Impact of Left Ventricular Mass on Mortality in Symptomatic Severe Aortic Stenosis: A Sex-Specific Analysis †
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AS | Aortic stenosis |
| LVM | Left ventricular mass |
| LVMi | Left ventricular mass index |
| LVEF | Left ventricular ejection fraction |
| AF | Atrial fibrillation |
| AVA | Aortic valve area |
| LVH | Left ventricular hypertrophy |
| AVR | Aortic valve replacement |
| TAVI | Transcatheter aortic valve implantation |
| CAD | Coronary artery disease |
| PASP | Pulmonary artery systolic pressure |
References
- Otto, C.M.; Burwash, I.G.; Legget, M.E.; Munt, B.I.; Fujioka, M.; Healy, N.L.; Kraft, C.D.; Miyake-Hull, C.Y.; Schwaegler, R.G. Prospective study of asymptomatic valvular aortic stenosis. Clinical, echocardiographic, and exercise predictors of outcome. Circulation 1997, 95, 2262–2270. [Google Scholar] [CrossRef] [PubMed]
- Rosenhek, R.; Binder, T.; Porenta, G.; Lang, I.; Christ, G.; Schemper, M.; Maurer, G.; Baumgartner, H. Predictors of outcome in severe, asymptomatic aortic stenosis. N. Engl. J. Med. 2000, 343, 611–617. [Google Scholar] [CrossRef]
- Pellikka, P.A.; Sarano, M.E.; Nishimura, R.A.; Malouf, J.F.; Bailey, K.R.; Scott, C.G.; Barnes, M.E.; Tajik, A.J. Outcome of 622 adults with asymptomatic, hemodynamically significant aortic stenosis during prolonged follow-up. Circulation 2005, 111, 3290–3295. [Google Scholar] [CrossRef]
- Avakian, S.D.; Grinberg, M.; Ramires, J.A.; Mansur, A.P. Outcome of adults with asymptomatic severe aortic stenosis. Int. J. Cardiol. 2008, 123, 322–327. [Google Scholar] [CrossRef]
- Bing, R.; Cavalcante, J.L.; Everett, R.J.; Clavel, M.-A.; Newby, D.E.; Dweck, M.R. Imaging and impact of myocardial fibrosis in aortic stenosis. JACC Cardiovasc. Imaging 2019, 12, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Dweck, M.R.; Boon, N.A.; Newby, D.E. Calcific aortic stenosis: A disease of the valve and the myocardium. J. Am. Coll. Cardiol. 2012, 60, 1854–1863. [Google Scholar] [CrossRef]
- De Biase, N.; Mazzola, M.; Del Punta, L.; Di Fiore, V.; De Carlo, M.; Giannini, C.; Costa, G.; Paneni, F.; Mengozzi, A.; Nesti, L.; et al. Haemodynamic and metabolic phenotyping of patients with aortic stenosis and preserved ejection fraction: A specific phenotype of heart failure with preserved ejection fraction? Eur. J. Heart Fail. 2023, 25, 1947–1958. [Google Scholar] [CrossRef]
