The Effect of Pupil Size on Cone Contrast Sensitivity
Abstract
1. Introduction
- Expanding the evaluable contrast range;
- Substituting letter stimuli with Landolt C stimuli;
- Implementing a four-direction keypad for responses;
- Introducing an “adaptive screening mode” for efficiency.
2. Materials and Methods
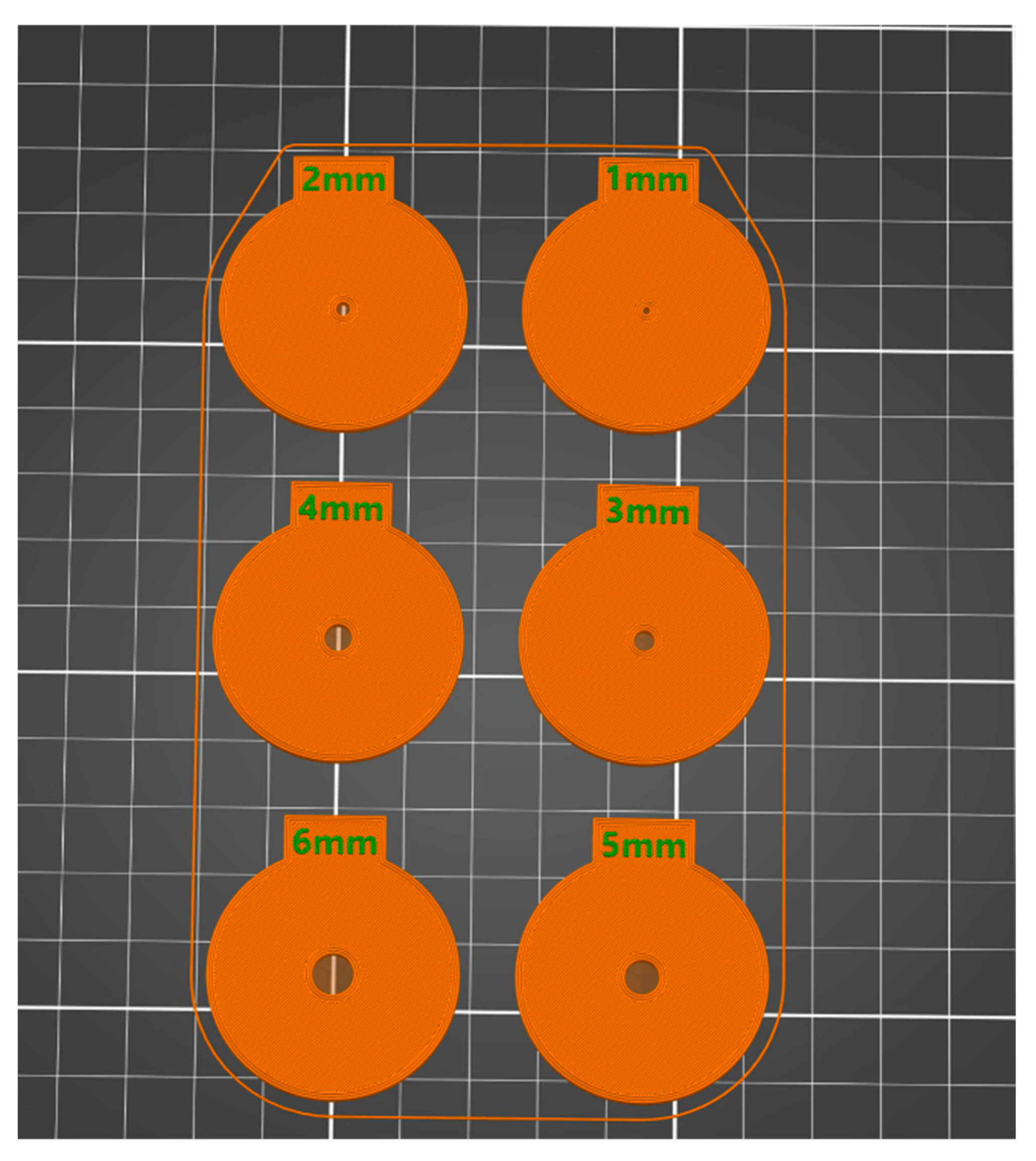
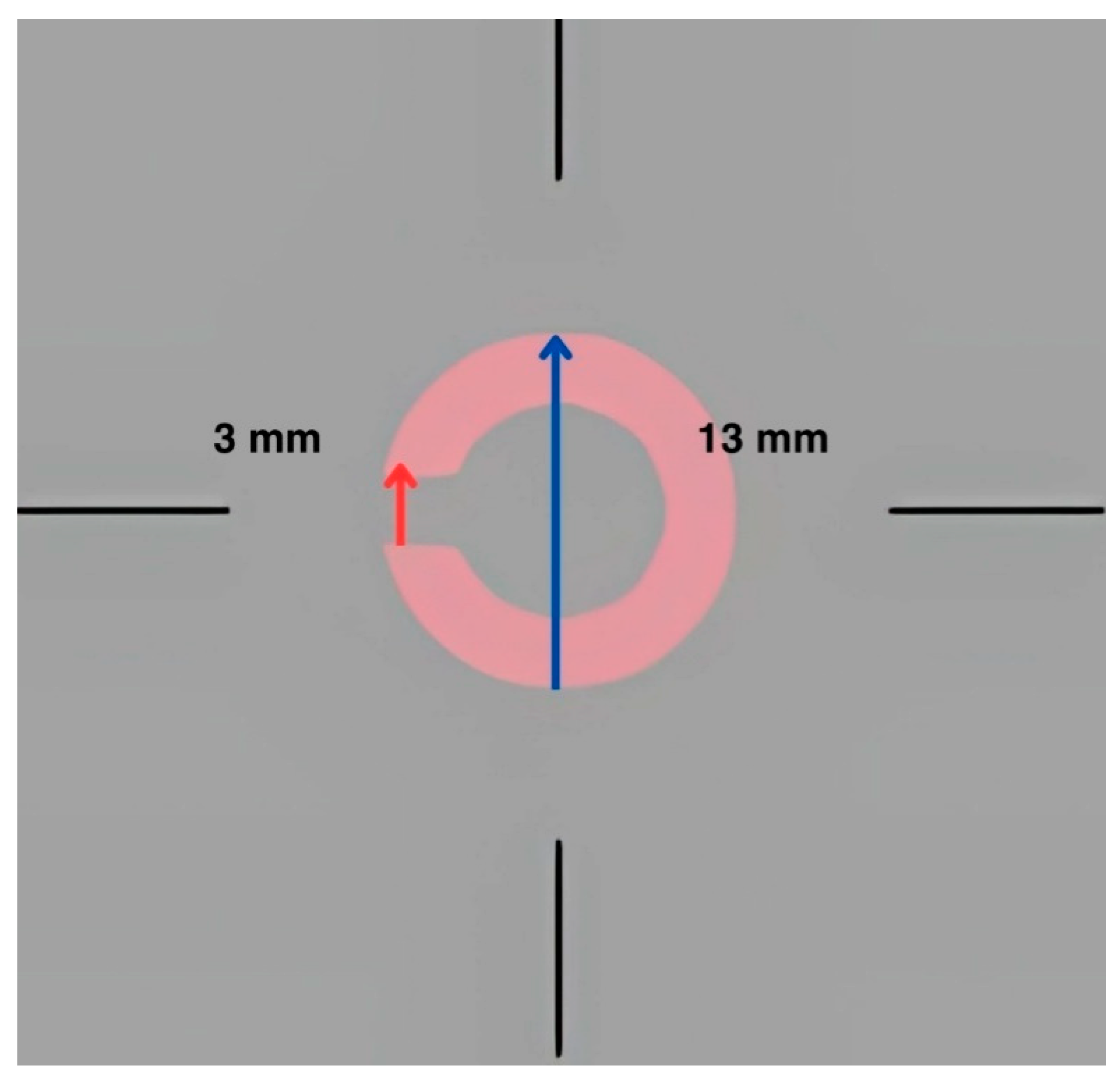
2.1. Instrumentation
2.2. Procedure
2.3. Statistical Analysis
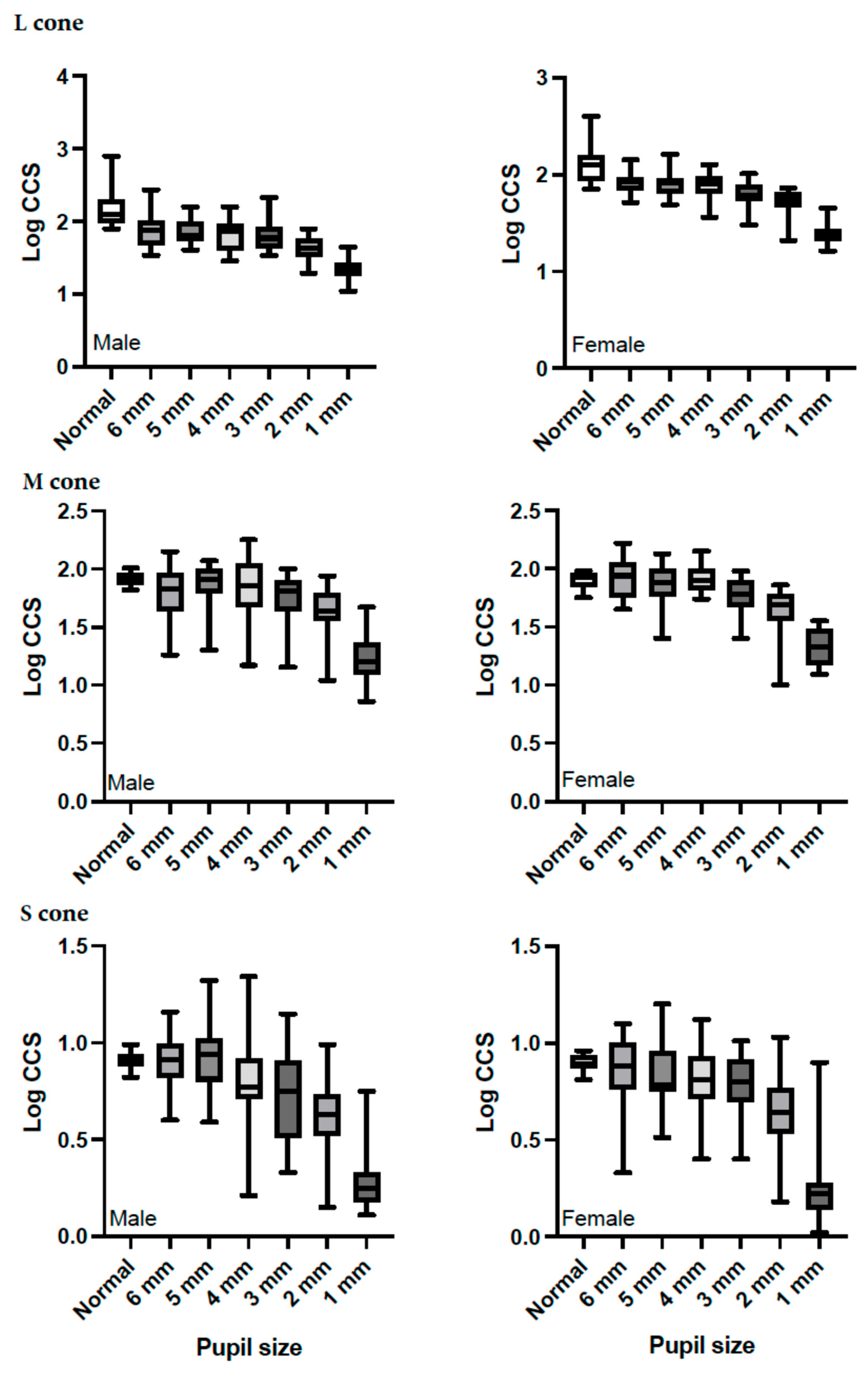
3. Results
4. Discussion
5. Strengths, Limitations, and Recommendations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Birch, J. Worldwide prevalence of red-green color deficiency. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 2012, 29, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Birch, J. Identification of red-green colour deficiency: Sensitivity of the Ishihara and American Optical Company (hard, Rand and Rittler) pseudo-isochromatic plates to identify slight anomalous trichromatism. Ophthalmic Physiol. Opt. 2010, 30, 667–671. [Google Scholar] [CrossRef]
- Vemala, R.; Sivaprasad, S.; Barbur, J.L. Detection of early loss of color vision in age-related macular degeneration—With emphasis on drusen and reticular pseudodrusen. Investig. Ophthalmol. Vis. Sci. 2017, 58, BIO247–BIO254. [Google Scholar] [CrossRef] [PubMed]
- Papaconstantinou, D.; Georgalas, I.; Kalantzis, G.; Karmiris, E.; Koutsandrea, C.; Diagourtas, A.; Ladas, I.; Georgopoulos, G. Acquired color vision and visual field defects in patients with ocular hypertension and early glaucoma. Clin. Ophthalmol. 2009, 3, 251–257. [Google Scholar]
- Melamud, A.; Hagstrom, S.; Traboulsi, E. Color vision testing. Ophthalmic Genet. 2004, 25, 159–187. [Google Scholar] [CrossRef] [PubMed]
