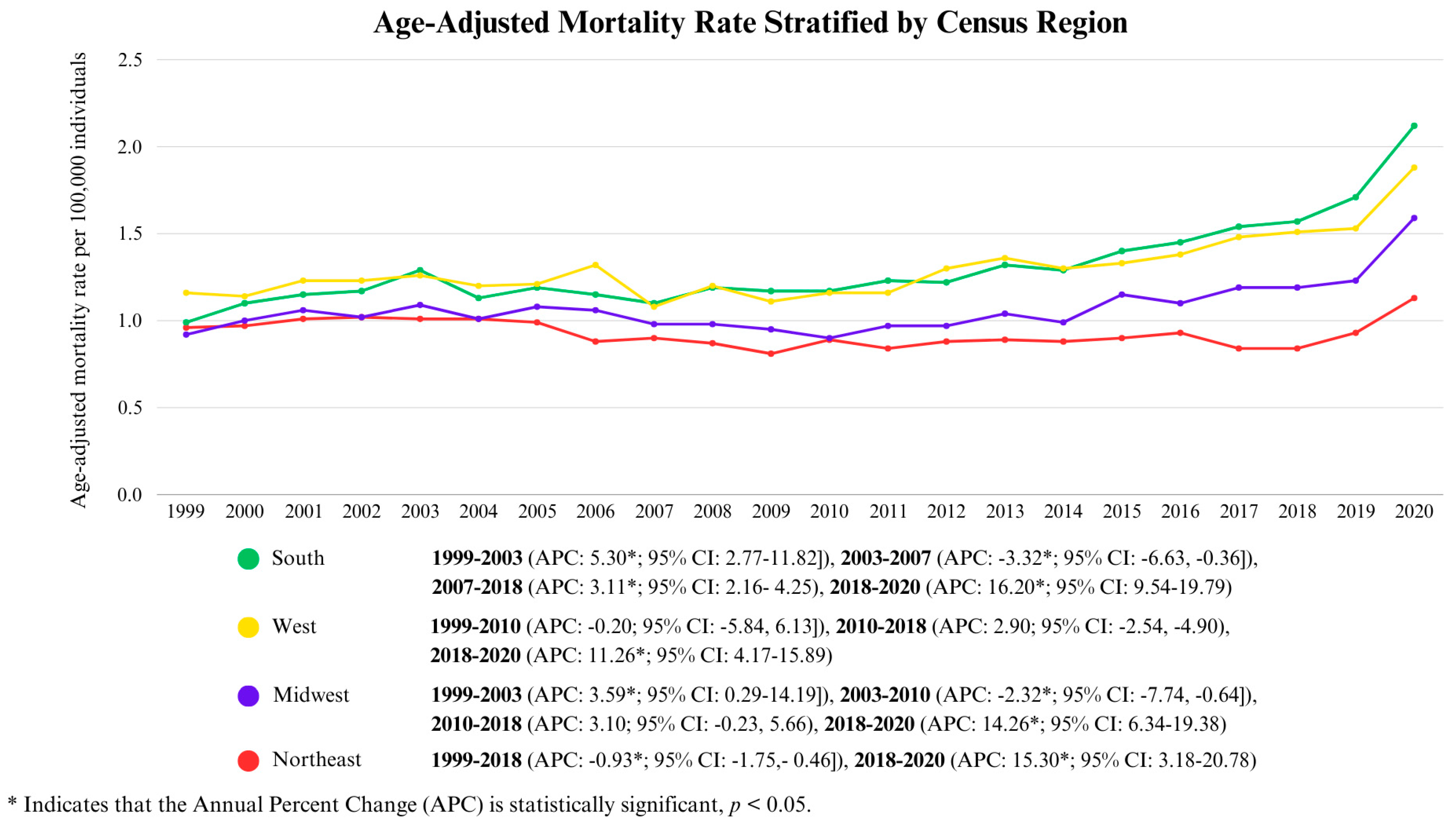
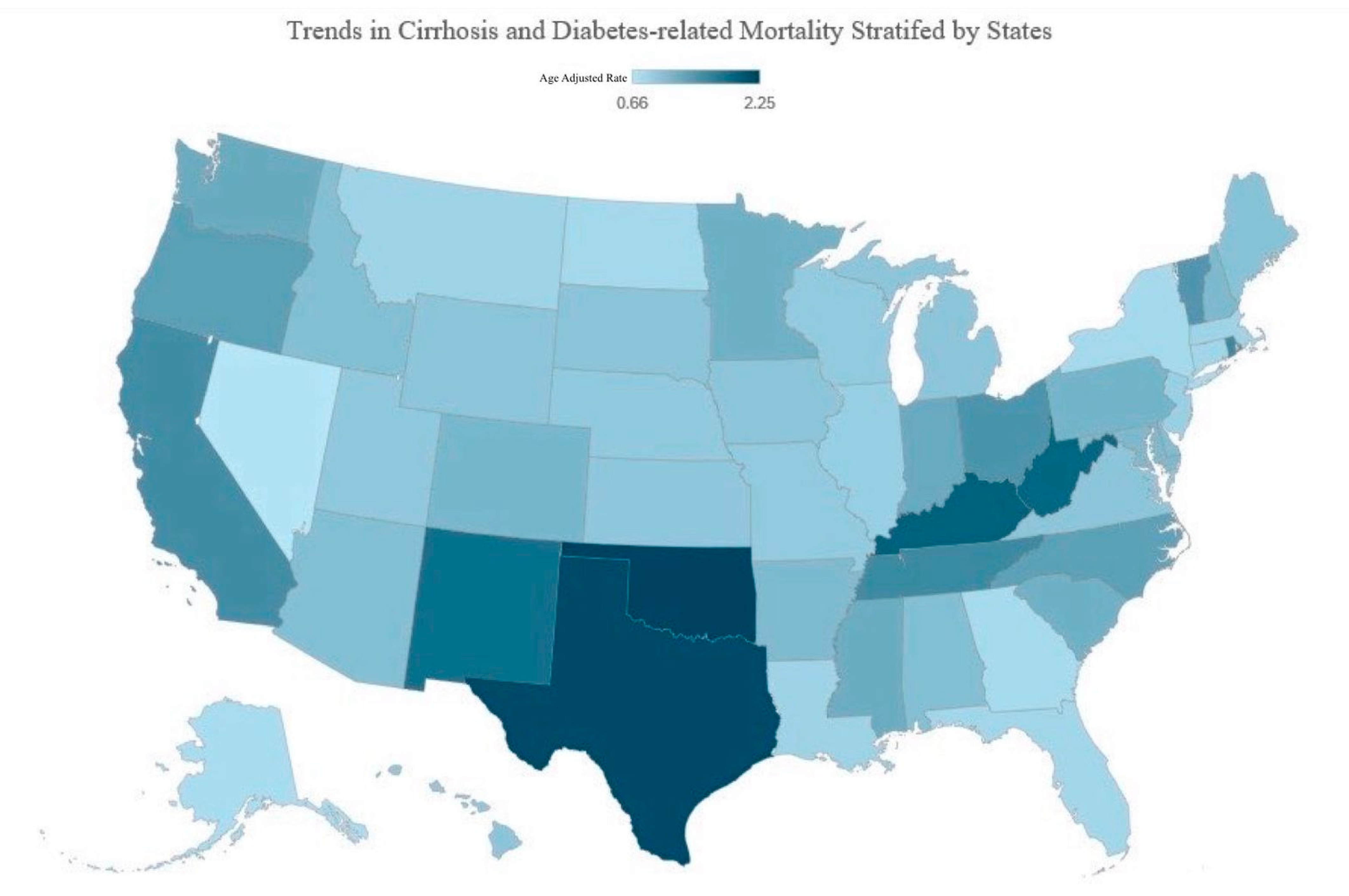
3.3. Cirrhosis and Diabetes Mellitus-Related Mortality Stratified by Region
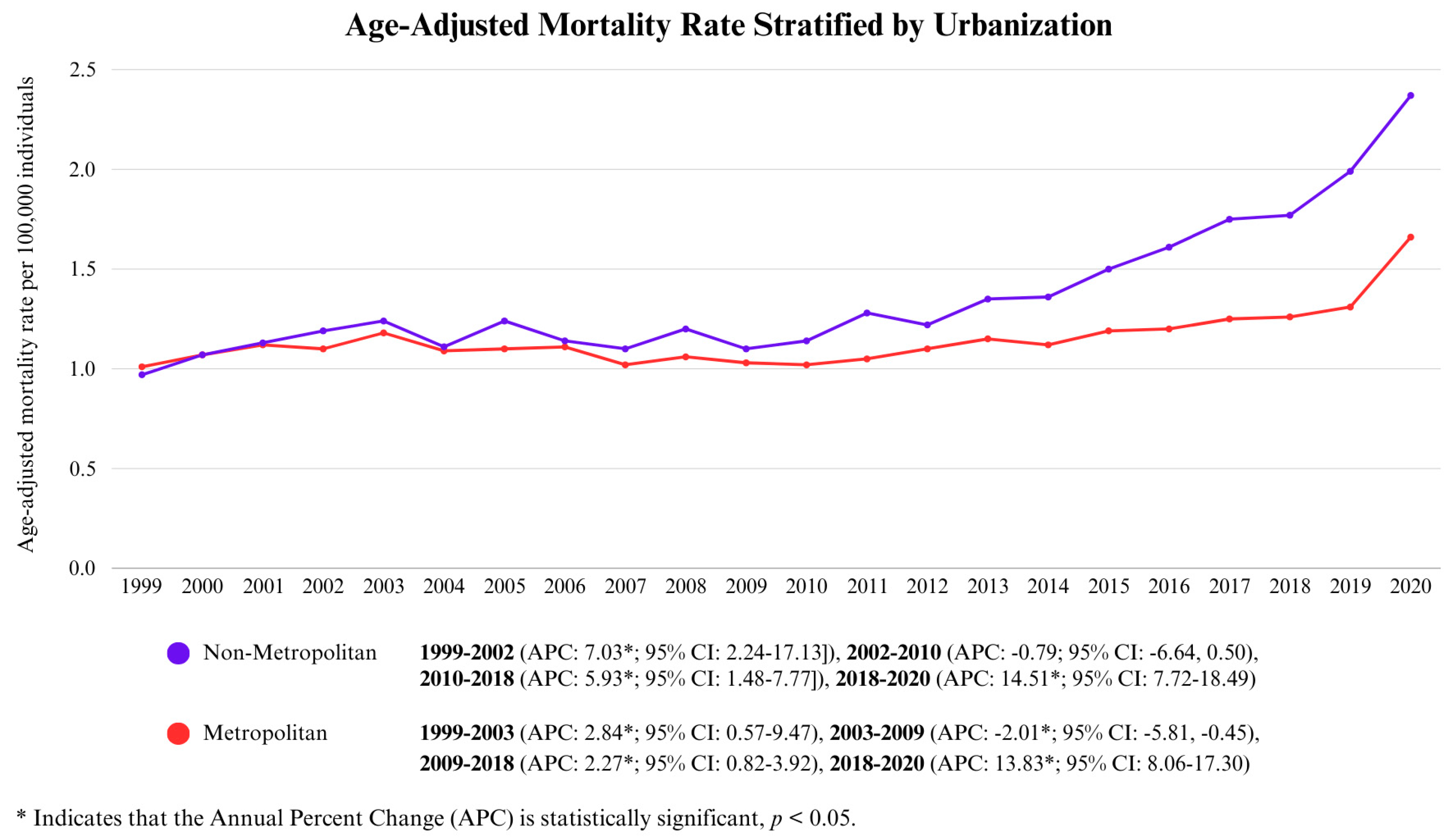
3.3.1. Urbanization-Wise Analysis
3.3.2. Census Region-Wise Analysis
3.3.3. State Wise Analysis
4. Discussion
4.1. Clinical Implications
4.2. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Harreiter, J.; Roden, M. Diabetes mellitus—Definition, Klassifikation, Diagnose, Screening und Prävention (Update 2023). Wien. Klin. Wochenschr. 2023, 135, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Definition & Facts for Cirrhosis—NIDDK. Available online: https://www.niddk.nih.gov/health-information/liver-disease/cirrhosis/definition-facts (accessed on 4 April 2025).
