Comparative Effects of Red and Blue LED Light on Melatonin Levels During Three-Hour Exposure in Healthy Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Setup Configuration
2.2. Theoretical Background
2.3. Sample Description
2.4. Saliva Sample Collection
2.5. Experimental Protocol
2.6. Determination of Melatonin in Saliva Samples
2.7. Data Processing and Statistical Analysis
3. Results
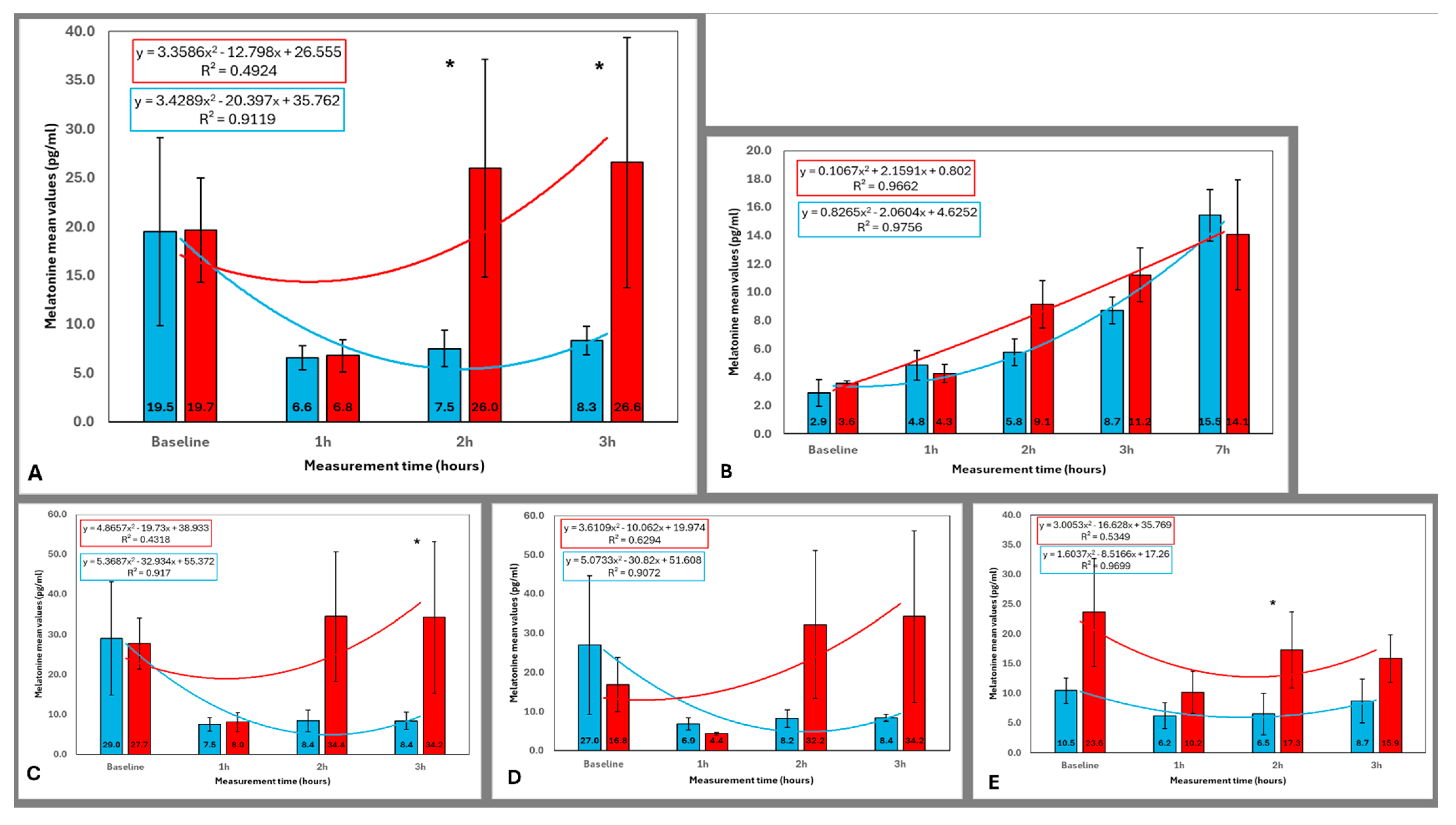
3.1. Melatonin Concentration Across All Participants
3.2. Melatonin Concentration in Older and Younger Participants
3.3. Melatonin Concentration in Female and Male Participants
3.4. Comparison of Melatonin Levels Across Time Points for Each Lighting Condition
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SCN | Suprachiasmatic nucleus |
| RHT | Retinohypothalamic tract |
| ipRGCs | Intrinsically photosensitive retinal ganglion cells |
| NIF | Non-image-forming |
| PLR | Pupillary light reflex |
| CIE | Commission International de l’Eclairage |
| mEDI | Melanopic equivalent daylight (D65) illuminance |
| EML | Equivalent melanopic lux |
| CLA | Circadian Light |
| CS | Circadian stimulus |
| SPD | Spectral power distribution |
| FWHM | Full width at half maximum |
| CSd | Circadian light dose |
| SE | Standard error |
References
- Wahl, S.; Engelhardt, M.; Schaupp, P.; Lappe, C.; Ivanov, I.V. The Inner Clock—Blue Light Sets the Human Rhythm. J. Biophotonics 2019, 12, e201900102. [Google Scholar] [CrossRef] [PubMed]
- Pundir, M.; Papagerakis, S.; De Rosa, M.C.; Chronis, N.; Kurabayashi, K.; Abdulmawjood, S.; Prince, M.E.P.; Lobanova, L.; Chen, X.; Papagerakis, P. Emerging Biotechnologies for Evaluating Disruption of Stress, Sleep, and Circadian Rhythm Mechanism Using Aptamer-Based Detection of Salivary Biomarkers. Biotechnol. Adv. 2022, 59, 107961. [Google Scholar] [CrossRef]
- Figueiro, M.G.; Nagare, R.; Price, L.L.A. Non-Visual Effects of Light: How to Use Light to Promote Circadian Entrainment and Elicit Alertness. Light. Res. Technol. 2018, 50, 38–62. [Google Scholar] [CrossRef] [PubMed]
- Berson, D.M.; Dunn, F.A.; Takao, M. Phototransduction by Retinal Ganglion Cells That Set the Circadian Clock. Science (1979) 2002, 295, 1070–1073. [Google Scholar] [CrossRef]
- Foster, R.G. Fundamentals of Circadian Entrainment by Light. Light. Res. Technol. 2021, 53, 377–393. [Google Scholar] [CrossRef]
- Provencio, I.; Rodriguez, I.R.; Jiang, G.; Hayes, W.P.; Moreira, E.F.; Rollag, M.D. A Novel Human Opsin in the Inner Retina. J. Neurosci. 2000, 20, 600–605. [Google Scholar] [CrossRef]
- Thapan, K.; Arendt, J.; Skene, D.J. An Action Spectrum for Melatonin Suppression: Evidence for a Novel Non-Rod, Non-Cone Photoreceptor System in Humans. J. Physiol. 2001, 535, 261–267. [Google Scholar] [CrossRef]
- Brainard, G.C.; Hanifin, J.P.; Greeson, J.M.; Byrne, B.; Glickman, G.; Gerner, E.; Rollag, M.D. Action Spectrum for Melatonin Regulation in Humans: Evidence for a Novel Circadian Photoreceptor. J. Neurosci. 2001, 21, 6405–6412. [Google Scholar] [CrossRef]