- Azevedo, C.F.; Nigri, M.; Higuchi, M.L.; Pomerantzeff, P.M.; Spina, G.S.; Sampaio, R.O.; Tarasoutchi, F.; Grinberg, M.; Rochitte, C.E. Prognostic significance of myocardial fibrosis quantification by histopathology and magnetic resonance imaging in patients with severe aortic valve disease. J. Am. Coll. Cardiol. 2010, 56, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Cioffi, G.; Faggiano, P.; Vizzardi, E.; Tarantini, L.; Cramariuc, D.; Gerdts, E.; de Simone, G. Prognostic effect of inappropriately high left ventricular mass in asymptomatic severe aortic stenosis. Heart 2011, 97, 301–307. [Google Scholar] [CrossRef]
- Stassen, J.; Ewe, S.H.; Hirasawa, K.; Butcher, S.C.; Singh, G.K.; Amanullah, M.R.; Sin, K.Y.K.; Ding, Z.P.; Pio, S.M.; Chew, N.W.S.; et al. Left ventricular remodelling patterns in patients with moderate aortic stenosis. Eur. Heart J. Cardiovasc. Imaging 2022, 23, 1326–1335. [Google Scholar] [CrossRef]
- Lorell, B.H.; Carabello, B.A. Left ventricular hypertrophy: Pathogenesis, detection, and prognosis. Circulation 2000, 102, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Duncan, A.I.; Lowe, B.S.; Garcia, M.J.; Xu, M.; Gillinov, A.M.; Mihaljevic, T.; Koch, C.G. Influence of concentric left ventricular remodeling on early mortality after aortic valve replacement. Ann. Thorac. Surg. 2008, 85, 2030–2039. [Google Scholar] [CrossRef]
- Burchfield, J.S.; Xie, M.; Hill, J.A. Pathological ventricular remodeling: Mechanisms: Part 1 of 2. Circulation 2013, 128, 388–400. [Google Scholar] [CrossRef]
- Minamino-Muta, E.; Kato, T.; Morimoto, T.; Taniguchi, T.; Inoko, M.; Haruna, T.; Izumi, T.; Miyamoto, S.; Nakane, E.; Sasaki, K.; et al. Impact of the left ventricular mass index on the outcomes of severe aortic stenosis. Heart 2017, 103, 1992–1999. [Google Scholar] [CrossRef]
- Ito, N.; Zen, K.; Takahara, M.; Tani, R.; Nakamura, S.; Fujimoto, T.; Takamatsu, K.; Yashige, M.; Kadoya, Y.; Yamano, M.; et al. Left ventricular hypertrophy as a predictor of cardiovascular outcomes after transcatheter aortic valve replacement. ESC Heart Fail. 2023, 10, 1336–1346. [Google Scholar] [CrossRef]
- DesJardin, J.T.; Chikwe, J.; Hahn, R.T.; Hung, J.W.; Delling, F.N. Sex Differences and Similarities in Valvular Heart Disease. Circ. Res. 2022, 130, 455–473. [Google Scholar] [CrossRef] [PubMed]
- Ong, J.Y.S.; Leow, A.S.T.; Ng, C.Y.; Loh, P.H.; Quek, S.C.; Kong, W.K.F.; Yeo, T.C.; Sia, C.H.; Poh, K.K. Longitudinal Outcomes of Patients with Aortic Stenosis Stratified by Sex: An Asian Perspective. J. Cardiovasc. Dev. Dis. 2025, 12, 32. [Google Scholar] [CrossRef] [PubMed]
- Devereux, R.B.; Alonso, D.R.; Lutas, E.M.; Gottlieb, G.J.; Campo, E.; Sachs, I.; Reichek, N. Echocardiographic assessment of left ventricular hypertrophy: Comparison to necropsy findings. Am. J. Cardiol. 1986, 57, 450–458. [Google Scholar] [CrossRef]
