Astaxanthin Alleviates the Decline of Sperm Quality Caused by Heat Stress in Mice via Reducing Oxidative Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Approval
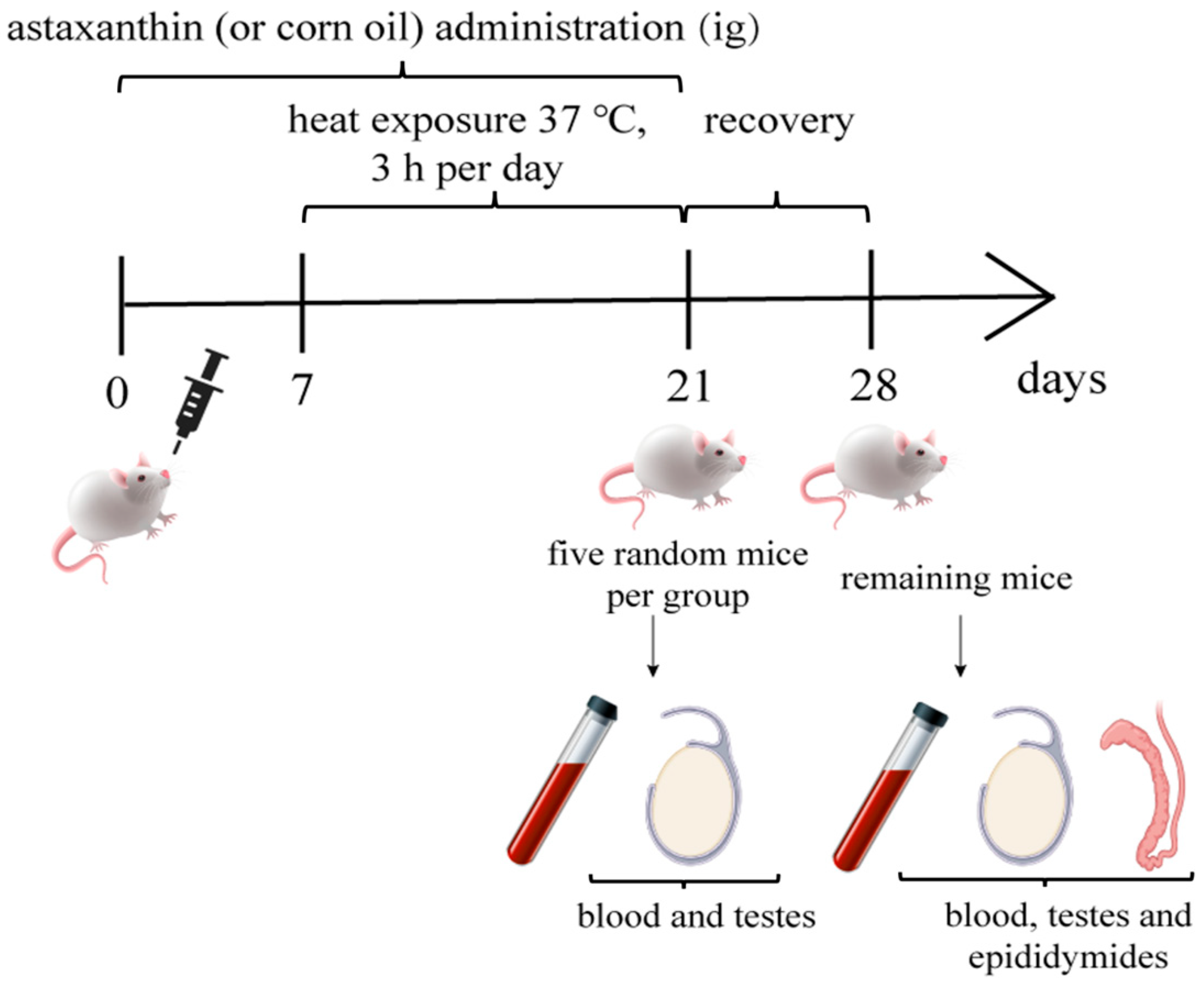
2.2. Animals and Experimental Design
2.3. Analysis of Sperm Motility, Viability, and Morphology
2.4. Determination of Testosterone Level in Serum
2.5. Determination of Oxidative Indexes Level in Testis and Serum
2.6. Statistical Analysis
3. Results
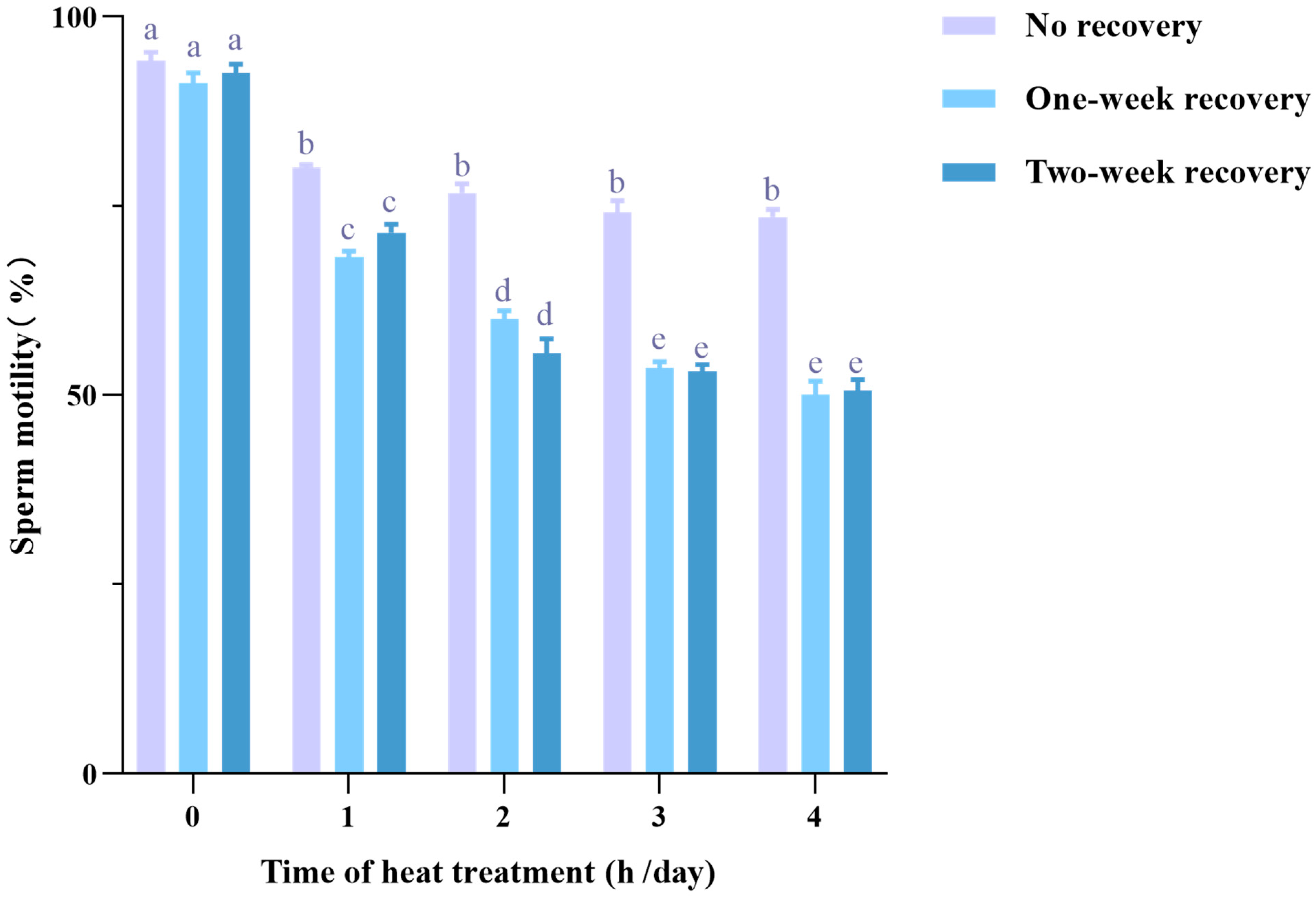
3.1. Heat Stress Decreases Sperm Motility with a Carryover Effect
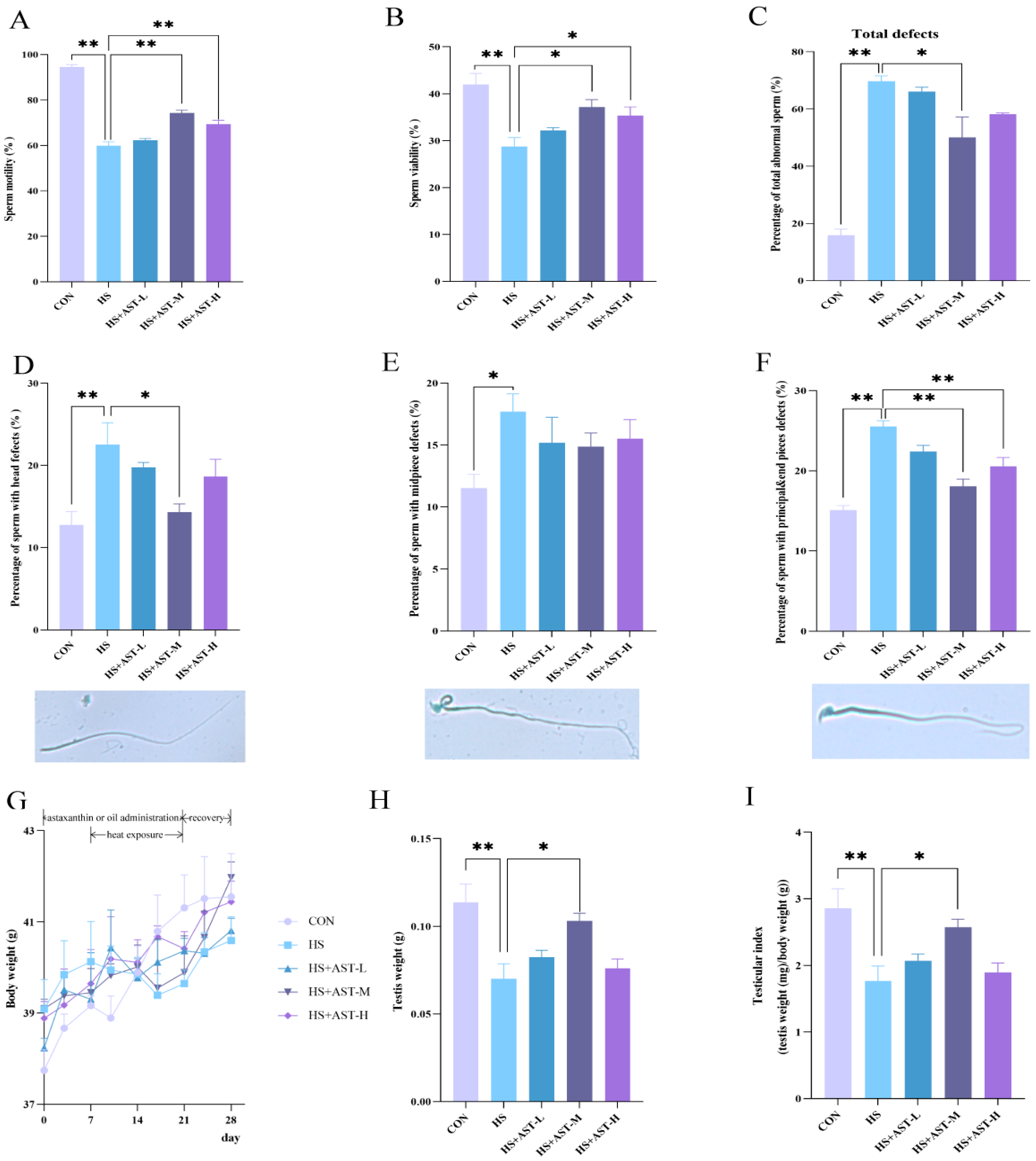
3.2. Astaxanthin Alleviates Heat Stress-Induced Decline of Sperm Quality
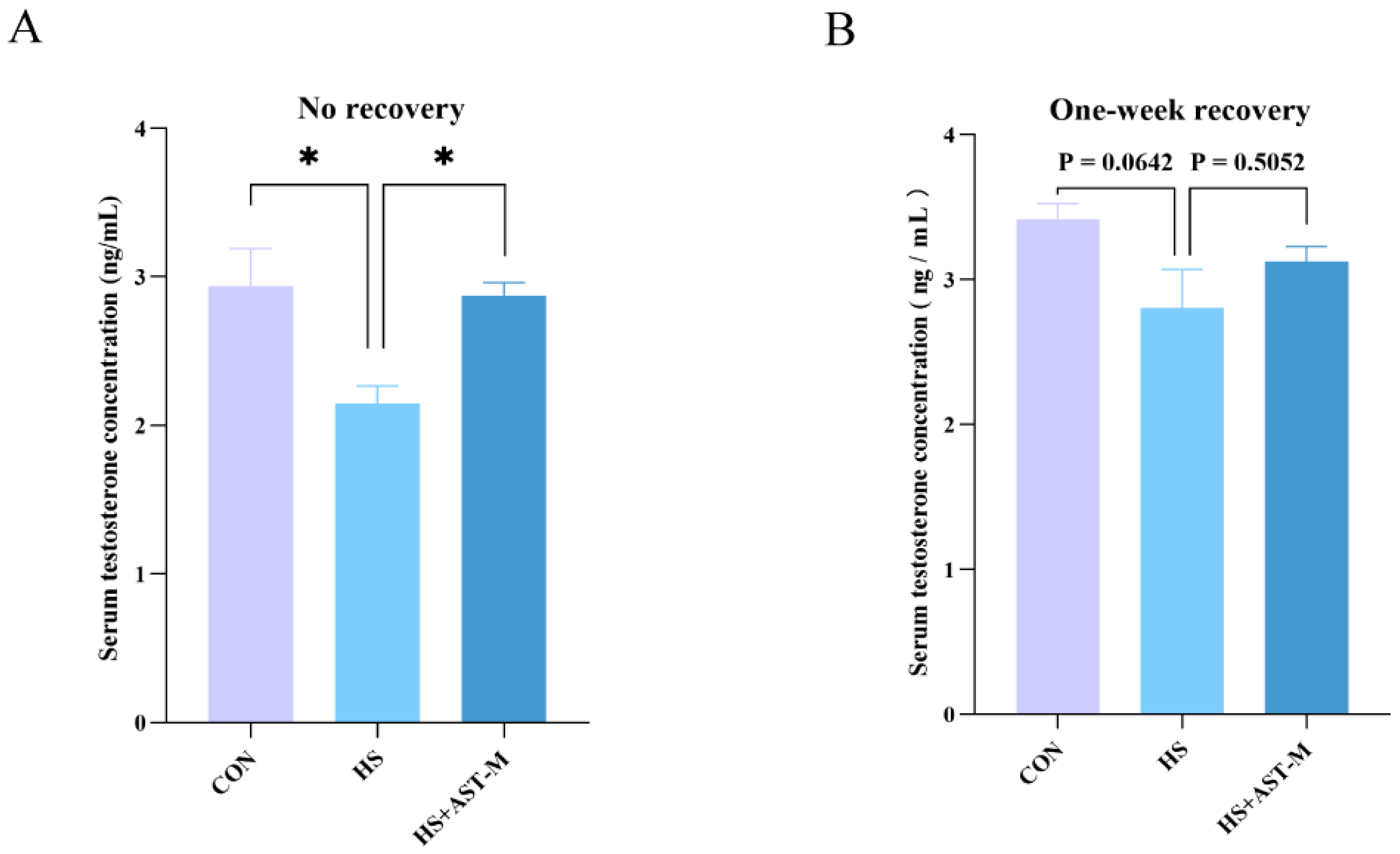
3.3. Astaxanthin Restores Serum Testosterone Level in Heat-Stressed Mice
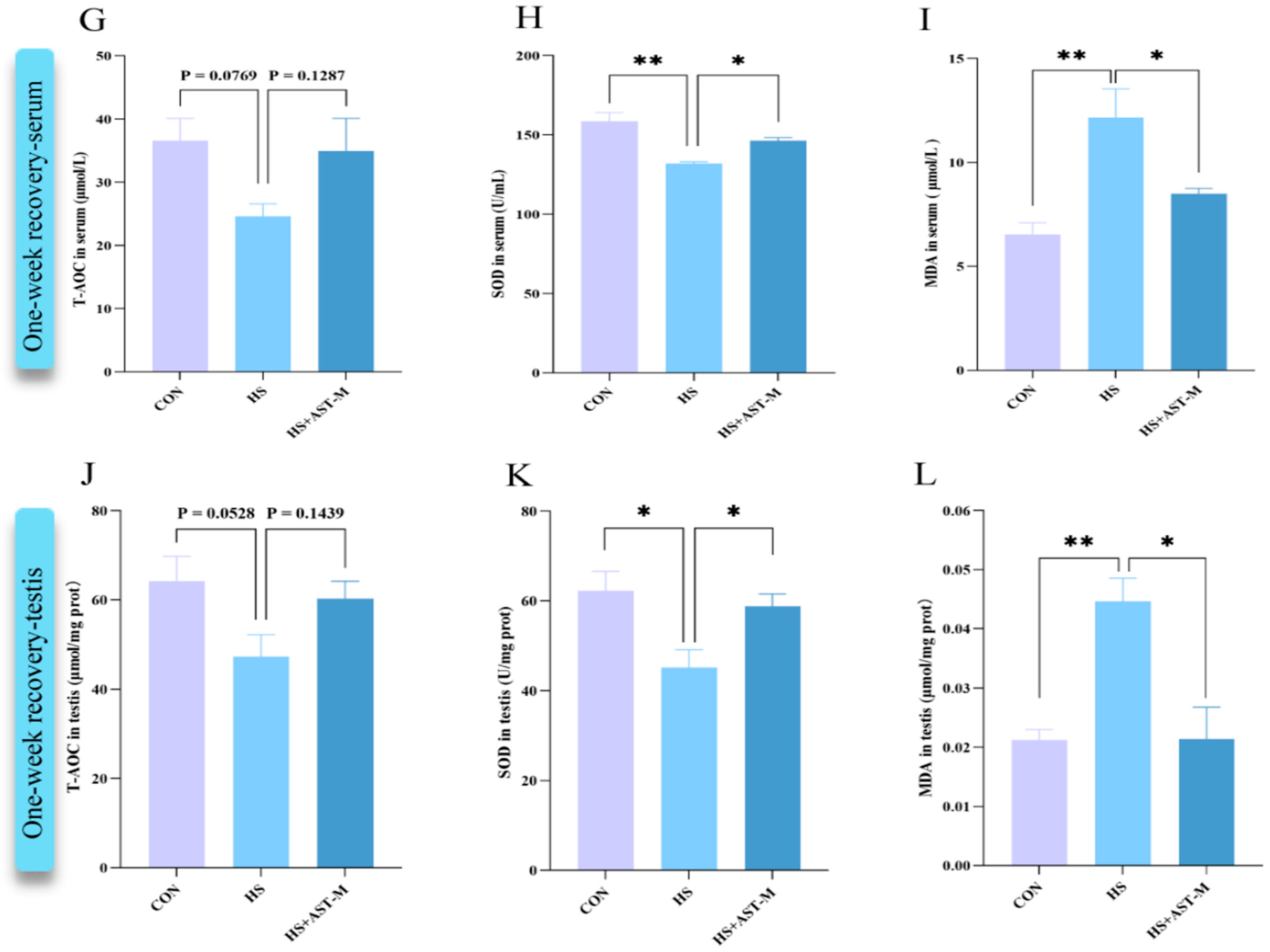
3.4. Astaxanthin Reduces Heat Stress-Induced Oxidative Stress
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lotti, F.; Maggi, M. Sexual dysfunction and male infertility. Nat. Rev. Urol. 2018, 15, 287–307. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Infertility Prevalence Estimates, 1990–2021; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- Hwang, K.; Walters, R.C.; Lipshultz, L.I. Contemporary concepts in the evaluation and management of male infertility. Nat. Rev. Urol. 2011, 8, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Jung, A.; Schuppe, H.C. Influence of genital heat stress on semen quality in humans. Andrologia 2007, 39, 203–215. [Google Scholar] [CrossRef]
- Mínguez-Alarcón, L.; Gaskins, A.J.; Chiu, Y.H.; Messerlian, C.; Williams, P.L.; Ford, J.B.; Souter, I.; Hauser, R.; Chavarro, J.E. Type of underwear worn and markers of testicular function among men attending a fertility center. Hum. Reprod. 2018, 33, 1749–1756. [Google Scholar] [CrossRef] [PubMed]