- Lee, W.G.; Wells, C.I.; McCall, J.L.; Murphy, R.; Plank, L.D. Prevalence of diabetes in liver cirrhosis: A systematic review and meta-analysis. Diabetes Metab. Res. Rev. 2019, 35, e3157. [Google Scholar] [CrossRef] [PubMed]
- Asrani, S.K.; Devarbhavi, H.; Eaton, J.; Kamath, P.S. Burden of liver diseases in the world. J. Hepatol. 2019, 70, 151–171. [Google Scholar] [CrossRef]
- Devarbhavi, H.; Asrani, S.K.; Arab, J.P.; Nartey, Y.A.; Pose, E.; Kamath, P.S. Global burden of liver disease: 2023 update. J. Hepatol. 2023, 79, 516–537. [Google Scholar] [CrossRef]
- Ma, C.; Qian, A.S.; Nguyen, N.H.; Stukalin, I.; Congly, S.E.; Shaheen, A.A.; Swain, M.G.; Teriaky, A.; Asrani, S.K.; Singh, S. Trends in the Economic Burden of Chronic Liver Diseases and Cirrhosis in the United States: 1996–2016. Am. J. Gastroenterol. 2021, 116, 2060–2067. [Google Scholar] [CrossRef]
- Ong, K.L.; Stafford, L.K.; McLaughlin, S.A.; Boyko, E.J.; Vollset, S.E.; Smith, A.E.; Dalton, B.E.; Duprey, J.; Cruz, J.A.; Hagins, H.; et al. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2023, 402, 203–234. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Compean, D.; Jaquez-Quintana, J.O.; Gonzalez-Gonzalez, J.A.; Maldonado-Garza, H. Liver cirrhosis and diabetes: Risk factors, pathophysiology, clinical implications and management. World J. Gastroenterol. 2009, 15, 280. [Google Scholar] [CrossRef]
- Perseghin, G.; Mazzaferro, V.; Sereni, L.P.; Regalia, E.; Benedini, S.; Bazzigaluppi, E.; Pulvirenti, A.; Leão, A.A.S.; Calori, G.; Romito, R.; et al. Contribution of reduced insulin sensitivity and secretion to the pathogenesis of hepatogenous diabetes: Effect of liver transplantation. Hepatology 2000, 31, 694–703. [Google Scholar] [CrossRef]
- Tietge, U.J.F.; Selberg, O.; Kreter, A.; Bahr, M.J.; Pirlich, M.; Burchert, W.; Müller, M.J.; Manns, M.P.; Böker, K.H.W. Alterations in glucose metabolism associated with liver cirrhosis persist in the clinically stable long-term course after liver transplantation. Liver Transpl. 2004, 10, 1030–1040. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef]
- Us_Regdiv.PDF. Available online: https://www2.census.gov/geo/pdfs/maps-data/maps/reference/us_regdiv.pdf (accessed on 4 April 2025).
- Aggarwal, R.; Chiu, N.; Loccoh, E.C.; Kazi, D.S.; Yeh, R.W.; Wadhera, R.K. Rural-Urban Disparities. J. Am. Coll. Cardiol. 2021, 77, 1480–1481. [Google Scholar] [CrossRef] [PubMed]
- Ingram, D.D.; Franco, S.J. 2013 NCHS Urban-Rural Classification Scheme for Counties. Google Books. Available online: https://books.google.com.pk/books?hl=en&lr=&id=uB5dsp2ZCdsC&oi=fnd&pg=PP8&ots=KJS11kniHA&sig=nUP7BVPJC04DelwKCDa05K-tcL4&redir_esc=y#v=onepage&q&f=false (accessed on 4 April 2025).
- Imran, R.; Aamir, Z.; Hasan, A.; Kasbati, M.; Iqbal, N.; Boyd, C.J. Trends in Iatrogenic Error–Related Mortality in the US From 1999 to 2020: Age–Period–CohortAnalysis. J. Surg. Res. 2025, 306, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Joinpoint Regression Program. Available online: https://surveillance.cancer.gov/joinpoint/ (accessed on 4 April 2025).