- Lang, R.M.; Biering, M.; Devereux, R.B.; Flachskampf, F.A.; Foster, E.; Pellikka, P.A.; Picard, M.H.; Roman, M.J.; Seward, J.; Shanewise, J.S.; et al. Recommendations for chamber quantification: A report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J. Am. Soc. Echocardiogr. 2005, 18, 1440–1463. [Google Scholar]
- Fuster, R.G.; Argudo, J.A.; Albarova, O.G.; Sos, F.H.; López, S.C.; Sorlıí, M.J.D.; Codoñer, M.B.; Miñano, J.A. Left ventricular mass index in aortic valve surgery: A new index for early valve replacement? Eur. J. Cardiothorac. Surg. 2003, 23, 696–702. [Google Scholar] [CrossRef]
- Stassen, J.; Pio, S.M.; Ewe, S.H.; Amanullah, M.R.; Hirasawa, K.; Butcher, S.C.; Singh, G.K.; Sin, K.Y.; Ding, Z.P.; Chew, N.W.; et al. Sex-Related Differences in Medically Treated Moderate Aortic Stenosis. Struct. Heart 2022, 6, 100042. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, H.; Douglas, P.S.; Pibarot, P.; Hahn, R.T.; Khalique, O.K.; Jaber, W.A.; Cremer, P.; Weissman, N.J.; Asch, F.M.; Zhang, Y.; et al. Left Ventricular Hypertrophy and Clinical Outcomes Over 5 Years After TAVR: An Analysis of the PARTNER Trials and Registries. JACC Cardiovasc. Interv. 2020, 13, 1329–1339. [Google Scholar] [CrossRef]
- Rader, F.; Sachdev, E.; Arsanjani, R.; Siegel, R.J. Left ventricular hypertrophy in valvular aortic stenosis: Mechanisms and clinical implications. Am. J. Med. 2015, 128, 344–352. [Google Scholar] [CrossRef]
- Gerdts, E.; Rossebø, A.B.; Pedersen, T.R.; Cioffi, G.; Lønnebakken, M.T.; Cramariuc, D.; Rogge, B.P.; Devereux, R.B. Relation of Left Ventricular Mass to Prognosis in Initially Asymptomatic Mild to Moderate Aortic Valve Stenosis. Circ. Cardiovasc. Imaging 2015, 8, e003644. [Google Scholar] [CrossRef]
- Douglas, P.S.; Otto, C.M.; Mickel, M.C.; Labovitz, A.; Reid, C.L.; Davis, K.B. Gender differences in left ventricle geometry and function in patients undergoing balloon dilatation of the aortic valve for isolated aortic stenosis. NHLBI Balloon Valvuloplasty Registry. Br. Heart J. 1995, 73, 548–554. [Google Scholar] [CrossRef][Green Version]
- Pighi, M.; Piazza, N.; Martucci, G.; Lachapelle, K.; Perrault, L.P.; Asgar, A.W.; Lauck, S.; Webb, J.G.; Popma, J.J.; Kim, D.H.; et al. Sex-Specific Determinants of Outcomes After Transcatheter Aortic Valve Replacement. Circ. Cardiovasc. Qual. Outcomes 2019, 12, e005363. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Musa, T.A.; Treibel, T.A.; Vassiliou, V.S.; Captur, G.; Chin, C.; Dobson, L.E.; Pica, S.; Loudon, M.; Malley, T.; et al. Sex differences in left ventricular remodelling, myocardial fibrosis and mortality after aortic valve replacement. Heart 2019, 105, 1818–1824. [Google Scholar] [CrossRef]
