Exploring the Link Between Nutritional and Functional Status and Short-Term Postoperative Outcomes in Patients Undergoing Pancreatic Cancer Surgery
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
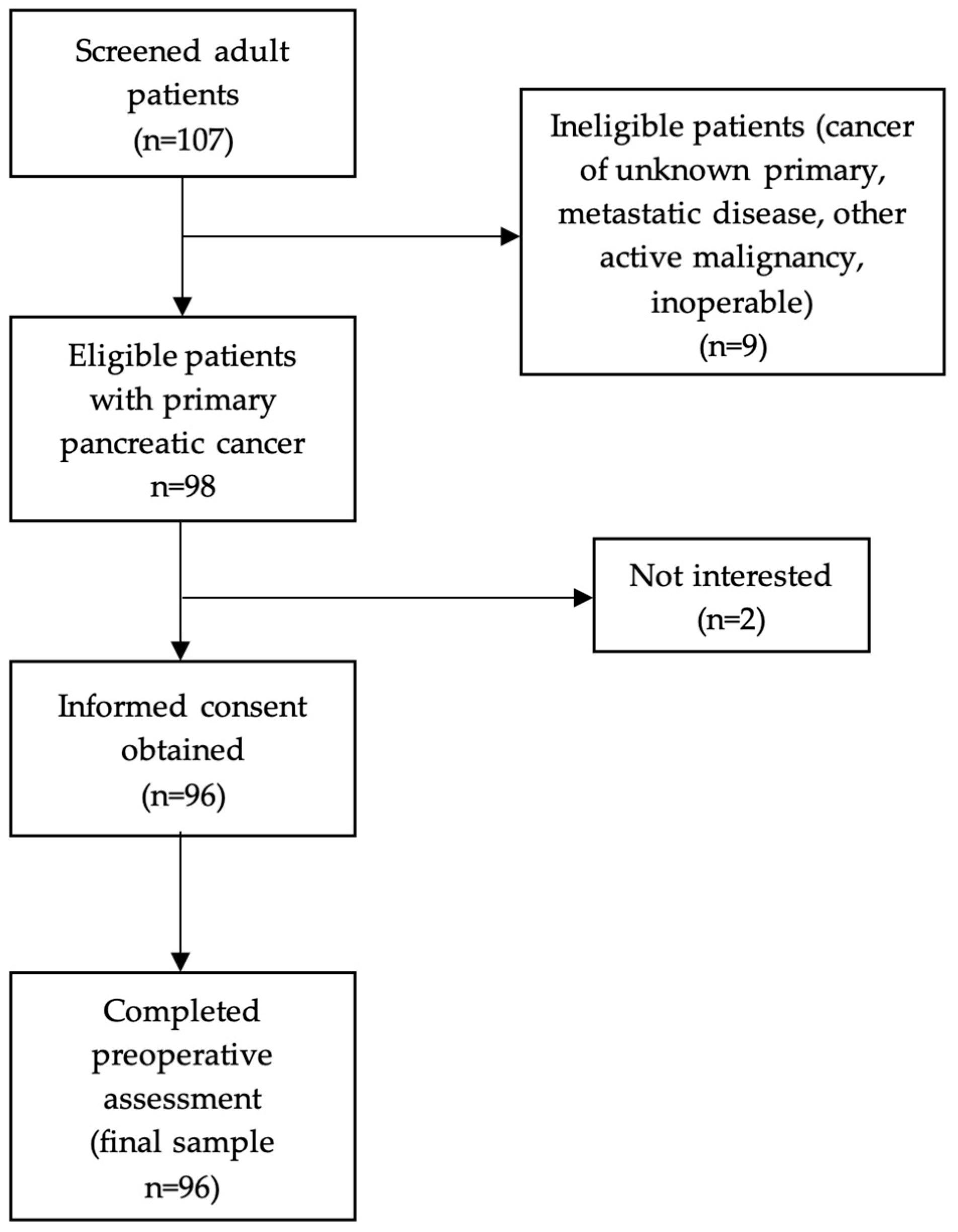
2.2. Patient Characteristics and Preoperative Assessment
2.3. Postoperative Outcomes Assessment
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Study Participants
3.2. Postoperative Outcomes
3.3. Determinants of Overall, Major Postoperative Complications and Mortality
3.4. Determinants of Length of Hospital Stay
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Puckett, Y.; Garfield, K. Pancreatic Cancer. StatPearls. 2022. Available online: https://www.statpearls.com (accessed on 26 September 2022).
