Milpa Diet for MASLD in Mesoamerican Populations: Feasibility, Advantages, and Future Perspectives
Abstract
1. Introduction
2. Current Treatment for MASLD
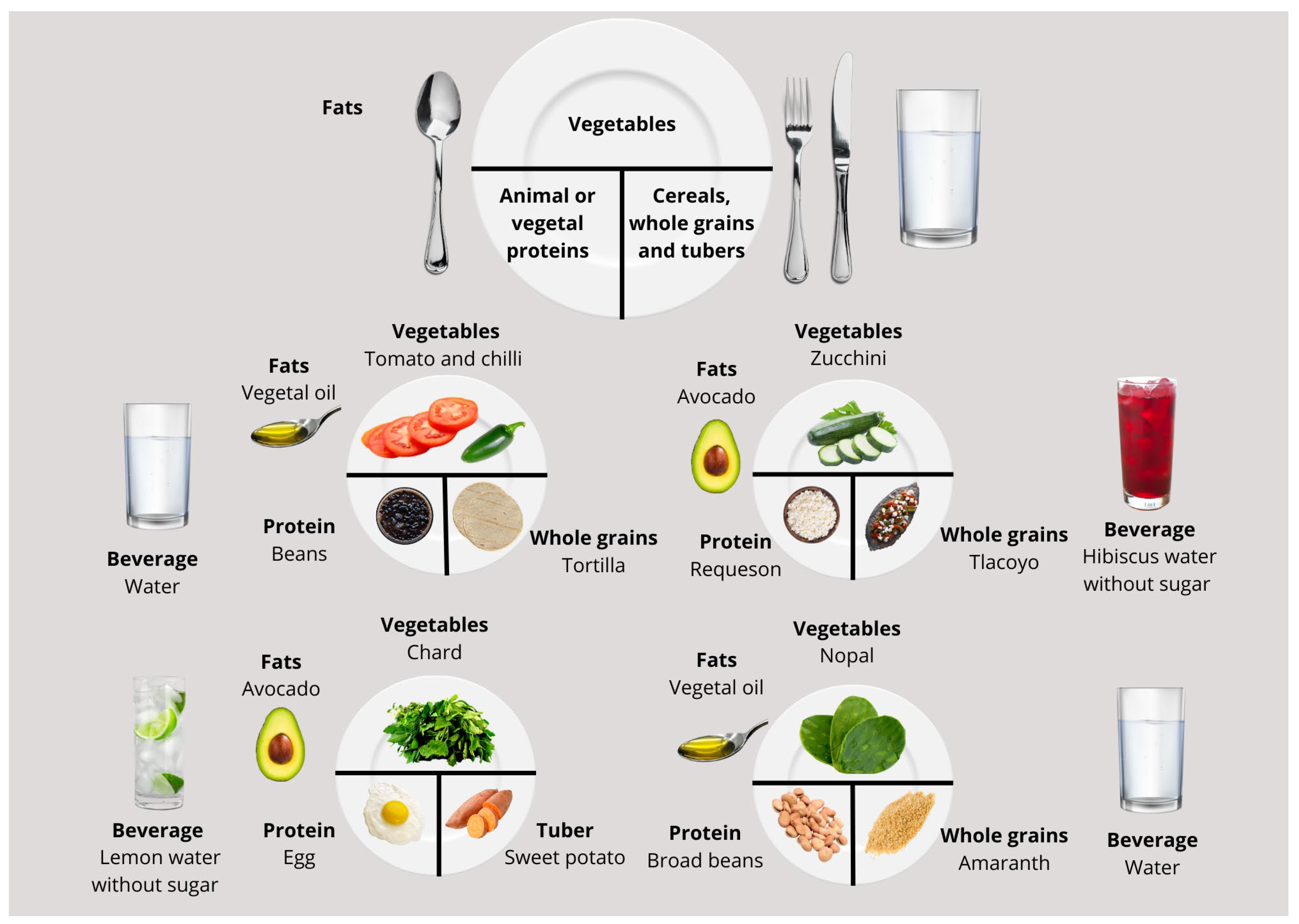
3. Milpa Diet Characteristics
| Aspect | Milpa Diet | Western Diet |
|---|---|---|
| Origin | The traditions of Mesoamerica are based on the millenial agricultural system | Predominant in industrialized countries, characterized by consuming processed and ultra-processed foods |
| Characteristic foods | Corn, beans, squash, chili, quelites, tomatoes, amaranth, ricotta cheese | Red meats, ultra-processed products, refined flours, added sugars, full-fat dairy products |
| Main source of proteins | Legumes (beans), insects, ricotta cheese, and lean meats | Red meats, sausages, full-fat dairy products, and animal proteins |
| Predominant carbohydrates | Nixtamalized corn and amaranth | Refined flours and added sugars |
| Fats | Predominantly unsaturated (seeds, avocado, zucchini, sunflower oil) | High in transfats and saturated fats |
| Fiber | High in fiber (whole grains, vegetables, pulses) | Low in fiber (diets high in processed and refined foods) |
| Caloric density | Moderate, based on natural and minimally processed ingredients | High, with rich calorie load from fats and sugars |
| Health impact | Promotes cardiovascular health, prevents metabolic diseases by reducing adipose tissue at the central level | Linked to obesity, type 2 diabetes, hypertension, and cardiovascular disease |
| Sustainability | Environmentally friendly, based on local production and polycultures | High environmental impact due to meat consumption and intensive monoculture |
| Food processing | Minimally processed, fresh, and natural food | High, with additives, preservatives, and excess sodium |
4. Potential Benefits of Milpa Diet Components
4.1. Suggested Protein Sources and Their Benefits
4.2. Benefits of Carbohydrates and Lipids in the Milpa Diet
| Food Group | Components |
|---|---|
| Whole grains and tubers | Corn, amaranth, oats, sweet potato, cassava, chayotextle, or chinchayote. |
| Vegetables | Nopales, quelites, quintoniles, purslane, green beans, romeritos, huauzontle, tomatoes, citlali tomatoes, tomatillo, miltomate, chili peppers, bell peppers, squash, chayote, chilacayote, colorines, izote flower, jicama, watercress, chaya, huitlacoche, achiote, epazote, vanilla, acuyo, mushrooms, and allspice, among others. |
| Legumes, seeds, and oilseeds | Beans, fava beans, chia seeds, chocolate, peanuts, and pumpkin seeds. |
| Fruits | Soursop, prickly pear, papaya, black zapote, chicozapote, mamey, guava, tejocote, capulín, pineapple, anona, xoconostle, cherimoya, nance, berries, yellow plum, and pitahaya. |
4.3. Antioxidants and Micronutrients
5. Discussion
6. Conclusions
7. Futures Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J. Hepatol. 2023, 79, 1542–1556. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.-K.; Chuah, K.-H.; Rajaram, R.B.; Lim, L.-L.; Ratnasingam, J.; Vethakkan, S.R. Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD): A State-of-the-Art Review. J. Obes. Metab. Syndr. 2023, 32, 197–213. [Google Scholar] [CrossRef] [PubMed]