- Walsh, D.V.; Robinson, J.; Jurek, G.M.; Capó-Aponte, J.E.; Riggs, D.W.; Temme, L.A. A performance comparison of color vision tests for military screening. Aerosp. Med. Hum. Perform. 2016, 87, 382–387. [Google Scholar] [CrossRef]
- Rabin, J. Cone-specific measures of human color vision. Investig. Ophthalmol. Vis. Sci. 1996, 37, 2771–2774. [Google Scholar]
- Rabin, J.; Gooch, J.; Ivan, D. Rapid quantification of color vision: The cone contrast test. Invest. Ophthalmol. Vis. Sci. 2011, 52, 816–820. [Google Scholar] [CrossRef]
- Rabin, J. Quantification of color vision with cone contrast sensitivity. Vis. Neurosci. 2004, 21, 483–485. [Google Scholar] [CrossRef]
- Rabin, J.C.; Kryder, A.C.; Lam, D. Diagnosis of normal and abnormal color vision with cone-specific VEPs. Transl. Vis. Sci. Technol. 2016, 5, 8. [Google Scholar] [CrossRef]
- Rabin, J.; Adams, A.J. Visual acuity and contrast sensitivity of the S cone pathway: Preliminary measures with letter charts. Optom. Vis. Sci. 1990, 67, 799–802. [Google Scholar] [CrossRef] [PubMed]
- Trieschmann, M.; van Kuijk, F.J.; Alexander, R.; Hermans, P.; Luthert, P.; Bird, A.C.; Pauleikhoff, D. Macular pigment in the human retina: Histological evaluation of localization and distribution. Eye 2008, 22, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Gaska, J.; Winterbottom, M.; van Atta, A. Operational Based Vision Assessment Cone Contrast Test: Description and Operation; Report No. FRL-SA-WP-SR-2016-0007; USAF School of Aerospace Medicine, Aeromedical Research Department: Wright-Patterson AFB, OH, USA, 2016. [Google Scholar]
- Cisarik, P.M.; Kampwerth, J.E. Cone-isolation contrast sensitivity–do pupil and stimulus sizes matter? Clin. Exp. Optom. 2024, 107, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Coletta, N.J.; Sharma, V. Effects of luminance and spatial noise on interferometric contrast sensitivity. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 1995, 12, 2244–2251. [Google Scholar] [CrossRef]