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.X.; Zhao, C.F.; Chen, W.B.; Liu, Q.C.; Li, Q.W.; Lin, Y.Y.; Gao, F. Pancreatic cancer: A review of epidemiology, trend, and risk factors. World J. Gastroenterol. 2021, 27, 4298–4321. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Andrén-Sandberg, A. Complications of pancreatic surgery. N. Am. J. Med. Sci. 2011, 3, 531–535. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gilliland, T.M.; Villafane-Ferriol, N.; Shah, K.P.; Shah, R.M.; Tran Cao, H.S.; Massarweh, N.N.; Silberfein, E.J.; Choi, E.A.; Hsu, C.; McElhany, A.L.; et al. Nutritional and metabolic derangements in pancreatic cancer and pancreatic resection. Nutrients 2017, 9, 243. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Yang, Y.F.; Han, P.; Ye, P.C.; Kong, H. Protein-energy malnutrition worsens hospitalization outcomes of patients with pancreatic cancer undergoing open pancreaticoduodenectomy. Updates Surg. 2022, 74, 1627–1636. [Google Scholar] [CrossRef]
- Jachnis, A.; Słodkowski, M.T. The relationship between nutritional status and body composition with clinical parameters, tumor stage, CA19-9, CEA levels in patients with pancreatic and periampullary tumors. Curr. Oncol. 2021, 28, 4805–4820. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, D.U.; Hastie, D.J.; Fan, G.H.; Addonizio, E.A.; Lee, K.J.; Han, J.; Karagozian, R. Effect of malnutrition on the postoperative outcomes of patients undergoing pancreatectomy for pancreatic cancer: Propensity score-matched analysis of 2011–2017 US hospitals. Nutr. Clin. Pract. 2022, 37, 117–129. [Google Scholar] [CrossRef]
- Meza-Valderrama, D.; Marco, E.; Dávalos-Yerovi, V.; Muns, M.D.; Tejero-Sánchez, M.; Duarte, E.; Sánchez-Rodríguez, D. Sarcopenia, malnutrition, and cachexia: Adapting definitions and terminology of nutritional disorders in older people with cancer. Nutrients 2021, 13, 761. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Argilés, J.M. Cancer-associated malnutrition. Eur. J. Oncol. Nurs. 2005, 9, 39–50. [Google Scholar] [CrossRef]
- Paiella, S.; Azzolina, D.; Trestini, I.; Malleo, G.; Nappo, G.; Ricci, C.; Ingaldi, C.; Vacca, P.G.; De Pastena, M.; Secchettin, E.; et al. Body composition parameters, immunonutritional indexes, and surgical outcome of pancreatic cancer patients resected after neoadjuvant therapy: A retrospective, multicenter analysis. Front. Nutr. 2023, 10, 1065294, Erratum in Front. Nutr. 2023, 10, 1212436. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yokoyama, Y.; Nagino, M.; Ebata, T. Importance of “muscle” and “intestine” training before major HPB surgery: A review. J. Hepatobiliary Pancreat. Sci. 2021, 28, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Planas, M.; Alvarez-Hernandez, J.; Leon-Sanz, M.; Celaya-Perez, S.; Araujo, K.; García de Lorenzo, A. Prevalence of hospital malnutrition in cancer patients: A sub-analysis of the PREDyCES study. Support. Care Cancer 2016, 24, 429–435. [Google Scholar] [CrossRef]