- Huh, Y.; Cho, Y.J.; Nam, G.E. Recent Epidemiology and Risk Factors of Nonalcoholic Fatty Liver Disease. J. Obes. Metab. Syndr. 2022, 31, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Huttasch, M.; Roden, M.; Kahl, S. Obesity and MASLD: Is weight loss the (only) key to treat metabolic liver disease? Metabolism 2024, 157, 155937. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines on the Management of Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). Obes Facts. 2024, 17, 374–444. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Zelber-Sagi, S.; Henry, L.; Gerber, L.H. Lifestyle interventions in nonalcoholic fatty liver disease. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 708–722. [Google Scholar] [CrossRef]
- Cai, H.; Zhang, J.; Liu, C.; Le, T.N.; Lu, Y.; Feng, F.; Feng, F.; Zhao, M. High-Fat Diet-Induced Decreased Circulating Bile Acids Contribute to Obesity Associated with Gut Microbiota in Mice. Foods 2024, 13, 699. [Google Scholar] [CrossRef]
- Pan, Y.; Yang, Y.; Wu, J.; Zhou, H.; Yang, C. Efficacy of probiotics, prebiotics, and synbiotics on liver enzymes, lipid profiles, and inflammation in patients with non-alcoholic fatty liver disease: A systematic review and meta-analysis of randomized controlled trials. BMC Gastroenterol. 2024, 24, 283. [Google Scholar] [CrossRef]
- Hayat, U.; Siddiqui, A.A.; Okut, H.; Afroz, S.; Tasleem, S.; Haris, A. The effect of coffee consumption on the non-alcoholic fatty liver disease and liver fibrosis: A meta-analysis of 11 epidemiological studies. Ann. Hepatol. 2021, 20, 100254. [Google Scholar] [CrossRef]
- Marti-Aguado, D.; Calleja, J.L.; Vilar-Gomez, E.; Iruzubieta, P.; Rodríguez-Duque, J.C.; Del Barrio, M.; Puchades, L.; Rivera-Esteban, J.; Perelló, C.; Puente, A.; et al. Low-to-moderate alcohol consumption is associated with increased fibrosis in individuals with metabolic dysfunction-associated steatotic liver disease. J. Hepatol. 2024, 81, 930–940. [Google Scholar] [CrossRef]
- Stine, J.G.; Long, M.T.; Corey, K.E.; Sallis, R.E.; Allen, A.M.; Armstrong, M.J.; Conroy, D.E.; Cuthbertson, D.J.; Duarte-Rojo, A.; Hallsworth, K.; et al. American College of Sports Medicine (ACSM) International Multidisciplinary Roundtable report on physical activity and nonalcoholic fatty liver disease. Hepatol. Commun. 2023, 7, e0108. [Google Scholar] [CrossRef] [PubMed]
- Cuthbertson, D.J.; Keating, S.E.; Pugh, C.J.A.; Owen, P.J.; Kemp, G.J.; Umpleby, M.; Geyer, N.G.; Chinchilli, V.M.; Stine, J.G. Exercise improves surrogate measures of liver histological response in metabolic dysfunction-associated steatotic liver disease. Liver Int. 2024, 44, 2368–2381. [Google Scholar] [CrossRef] [PubMed]
- Zizumbo-Villarreal, D.; Colunga-GarcíaMarín, P. La milpa del occidente de Mesoamérica: Profundidad histórica, dinámica evolutiva y rutas de dispersión a Suramérica. Rev. Geogr. Agríc. 2017, 58, 33–46. [Google Scholar]
- Sánchez-Velázquez, O.A.; Luna-Vital, D.A.; Morales-Hernandez, N.; Contreras, J.; Villaseñor-Tapia, E.C.; Fragoso-Medina, J.A.; Mojica, L. Nutritional, bioactive components and health properties of the milpa triad system seeds (corn, common bean and pumpkin). Front. Nutr. 2023, 10, 1169675. [Google Scholar] [CrossRef] [PubMed]
- Secretaría de Salud. Dieta de la Milpa. Modelo de Alimentación Saludable y Culturalmente Pertinente. Fortalecimiento de la Salud con Comida, Ejercicio y Buen Humor. June 2020. Available online: https://www.gob.mx/cms/uploads/attachment/file/715861/01_Documento_de_La_dieta_de_la_milpa.pdf (accessed on 1 March 2025).