- Hastings, G.D.; Marsack, J.D.; Thibos, L.N.; Applegate, R.A. Combining optical and neural components in physiological visual image quality metrics as functions of luminance and age. J. Vis. 2020, 20, 20. [Google Scholar] [CrossRef]
- Pelli, D.G.; Bex, P. Measuring contrast sensitivity. Vis. Res. 2013, 90, 10–14. [Google Scholar] [CrossRef]
- Owsley, C. Contrast sensitivity. Ophthalmol. Clin. N. Am. 2003, 16, 171–177. [Google Scholar] [CrossRef]
- Nio, Y.K.; Jansonius, N.M.; Fidler, V.; Geraghty, E.; Norrby, S.; Kooijman, A.C. Age-related changes of defocus-specific contrast sensitivity in healthy subjects. Ophthalmic Physiol. Opt. 2000, 20, 323–334. [Google Scholar] [CrossRef]
- Wachler, B.S. Effect of pupil size on visual function under monocular and binocular conditions in LASIK and non-LASIK patients. J. Cataract. Refract. Surg. 2003, 29, 275–278. [Google Scholar] [CrossRef]
- Alfonso, J.F.; Fernández-Vega, L.; Baamonde, B.M.; Montés-Micó, R. Correlation of pupil size with visual acuity and contrast sensitivity after implantation of an apodized diffractive intraocular lens. J. Cataract. Refract. Surg. 2007, 33, 430–438. [Google Scholar] [CrossRef]
- Gaska, J.; Winterbottom, M.; Hadley, S.; O’Keefe, E.; Shoda, E.; Van Atta, A. Evaluation of Konan Medical CCT HD [White Paper—Internet]; Air Force Research Laboratory: Wright-Patterson AFB, OH, USA, 2021. [Google Scholar]
- Almustanyir, A. Assessment of Current and Next Generation of Colour Vision Tests for Occupational Use. Ph.D. Thesis, University of Waterloo, Waterloo, ON, Canada, 2018. [Google Scholar]