- Elkrief, L.; Rautou, P.; Sarin, S.; Valla, D.; Paradis, V.; Moreau, R. Diabetes mellitus in patients with cirrhosis: Clinical implications and management. Liver Int. 2016, 36, 936–948. [Google Scholar] [CrossRef]
- Holstein, A.; Hinze, S.; THIEßEn, E.; Plaschke, A.; Egberts, E. Clinical implications of hepatogenous diabetes in liver cirrhosis. J. Gastroenterol. Hepatol. 2002, 17, 677–681. [Google Scholar] [CrossRef]
- Nishida, T.; Tsuji, S.; Tsujii, M.; Arimitsu, S.; Haruna, Y.; Imano, E.; Suzuki, M.; Kanda, T.; Kawano, S.; Hiramatsu, N.; et al. Oral Glucose Tolerance Test Predicts Prognosis of Patients with Liver Cirrhosis. Am. J. Gastroenterol. 2006, 101, 70–75. [Google Scholar] [CrossRef]
- Bianchi, G. Prognostic significance of diabetes in patients with cirrhosis*1. Hepatology 1994, 20, 119–125. [Google Scholar] [CrossRef]
- Moreau, R.; Delègue, P.; Pessione, F.; Hillaire, S.; Durand, F.; Lebrec, D.; Valla, D. Clinical characteristics and outcome of patients with cirrhosis and refractory ascites. Liver Int. 2004, 24, 457–464. [Google Scholar] [CrossRef]
- Kolaczynski, J.W.; Carter, R.; Soprano, K.J.; Moscicki, R.; Boden, G. Insulin binding and degradation by rat liver Kupffer and endothelial cells. Metabolism 1993, 42, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Seki, E.; Schwabe, R.F. Hepatic inflammation and fibrosis: Functional links and key pathways. Hepatology 2015, 61, 1066–1079. [Google Scholar] [CrossRef]
- Anty, R.; Lemoine, M. Liver fibrogenesis and metabolic factors. Clin. Res. Hepatol. Gastroenterol. 2011, 35, S10–S20. [Google Scholar] [CrossRef]
- García-Compeán, D.; Jáquez-Quintana, J.O.; Lavalle-González, F.J.; González-González, J.A.; Maldonado-Garza, H.J.; Villarreal-Pérez, J.Z. Plasma cytokine levels imbalance in cirrhotic patients with impaired glucose tolerance and diabetes mellitus. A prospective study. Ann. Hepatol. 2014, 13, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Bertolani, C.; Marra, F. The role of adipokines in liver fibrosis. Pathophysiology 2008, 15, 91–101. [Google Scholar] [CrossRef]
- Adams, L.A.; Sanderson, S.; Lindor, K.D.; Angulo, P. The histological course of nonalcoholic fatty liver disease: A longitudinal study of 103 patients with sequential liver biopsies. J. Hepatol. 2005, 42, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Steppan, C.M.; Bailey, S.T.; Bhat, S.; Brown, E.J.; Banerjee, R.R.; Wright, C.M.; Patel, H.R.; Ahima, R.S.; Lazar, M.A. The hormone resistin links obesity to diabetes. Nature 2001, 409, 307–312. [Google Scholar] [CrossRef]
- Nielsen, M.F.; Caumo, A.; Aagaard, N.K.; Chandramouli, V.; Schumann, W.C.; Landau, B.R.; Schmitz, O.; Vilstrup, H. Contribution of defects in glucose uptake to carbohydrate intolerance in liver cirrhosis: Assessment during physiological glucose and insulin concentrations. Am. J. Physiol.-Gastrointest. Liver Physiol. 2005, 288, G1135–G1143. [Google Scholar] [CrossRef]
- Takai, S.; Inoue, J.; Kogure, T.; Kakazu, E.; Ninomiya, M.; Iwata, T.; Umetsu, T.; Nakamura, T.; Sano, A.; Shimosegawa, T. Acute-onset Autoimmune Hepatitis in a Young Woman with Type 1 Diabetes Mellitus. Intern. Med. Tokyo Jpn. 2018, 57, 1591–1596. [Google Scholar] [CrossRef] [PubMed]
- Fehrenbach, H.; Weiskirchen, R.; Kasper, M.; Gressner, A.M. Up-regulated expression of the receptor for advanced glycation end products in cultured rat hepatic stellate cells during transdifferentiation to myofibroblasts. Hepatology 2001, 34, 943–952. [Google Scholar] [CrossRef]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef]