- Wang, L.-C.; Yu, Y.-Q.; Fang, M.; Zhan, C.-G.; Pan, H.-Y.; Wu, Y.-N.; Gong, Z.Y. Antioxidant and antigenotoxic activity of bioactive extracts from corn tassel. J. Huazhong Univ. Sci. Technol. Med. Sci. 2014, 34, 131–136. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef]
- Ballal, K.; Wilson, C.R.; Harmancey, R.; Taegtmeyer, H. Obesogenic high fat western diet induces oxidative stress and apoptosis in rat heart. Mol. Cell. Biochem. 2010, 344, 221–230. [Google Scholar] [CrossRef]
- Christ, A.; Lauterbach, M.; Latz, E. Western Diet and the Immune System: An Inflammatory Connection. Immunity 2019, 51, 794–811. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Beltrán-Velasco, A.I.; Redondo-Flórez, L.; Martín-Rodríguez, A.; Tornero-Aguilera, J.F. Global Impacts of Western Diet and Its Effects on Metabolism and Health: A Narrative Review. Nutrients 2023, 15, 2749. [Google Scholar] [CrossRef]
- Castellanos-Gutiérrez, A.; Sánchez-Pimienta, T.G.; Batis, C.; Willett, W.; Rivera, J.A. Toward a healthy and sustainable diet in Mexico: Where are we and how can we move forward? Am. J. Clin. Nutr. 2021, 113, 1177–1184. [Google Scholar] [CrossRef]
- Meza-Rios, A.; López-Villalobos, E.F.; Anguiano-Sevilla, L.A.; Ruiz-Quezada, S.L.; Velazquez-Juarez, G.; López-Roa, R.I.; Marin-Molina, A.L.; Zepeda-Morales, A.S.M. Effects of Foods of Mesoamerican Origin in Adipose Tissue and Liver-Related Metabolism. Medicina 2023, 59, 1907. [Google Scholar] [CrossRef] [PubMed]
- Castelnuovo, G.; Perez-Diaz-del-Campo, N.; Rosso, C.; Armandi, A.; Caviglia, G.P.; Bugianesi, E. A Healthful Plant-Based Diet as an Alternative Dietary Approach in the Management of Metabolic Dysfunction-Associated Steatotic Liver Disease. Nutrients 2024, 16, 2027. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, R.M.; Juszczak, H.M.; Wong, M.A. Scoping review of the association of plant-based diet quality with health outcomes. Front. Nutr. 2023, 10, 1211535. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Rong, S.; Deng, Y.; Bao, W.; Xia, Y.; Chen, L. Plant-based diets, genetic predisposition and risk of non-alcoholic fatty liver disease. BMC Med. 2023, 21, 351. [Google Scholar] [CrossRef]
- Boye, J.; Wijesinha-Bettoni, R.; Burlingame, B. Protein quality evaluation twenty years after the introduction of the protein digestibility corrected amino acid score method. Br. J. Nutr. 2012, 108, S183–S211. [Google Scholar] [CrossRef]
- Bahrami, A.; Teymoori, F.; Eslamparast, T.; Sohrab, G.; Hejazi, E.; Poustchi, H.; Hekmatdoost, A. Legume intake and risk of nonalcoholic fatty liver disease. Indian J. Gastroenterol. 2019, 38, 55–60. [Google Scholar] [CrossRef]
- Cardoso, B.R.; Tan, S.Y.; Daly, R.M.; Via, J.D.; Georgousopoulou, E.N.; George, E.S. Intake of Nuts and Seeds Is Associated with a Lower Prevalence of Nonalcoholic Fatty Liver Disease in US Adults: Findings from 2005–2018 NHANES. J. Nutr. 2021, 151, 3507–3515. [Google Scholar] [CrossRef]
- Hussain, A.; Kausar, T.; Jamil, M.A.; Noreen, S.; Iftikhar, K.; Rafique, A.; Iqbal, M.A.; Majeed, M.A.; Quddoos, M.Y.; Aslam, J.; et al. Role of Pumpkin Parts as Pharma-Foods: Antihyperglycemic and Antihyperlipidemic Activities of Pumpkin Peel, Flesh, and Seed Powders, in Alloxan-Induced Diabetic Rats. Int. J. Food Sci. 2022, 2022, 4804408. [Google Scholar] [CrossRef]
- Yuzbashian, E.; Fernando, D.N.; Pakseresht, M.; Eurich, D.T.; Chan, C.B. Dairy product consumption and risk of non-alcoholic fatty liver disease: A systematic review and meta-analysis of observational studies. Nutr. Metab. Cardiovasc. Dis. 2023, 33, 1461–1471. [Google Scholar] [CrossRef]
- Kaenkumchorn, T.K.; Merritt, M.A.; Lim, U.; Le Marchand, L.; Boushey, C.J.; Shepherd, J.A.; Wilkens, L.R.; Ernst, T.; Lampe, J.W. Diet and Liver Adiposity in Older Adults: The Multiethnic Cohort Adiposity Phenotype Study. J. Nutr. 2021, 151, 3579–3587. [Google Scholar] [CrossRef]