- Aaldriks, A.A.; van der Geest, L.G.M.; Giltay, E.J.; Le Cessie, S.; Portielje, J.E.A.; Tanis, B.C.; Nortier, J.W.R.; Maartense, E. Frailty and malnutrition predictive of mortality risk in older patients with advanced colorectal cancer receiving chemotherapy. J. Geriatr. Oncol. 2013, 4, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.; Senesse, P.; Gioulbasanis, I.; Antoun, S.; Bozzetti, F.; Deans, C.; Strasser, F.; Thoresen, L.; Jagoe, R.T.; Chasen, M.; et al. Diagnostic criteria for the classification of cancer-associated weight loss. J. Clin. Oncol. 2015, 33, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Mintziras, I.; Wächter, S.; Manoharan, J.; Kanngiesser, V.; Maurer, E.; Bartsch, D.K. Postoperative morbidity following pancreatic cancer surgery is significantly associated with worse overall patient survival: A systematic review and meta-analysis. Surg. Oncol. 2021, 38, 101573. [Google Scholar] [CrossRef]
- Amin, M.B.; Edge, S.; Greene, F.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C.; et al. AJCC Cancer Staging Manual, 8th ed.; Springer International Publishing: Berlin/Heidelberg, Germany; American Joint Commission on Cancer: Chicago, IL, USA, 2017. [Google Scholar]
- Jager-Wittenaar, H.; Ottery, F.D. Assessing nutritional status in cancer: Role of the patient-generated subjective global assessment. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 322–329. [Google Scholar] [CrossRef]
- Lidoriki, I.; Jager-Wittenaar, H.; Papapanou, M.; Routsi, E.; Frountzas, M.; Mylonas, K.S.; Ottery, F.D.; Schizas, D. Greek translation and cultural adaptation of the scored patient-generated subjective global assessment: A nutritional assessment tool suitable for cancer patients. Clin. Nutr. ESPEN 2021, 43, 322–328. [Google Scholar] [CrossRef]
- Luna-Heredia, E.; Martín-Peña, G.; Ruiz-Galiana, J. Handgrip dynamometry in healthy adults. Clin. Nutr. 2005, 24, 250–258. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Bouillanne, O.; Morineau, G.; Dupont, C.; Coulombel, I.; Vincent, J.P.; Nicolis, I.; Benazeth, S.; Cynober, L.; Aussel, C. Geriatric Nutritional Risk Index: A new index for evaluating at-risk elderly medical patients. Am. J. Clin. Nutr. 2005, 82, 777–783. [Google Scholar] [CrossRef]
- Onodera, T.; Goseki, N.; Kosaki, G. Prognostic Nutritional Index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi 1984, 85, 1001–1005. [Google Scholar]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- National Cancer Institute. Pancreatic Cancer [Cancer Statistics]. SEER Cancer Statistics. Available online: https://seer.cancer.gov/statfacts/html/pancreas.html (accessed on 19 February 2025).
- Anker, M.S.; Holcomb, R.; Muscaritoli, M.; von Haehling, S.; Haverkamp, W.; Jatoi, A.; Morley, J.E.; Strasser, F.; Landmesser, U.; Coats, A.J.S.; et al. Orphan disease status of cancer cachexia in the USA and in the European Union: A systematic review. J. Cachexia Sarcopenia Muscle 2019, 10, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Chou, Y.J.; Liou, Y.T.; Lai, S.R.; Tien, Y.W.; Kuo, H.J.; Yang, H.Y.; Shun, S.C. Role of preoperative malnutrition and symptom severity in anorexia-cachexia-related quality of life in patients with operable pancreatic cancer. Eur. J. Oncol. Nurs. 2023, 66, 102352. [Google Scholar] [CrossRef]