- Garolla, A.; Torino, M.; Miola, P.; Caretta, N.; Pizzol, D.; Menegazzo, M.; Bertoldo, A.; Foresta, C. Twenty-four-hour monitoring of scrotal temperature in obese men and men with a varicocele as a mirror of spermatogenic function. Hum. Reprod. 2015, 30, 1006–1013. [Google Scholar] [CrossRef]
- Durairajanayagam, D.; Agarwal, A.; Ong, C. Causes, effects and molecular mechanisms of testicular heat stress. Reprod. Biomed. Online 2015, 30, 14–27. [Google Scholar] [CrossRef]
- Abdelhamid, M.H.M.; Fellah, A.A.; Elmarghani, A.; Al Msellati, I.A. An Assessment of Men Semen Alterations in SARS-CoV-2: Is Fever the Principal Concern? Reprod. Sci. 2023, 30, 72–80. [Google Scholar] [CrossRef]
- Budzinska, M.; Kamieniczna, M.; Wojnar, L.; Gill, K.; Piasecka, M.; Kups, M.; Fraczek, M. The role of the intrinsic pathway of apoptosis in human ejaculated sperm damage under a state of scrotal heat stress. J. Assist. Reprod. Genet. 2024, 41, 99–108. [Google Scholar] [CrossRef]
- Jones, R.; Mann, T.; Sherins, R. Peroxidative breakdown of phospholipids in human spermatozoa, spermicidal properties of fatty acid peroxides, and protective action of seminal plasma. Fertil. Steril. 1979, 31, 531–537. [Google Scholar] [CrossRef]
- Aitken, R.J.; Whiting, S.; De Iuliis, G.N.; McClymont, S.; Mitchell, L.A.; Baker, M.A. Electrophilic aldehydes generated by sperm metabolism activate mitochondrial reactive oxygen species generation and apoptosis by targeting succinate dehydrogenase. J. Biol. Chem. 2012, 287, 33048–33060. [Google Scholar] [CrossRef]
- Mularczyk, M.; Michalak, I.; Marycz, K. Astaxanthin and other Nutrients from Haematococcus pluvialis-Multifunctional Applications. Mar. Drugs 2020, 18, 459. [Google Scholar] [CrossRef] [PubMed]