- Kontsevich, L.L.; Tyler, C.W. Bayesian adaptive estimation of psychometric slope and threshold. Vision. Res. 1999, 39, 2729–2737. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, H.; Charman, W.N.; Gilmartin, B.; Jayakumar, J.; Dhanasekaran, S.; Sundaram, V.; Kumaran, R.; Rajendran, K.; Subramanian, M.; Krishnan, A.; et al. The effect of pupil size on visual acuity and contrast sensitivity with multifocal contact lenses. Ophthalmic Physiol. Opt. 2004, 24, 454–463. [Google Scholar]
- Jacobs, R.J.; Bailey, I.L.; Bullimore, M.A. Artificial pupils and Maxwellian view. Appl. Opt. 1992, 31, 3668–3677. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gawne, T.J.; Banks, M.S. The role of chromatic aberration in vision. Annu. Rev. Vis. Sci. 2024, 10, 199–212. [Google Scholar] [CrossRef]
- Moldvar, E. Photopic, Mesopic, Scotopic—Concepts [Internet]; Lighting Analysts, Inc.: Littleton, CO, USA, 2021; Available online: https://docs.agi32.com/AGi32/Content/references/Photopic_Mesopic_Scotopic_-_Concepts.htm (accessed on 5 April 2023).
- Lin, J.B.; Tsubota, K.; Apte, R.S. A glimpse at the aging eye. NPJ Aging Mech. Dis. 2016, 2, 16003. [Google Scholar] [CrossRef]
- National Research Council (US) Committee on Disability Determination for Individuals with Visual Impairments. Visual Impairments: Determining Eligibility for Social Security benefits: 2, tests of Visual Functions [Internet]; Lennie, P., Van Hemel, S.B., Eds.; National Academies Press: Washington, DC, USA, 2002. Available online: https://www.ncbi.nlm.nih.gov/books/NBK207559/ (accessed on 4 November 2022).
| Gender | Pupil Size (mm) | Mean ± SD | 95% Confidence Interval | Mean Difference from Normal | p-Value |
|---|---|---|---|---|---|
| Males | Normal | 2.183 ± 0.044 | 2.095–2.270 | — | — |
| 1 mm | 1.341 ± 0.025 | 1.292–1.390 | 0.842 | <0.001 | |
| 2 mm | 1.641 ± 0.028 | 1.585–1.697 | 0.542 | <0.001 | |
| 3 mm | 1.789 ± 0.032 | 1.724–1.853 | 0.394 | <0.001 | |