- Gheorghe, G.; Bungau, S.; Ilie, M.; Behl, T.; Vesa, C.M.; Brisc, C.; Bacalbasa, N.; Turi, V.; Costache, R.S.; Diaconu, C.C. Early Diagnosis of Pancreatic Cancer: The Key for Survival. Diagnostics 2020, 10, 869. [Google Scholar] [CrossRef]
- Muscaritoli, M.; Arends, J.; Aapro, M. From guidelines to clinical practice: A roadmap for oncologists for nutrition therapy for cancer patients. Ther. Adv. Med. Oncol. 2019, 11, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Na, B.G.; Han, S.S.; Cho, Y.A.; Wie, G.A.; Kim, J.Y.; Lee, J.M.; Lee, S.D.; Kim, S.H.; Park, S.J. Nutritional status of patients with cancer: A prospective cohort study of 1,588 hospitalized patients. Nutr. Cancer 2018, 70, 1228–1236. [Google Scholar] [CrossRef] [PubMed]
- Loan, B.T.H.; Nakahara, S.; Tho, B.A.; Dang, T.N.; Anh, L.N.; Huy, N.D.; Ichikawa, M. Nutritional status and postoperative outcomes in patients with gastrointestinal cancer in Vietnam: A retrospective cohort study. Nutrition 2018, 48, 117–121. [Google Scholar] [CrossRef]
- Garth, A.K.; Newsome, C.M.; Simmance, N.; Crowe, T.C. Nutritional status, nutrition practices and post-operative complications in patients with gastrointestinal cancer. J. Hum. Nutr. Diet. 2010, 23, 393–401. [Google Scholar] [CrossRef]
- Ścisło, L.; Bodys-Cupak, I.; Walewska, E.; Kózka, M. Nutritional status indicators as predictors of postoperative complications in the elderly with gastrointestinal cancer. Int. J. Environ. Res. Public Health 2022, 19, 13453. [Google Scholar] [CrossRef]
- Lidoriki, I.; Schizas, D.; Frountzas, M.; Machairas, N.; Prodromidou, A.; Kapelouzou, A.; Karavokyros, I.; Pikoulis, E.; Kales, S.N.; Liakakos, T. GNRI as a prognostic factor for outcomes in cancer patients: A systematic review of the literature. Nutr. Cancer 2021, 73, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Li, X.; Chen, M.; Liu, L.; Yao, T.; Li, J.; Su, W. Prognostic potential of nutritional risk screening and assessment tools in predicting survival of patients with pancreatic neoplasms: A systematic review. Nutr. J. 2024, 23, 17. [Google Scholar] [CrossRef]
- Ye, W.K.; Wang, J.; Zheng, J.; Jiang, M.; Zhou, Y.N.; Wu, Z.X. Predictive value of the nutritional risk index for postoperative complications in individuals with pancreatic cancer undergoing pancreaticoduodenectomy. Geriatr. Nurs. 2025, 61, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y.; Lin, S.Q.; Ruan, G.T.; Zheng, X.; Chen, Y.; Zhang, H.Y.; Liu, T.; Xie, H.L.; Shi, H.P. The prognostic utility of the triceps skinfold thickness albumin index in colorectal cancer patients with cachexia. Nutr. Cancer 2025, 77, 265–275. [Google Scholar] [CrossRef]
- Shi, G.; Gao, T.; Du, P.; Guo, J.; Dong, Y.; Mao, J. Association between different patterns of obesity and the short-term outcomes of gastric cancer surgery. Eur. J. Cancer Prev. 2024, 10.1097/CEJ, 0926. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yang, R.; Xu, J.; Fang, K.; Abdelrahim, M.; Chang, L. Sarcopenia as a predictor of postoperative risk of complications, mortality, and length of stay following gastrointestinal oncological surgery. Ann. R. Coll. Surg. Engl. 2021, 103, 630–637. [Google Scholar] [CrossRef] [PubMed]