- Davinelli, S.; Nielsen, M.E.; Scapagnini, G. Astaxanthin in Skin Health, Repair, and Disease: A Comprehensive Review. Nutrients 2018, 10, 522. [Google Scholar] [CrossRef] [PubMed]
- Brotosudarmo, T.H.P.; Limantara, L.; Setiyono, E.; Heriyanto. Structures of Astaxanthin and Their Consequences for Therapeutic Application. Int. J. Food Sci. 2020, 2020, 2156582. [Google Scholar] [CrossRef]
- Dede, G.; Saylan, A. The effect of astaxanthin on human sperm parameters after cryopreservation. Can. Urol. Assoc. J. 2022, 16, E552–E557. [Google Scholar] [CrossRef] [PubMed]
- Basioura, A.; Tsakmakidis, I.A.; Martinez, E.A.; Roca, J.; Li, J.; Molina, M.F.; Theodoridis, A.; Boscos, C.M.; Parrilla, I. Effect of astaxanthin in extenders on sperm quality and functional variables of frozen-thawed boar semen. Anim. Reprod. Sci. 2020, 218, 106478. [Google Scholar] [CrossRef]
- Najafi, D.; Taheri, R.A.; Najafi, A.; Shamsollahi, M.; Alvarez-Rodriguez, M. Effect of astaxanthin nanoparticles in protecting the post-thawing quality of rooster sperm challenged by cadmium administration. Poult. Sci. 2020, 99, 1678–1686. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Zhao, B.-X.; Long, C.; Heng, N.; Guo, Y.; Sheng, X.-H.; Wang, X.-G.; Xing, K.; Xiao, L.-F.; Ni, H.-M.; et al. Natural Astaxanthin Improves Testosterone Synthesis and Sperm Mitochondrial Function in Aging Roosters. Antioxidants 2022, 11, 1684. [Google Scholar] [CrossRef]
- Andrisani, A.; Donà, G.; Tibaldi, E.; Brunati, A.M.; Sabbadin, C.; Armanini, D.; Alvisi, G.; Gizzo, S.; Ambrosini, G.; Ragazzi, E.; et al. Astaxanthin Improves Human Sperm Capacitation by Inducing Lyn Displacement and Activation. Mar. Drugs 2015, 13, 5533–5551. [Google Scholar] [CrossRef]