| 4 mm | 1.812 ± 0.033 | 1.746–1.878 | 0.371 | <0.001 | |
| 5 mm | 1.861 ± 0.028 | 1.805–1.917 | 0.322 | <0.001 | |
| 6 mm | 1.872 ± 0.035 | 1.801–1.943 | 0.311 | <0.001 | |
| Females | Normal | 2.092 ± 0.051 | 1.989–2.195 | — | — |
| 1 mm | 1.389 ± 0.029 | 1.331–1.447 | 0.703 | <0.001 | |
| 2 mm | 1.726 ± 0.033 * | 1.660–1.792 | 0.366 | <0.001 | |
| 3 mm | 1.798 ± 0.037 | 1.722–1.873 | 0.294 | <0.001 | |
| 4 mm | 1.880 ± 0.039 | 1.803–1.958 | 0.211 | 0.026 | |
| 5 mm | 1.905 ± 0.033 | 1.839–1.971 | 0.187 | 0.050 | |
| 6 mm | 1.910 ± 0.042 | 1.827–1.994 | 0.181 | 0.089 |
| Gender | Pupil Size (mm) | Mean ± SD | 95% Confidence Interval | Mean Difference from Normal | p-Value |
|---|---|---|---|---|---|
| Males | Normal | 1.917 ± 0.012 | 1.893–1.942 | — | — |
| 1 mm | 1.226 ± 0.033 | 1.160–1.292 | 0.691 | <0.001 | |
| 2 mm | 1.637 ± 0.037 | 1.563–1.710 | 0.280 | <0.001 | |
| 3 mm | 1.768 ± 0.031 | 1.705–1.831 | 0.149 | <0.001 | |
| 4 mm | 1.832 ± 0.037 | 1.756–1.907 | 0.086 | 0.037 | |
| 5 mm | 1.853 ± 0.037 | 1.779–1.928 | 0.064 | 0.120 | |
| 6 mm | 1.819 ± 0.036 | 1.746–1.891 | 0.099 | 0.013 | |
| Females | Normal | 1.899 ± 0.014 | 1.870–1.928 | — | — |
| 1 mm | 1.332 ± 0.039 * | 1.255–1.410 | 0.566 | <0.001 | |
| 2 mm | 1.638 ± 0.043 | 1.551–1.724 | 0.261 | <0.001 | |
| 3 mm | 1.779 ± 0.037 | 1.704–1.853 | 0.120 | 0.007 | |
| 4 mm | 1.919 ± 0.044 | 1.831–2.007 | −0.020 | 0.664 | |
| 5 mm | 1.847 ± 0.043 | 1.759–1.934 | 0.052 | 0.279 | |
| 6 mm | 1.930 ± 0.043 | 1.845–2.016 | −0.032 | 0.481 |
| Gender | Pupil Size (mm) | Mean ± SD | 95% Confidence Interval | Mean Difference from Normal | p-Value |
|---|---|---|---|---|---|
| Males | Normal | 0.904 ± 0.029 | 0.845–0.963 | — | — |
| 1 mm | 0.907 ± 0.008 | 0.892–0.922 | −0.003 | 1.0 | |
| 2 mm | 0.280 ± 0.03 | 0.220–0.341 | 0.623 | <0.001 | |