- Capoulade, R.; Clavel, M.A.; Le Ven, F.; Dahou, A.; Thébault, C.; Tastet, L.; Shen, M.; Arsenault, M.; Bédard, É.; Beaudoin, J.; et al. Impact of left ventricular remodelling patterns on outcomes in patients with aortic stenosis. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 1378–1387. [Google Scholar] [CrossRef] [PubMed]
- Gavina, C.; Falcao-Pires, I.; Pinho, P.; Manso, M.-C.; Gonçalves, A.; Rocha-Gonçalves, F.; Leite-Moreira, A. Relevance of residual left ventricular hypertrophy after surgery for isolated aortic stenosis. Eur. J. Cardiothorac. Surg. 2016, 49, 952–959. [Google Scholar] [CrossRef]
- Hachicha, Z.; Dumesnil, J.G.; Bogaty, P.; Pibarot, P. Paradoxical low-flow, low-gradient severe aortic stenosis despite preserved ejection fraction is associated with higher afterload and reduced survival. Circulation 2007, 115, 2856–2864. [Google Scholar] [CrossRef]
- Tastet, L.; Kwiecinski, J.; Pibarot, P.; Capoulade, R.; Everett, R.J.; Newby, D.E.; Shen, M.; Guzzetti, E.; Arsenault, M.; Bédard, É.; et al. Sex-Related Differences in the Extent of Myocardial Fibrosis in Patients With Aortic Valve Stenosis. JACC Cardiovasc. Imaging 2020, 13, 699–711. [Google Scholar] [CrossRef] [PubMed]
- Kararigas, G.; Dworatzek, E.; Petrov, G.; Summer, H.; Schulze, T.M.; Baczko, I.; Knosalla, C.; Golz, S.; Hetzer, R.; Regitz-Zagrosek, V. Sex dependent regulation of fibrosis and inflammation in human left ventricular remodelling under pressure overload. Eur. J. Heart Fail. 2014, 16, 1160–1167. [Google Scholar] [CrossRef]
- Naoum, C.; Blanke, P.; Dvir, D.; Pibarot, P.; Humphries, K.; Webb, J.; Leipsic, J. Clinical Outcomes and Imaging Findings in Women Undergoing TAVR. JACC Cardiovasc. Imaging 2016, 9, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Treibel, T.A.; Kozor, R.; Fontana, M.; Torlasco, C.; Reant, P.; Badiani, S.; Espinoza, M.; Yap, J.; Diez, J.; Hughes, A.D.; et al. Sex Dimorphism in the Myocardial Response to Aortic Stenosis. JACC Cardiovasc. Imaging 2018, 11, 962–973. [Google Scholar] [CrossRef] [PubMed]
- Kwak, S.; Singh, A.; Everett, R.J.; Treibel, T.A.; Lim, J.; Won, S.; Williams, M.C.; Loganathan, K.; Bing, R.; Craig, N.; et al. Sex-Specific Association of Myocardial Fibrosis With Mortality in Patients With Aortic Stenosis. JAMA Cardiol. 2025, 19, e245593. [Google Scholar] [CrossRef]
- Zwadlo, C.; Schmidtmann, E.; Szaroszyk, M.; Kattih, B.; Froese, N.; Hinz, H.; Schmitto, J.D.; Widder, J.; Batkai, S.; Bähre, H.; et al. Antiandrogenic therapy with finasteride attenuates cardiac hypertrophy and left ventricular dysfunction. Circulation 2015, 131, 1071–1081. [Google Scholar] [CrossRef]