- Cooke, H.J.; Saunders, P.T. Mouse models of male infertility. Nat. Rev. Genet. 2002, 3, 790–801. [Google Scholar] [CrossRef]
- Aldahhan, R.A.; Stanton, P.G. Heat stress response of somatic cells in the testis. Mol. Cell. Endocrinol. 2021, 527, 111216. [Google Scholar] [CrossRef]
- Pfaff, D.W. (Ed.) Knobil and Neill’s The Physiology of Reproduction, 3rd ed.; Elsevier/Academic Press: San Diego, CA, USA, 2006. [Google Scholar]
- Chen, F.; Wu, Y.; Ke, L.; Lin, X.; Wang, F.; Qin, Y. ARHGEF15 in Sertoli cells contributes to germ cell development and testicular immune privilege. Biol. Reprod. 2022, 107, 1565–1579. [Google Scholar] [CrossRef] [PubMed]
- Ke, L.; Lin, X.; Luo, Y.; Tao, S.; Yan, C.; He, Y.; Wu, Y.; Liu, N.; Qin, Y. Autophagy core protein BECN1 is vital for spermatogenesis and male fertility in mice. Biol. Reprod. 2024, 110, 599–614. [Google Scholar] [CrossRef] [PubMed]
- Cao, K.X.; Deng, Z.C.; Liu, M.; Huang, Y.X.; Yang, J.C.; Sun, L.H. Heat Stress Impairs Male Reproductive System with Potential Disruption of Retinol Metabolism and Microbial Balance in the Testis of Mice. J. Nutr. 2023, 153, 3373–3381. [Google Scholar] [CrossRef]
- Shahat, A.M.; Rizzoto, G.; Kastelic, J.P. Amelioration of heat stress-induced damage to testes and sperm quality. Theriogenology 2020, 158, 84–96. [Google Scholar] [CrossRef]
- Wu, Y.; Pei, Y.; Qin, Y. Developmental expression of heat shock proteins 60, 70, 90, and A2 in rabbit testis. Cell Tissue Res. 2011, 344, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Herbut, P.; Angrecka, S.; Walczak, J. Environmental parameters to assessing of heat stress in dairy cattle-a review. Int. J. Biometeorol. 2018, 62, 2089–2097. [Google Scholar] [CrossRef]