- Wielsøe, S.; Sundberg, A.; Kristensen, T.S.; Christensen, J.; Sillesen, M.; Hansen, C.P.; Burgdorf, S.K.; Pedersen, B.K.; Suetta, C.; Christensen, J.F.; et al. Impact of sarcopenia and muscle strength on postoperative complication risk following pancreatic resection. Clin. Nutr. ESPEN 2024, 64, 263–273. [Google Scholar] [CrossRef]
- Kusama, N.; Mitobe, Y.; Hyodo, N.; Miyashita, T.; Baba, Y.; Hashimoto, T.; Inagaki, Y. Preoperative risk factors in patients with pancreatic cancer. J. Clin. Med. Res. 2023, 15, 300–309. [Google Scholar] [CrossRef]
- Kryvoruchko, I.A.; Staikov, P.; Boyko, V.V.; Sartelli, M.; Ivanova, Y.V.; Honcharov, A.; Gramatiuk, S.; Sargsyan, K. Physiological stress level and screening for malnutrition as preoperative predictors of postoperative complications in pancreatic surgery: A retrospective study. BMC Surg. 2023, 23, 156. [Google Scholar] [CrossRef]
- Pecorelli, N.; Guarneri, G.; Quattromani, R.; Arru, G.G.; Gozzini, L.; Lee, Y.H.; Vallorani, A.; Turi, S.; Partelli, S.; Crippa, S.; et al. The impact of preoperative anemia on pancreatic resection outcomes. HPB 2022, 24, 717–726. [Google Scholar] [CrossRef]
- Leichtle, S.W.; Mouawad, N.J.; Lampman, R.; Singal, B.; Cleary, R.K. Does preoperative anemia adversely affect colon and rectal surgery outcomes? J. Am. Coll. Surg. 2011, 212, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Funakoshi, S.; Suzuki, Y.; Yoshida, M.; Momose, H.; Matsuki, R.; Kogure, M.; Abe, N.; Sunami, E.; Sakamoto, Y. Advantage of postoperative inflammatory status after laparoscopic distal pancreatectomy. Dig. Surg. 2024, 41, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Gaspar-Figueiredo, S.; Labgaa, I.; Demartines, N.; Schäfer, M.; Joliat, G.R. Assessment of the predictive value of preoperative serum albumin and postoperative albumin drop (ΔAlb) for complications after pancreas surgery: A single-center cross-sectional study. J. Clin. Med. 2023, 12, 972. [Google Scholar] [CrossRef] [PubMed]
- Putila, E.; Helminen, O.; Helmiö, M.; Huhta, H.; Jalkanen, A.; Junttila, A.; Kallio, R.; Koivukangas, V.; Kokkola, A.; Lietzen, E.; et al. Preoperative predictors of postoperative complications after gastrectomy for gastric cancer, a population-based study in Finland. Eur. J. Surg. Oncol. 2025, 51, 109682. [Google Scholar] [CrossRef]
| Characteristic | Patients (n = 96) |
|---|---|
| Age (years) | 65.0 ± 12.3 65.0 ± 12.3 (Median: 67, Min: 27, Max: 89) |
| Sex | n (%) |
| Male | 59 (61.5) |
| Female | 37 (38.5) |
| Type of cancer (n = 89) | n (%) |
| Adenocarcinoma | 83 (93.3) |
| NET | 4 (4.5) |
| Mixed adenoneuroendocrine carcinoma | 2 (2.2) |
| Surgical procedure | n (%) |
| Pancreaticoduodenectomy | 66 (68.8) |
| Distal pancreatectomy | 26 (27.1) |
| Wedge resection | 1 (1.0) |
| Non specific | 3 (3.1%) |
| Neoadjuvant Chemotherapy | n (%) |
| Yes | 20 (20.8) |
| No | 76 (79.2) |
| Neoadjuvant Radiotherapy | n (%) |
| Yes | 2 (2.1) |
| No | 94 (97.9) |
| Stage (n = 89) | n (%) |
| in situ | 5 (5.6) |
| I | 17 (19.1) |
| II | 44 (49.4) |
| III | 19 (21.3) |
| IV | 4 (4.5) |
| Adjuvant Chemotherapy (n = 76) | n (%) |
| Yes | 47 (61.8) |
| No | 29 (38.2) |
| Adjuvant Radiotherapy (n = 76) | n (%) |
| Yes | 3 (3.9) |
| No | 73 (96.1) |
| Albumin (g/dL) | 3.8 ± 0.8 |
| Hb (g/dL) | 12.4 ± 1.9 |
| HCT (%) | 37.4 ± 5.2 |