- Chehab, O.; Shabani, M.; Varadarajan, V.; Wu, C.; E Watson, K.; Yeboah, J.; Post, W.S.; Ambale-Venkatesh, B.; A Bluemke, D.; Michos, E.D.; et al. Endogenous sex hormone levels and myocardial fibrosis in men and postmenopausal women. JACC Adv. 2023, 2, 100320. [Google Scholar] [CrossRef]
- Greiten, L.E.; Holditch, S.J.; Arunachalam, S.P.; Miller, V.M. Should there be sex-specific criteria for the diagnosis and treatment of heart failure? J. Cardiovasc. Transl. Res. 2014, 7, 139–155. [Google Scholar] [CrossRef]
- Shub, C.; Klein, A.L.; Zachariah, P.K.; Bailey, K.R.; Tajik, A.J. Determination of left ventricular mass by echocardiography in a normal population: Effect of age and sex in addition to body size. Mayo Clin. Proc. 1994, 69, 205–211. [Google Scholar] [CrossRef]
- Sickinghe, A.A.; Korporaal, S.J.A.; den Ruijter, H.M.; Kessler, E.L. Estrogen contributions to microvascular dysfunction evolving to heart failure with preserved ejection fraction. Front. Endocrinol. 2019, 10, 442. [Google Scholar] [CrossRef]
- Petrov, G.; Regitz-Zagrosek, V.; Lehmkuhl, E.; Krabatsch, T.; Dunkel, A.; Dandel, M.; Dworatzek, E.; Mahmoodzadeh, S.; Schubert, C.; Becher, E.; et al. Regression of myocardial hypertrophy after aortic valve replacement: Faster in women? Circulation 2010, 122 (Suppl. 11), S23–S28. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Shao, Y.; Huang, Y.; Yao, T.; Lu, L.M. 17-Estradiol inhibits angiotensin II-induced collagen synthesis of cultured rat cardiac fibroblasts via modulating angiotensin II receptors. Eur. J. Pharmacol. 2007, 567, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Michail, M.; Davies, J.E.; Cameron, J.D.; Parker, K.H.; Brown, A.J. Pathophysiological coronary and microcirculatory flow alterations in aortic stenosis. Nat. Rev. Cardiol. 2018, 15, 420–431. [Google Scholar] [CrossRef] [PubMed]
- McConkey, H.Z.R.; Marber, M.; Chiribiri, A.; Pibarot, P.; Redwood, S.R.; Prendergast, B.D. Coronary Microcirculation in Aortic Stenosis. Circ. Cardiovasc. Interv. 2019, 12, e007547. [Google Scholar] [CrossRef]
- Reynolds, H.R.; Bairey Merz, C.N.; Berry, C.; Samuel, R.; Saw, J.; Smilowitz, N.R.; de Souza, A.C.D.A.; Sykes, R.; Taqueti, V.R.; Wei, J. Coronary Arterial Function and Disease in Women With No Obstructive Coronary Arteries. Circ. Res. 2022, 130, 529–551. [Google Scholar] [CrossRef]
- Oguz, D.; Huntley, G.D.; El-Am, E.A.; Scott, C.G.; Thaden, J.J.; Pislaru, S.V.; Fabre, K.L.; Singh, M.; Greason, K.L.; Crestanello, J.A.; et al. Impact of atrial fibrillation on outcomes in asymptomatic severe aortic stenosis: A propensity-matched analysis. Front. Cardiovasc. Med. 2023, 10, 1195123. [Google Scholar] [CrossRef]