- Zhou, Q.; Hu, H.; Hu, L.; Liu, S.; Chen, J.; Tong, S. Association between processed and unprocessed red meat consumption and risk of nonalcoholic fatty liver disease: A systematic review and dose-response meta-analysis. J Glob. Health 2024, 14, 04060. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yoshimura, S.M.; Duarte, S.M.B.; Stefano, J.T.; Mazo, D.F.C.; Pinho, J.R.R.; Oliveira, C.P. PNPLA3 gene polymorphism and red meat consumption increased fibrosis risk in nash biopsy-proven patients under medical follow-up in a tertiary center in southwest Brazil. Arq. Gastroenterol. 2023, 60, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Langmann, F.; Ibsen, D.B.; Johnston, L.W.; Perez-Cornago, A.; Dahm, C.C. Legumes as a Substitute for Red and Processed Meat, Poultry or Fish, and the Risk of Non-Alcoholic Fatty Liver Disease in a Large Cohort. J. Hum. Nutr. Diet. 2025, 38, e70004. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, D.; Zhou, S.; Duan, H.; Guo, J.; Yan, W. Nutritional Composition, Health Benefits, and Application Value of Edible Insects: A Review. Foods 2022, 11, 3961. [Google Scholar] [CrossRef]
- Quah, Y.; Tong, S.R.; Bojarska, J.; Giller, K.; Tan, S.A.; Ziora, Z.M.; Esatbeyoglu, T.; Chai, T.-T. Bioactive Peptide Discovery from Edible Insects for Potential Applications in Human Health and Agriculture. Molecules 2023, 28, 1233. [Google Scholar] [CrossRef]
- Scoditti, E.; Sabatini, S.; Carli, F.; Gastaldelli, A. Hepatic glucose metabolism in the steatotic liver. Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 319–334. [Google Scholar] [CrossRef]
- The Lancet Diabetes Endocrinology. edefining obesity: Advancing care for better lives. RLancet. Diabetes Endocrinol. 2025, 13, 75.
- Montemayor, S.; Mascaró, C.M.; Ugarriza, L.; Casares, M.; Llompart, I.; Abete, I.; Zulet, M.Á.; Martínez, J.A.; Tur, J.A.; Bouzas, C. Adherence to Mediterranean Diet and NAFLD in Patients with Metabolic Syndrome: The FLIPAN Study. Nutrients 2022, 14, 3186. [Google Scholar] [CrossRef]
- Hamamah, S.; Iatcu, O.C.; Covasa, M. Dietary Influences on Gut Microbiota and Their Role in Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). Nutrients 2024, 17, 143. [Google Scholar] [CrossRef]
- Mogna-Peláez, P.; Riezu-Boj, J.I.; Milagro, F.I.; Herrero, J.I.; Elorz, M.; Benito-Boillos, A.; Tobaruela-Resola, A.L.; Tur, J.A.; Martínez, J.A.; Abete, I.; et al. Inflammatory markers as diagnostic and precision nutrition tools for metabolic dysfunction-associated steatotic liver disease: Results from the Fatty Liver in Obesity trial. Clin. Nutr. 2024, 43, 1770–1781. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, S.; Wang, J.; Liu, W.; Gong, H.; Zhang, Z.; Lyu, B.; Yu, H. Insoluble dietary fiber from soybean residue (okara) exerts anti-obesity effects by promoting hepatic mitochondrial fatty acid oxidation. Foods 2023, 12, 2081. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Sun, W.; Swallah, M.S.; Amin, K.; Lyu, B.; Fan, H.; Zhang, Z.; Yu, H. Preparation and characterization of soybean insoluble dietary fiber and its prebiotic effect on dyslipidemia and hepatic steatosis in high fat-fed C57BL/6J mice. Food Funct. 2021, 12, 8760–8773. [Google Scholar] [CrossRef]
- Wu, Y.; Pan, X.; Zhang, S.; Wang, W.; Cai, M.; Li, Y.; Yang, F.; Guo, H. Protective effect of corn peptides against alcoholic liver injury in men with chronic alcohol consumption: A randomized double-blind placebo-controlled study. Lipids Health Dis. 2014, 13, 192. [Google Scholar] [CrossRef] [PubMed]
- Dang, J.; Cai, T.; Tuo, Y.; Peng, S.; Wang, J.; Gu, A.; Li, J.; Ding, L.; Du, S.; Wang, L. Corn peptides alleviate nonalcoholic fatty liver fibrosis in mice by inhibiting NLRP3 inflammasome activation and regulating gut Microbiota. J. Agric. Food Chem. 2024, 72, 19378–19394. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.-X.; Luo, Y.; Mao, Y.-Y.; Yuan, K.; Jin, S.-H.; Zhu, X.-T.; Zhong, B. Purified anthocyanins from Zea mays L. cob ameliorates chronic liver injury in mice via modulating of oxidative stress and apoptosis. J. Sci. Food Agric. 2021, 101, 4672–4680. [Google Scholar] [CrossRef]
- Lee-Martínez, S.N.; Luzardo-Ocampo, I.; Vergara-Castañeda, H.A.; Vasco-Leal, J.F.; Gaytán-Martínez, M.; Cuellar-Nuñez, M.L. Native corn (Zea mays L., cv. “Elotes Occidentales”) polyphenols extract reduced total cholesterol and triglycerides levels, and decreased lipid accumulation in mice fed a high-fat diet. Biomed. Pharmacother. 2024, 180, 117610. [Google Scholar] [CrossRef]
- Lucero López, V.R.; Razzeto, G.S.; Escudero, N.L.; Gimenez, M.S. Biochemical and molecular study of the influence of Amaranthus hypochondriacus flour on serum and liver lipids in rats treated with ethanol. Plant Foods Hum. Nutr. 2013, 68, 396–402. [Google Scholar] [CrossRef]