| TLC (K/μL) | 2.0 ± 1.8 |
| NLR (n = 94) | 3.35 ± 4.08 |
| PLR (n = 95) | 170.8 ± 145.4 |
| PG-SGA (n = 92) | 8.8 ± 6.5 |
| PG-SGA category (n = 92) | n (%) |
| A | 30 (32.6) |
| B | 29 (31.5) |
| C | 33 (35.9) |
| GNRI (n = 95) | 103.7 ± 17.9 |
| PNI (n = 95) | 38.8 ± 7.0 |
| BMI (kg/m2) | 25.8 ± 3.8 |
| WC (cm) | 93.9 ± 11.6 |
| HC (cm) | 98.0 ± 10.1 |
| WHR | 0.96 ± 0.07 |
| TSF (mm) | 10.3 ± 11.7 |
| Weight Loss (%) | 5.9 ± 5.9 |
| Handgrip strength (kg) | 27.1 ± 10.6 |
| Handgrip strength category (n = 89) | n (%) |
| Low | 24 (27.0) |
| Normal | 65 (73.0) |
| Gait speed (m/s) (n = 58) | 0.97 ± 0.32 |
| Gait speed category (n = 87) | n (%) |
| Low | 21 (24.1) |
| Normal | 66 (75.9) |
| Postoperative Complications | Patients (n = 96) |
|---|---|
| Complication | n (%) |
| Yes | 69 (71.9) |
| No | 27 (28.1) |
| Clavien grade | n (%) |
| 0 | 27 (28.1) |
| I | 6 (6.3) |
| II | 35 (36.5) |
| III | 17 (17.7) |
| IV | 2 (2.1) |
| V | 9 (9.4) |
| Minor complications (Ι–ΙΙ) | 41 (42.7) |
| Major complications (ΙΙΙ–V) | 28 (29.2) |
| Mortality | |
| Yes | 9 (9.4%) |
| No | 87 (90.6%) |
| LOS (days) | 17.5 ± 8.9 |
| Parameter | Complications | Major Complications | Mortality | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Yes (n = 69) | No (n = 27) | p-value | Yes (n = 28) | No (n = 68) | p-value | Yes (n = 9) | No (n = 87) | p-value |
| Age (years) | 65.5 ± 12.8 | 63.8 ± 2.2 | 0.553 | 67.29 ± 13.6 | 64.0 ± 11.7 | 0.241 | 72.2 ± 9.5 | 64.2 ± 12.3 | 0.063 |
| Sex | n (%) | n (%) | 0.303 | n (%) | n (%) | 0.449 | n (%) | n (%) | 1.000 |
| Male | 44 (74.6) | 15 (25.4) | 18 (30.5) | 41 (69.5) | 6 (10.2) | 53 (89.8) | |||
| Female | 25 (67.6) | 12 (32.4) | 10 (27.0) | 27 (73.0) | 3 (8.1) | 34 (91.9) | |||
| Type of cancer (n = 89) | n (%) | n (%) | 0.072 | n (%) | n (%) | 0.324 | n (%) | n (%) | 0.382 |
| Adenocarcinoma | 65 (78.3) | 18 (21.7) | 25 (30.1) | 58 (69.9) | 8 (9.6) | 75 (90.4) | |||
| NET | 2 (50.0) | 2 (50.0) | 1 (25.0) | 3 (75.0) | 0 (0) | 4 (100.0) | |||
| Mixed adenoneuroendocrine carcinoma | 2 (100) | 0 (0) | 2 (100) | 0 (0) | 1 (50.0) | 1 (50.0) | |||
| Surgical procedure | n (%) | n (%) | 0.595 | n (%) | n (%) | 0.512 | n (%) | n (%) | 0.943 |
| Pancreaticoduodenectomy | 45 (68.2) | 21 (31.8) | 19 (28.8) | 47 (71.2) | 7 (10.6) | 59 (89.4) | |||
| Distal Pancreatectomy | 21 (80.8) | 5 (19.2) | 7 (26.9) | 19 (73.1) | 2 (7.7) | 24 (92.3) | |||
| Wedge resection | 1 (100.0) | 0 (0) | 0 (0) | 1 (100) | 0 (0) | 1 (100) | |||
| Non specific | 2 (66.7) | 1 (33.3) | 2 (66.7) | 1 (33.3) | 0 (0) | 3 (100) | |||
| Neoadjuvant Chemotherapy | n (%) | n (%) | 0.412 | n (%) | n (%) | 0.375 | n (%) | n (%) | 0.291 |
| Yes | 16 (80.0) | 4 (20.0) | 7 (35.0) | 13 (65.0) | 3 (15.0) | 17 (85.0) | |||
| No | 53 (69.7) | 23 (30.3) | 21 (27.6) | 55 (72.4) | 6 (7.9) | 70 (92.1) | |||
| Neoadjuvant Radiotherapy | n (%) | n (%) | 0.486 | n (%) | n (%) | 0.500 | n (%) | n (%) | 1.000 |
| Yes | 1 (50.0) | 1 (50.0) | 1 (50.0) | 1 (50.0) | 0 (0) | 2 (100) | |||
| No | 68 (72.3) | 26 (27.7) | 27 (28.7) | 67 (71.3) | 9 (9.6) | 85 (90.4) | |||
| Stage (n = 89) | n (%) | n (%) | 0.433 | n (%) | n (%) | 0.024 | n (%) | n (%) | 0.006 |