- Rizzoto, G.; Ferreira, J.C.P.; Codognoto, V.M.; Oliveira, K.C.; Mogollón García, H.D.; Pupulim, A.G.R.; Teixeira-Neto, F.J.; Castilho, A.; Nunes, S.G.; Thundathil, J.C.; et al. Testicular hyperthermia reduces testosterone concentrations and alters gene expression in testes of Nelore bulls. Theriogenology 2020, 152, 64–68. [Google Scholar] [CrossRef]
- Fadl, A.M.; Abdelnaby, E.A.; El-Sherbiny, H.R. Supplemental dietary zinc sulphate and folic acid combination improves testicular volume and haemodynamics, testosterone levels and semen quality in rams under heat stress conditions. Reprod. Domest. Anim. 2022, 57, 567–576. [Google Scholar] [CrossRef]
- Liu, J.; Xu, S.; Liu, S.; Chen, B. miR-3613-3p/MAP3K2/p38/caspase-3 pathway regulates the heat-stress-induced apoptosis of endothelial cells. Mol. Med. Rep. 2021, 24, 633. [Google Scholar] [CrossRef]
- Li, Z.; Tian, J.; Cui, G.; Wang, M.; Yu, D. Effects of local testicular heat treatment on Leydig cell hyperplasia and testosterone biosynthesis in rat testes. Reprod. Fertil. Dev. 2015, 28, 1424–1432. [Google Scholar] [CrossRef]
- Hein, K.G.; Allrich, R.D. Influence of exogenous adrenocorticotropic hormone on estrous behavior in cattle. J. Anim. Sci. 1992, 70, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Gan, M.; Jing, Y.; Xie, Z.; Ma, J.; Chen, L.; Zhang, S.; Zhao, Y.; Niu, L.; Wang, Y.; Li, X.; et al. Potential Function of Testicular MicroRNAs in Heat-Stress-Induced Spermatogenesis Disorders. Int. J. Mol. Sci. 2023, 24, 8809. [Google Scholar] [CrossRef]
- Xiong, Y.; Li, B.; Wang, K.; Li, J.; He, S. Betaine ameliorates heat stress-induced apoptosis by affecting oxidative and endoplasmic reticulum stress in mouse Leydig cells. Biosci. Biotechnol. Biochem. 2023, 88, 53–62. [Google Scholar] [CrossRef]