- Yang, Y.; Fukui, R.; Jia, H.; Kato, H. Amaranth supplementation improves hepatic lipid dysmetabolism and modulates gut Microbiota in mice fed a high-fat diet. Foods 2021, 10, 1259. [Google Scholar] [CrossRef]
- Rjeibi, I.; Ben Saad, A.; Hfaiedh, N. Oxidative damage and hepatotoxicity associated with deltamethrin in rats: The protective effects of Amaranthus spinosus seed extract. Biomed. Pharmacother. 2016, 84, 853–860. [Google Scholar] [CrossRef]
- Escudero, N.L.; Albarracín, G.J.; Lucero López, R.V.; Giménez, M.S. Antioxidant activity and phenolic content of flour and protein concentrate of Amaranthus cruentus seeds. J. Food Biochem. 2011, 35, 1327–1341. [Google Scholar] [CrossRef]
- Valerino-Perea, S.; Lara-Castor, L.; Armstrong, M.E.G.; Papadaki, A. Definition of the traditional Mexican diet and its role in health: A Systematic review. Nutrients 2019, 11, 2803. [Google Scholar] [CrossRef] [PubMed]
- Simancas-Racines, D.; Annunziata, G.; Verde, L.; Fascì-Spurio, F.; Reytor-González, C.; Muscogiuri, G.; Frias-Toral, E.; Barrea, L. Nutritional Strategies for Battling Obesity-Linked Liver Disease: The Role of Medical Nutritional Therapy in Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD) Management. Curr. Obes. Rep. 2025, 14, 7. [Google Scholar] [CrossRef] [PubMed]
- García-Berumen, C.I.; Vargas-Vargas, M.A.; Ortiz-Avila, O.; Piña-Zentella, R.M.; Ramos-Gómez, M.; Figueroa-García, M.D.C.; Mejía-Zepeda, R.; Rodríguez–Orozco, A.R.; Saavedra–Molina, A.; Cortés-Rojo, C. Avocado oil alleviates non-alcoholic fatty liver disease by improving mitochondrial function, oxidative stress and inflammation in rats fed a high fat-High fructose diet. Front. Pharmacol. 2022, 13, 1089130. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Avila, O.; Gallegos-Corona, M.A.; Sánchez-Briones, L.A.; Calderón-Cortés, E.; Montoya-Pérez, R.; Rodriguez-Orozco, A.R.; Campos-García, J.; Saavedra-Molina, A.; Mejía-Zepeda, R.; Cortés-Rojo, C. Protective effects of dietary avocado oil on impaired electron transport chain function and exacerbated oxidative stress in liver mitochondria from diabetic rats. J. Bioenerg. Biomembr. 2015, 47, 337–353. [Google Scholar] [CrossRef]
- Pacheco, L.S.; Bradley, R.D.; Anderson, C.A.M.; Allison, M.A. Changes in Biomarkers of Non-Alcoholic Fatty Liver Disease (NAFLD) upon Access to Avocados in Hispanic/Latino Adults: Secondary Data Analysis of a Cluster Randomized Controlled Trial. Nutrients 2022, 14, 2744. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3 fatty acids and metabolic partitioning of fatty acids within the liver in the context of nonalcoholic fatty liver disease. Curr. Opin. Clin. Nutr. Metab. Care 2022, 25, 248–255. [Google Scholar] [CrossRef]
- Musazadeh, V.; Karimi, A.; Malekahmadi, M.; Ahrabi, S.S.; Dehghan, P. Omega-3 polyunsaturated fatty acids in the treatment of non-alcoholic fatty liver disease: An umbrella systematic review and meta-analysis. Clin. Exp. Pharmacol. Physiol. 2023, 50, 327–334. [Google Scholar] [CrossRef]
- Agarwal, A.; Rizwana Tripathi, A.D.; Kumar, T.; Sharma, K.P.; Patel, S.K.S. Nutritional and Functional New Perspectives and Potential Health Benefits of Quinoa and Chia Seeds. Antioxidants 2023, 12, 1413. [Google Scholar] [CrossRef]
- Lee, S.; Choi, Y.; Jeong, H.S.; Lee, J.; Sung, J. Effect of different cooking methods on the content of vitamins and true retention in selected vegetables. Food Sci. Biotechnol. 2017, 27, 333. [Google Scholar] [CrossRef]
- Aburto, T.C.; Batis, C.; Pedroza-Tobías, A.; Pedraza, L.S.; Ramírez-Silva, I.; Rivera, J.A. Dietary intake of the Mexican population: Comparing food group contribution to recommendations, 2012–2016. Salud Publica Mex. 2022, 64, 267–279. [Google Scholar] [CrossRef]
- Huang, X.; Gan, D.; Fan, Y.; Fu, Q.; He, C.; Liu, W.; Li, F.; Ma, L.; Wang, M.; Zhang, W. The Associations between Healthy Eating Patterns and Risk of Metabolic Dysfunction-Associated Steatotic Liver Disease: A Case-Control Study. Nutrients 2024, 16, 1956. [Google Scholar] [CrossRef] [PubMed]
- Donghia, R.; Campanella, A.; Bonfiglio, C.; Cuccaro, F.; Tatoli, R.; Giannelli, G. Protective Role of Lycopene in Subjects with Liver Disease: NUTRIHEP Study. Nutrients 2024, 16, 562. [Google Scholar] [CrossRef] [PubMed]