- Vos, T.; Lim, S.S.; Abbafati, C.; Abbas, K.M.; Abbasi, M.; Abbasifard, M.; Abbasi-Kangevari, M.; Abbastabar, H.; Abd-Allah, F.; Abdelalim, A.; et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Ge, J.; Najafi, N.; Zhao, W.; Somsouk, M.; Fang, M.; Lai, J.C. A Methodology to Generate Longitudinally Updated Acute-On-Chronic Liver Failure Prognostication Scores From Electronic Health Record Data. Hepatol. Commun. 2021, 5, 1069–1080. [Google Scholar] [CrossRef]
- Wang, H.; Paulson, K.R.; Pease, S.A.; Watson, S.; Comfort, H.; Zheng, P.; Aravkin, A.Y.; Bisignano, C.; Barber, R.M.; Alam, T.; et al. Estimating excess mortality due to the COVID-19 pandemic: A systematic analysis of COVID-19-related mortality, 2020–2021. Lancet 2022, 399, 1513–1536. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.L.; Kochanek, K.D.; Xu, J.; Arias, E. Deaths: Final Data for 2021; National Center for Health Statistics: Hyattsville, MD, USA, 2024. [Google Scholar]
- Standards of Medical Care in Diabetes—2012. Diabetes Care 2012, 35 (Suppl. 1), S11–S63. [CrossRef]
- Younossi, Z.M.; Golabi, P.; Paik, J.M.; Henry, A.; Van Dongen, C.; Henry, L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): A systematic review. Hepatology 2023, 77, 1335–1347. [Google Scholar] [CrossRef] [PubMed]
- Evert, A.B.; Franz, M.J. American Diabetes Association Guide to Nutrition Therapy for Diabetes, 3rd ed.; American Diabetes Association: Crystal City, VA, USA, 2017; Available online: https://diabetesjournals.org/books/book/44/American-Diabetes-Association-Guide-to-Nutrition?searchresult=1 (accessed on 18 May 2025).
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wai-Sun Wong, V.; Dufour, J.-F.; Schattenberg, J.M.; et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Adams, L.A.; Anstee, Q.M.; Tilg, H.; Targher, G. Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut 2017, 66, 1138–1153. [Google Scholar] [CrossRef]
- Stefan, N.; Cusi, K. A global view of the interplay between non-alcoholic fatty liver disease and diabetes. Lancet Diabetes Endocrinol. 2022, 10, 284–296. [Google Scholar] [CrossRef]
- Sacharidou, A.; Chambliss, K.; Peng, J.; Barrera, J.; Tanigaki, K.; Luby-Phelps, K.; Özdemir, İ.; Khan, S.; Sirsi, S.R.; Kim, S.H.; et al. Endothelial ERα promotes glucose tolerance by enhancing endothelial insulin transport to skeletal muscle. Nat. Commun. 2023, 14, 4989. [Google Scholar] [CrossRef]
- Ezhilarasan, D. Critical role of estrogen in the progression of chronic liver diseases. Hepatobiliary Pancreat. Dis. Int. 2020, 19, 429–434. [Google Scholar] [CrossRef]
- Marjot, T.; Webb, G.J.; Barritt, A.S.; Moon, A.M.; Stamataki, Z.; Wong, V.W.; Barnes, E. COVID-19 and liver disease: Mechanistic and clinical perspectives. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 348–364. [Google Scholar] [CrossRef]
- Hales, C.M.; Carroll, M.D.; Fryar, C.D.; Ogden, C.L. Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017–2018; NCHS Data Brief No. 360; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2020; pp. 1–8. [Google Scholar]
- Adult Obesity Prevalence Map | Obesity | CDC. Available online: https://www.cdc.gov/obesity/data-and-statistics/adult-obesity-prevalence-maps.html (accessed on 4 April 2025).
- Health and Health Coverage in the South: A Data Update | KFF. Available online: https://www.kff.org/racial-equity-and-health-policy/issue-brief/health-and-health-coverage-in-the-south-a-data-update (accessed on 18 May 2025).
- The Commonwealth Fund—2020 Scorecard on State Health System Performance. Available online: https://2020scorecard.commonwealthfund.org/ (accessed on 4 April 2025).