- Avakian, S.D.; Tarasoutchi, F.; Mansur, A. Left ventricular mass index and prognosis of symptomatic severe aortic stenosis in women and men. In Proceedings of the European Congress of Cardiology, London, UK, 30 August–2 September 2024. [Google Scholar]
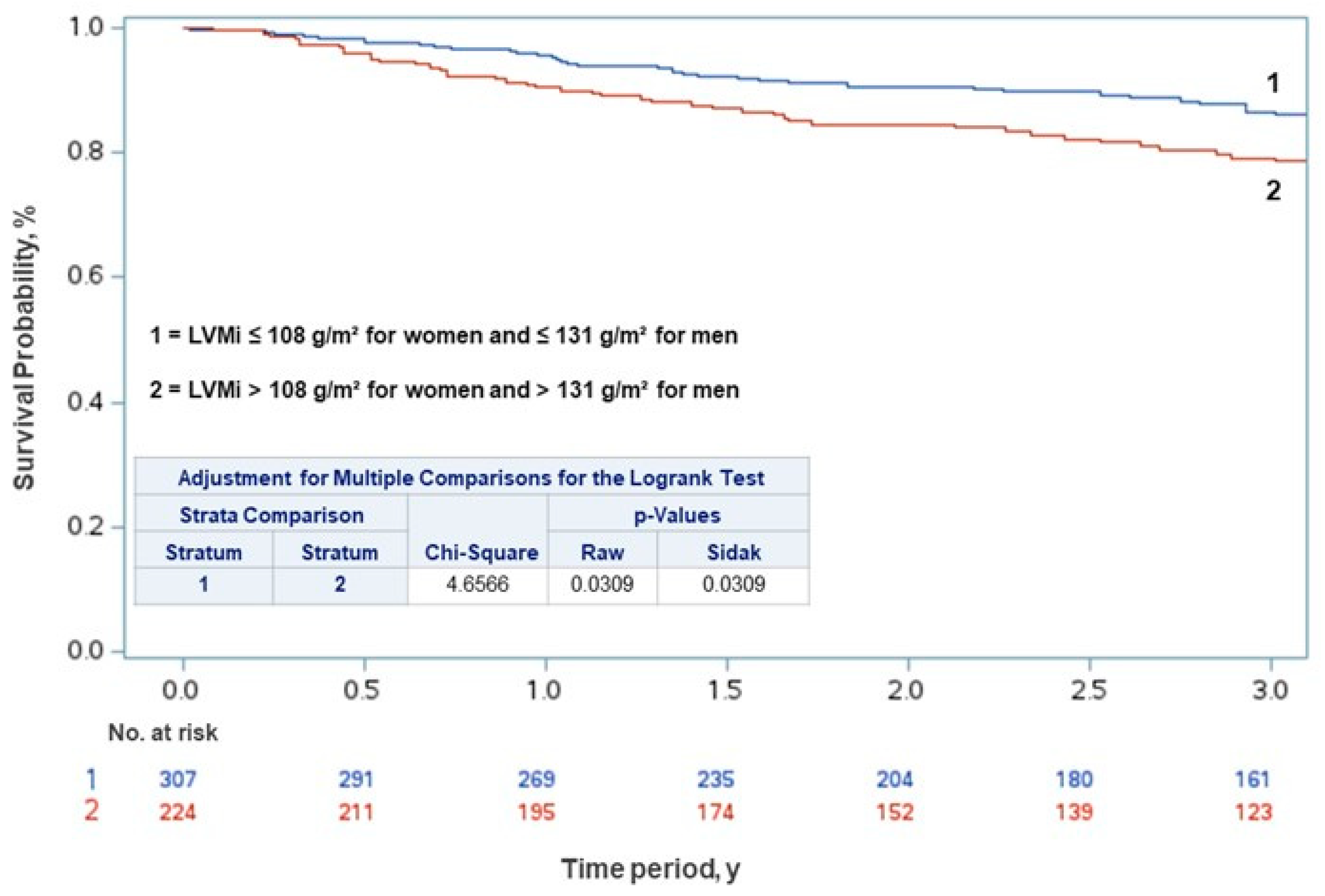
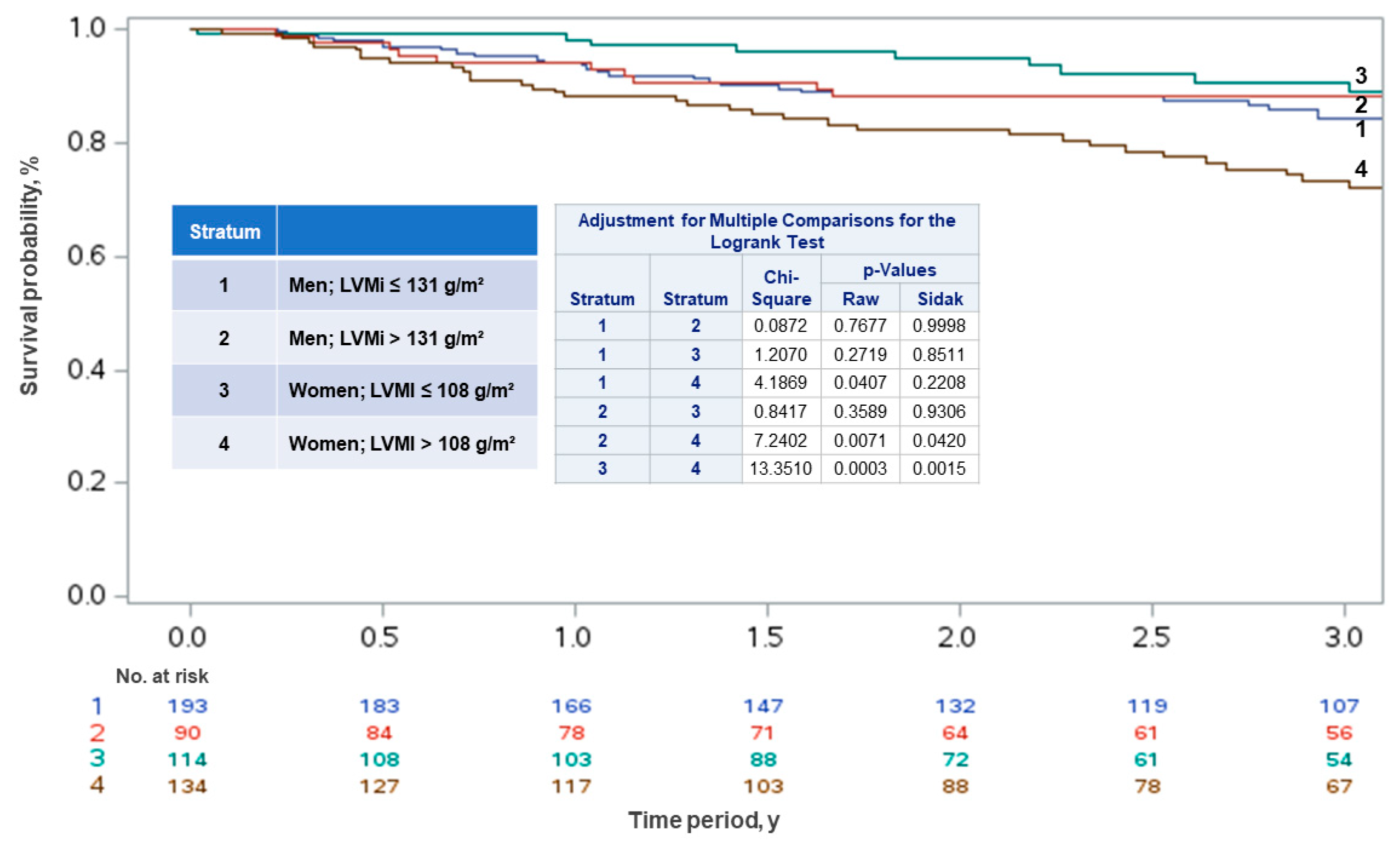
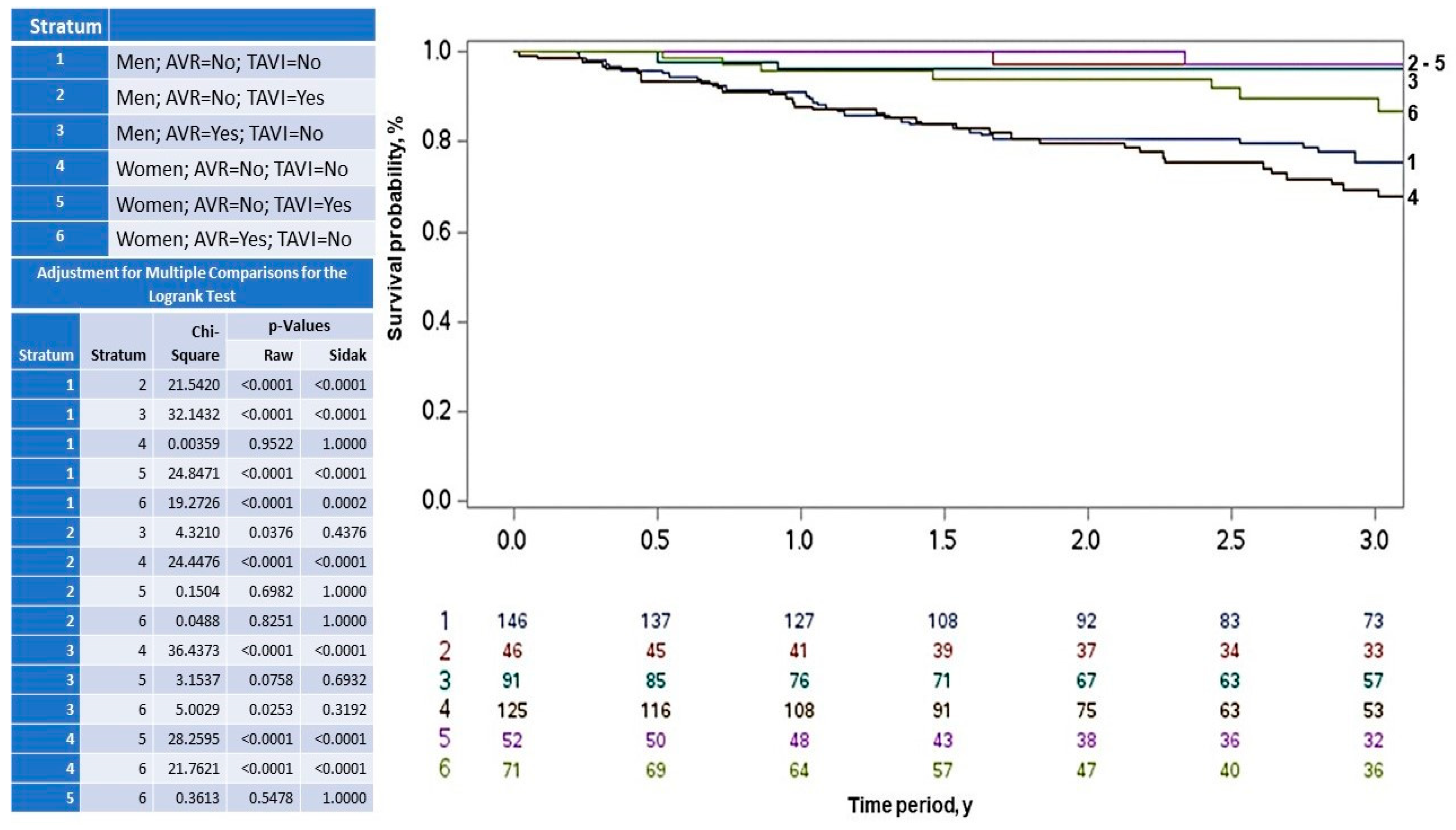
| All Patients N = 531 (%) | Men N = 283 (53.3) | Women N = 248 (46.7) | p | |
|---|---|---|---|---|
| Age, mean (SD), y | 74.7 (11.6) | 74.1 (12.6) | 75.5 (10.2) | 0.149 |
| Time since baseline, mean (SD), y | 2.7 (1.2) | 2.8 (1.3) | 2.6 (1.2) | 0.138 |
| Dyslipidemia, No. (%) | 177 (33.3) | 81 (28.6) | 96 (38.7) | 0.014 |
| Hypertension, No. (%) | 390 (73.6) | 192 (68.1) | 198 (79.8) | 0.002 |
| Diabetes, No. (%) | 153 (28.8) | 85 (30.0) | 68 (27.4) | 0.507 |
| Smoking, No. (%) | 43 (8.1) | 23 (8.1) | 20 (8.1) | 0.979 |
| Anemia, No. (%) | 145 (27.3) | 81 (28.6) | 64 (25.8) | 0.468 |
| Syncope, No. (%) | 46 (8.7) | 32 (11.3) | 14 (5.7) | 0.021 |
| Angina, No. (%) | 197 (37.1) | 112 (39.6) | 85 (34.3) | 0.207 |
| Dyspnea, No. (%) | 475 (89.5) | 247 (87.3) | 228 (91.9) | 0.081 |
| AF, No. (%) | 33 (6.2) | 24 (8.5) | 9 (3.6) | 0.021 |
| Weight, mean (SD), Kg | 74.3 (14.3) | 79.1 (13.0) | 68.6 (13.6) | <0.001 |
| BSA, mean (SD), m2 | 1.8 (0.2) | 1.9 (0.2) | 1.7 (0.2) | <0.001 |
| LVEF, mean (SD), % | 60.6 (9.6) | 58.7 (10.7) | 62.8 (7.6) | <0.001 |
| LVMi, mean (SD), g/m2 | 118.6 (30.6) | 122.1 (29.9) | 114.5 (30.9) | 0.004 |
| LVMi moderate + severe | 224 (42.2) | 90 (31.8) | 134 (54.0) | <0.001 |
| LA volume, mean (SD), mL | 44.9 (15.2) | 45.3 (14.5) | 44.5 (16.0) | 0.666 |
| Peak gradient, mean (SD), mmHg | 79.8 (22.9) | 76.9 (21.4) | 83.1 (24.2) | 0.002 |
| Mean gradient, mean (SD), mmHg | 50.5 (15.6) | 48.6 (14.7) | 52.6 (16.3) | 0.003 |
| Valve area, mean (SD), cm2 | 0.87 (2.42) | 1.0 (3.3) | 0.72 (0.19) | 0.155 |
| Peak jet velocity, mean (SD), m/s | 4.4 (0.6) | 4.3 (0.6) | 4.5 (0.6) | 0.002 |
| Bicuspid/Tricuspid, No. (%) | 54 (10.2)/477 (89.8) | 30 (10.6)/253 (89.4) | 24 (9.68)/224 (90.3) | 0.725 |
| CAD, No. (%) | 279 (52.5) | 172 (60.78) | 107 (43.2) | <0.001 |
| AVR, No. (%) | 162 (30.5) | 91 (32.2) | 71 (28.6) | 0.378 |
| Valvuloplasty, No. (%) | 13 (2.45) | 10 (3.5) | 3 (1.21) | 0.084 |
| TAVI, No. (%) | 98 (18.5) | 46 (16.3) | 52 (21.0) | 0.163 |
| Death, No. (%) | 165 (31.1) | 89 (31.5) | 76 (30.7) | 0.842 |
| Cardiovascular death, No. (%) | 148 (27.9) | 79 (27.9) | 69 (27.8) | 0.921 |
| All Patients N = 531 (%) | Survivors N = 366 (68.9) | Non-Survivors N = 165 (31.1) | p | |
|---|---|---|---|---|
| Age, mean (SD), y | 74.7 (11.6) | 74.4 (11.3) | 75.5 (12.1) | 0.133 |
| Female, No. (%) | 366 (68.9) | 172 (47.0) | 76 (46.1) | 0.841 |