- Morán-Ramos, S.; Avila-Nava, A.; Tovar, A.R.; Pedraza-Chaverri, J.; López-Romero, P.; Torres, N. Opuntia ficus indica (nopal) attenuates hepatic steatosis and oxidative stress in obese Zucker (fa/fa) rats. J. Nutr. 2012, 142, 1956–1963. [Google Scholar] [CrossRef]
- Lee, D.; Chiavaroli, L.; Ayoub-Charette, S.; Khan, T.A.; Zurbau, A.; Au-Yeung, F.; Cheung, A.; Liu, Q.; Qi, X.; Ahmed, A.; et al. Important Food Sources of Fructose-Containing Sugars and Non-Alcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis of Controlled Trials. Nutrients 2022, 14, 2846. [Google Scholar] [CrossRef]
- Shin, M.K.; Yang, S.M.; Han, I.S. Capsaicin suppresses liver fat accumulation in high-fat diet-induced NAFLD mice. Anim. Cells Syst. 2020, 24, 214–219. [Google Scholar] [CrossRef]
- Li, S.; Hao, L.; Yu, F.; Li, N.; Deng, J.; Zhang, J.; Xiong, S.; Hu, X. Capsaicin: A spicy way in liver disease. Front. Pharmacol. 2024, 15, 1451084. [Google Scholar] [CrossRef]
- Sun, M.; Gu, Y.; Glisan, S.L.; Lambert, J.D. Dietary cocoa ameliorates non-alcoholic fatty liver disease and increases markers of antioxidant response and mitochondrial biogenesis in high fat-fed mice. J. Nutr. Biochem. 2021, 92, 108618. [Google Scholar] [CrossRef]
- Rebollo-Hernanz, M.; Aguilera, Y.; Martin-Cabrejas, M.A.; de Mejia, E.G. Phytochemicals from the Cocoa Shell Modulate Mitochondrial Function, Lipid and Glucose Metabolism in Hepatocytes via Activation of FGF21/ERK, AKT, and mTOR Pathways. Antioxidants 2022, 11, 136. [Google Scholar] [CrossRef]
- Askari, F.; Rashidkhani, B.; Hekmatdoost, A. Cinnamon may have therapeutic benefits on lipid profile, liver enzymes, insulin resistance, and high-sensitivity C-reactive protein in nonalcoholic fatty liver disease patients. Nutr. Res. 2014, 34, 143–148. [Google Scholar] [CrossRef]
- Mackonochie, M.; Rodriguez-Mateos, A.; Mills, S.; Rolfe, V. A Scoping Review of the Clinical Evidence for the Health Benefits of Culinary Doses of Herbs and Spices for the Prevention and Treatment of Metabolic Syndrome. Nutrients 2023, 15, 4867. [Google Scholar] [CrossRef]
- Ranasinghe, P.; Jayawardana, R.; Galappaththy, P.; Constantine, G.R.; de Vas Gunawardana, N.; Katulanda, P. Efficacy and safety of “true” cinnamon (Cinnamomum zeylanicum) as a pharmaceutical agent in diabetes: A systematic review and meta-analysis. Diabet. Med. 2012, 29, 1480–1492. [Google Scholar] [CrossRef] [PubMed]
- Bandara, T.; Uluwaduge, I.; Jansz, E.R. Bioactivity of cinnamon with special emphasis on diabetes mellitus: A review. Int. J. Food Sci. Nutr. 2012, 63, 380–386. [Google Scholar] [CrossRef]
- Ranasinghe, P.; Pigera, S.; Premakumara, G.A.S.; Galappaththy, P.; Constantine, G.R.; Katulanda, P. Medicinal properties of “true” cinnamon (Cinnamomum zeylanicum): A systematic review. BMC Complement. Altern. Med. 2013, 13, 275. [Google Scholar] [CrossRef]
- Eidi, A.; Mortazavi, P.; Bazargan, M.; Zaringhalam, J. Hepatoprotective activity of cinnamon ethanolic extract against CCI4-induced liver injury in rats. Excli J. 2012, 11, 495–507. [Google Scholar]
- De Silva, D.A.M.; Jeewanthi, R.K.C.; Rajapaksha, R.H.N.; Wmtb, W.; Hirotsu, N.; Shimizu, B.I.; Munasinghe, M.A.J.P. Clean vs dirty labels: Transparency and authenticity of the labels of Ceylon cinnamon. PLoS ONE 2021, 16, e0260474. [Google Scholar] [CrossRef]
- Li, H.-Y.; Gan, R.-Y.; Shang, A.; Mao, Q.-Q.; Sun, Q.-C.; Wu, D.-T.; Geng, F.; He, X.Q.; Li, H.B. Plant-Based Foods and Their Bioactive Compounds on Fatty Liver Disease: Effects, Mechanisms, and Clinical Application. Oxidative Med. Cell. Longev. 2021, 2021, 6621644. [Google Scholar] [CrossRef]
- Mohammadian, K.; Fakhar, F.; Keramat, S.; Stanek, A. The role of antioxidants in the treatment of metabolic dysfunction-associated fatty liver disease: A systematic review. Antioxidants 2024, 13, 797. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Uscanga, A.; Loarca-Piña, G.; Gonzalez de Mejia, E. Baked corn (Zea mays L.) and bean (Phaseolus vulgaris L.) snack consumption lowered serum lipids and differentiated liver gene expression in C57BL/6 mice fed a high-fat diet by inhibiting PPARγ and SREBF2. J. Nutr. Biochem. 2017, 50, 1–15. [Google Scholar] [CrossRef]