| 3 mm | 0.612 ± 0.038 | 0.535–0.689 | 0.292 | <0.001 | |
| 4 mm | 0.713 ± 0.039 | 0.635–0.792 | 0.190 | <0.001 | |
| 5 mm | 0.810 ± 0.036 | 0.737–0.883 | 0.094 | 0.29 | |
| 6 mm | 0.910 ± 0.031 | 0.853–0.979 | −0.012 | 1.0 | |
| Females | Normal | 0.851 ± 0.034 | 0.782–0.921 | — | — |
| 1 mm | 0.899 ± 0.009 | 0.881–0.916 | −0.047 | 1.0 | |
| 2 mm | 0.242 ± 0.035 | 0.171–0.314 | 0.609 | <0.001 | |
| 3 mm | 0.640 ± 0.045 | 0.549–0.731 | 0.211 | <0.001 | |
| 4 mm | 0.771 ± 0.046 | 0.679–0.864 | 0.080 | 1.0 | |
| 5 mm | 0.798 ± 0.043 | 0.712–0.883 | 0.054 | 1.0 | |
| 6 mm | 0.841 ± 0.037 | 0.768–0.915 | 0.010 | 1.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almustanyir, A.; Almutairi, M.S.; Aldrwish, A.; Hasrod, N.; Alqhtani, B.A.; Alqahtani, T.; Alanazi, M.; Alghamdi, M.; Almutleb, E.; Alabdulkader, B.; et al. The Effect of Pupil Size on Cone Contrast Sensitivity. Life 2025, 15, 801. https://doi.org/10.3390/life15050801
Almustanyir A, Almutairi MS, Aldrwish A, Hasrod N, Alqhtani BA, Alqahtani T, Alanazi M, Alghamdi M, Almutleb E, Alabdulkader B, et al. The Effect of Pupil Size on Cone Contrast Sensitivity. Life. 2025; 15(5):801. https://doi.org/10.3390/life15050801
Chicago/Turabian StyleAlmustanyir, Ali, Meznah S. Almutairi, Amal Aldrwish, Nabeela Hasrod, Bader A. Alqhtani, Tahani Alqahtani, Muteb Alanazi, Mansour Alghamdi, Essam Almutleb, Balsam Alabdulkader, and et al. 2025. "The Effect of Pupil Size on Cone Contrast Sensitivity" Life 15, no. 5: 801. https://doi.org/10.3390/life15050801
APA StyleAlmustanyir, A., Almutairi, M. S., Aldrwish, A., Hasrod, N., Alqhtani, B. A., Alqahtani, T., Alanazi, M., Alghamdi, M., Almutleb, E., Alabdulkader, B., Fakhouri, F., & Alhassan, M. (2025). The Effect of Pupil Size on Cone Contrast Sensitivity. Life, 15(5), 801. https://doi.org/10.3390/life15050801