| Time since baseline, mean (SD), y | 2.7 (1.2) | 2.73 (1.2) | 2.55 (1.3) | 0.146 |
| Dyslipidemia, No. (%) | 177 (33.3) | 124 (33.4) | 53 (32.1) | 0.691 |
| Hypertension, No. (%) | 390 (73.6) | 270 (73.8) | 120 (73.2) | 0.885 |
| Diabetes, No. (%) | 153 (28.8) | 101 (27.6) | 52 (31.5) | 0.356 |
| Smoking, No. (%) | 43 (8.1) | 26 (7.1) | 17 (10.3) | 0.211 |
| Anemia, No. (%) | 145 (27.3) | 96 (26.2) | 49 (29.7) | 0.410 |
| Syncope, No. (%) | 46 (8.7) | 35 (9.6) | 11 (6.7) | 0.272 |
| Angina, No. (%) | 197 (37.1) | 146 (39.9) | 51 (30.9) | 0.047 |
| Dyspnea, No. (%) | 475 (89.5) | 325 (88.8) | 150 (90.9) | 0.463 |
| AF, No. (%) | 33 (6.2) | 15 (4.1) | 18 (10.9) | 0.003 |
| Weight, mean (SD), Kg | 74.3 (14.3) | 74.7 (14.2) | 73.4 (14.6) | 0.334 |
| BSA, mean (SD), m2 | 1.8 (0.21) | 1.8 (0.2) | 1.7 (0.2) | 0.205 |
| LVEF, mean (SD), % | 60.6 (9.6) | 61.4 (8.9) | 58.8 (10.8) | 0.008 |
| LVMi, mean (SD), g/m2 | 118.6 (30.6) | 115.5 (29.3) | 125.3 (32.5) | <0.001 |
| LVMi moderate + severe | 224 (42.2) | 136 (37.2) | 88 (53.3) | <0.001 |
| LA volume, mean (SD), mL | 44.9 (15.2) | 44.4 (16.1) | 46.3 (12.8) | 0.246 |
| Peak gradient, mean (SD), mmHg | 79.8 (22.9) | 80.4 (23.3) | 78.4 (22.0) | 0.365 |
| Mean gradient, mean (SD), mmHg | 50.5 (15.6) | 50.6 (15.7) | 50.4 (15.3) | 0.894 |
| Valve area, mean (SD), cm2 | 0.87 (2.42) | 0.9 (2.9) | 0.7 (0.2) | 0.258 |
| Peak jet velocity, mean (SD), m/s | 4.4 (0.6) | 4.4 (0.6) | 4.4 (0.6) | 0.359 |
| Bicuspid/Tricuspid, No. (%) | 54 (10.2)/477 (89.8) | 42 (11.5)/324 (88.5) | 12 (7.2)/153 (92.7) | 0.138 |
| CAD, No. (%) | 279 (52.5) | 183 (50.0) | 96 (58.1) | 0.081 |
| AVR, No. (%) | 162 (30.5) | 142 (38.8) | 20 (12.1) | <0.001 |
| Valvuloplasty, No. (%) | 13 (2.45) | 5 (1.37) | 8 (4.9) | 0.016 |
| TAVI, No. (%) | 98 (18.5) | 85 (23.2) | 13 (7.9) | <0.001 |
| Survivors | p | Non-Survivors | p | |||
|---|---|---|---|---|---|---|
| Men N = 194 (53) | Women N = 172 (47) | Men N = 89 (53.9) | Women N = 76 (46.1) | |||
| Age, mean (SD), y | 75.1 (10.5) | 75.1 (10.8) | 0.792 | 73.9 (15.0) | 73.0 (11.2) | 0.650 |
| Time since baseline, mean (SD), y | 2.8 (1.2) | 2.7 (1.1) | 0.284 | 2.7 (1.4) | 2.4 (1.3) | 0.289 |
| Dyslipidemia, No. (%) | 59 (30.4) | 65 (37.8) | 0.137 | 22 (24.7) | 31 (40.8) | 0.028 |
| Hypertension, No. (%) | 127 (65.5) | 143 (83.1) | <0.001 | 65 (73.9) | 55 (72.4) | 0.829 |
| Diabetes, No. (%) | 50 (25.8) | 51 (29.7) | 0.408 | 35 (39.3) | 17 (22.4) | 0.019 |
| Smoking, No. (%) | 16 (8.3) | 10 (5.8) | 0.366 | 7 (7.9) | 10 (13.2) | 0.265 |
| Anemia, No. (%) | 52 (26.8) | 44 (25.6) | 0.791 | 29 (32.6) | 20 (26.3) | 0.380 |
| Syncope, No. (%) | 25 (12.9) | 10 (5.8) | 0.022 | 7 (7.9) | 4 (5.3) | 0.506 |