- Kim, D.; Alshuwaykh, O.; Dennis, B.B.; Cholankeril, G.; Knowles, J.W.; Ahmed, A. Chronic liver disease-related mortality in diabetes before and during the COVID-19 in the United States. Dig. Liver Dis. 2023, 55, 3–10. [Google Scholar] [CrossRef]
- Thompson, A.E.; Anisimowicz, Y.; Miedema, B.; Hogg, W.; Wodchis, W.P.; Aubrey-Bassler, K. The influence of gender and other patient characteristics on health care-seeking behaviour: A QUALICOPC study. BMC Fam. Pract. 2016, 17, 38. [Google Scholar] [CrossRef]
- Adakai, M.; Sandoval-Rosario, M.; Xu, F.; Aseret-Manygoats, T.; Allison, M.; Greenlund, K.J.; Barbour, K.E. Health Disparities Among American Indians/Alaska Natives—Arizona, 2017. MMWR Morb. Mortal. Wkly. Rep. 2018, 67, 1314–1318. [Google Scholar] [CrossRef] [PubMed]
- American Indian Health Disparities. Available online: https://www.nicoa.org/elder-resources/health-disparities/ (accessed on 4 April 2025).
- Delker, E.; Brown, Q.; Hasin, D.S. Alcohol Consumption in Demographic Subpopulations: An Epidemiologic Overview. Alcohol. Res. Curr. Rev. 2016, 38, 7–15. [Google Scholar]
- Landen, M.; Roeber, J.; Naimi, T.; Nielsen, L.; Sewell, M. Alcohol-Attributable Mortality Among American Indians and Alaska Natives in the United States, 1999–2009. Am. J. Public Health 2014, 104, S343–S349. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.; Dang, K.; Ladhani, S.; Singal, A.K.; Wong, R.J. Prevalence of Alcoholic Fatty Liver Disease Among Adults in the United States, 2001–2016. JAMA 2019, 321, 1723. [Google Scholar] [CrossRef]
- Levy, R.E.; Catana, A.M.; Durbin-Johnson, B.; Halsted, C.H.; Medici, V. Ethnic Differences in Presentation and Severity of Alcoholic Liver Disease. Alcohol. Clin. Exp. Res. 2015, 39, 566–574. [Google Scholar] [CrossRef]
- Martínez, L.A.; Larrieta, E.; Calva, J.J.; Kershenobich, D.; Torre, A. The Expression of PNPLA3 Polymorphism could be the Key for Severe Liver Disease in NAFLD in Hispanic Population. Ann. Hepatol. 2017, 16, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Stokowski, R.P.; Kershenobich, D.; Ballinger, D.G.; Hinds, D.A. Variant in PNPLA3 is associated with alcoholic liver disease. Nat. Genet. 2010, 42, 21–23. [Google Scholar] [CrossRef]
- Income and Poverty in the United States: 2020. Available online: https://www.census.gov/library/publications/2021/demo/p60-273.html (accessed on 18 May 2025).
- Rural Health Disparities Overview—Rural Health Information Hub. Available online: https://www.ruralhealthinfo.org/topics/rural-health-disparities (accessed on 4 April 2025).
- Chen, V.L.; Tedesco, N.R.; Hu, J.; Jasty, V.S.J.; Perumalswami, P.V. Rurality and Neighborhood Socioeconomic Status are Associated With Overall and Cause-Specific Mortality and Hepatic Decompensation in Type 2 Diabetes. Am. J. Med. 2025, 138, 809–818.e10. [Google Scholar] [CrossRef]
- Oberti, F.; Valsesia, E.; Pilette, C.; Rousselet, M.; Bedossa, P.; Aube, C.; Gallois, Y.; Rifflet, H.; Maiga, M.; Penneau-Fontbonne, D.; et al. Noninvasive diagnosis of hepatic fibrosis or cirrhosis. Gastroenterology 1997, 113, 1609–1616. [Google Scholar] [CrossRef]
- Sandrin, L.; Fourquet, B.; Hasquenoph, J.-M.; Yon, S.; Fournier, C.; Mal, F.; Christidis, C.; Ziol, M.; Poulet, B.; Kazemi, F.; et al. Transient elastography: A new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med. Biol. 2003, 29, 1705–1713. [Google Scholar] [CrossRef] [PubMed]
- Castera, L.; Cusi, K. Diabetes and cirrhosis: Current concepts on diagnosis and management. Hepatology 2023, 77, 2128–2146. [Google Scholar] [CrossRef]
- Bergman, M.; Abdul-Ghani, M.; DeFronzo, R.A.; Manco, M.; Sesti, G.; Fiorentino, T.V.; Ceriello, A.; Rhee, M.; Phillips, L.S.; Chung, S.; et al. Review of methods for detecting glycemic disorders. Diabetes Res. Clin. Pract. 2020, 165, 108233. [Google Scholar] [CrossRef] [PubMed]
- Tsoris, A.; Marlar, C.A. Use of the Child Pugh Score in Liver Disease; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: http://www.ncbi.nlm.nih.gov/books/NBK542308/ (accessed on 4 April 2025).