- Pintó, X.; Fanlo-Maresma, M.; Corbella, E.; Corbella, X.; Mitjavila, M.T.; Moreno, J.J.; Casas, R.; Estruch, R.; Corella, D.; Bulló, M.; et al. A Mediterranean Diet Rich in Extra-Virgin Olive Oil Is Associated with a Reduced Prevalence of Nonalcoholic Fatty Liver Disease in Older Individuals at High Cardiovascular Risk. J. Nutr. 2019, 149, 1920–1929. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, S.; Lee, Y.; Kwon, Y.J.; Lee, J.W. Higher Adherence to the Mediterranean Diet Is Associated with a Lower Risk of Steatotic, Alcohol-Related, and Metabolic Dysfunction-Associated Steatotic Liver Disease: A Retrospective Analysis. Nutrients 2024, 16, 3551. [Google Scholar] [CrossRef]
- Ad, C.C.; García, C.M.; Gp, M.P.; Aa, O.L.; Francisco, M.D.R.; Ordaz, Á.H.; Dietlen, F.R.; Remes-Troche, J.M. Efficacy of the regional Mexican diet versus the Mediterranean diet in patients with MASLD: A 24-week non-inferiority trial. Rev. Esp. de Enfermedades Dig. 2024. [Google Scholar] [CrossRef]
- Wikan, N.; Tocharus, J.; Oka, C.; Sivasinprasasn, S.; Chaichompoo, W.; Suksamrarn, A.; Tocharus, C. The capsaicinoid nonivamide suppresses the inflammatory response and attenuates the progression of steatosis in a NAFLD-rat model. J. Biochem. Mol. Toxicol. 2023, 37, e23279. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.X.; Teng, Y.Y.; Zhu, Q.D.; Zhang, Q.Y.; Tang, Y.H. Inhibitory effects of capsaicin on hepatic stellate cells and liver fibrosis. Biochem. Cell Biol. 2014, 92, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Goto, T.; Han, I.S.; Kawada, T.; Kim, Y.M.; Yu, R. Dietary capsaicin reduces obesity-induced insulin resistance and hepatic steatosis in obese mice fed a high-fat diet. Obesity 2010, 18, 780–787. [Google Scholar] [CrossRef]
- Pipitone, R.M.; Zito, R.; Gambino, G.; Di Maria, G.; Javed, A.; Lupo, G.; Giglia, G.; Sardo, P.; Ferraro, G.; Rappa, F. Red and golden tomato administration improves fat diet-induced hepatic steatosis in rats by modulating HNF4α, Lepr, and GK expression. Front. Nutr. 2023, 10, 1221013. [Google Scholar] [CrossRef]
- Baz, L.; Algarni, S.; Al-thepyani, M.; Aldairi, A.; Gashlan, H. Lycopene Improves Metabolic Disorders and Liver Injury Induced by a Hight-Fat Diet in Obese Rats. Molecules 2022, 27, 7736. [Google Scholar] [CrossRef]
- García-Alonso, F.J.; González-Barrio, R.; Martín-Pozuelo, G.; Hidalgo, N.; Navarro-González, I.; Masuero, D.; Soini, E.; Vrhovsek, U.; Periago, M.J. A study of the prebiotic-like effects of tomato juice consumption in rats with diet-induced non-alcoholic fatty liver disease (NAFLD). Food Funct. 2017, 8, 3542–3552. [Google Scholar] [CrossRef]
- Pannunzio, A.; Baratta, F.; Maggio, E.; Palumbo, I.M.; Magna, A.; Trivigno, C.; Carnevale, R.; Simona, B.; Cammisotto, V.; Vidili, G.; et al. Dark chocolate’s impact on low-grade endotoxemia in metabolic dysfunction-associated steatohepatitis. Nutrition 2025, 131, 112643. [Google Scholar] [CrossRef]
- Loffredo, L.; Baratta, F.; Ludovica, P.; Battaglia, S.; Carnevale, R.; Nocella, C.; Novo, M.; Pannitteri, G.; Ceci, F.; Angelico, F.; et al. Effects of dark chocolate on endothelial function in patients with non-alcoholic steatohepatitis. Nutr. Metab. Cardiovasc. Dis. NMCD 2017, 28, 143–149. [Google Scholar] [CrossRef]
- Cordero-Herrera, I.; Martín, M.Á.; Escrivá, F.; Álvarez, C.; Goya, L.; Ramos, S. Cocoa-rich diet ameliorates hepatic insulin resistance by modulating insulin signaling and glucose homeostasis in Zucker diabetic fatty rats. J. Nutr. Biochem. 2015, 26, 704–712. [Google Scholar] [CrossRef]
- Fidaleo, M.; Fracassi, A.; Zuorro, A.; Lavecchia, R.; Moreno, S.; Sartori, C. Cocoa protective effects against abnormal fat storage and oxidative stress induced by a high-fat diet involve PPARα signalling activation. Food Funct. 2014, 5, 2931–2939. [Google Scholar] [CrossRef] [PubMed]
- Besné-Eseverri, I.; Martín, M.Á.; Lobo, G.; Cano, M.P.; Portillo, M.P.; Trepiana, J. Antioxidant and Anti-Inflammatory Effects of Opuntia Extracts on a Model of Diet-Induced Steatosis. Antioxidants 2024, 13, 1416. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Tapia, M.; Aguilar-López, M.; Pérez-Cruz, C.; Pichardo-Ontiveros, E.; Wang, M.; Donovan, S.M.; Tovar, A.R.; Torres, N. Nopal (Opuntia ficus indica) protects from metabolic endotoxemia by modifying gut microbiota in obese rats fed high fat/sucrose diet. Sci. Rep. 2017, 7, 4716. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Li, J.; Hu, S. Cinnamon could improve hepatic steatosis caused by a high-fat diet via enhancing hepatic beta-oxidation and inhibiting hepatic lipogenesis, oxidative damage, and inflammation in male rats. J. Food Biochem. 2022, 46, e14077. [Google Scholar] [CrossRef]