| Angina, No. (%) | 86 (44.3) | 60 (34.8) | 0.066 | 26 (29.2) | 25 (32.9) | 0.610 |
| Dyspnea, No. (%) | 169 (87.1) | 156 (90.7) | 0.279 | 78 (87.6) | 72 (94.7) | 0.114 |
| AF, No. (%) | 12 (6.19) | 3 (1.74) | 0.032 | 12 (13.5) | 6 (7.9) | 0.251 |
| Weight, mean (SD), Kg | 79.7 (12.6) | 69.0 (13.7) | <0.001 | 27.9 (13.9) | 67.6 (13.5) | <0.001 |
| BSA, mean (SD), m2 | 1.9 (0.2) | 1.7 (0.2) | <0.001 | 1.9 (0.2) | 1.7 (0.2) | <0.001 |
| LVEF, mean (SD), % | 59.6 (9.7) | 63.4 (6.9) | <0.001 | 56.7 (12.0) | 61.3 (8.7) | 0.006 |
| LVMi, mean (SD), g/m2 | 120.3 (29.1) | 110.1 (28.5) | <0.001 | 125.9 (31.5) | 124.5 (33.8) a | 0.784 |
| LVMi moderate + severe | 60 (30.9) | 76 (44.2) | 0.009 | 30 (33.7) | 58 (76.3) a | <0.001 |
| LA volume, mean (SD), mL | 44.8 (15.4) | 43.8 (16.9) | 0.619 | 46.6 (12.2) | 46.3 (13.7) | 0.995 |
| Peak gradient, mean (SD), mmHg | 77.6 (20.5) | 83.6 (25.8) | 0.015 | 75.4 (23.1) | 82.0 (20.2) | 0.057 |
| Mean gradient, mean (SD), mmHg | 48.9 (14.0) | 52.5 (17.3) | 0.030 | 48.1 (16.1) | 53.1 (13.9) | 0.038 |
| Valve area, mean (SD), cm2 | 1.1 (4.0) | 0.7 (0.2) | 0.200 | 0.8 (1.2) | 0.7 (0.2) | <0.001 |
| Peak jet velocity, mean (SD), m/s | 4.4 (0.6) | 4.5 (0.7) | 0.023 | 4.3 (0.7) | 4.5 (0.6) | 0.039 |
| Bicuspid/Tricuspid, No. (%) | 23 (11.9)/171 (88.1) | 19 (11.1)/153 (88.9) | 0.809 | 7 (7.9)/82 (92.1) | 5 (6.6)/71 (93.4) | 0.751 |
| CAD, No. (%) | 114 (58.8) | 69 (40.1) | <0.001 | 58 (65.2) | 38 (50) | 0.049 |
| AVR, No. (%) | 82 (42.3) | 60 (34.9) | 0.148 | 9 (10.1) | 11 (14.5) | 0.392 |
| Valvuloplasty, No. (%) | 4 (2.1) | 1 (0.6) | 0.223 | 6 (6.7) | 2 (2.6) | 0.220 |
| TAVI, No. (%) | 38 (19.6) | 47 (27.3) | 0.081 | 8 (9.0) | 5 (6.6) | 0.567 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avakian, S.D.; Tarasoutchi, F.; Mansur, A.d.P. Impact of Left Ventricular Mass on Mortality in Symptomatic Severe Aortic Stenosis: A Sex-Specific Analysis. Life 2025, 15, 814. https://doi.org/10.3390/life15050814
Avakian SD, Tarasoutchi F, Mansur AdP. Impact of Left Ventricular Mass on Mortality in Symptomatic Severe Aortic Stenosis: A Sex-Specific Analysis. Life. 2025; 15(5):814. https://doi.org/10.3390/life15050814
Chicago/Turabian StyleAvakian, Solange Desirée, Flávio Tarasoutchi, and Antonio de Padua Mansur. 2025. "Impact of Left Ventricular Mass on Mortality in Symptomatic Severe Aortic Stenosis: A Sex-Specific Analysis" Life 15, no. 5: 814. https://doi.org/10.3390/life15050814
APA StyleAvakian, S. D., Tarasoutchi, F., & Mansur, A. d. P. (2025). Impact of Left Ventricular Mass on Mortality in Symptomatic Severe Aortic Stenosis: A Sex-Specific Analysis. Life, 15(5), 814. https://doi.org/10.3390/life15050814