| ≤II | 45 (70.3) | 19 (29.7) | 15 (23.4) | 49 (76.6) | 2 (3.1) | 62 (96.9) | |||
| III–IV | 20 (80.0) | 5 (20.0) | 12 (48.0) | 13 (52.0) | 6 (24.0) | 19 (76.0) | |||
| Hb (g/dL) | 12.2 ± 1.9 | 13.01 ± 1.6 | 0.052 | 11.5 ± 1.9 | 12.8 ± 1.7 | 0.001 | 10.6 ± 2.1 | 12.6 ± 1.7 | 0.002 |
| HCT (%) | 36.7 ± 5.4 | 39.0 ± 4.3 | 0.055 | 34.7 ± 5.4 | 38.4 ± 4.7 | 0.001 | 32.2 ± 6.0 | 37.9 ± 4.8 | 0.002 |
| Albumin (g/dL) | 3.7 ± 0.9 | 4.1 ± 0.5 | 0.058 | 3.7 ± 0.8 | 3.9 ± 0.8 | 0.348 | 3.4 ± 1.1 | 3.9 ± 0.8 | 0.066 |
| TLC (K/μL) | 1.8 ± 0.8 | 2.4 ± 3.2 | 0.142 | 1.7 ± 0.9 | 2.1 ± 2.0 | 0.260 | 2.3 ± 1.2 | 2.0 ± 1.9 | 0.608 |
| NLR | 3.26 ± 2.35 | 3.59 ± 6.7 | 0.724 | 3.78 ± 4.59 | 3.17 ± 4.59 | 0.516 | 4.08 ± 3.13 | 3.28 ± 4.18 | 0.575 |
| PLR | 155.52 ± 71.16 | 209.15 ± 47.64 | 0.105 | 178.95 ± 77.43 | 167.34 ± 166.23 | 0.725 | 130.77 ± 71.95 | 174.95 ± 150.72 | 0.389 |
| PG-SGA (n = 92) | 9.6 ± 6.9 | 6.6 ± 4.9 | 0.041 | 10.4 ± 7.1 | 8.0 ± 6.1 | 0.106 | 12.9 ± 6.7 | 8.3 ± 6.3 | 0.045 |
| PG-SGA category (n = 92) | n (%) | n (%) | 0.015 | n (%) | n (%) | 0.05 | n (%) | n (%) | 0.065 |
| A + B | 37 (62.7) | 22 (37.3) | 14 (23.7) | 45 (76.3) | 3 (5.1) | 56 (94.9) | |||
| C | 29 (87.9) | 4 (12.1) | 14 (42.4) | 19 (57.6) | 6 (18.2) | 27 (81.8) | |||
| GNRI (n = 95) | 102.7 ± 19.0 | 106.3 ± 14.8 | 0.382 | 104.9 ± 15.8 | 103.2 ± 18.8 | 0.674 | 98.3 ± 20.1 | 104.3 ± 17.7 | 0.340 |
| GNRI category (n = 95) | n (%) | n (%) | 0.023 | n (%) | n (%) | 0.425 | n (%) | n (%) | 0.105 |
| high (≥92) | 49 (66.2) | 25 (33.8) | 21 (28.4) | 53 (71.6) | 5 (6.8) | 69 (93.2) | |||
| low (<92) | 19 (90.5) | 2 (9.5) | 7 (33.3) | 14 (66.7) | 4 (19.0) | 17 (81.0) | |||
| PNI | 38.0 ± 7.4 | 40.9 ± 5.3 | 0.069 | 37.2 ± 8.0 | 39.5 ± 6.5 | 0.149 | 33.7 ± 10.2 | 39.3 ± 6.4 | 0.141 |
| BMI (kg/m2) | 26.1 ± 3.9 | 25.2 ± 3.8 | 0.357 | 26.5 ± 3.7 | 25.5 ± 3.9 | 0.276 | 25.7 ± 4.2 | 25.8 ± 3.9 | 0.941 |
| WC (cm) | 94.7 ± 11.4 | 96.7 ± 11.5 | 0.349 | 97.4 ± 11.3 | 92.5 ± 11.6 | 0.079 | 102.7 ± 14.0 | 93.2 ± 11.3 | 0.049 |
| HC (cm) | 98.6 ± 9.4 | 96.7 ± 11.5 | 0.423 | 99.9 ± 9.7 | 97.3 ± 10.2 | 0.290 | 96.4 ± 13.4 | 98.1 ± 10.0 | 0.712 |
| TSF (mm) | 11.1 ± 12.5 | 8.5 ± 9.6 | 0.342 | 13.0 ± 13.5 | 9.2 ± 10.8 | 0.160 | 12.3 ± 11.4 | 10.1 ± 11.8 | 0.617 |
| Weight Loss (%) | 6.5 ± 5.4 | 4.5 ± 7.0 | 0.153 | 6.9 ± 5.1 | 5.4 ± 6.2 | 0.282 | 8.8 ± 3.7 | 5.6 ± 6.0 | 0.128 |
| Handgrip strength (kg) | 26.3 ± 10.8 | 28.9 ± 10.2 | 0.284 | 25.6 ± 12.2 | 27.6 ± 10.0 | 0.418 | 21.4 ± 15.6 | 27.5 ± 10.1 | 0.144 |
| Handgrip strength category (n = 89) | n (%) | n (%) | 0.037 | n (%) | n (%) | 0.097 | n (%) | n (%) | 0.082 |
| Low | 22 (91.7) | 2 (8.3) | 10(41.7) | 14 (58.3) | 4 (16.7) | 20 (83.3) | |||
| Normal | 40 (61.5) | 25 (38.4) | 16(24.6) | 49 (75.4) | 3 (4.6) | 62 (95.4) | |||
| Gait speed (m/s) | 0.91 ± 0.34 | 1.10 ± 0.23 | 0.004 | 0.92 ± 0.36 | 0.99 ± 0.30 | 0.329 | 1.02 ± 0.48 | 0.97 ± 0.31 | 0.698 |
| Gait speed category (n = 87) | n (%) | n (%) | 0.027 | n (%) | n (%) | 0.088 | n (%) | n (%) | 0.628 |
| Low | 19 (90.5) | 2 (9.5) | 9 (42.9) | 12 (57.1) | 2 (9.5) | 19 (90.5) | |||
| Normal | 42 (63.6) | 24 (36.4) | 16 (24.2) | 50 (75.8) | 4 (6.1) | 62 (93.9) | |||
| Parameter | LOS | p-Value |
|---|---|---|