- Ramírez-Jiménez, A.K.; Luzardo-Ocampo, I.; Cuellar-Nuñez, M.L.; Anaya-Loyola, M.A.; León-Galván, M.F.; Loarca-Piña, G. Daily Intake of a Phaseolus vulgaris L. Snack Bar Attenuates Hypertriglyceridemia and Improves Lipid Metabolism-Associated Plasma Proteins in Mexican Women: A Randomized Clinical Trial. Front. Nutr. 2022, 9, 890136. [Google Scholar] [CrossRef]
- Pérez-Ramírez, I.F.; Becerril-Ocampo, L.J.; Reynoso-Camacho, R.; Herrera Guzmán-Maldonado, S.H.; Cruz-Bravo, R.K. Cookies elaborated with oat and common bean flours improved serum markers in diabetic rats. J. Sci. Food Agric. 2018, 98, 998–1007. [Google Scholar] [CrossRef]
- Ou, J.Y.; Huang, J.Q.; Song, Y.; Yao, S.W.; Peng, X.C.; Wang, M.F.; Ou, S.-Y. Feruloylated Oligosaccharides from Maize Bran Modulated the Gut Microbiota in Rats. Plant Foods Hum. Nutr. 2016, 71, 123–128. [Google Scholar] [CrossRef]
- Gu, M.J.; Ahn, Y.; Lee, Y.R.; Yoo, G.; Kim, Y.; Choi, I.; Ha, S.K.; Kim, D. Coriandrum sativum L. Leaf Extract Ameliorates Metabolic Dysfunction-Associated Steatotic Liver Disease by Modulating the AMPK Pathway in High Fat-Fed C57BL/6 Mice. Nutrients 2024, 16, 4165. [Google Scholar] [CrossRef]
- Ortiz-Avila, O.; Esquivel-Martínez, M.; Olmos-Orizaba, B.E.; Saavedra-Molina, A.; Rodriguez-Orozco, A.R.; Cortés-Rojo, C. Avocado Oil Improves Mitochondrial Function and Decreases Oxidative Stress in Brain of Diabetic Rats. J. Diabetes Res. 2015, 2015, 485759. [Google Scholar] [CrossRef]
- Escobar-Ortiz, A.; Hernández-Saavedra, D.; Lizardi-Mendoza, J.; Pérez-Ramírez, I.F.; Mora-Izaguirre, O.; Ramos-Gómez, M.; Reynoso-Camacho, R. Consumption of cricket (Acheta domesticus) flour decreases insulin resistance and fat accumulation in rats fed with high-fat and -fructose diet. J. Food Biochem. 2022, 46, e14269. [Google Scholar] [CrossRef]
- Medina-Urrutia, A.X.; Jorge-Galarza, E.; El Hafidi, M.; Reyes-Barrera, J.; Páez-Arenas, A.; Masso-Rojas, F.A.; Martínez-Sánchez, F.D.; López-Uribe, Á.R.; González-Salazar, M.D.C.; Torres-Tamayo, M.; et al. Effect of dietary chia supplementation on glucose metabolism and adipose tissue function markers in non-alcoholic fatty liver disease subjects. Nutr. Hosp. 2022, 39, 1280–1288. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.N.; Kang, S.G.; Roh, Y.K.; Choi, M.K.; Song, S.W. Efficacy and safety of fermented garlic extract on hepatic function in adults with elevated serum gamma-glutamyl transpeptidase levels: A double-blind, randomized, placebo-controlled trial. Eur. J. Nutr. 2017, 56, 1993–2002. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.S.; Chen, W.C.; Ho, C.T.; Lu, K.H.; Lin, S.H.; Tseng, H.C.; Lin, S.-Y.; Sheen, L.-Y. Garlic essential oil protects against obesity-triggered nonalcoholic fatty liver disease through modulation of lipid metabolism and oxidative stress. J. Agric. Food Chem. 2014, 62, 5897–5906. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huerta-Álvarez, A.; Arellano, M.; Chávez-Méndez, C.A.; Carpinteyro-Espin, P.; Palacios-Reyes, C.; Pérez-Escobar, J. Milpa Diet for MASLD in Mesoamerican Populations: Feasibility, Advantages, and Future Perspectives. Life 2025, 15, 812. https://doi.org/10.3390/life15050812
Huerta-Álvarez A, Arellano M, Chávez-Méndez CA, Carpinteyro-Espin P, Palacios-Reyes C, Pérez-Escobar J. Milpa Diet for MASLD in Mesoamerican Populations: Feasibility, Advantages, and Future Perspectives. Life. 2025; 15(5):812. https://doi.org/10.3390/life15050812
Chicago/Turabian StyleHuerta-Álvarez, Aline, Mariana Arellano, Clyo Anahí Chávez-Méndez, Paulina Carpinteyro-Espin, Carmen Palacios-Reyes, and Juanita Pérez-Escobar. 2025. "Milpa Diet for MASLD in Mesoamerican Populations: Feasibility, Advantages, and Future Perspectives" Life 15, no. 5: 812. https://doi.org/10.3390/life15050812
APA StyleHuerta-Álvarez, A., Arellano, M., Chávez-Méndez, C. A., Carpinteyro-Espin, P., Palacios-Reyes, C., & Pérez-Escobar, J. (2025). Milpa Diet for MASLD in Mesoamerican Populations: Feasibility, Advantages, and Future Perspectives. Life, 15(5), 812. https://doi.org/10.3390/life15050812