- American Diabetes Association. 8. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes—2018. Diabetes Care 2018, 41 (Suppl. 1), S73–S85. [Google Scholar] [CrossRef]
- Wlazlo, N.; Beijers, H.J.B.H.; Schoon, E.J.; Sauerwein, H.P.; Stehouwer, C.D.A.; Bravenboer, B. High prevalence of diabetes mellitus in patients with liver cirrhosis. Diabet. Med. 2010, 27, 1308–1311. [Google Scholar] [CrossRef] [PubMed]
- Fukui, H.; Saito, H.; Ueno, Y.; Uto, H.; Obara, K.; Sakaida, I.; Shibuya, A.; Seike, M.; Nagoshi, S.; Segawa, M.; et al. Evidence-based clinical practice guidelines for liver cirrhosis 2015. J. Gastroenterol. 2016, 51, 629–650. [Google Scholar] [CrossRef]
- Merli, M.; Leonetti, F.; Riggio, O.; Valeriano, V.; Ribaudo, M.C.; Sprati, F.; Tisone, G.; Casciani, C.U.; Capocaccia, L. Glucose intolerance and insulin resistance in cirrhosis are normalized after liver transplantation. Hepatology 1999, 30, 649–654. [Google Scholar] [CrossRef]
- D’Amico, G.; Garcia-Tsao, G.; Pagliaro, L. Natural history and prognostic indicators of survival in cirrhosis: A systematic review of 118 studies. J. Hepatol. 2006, 44, 217–231. [Google Scholar] [CrossRef]
- Facts About, U.S. Deaths from Excessive Alcohol Use | Alcohol Use | CDC. Available online: https://www.cdc.gov/alcohol/facts-stats/index.html (accessed on 4 April 2025).

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Irfan, M.; Ahmad, R.; Ahmed, M.A.; Khan, A.M.; Aamir, Z.; Imran, R.; Ahmed, R. Trends in Liver Cirrhosis and Diabetes-Related Mortality Among Adults in the United States: A CDC WONDER Database Analysis (1999–2020). Life 2025, 15, 852. https://doi.org/10.3390/life15060852
Irfan M, Ahmad R, Ahmed MA, Khan AM, Aamir Z, Imran R, Ahmed R. Trends in Liver Cirrhosis and Diabetes-Related Mortality Among Adults in the United States: A CDC WONDER Database Analysis (1999–2020). Life. 2025; 15(6):852. https://doi.org/10.3390/life15060852
Chicago/Turabian StyleIrfan, Manahil, Rameesha Ahmad, Mariam Arshad Ahmed, Ayan Mohammed Khan, Zoya Aamir, Rayaan Imran, and Raheel Ahmed. 2025. "Trends in Liver Cirrhosis and Diabetes-Related Mortality Among Adults in the United States: A CDC WONDER Database Analysis (1999–2020)" Life 15, no. 6: 852. https://doi.org/10.3390/life15060852
APA StyleIrfan, M., Ahmad, R., Ahmed, M. A., Khan, A. M., Aamir, Z., Imran, R., & Ahmed, R. (2025). Trends in Liver Cirrhosis and Diabetes-Related Mortality Among Adults in the United States: A CDC WONDER Database Analysis (1999–2020). Life, 15(6), 852. https://doi.org/10.3390/life15060852