- Xue, H.; Huo, Y.; Hu, Y.; Zhang, J.; Deng, C.; Zhang, J.; Wang, X. The role of ALOX15B in heat stress-induced apoptosis of porcine sertoli cells. Theriogenology 2022, 185, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Cooke, H.J.; Rhee, K. DAZL is essential for stress granule formation implicated in germ cell survival upon heat stress. Development 2012, 139, 568–578. [Google Scholar] [CrossRef]
- Lue, Y.H.; Hikim, A.P.; Swerdloff, R.S.; Im, P.; Taing, K.S.; Bui, T.; Leung, A.; Wang, C. Single exposure to heat induces stage-specific germ cell apoptosis in rats: Role of intratesticular testosterone on stage specificity. Endocrinology 1999, 140, 1709–1717. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.K.; Pandey, A.; Kumar, L.; Devi, A.; Kushwaha, B.; Vishvkarma, R.; Maikhuri, J.P.; Rajender, S.; Gupta, G. The thermo-sensitive gene expression signatures of spermatogenesis. Reprod. Biol. Endocrinol. 2018, 16, 56. [Google Scholar] [CrossRef]
- Singh, R.; Letai, A.; Sarosiek, K. Regulation of apoptosis in health and disease: The balancing act of BCL-2 family proteins. Nat. Rev. Mol. Cell Biol. 2019, 20, 175–193. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.X. Temperature control of spermatogenesis and prospect of male contraception. Front. Biosci. (Schol. Ed.) 2010, 2, 730–755. [Google Scholar]
- Peña-Blanco, A.; García-Sáez, A.J. Bax, Bak and beyond-mitochondrial performance in apoptosis. FEBS J. 2018, 285, 416–431. [Google Scholar] [CrossRef]
- Ma, W.; An, L.; Wu, Z.; Wang, X.; Guo, M.; Miao, K.; Ma, W.; Tian, J. Efficient and safe recipient preparation for transplantation of mouse spermatogonial stem cells: Pretreating testes with heat shock. Biol. Reprod. 2011, 85, 670–677. [Google Scholar] [CrossRef][Green Version]