| Age (years) | 0.061 | 0.557 |
| Sex | 0.101 | |
| Male | 19.3 ±10.8 | |
| Female | 16.3 ± 7.2 | |
| Type of cancer (n = 89) | 0.254 | |
| Adenocarcinoma | 16.7 ± 8.8 | |
| NET | 17.8 ± 8.7 | |
| Mixed adenoneuroendocrine carcinoma | 17.9 ± 9.2 | |
| Surgical procedure | 0.717 | |
| Pancreaticoduodenectomy | 16.4 ± 7.4 | |
| Distal Pancreatectomy | 13.0 ± 0.0 | |
| Wedge resection | 21.7 ±13.6 | |
| Non specific | 17.7 ± 9.3 | |
| Neoadjuvant Chemotherapy | 0.688 | |
| Yes | 18.3 ± 9.5 | |
| No | 17.4 ± 8.8 | |
| Neoadjuvant Radiotherapy | 0.023 | |
| Yes | 31.5 ±3.5 | |
| No | 17.2 ± 8.7 | |
| Stage (n = 89) | 0.695 | |
| ≤II | 17.8 ± 8.3 | |
| III–IV | 17.0 ± 10.6 | |
| Hb (g/dL) | −0.142 | 0.169 |
| HCT (%) | −0.175 | 0.089 |
| Albumin (g/dL) | −0.031 | 0.767 |
| TLC (K/μL) | −0.070 | 0.502 |
| NLR | −0.066 | 0.529 |
| PLR | −0.004 | 0.970 |
| PG-SGA (n = 92) | 0.039 | 0.709 |
| PG-SGA category (n = 92) | 0.580 | |
| A + B | 18.0 ± 9.2 | |
| C | 16.9 ± 8.8 | |
| GNRI (n = 95) | 0.019 | 0.858 |
| GNRI category (n = 95) | 0.979 | |
| high (≥92) | 17.5 ± 8.8 | |
| low (<92) | 17.6 ± 9.2 | |
| PNI | −0.065 | 0.532 |
| BMI (kg/m2) | 0.003 | 0.975 |
| WC (cm) | 0.132 | 0.229 |
| HC (cm) | 0.175 | 0.111 |
| TSF (mm) | 0.069 | 0.514 |
| Weight Loss (%) | −0.157 | 0.270 |
| Handgrip strength (kg) | −0.210 | 0.049 |
| Handgrip strength category (n = 89) | 0.013 | |
| Low | 21.8 ± 10.7 | |
| Normal | 16.5 ± 7.9 | |
| Gait speed (m/s) | −0.223 | 0.038 |
| Gait speed category (n = 87) | 0.151 | |
| Low | 20.2 ± 10.1 | |
| Normal | 16.9 ± 8.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lidoriki, I.; Frountzas, M.; Karanikki, E.; Mylonakis, A.; Kozadinos, A.; Tsikrikou, I.; Kyriakidou, M.; Karydakis, L.; Stefanoudakis, D.; Lampou, M.; et al. Exploring the Link Between Nutritional and Functional Status and Short-Term Postoperative Outcomes in Patients Undergoing Pancreatic Cancer Surgery. Life 2025, 15, 803. https://doi.org/10.3390/life15050803
Lidoriki I, Frountzas M, Karanikki E, Mylonakis A, Kozadinos A, Tsikrikou I, Kyriakidou M, Karydakis L, Stefanoudakis D, Lampou M, et al. Exploring the Link Between Nutritional and Functional Status and Short-Term Postoperative Outcomes in Patients Undergoing Pancreatic Cancer Surgery. Life. 2025; 15(5):803. https://doi.org/10.3390/life15050803
Chicago/Turabian StyleLidoriki, Irene, Maximos Frountzas, Eva Karanikki, Adam Mylonakis, Alexandros Kozadinos, Iliana Tsikrikou, Maria Kyriakidou, Lysandros Karydakis, Dimitrios Stefanoudakis, Maria Lampou, and et al. 2025. "Exploring the Link Between Nutritional and Functional Status and Short-Term Postoperative Outcomes in Patients Undergoing Pancreatic Cancer Surgery" Life 15, no. 5: 803. https://doi.org/10.3390/life15050803
APA StyleLidoriki, I., Frountzas, M., Karanikki, E., Mylonakis, A., Kozadinos, A., Tsikrikou, I., Kyriakidou, M., Karydakis, L., Stefanoudakis, D., Lampou, M., Vailas, M., Felekouras, E., Toutouzas, K. G., & Schizas, D. (2025). Exploring the Link Between Nutritional and Functional Status and Short-Term Postoperative Outcomes in Patients Undergoing Pancreatic Cancer Surgery. Life, 15(5), 803. https://doi.org/10.3390/life15050803