- Eslami, M.; Esfandyari, S.; Aghahosseini, M.; Rashidi, Z.; Hosseinishental, S.H.; Brenjian, S.; Sobhani, A.; Amidi, F. Astaxanthin Protects Human Granulosa Cells against Oxidative Stress through Activation of NRF2/ARE Pathway and Its Downstream Phase II Enzymes. Cell J. 2021, 23, 319–328. [Google Scholar] [PubMed]
- Allen, Q.M.; Febres, V.J.; Rathinasabapathi, B.; Chaparro, J.X. Engineering a Plant-Derived Astaxanthin Synthetic Pathway Into Nicotiana benthamiana. Front. Plant Sci. 2022, 12, 831785. [Google Scholar] [CrossRef]
- Bašković, M.; Bojanac, A.K.; Sinčić, N.; Perić, M.H.; Krsnik, D.; Ježek, D. The effect of astaxanthin on testicular torsion-detorsion injury in rats—Detailed morphometric evaluation of histological sections. J. Pediatr. Urol. 2021, 17, e1–e439. [Google Scholar] [CrossRef]
- Chen, Y.; Tang, J.; Zhang, Y.; Du, J.; Wang, Y.; Yu, H.; He, Y. Astaxanthin alleviates gestational diabetes mellitus in mice through suppression of oxidative stress. Naunyn Schmiedebergs Arch. Pharmacol. 2020, 393, 2517–2527. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z. Astaxanthin improves serum cytokine expression and semen quality of diabetes mellitus KKAy mice. Chem. Biol. Interact. 2020, 332, 109303. [Google Scholar] [CrossRef]
- Brendler, T.; Williamson, E.M. Astaxanthin: How much is too much? A safety review. Phytother. Res. 2019, 33, 3090–3111. [Google Scholar] [CrossRef] [PubMed]
- Showalter, L.A.; Weinman, S.A.; Østerlie, M.; Lockwood, S.F. Plasma appearance and tissue accumulation of non-esterified, free astaxanthin in C57BL/6 mice after oral dosing of a disodium disuccinate diester of astaxanthin (Heptax). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2004, 137, 227–236. [Google Scholar] [CrossRef]
- Choi, H.D.; Kang, H.E.; Yang, S.H.; Lee, M.G.; Shin, W.G. Pharmacokinetics and first-pass metabolism of astaxanthin in rats. Br. J. Nutr. 2011, 105, 220–227. [Google Scholar] [CrossRef]
- Han, Y.; Gao, Z.; Chen, L.; Kang, L.; Huang, W.; Jin, M.; Wang, Q.; Bae, Y.H. Multifunctional oral delivery systems for enhanced bioavailability of therapeutic peptides/proteins. Acta Pharm. Sin. B. 2019, 9, 902–922. [Google Scholar] [CrossRef]
- Gao, Y.; Kim, S.; Lee, Y.I.; Lee, J. Cellular Stress-Modulating Drugs Can Potentially Be Identified by in Silico Screening with Connectivity Map (CMap). Int. J. Mol. Sci. 2019, 20, 5601. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Pastor, R.; Burchfiel, E.T.; Thiele, D.J. Regulation of heat shock transcription factors and their roles in physiology and disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 4–19. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Luo, Y.; He, Y.; Li, W.; Qin, Y.; Wu, Y. Astaxanthin Alleviates the Decline of Sperm Quality Caused by Heat Stress in Mice via Reducing Oxidative Stress. Life 2025, 15, 851. https://doi.org/10.3390/life15060851
Wang J, Luo Y, He Y, Li W, Qin Y, Wu Y. Astaxanthin Alleviates the Decline of Sperm Quality Caused by Heat Stress in Mice via Reducing Oxidative Stress. Life. 2025; 15(6):851. https://doi.org/10.3390/life15060851
Chicago/Turabian StyleWang, Jing, Yuchuan Luo, Yifeilong He, Wanzhen Li, Yinghe Qin, and Yingjie Wu. 2025. "Astaxanthin Alleviates the Decline of Sperm Quality Caused by Heat Stress in Mice via Reducing Oxidative Stress" Life 15, no. 6: 851. https://doi.org/10.3390/life15060851
APA StyleWang, J., Luo, Y., He, Y., Li, W., Qin, Y., & Wu, Y. (2025). Astaxanthin Alleviates the Decline of Sperm Quality Caused by Heat Stress in Mice via Reducing Oxidative Stress. Life, 15(6), 851. https://doi.org/10.3390/life15060851