- Lucas, R.J.; Peirson, S.N.; Berson, D.M.; Brown, T.M.; Cooper, H.M.; Czeisler, C.A.; Figueiro, M.G.; Gamlin, P.D.; Lockley, S.W.; O’Hagan, J.B.; et al. Measuring and Using Light in the Melanopsin Age. Trends Neurosci. 2014, 37, 1–9. [Google Scholar] [CrossRef]
- Brown, T.M.; Thapan, K.; Arendt, J.; Revell, V.L.; Skene, D.J. S-Cone Contribution to the Acute Melatonin Suppression Response in Humans. J. Pineal. Res. 2021, 71, e12719. [Google Scholar] [CrossRef]
- Novotny, P.; Plischke, H. Pupillary Light Reflex and Circadian Synchronization in the Elderly. Psych. J. 2017, 6, 292–293. [Google Scholar] [CrossRef] [PubMed]
- Mure, L.S. Intrinsically Photosensitive Retinal Ganglion Cells of the Human Retina. Front. Neurol. 2021, 12, 636330. [Google Scholar] [CrossRef]
- Najjar, R.P.; Prayag, A.S.; Gronfier, C. Melatonin Suppression by Light Involves Different Retinal Photoreceptors in Young and Older Adults. J. Pineal. Res. 2024, 76, e12930. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.M.; Brainard, G.C.; Cajochen, C.; Czeisler, C.A.; Hanifin, J.P.; Lockley, S.W.; Lucas, R.J.; Münch, M.; OHagan, J.B.; Peirson, S.N.; et al. Recommendations for Daytime, Evening, and Nighttime Indoor Light Exposure to Best Support Physiology, Sleep, and Wakefulness in Healthy Adults. PLoS Biol. 2022, 20, e3001571. [Google Scholar] [CrossRef]
- Chellappa, S.L. Individual Differences in Light Sensitivity Affect Sleep and Circadian Rhythms. Sleep 2021, 44, zsaa214. [Google Scholar] [CrossRef] [PubMed]
- Nagare, R.; Plitnick, B.; Figueiro, M.G. Effect of Exposure Duration and Light Spectra on Nighttime Melatonin Suppression in Adolescents and Adults. Light. Res. Technol. 2019, 51, 530–543. [Google Scholar] [CrossRef] [PubMed]
- Eto, T.; Higuchi, S. Review on Age-Related Differences in Non-Visual Effects of Light: Melatonin Suppression, Circadian Phase Shift and Pupillary Light Reflex in Children to Older Adults. J. Physiol. Anthropol. 2023, 42, 11. [Google Scholar] [CrossRef]
- Lee, S.-I.; Kinoshita, S.; Noguchi, A.; Eto, T.; Ohashi, M.; Nishimura, Y.; Maeda, K.; Motomura, Y.; Awata, Y.; Higuchi, S. Melatonin Suppression during a Simulated Night Shift in Medium Intensity Light Is Increased by 10-Minute Breaks in Dim Light and Decreased by 10-Minute Breaks in Bright Light. Chronobiol. Int. 2020, 37, 897–909. [Google Scholar] [CrossRef]
- Swope, C.B.; Rong, S.; Campanella, C.; Vaicekonyte, R.; Phillips, A.J.K.; Cain, S.W.; McGlashan, E.M. Factors Associated with Variability in the Melatonin Suppression Response to Light: A Narrative Review. Chronobiol. Int. 2023, 40, 542–556. [Google Scholar] [CrossRef]
- Ju, J.; He, L.; Li, Z.; Wu, Z.; He, Z.; Liang, R. Biological Effects of Color Lighting for Different Ages. Appl. Mech. Mater. 2012, 236–237, 787–791. [Google Scholar] [CrossRef]
- Reutrakul, S.; Crowley, S.J.; Park, J.C.; Chau, F.Y.; Priyadarshini, M.; Hanlon, E.C.; Danielson, K.K.; Gerber, B.S.; Baynard, T.; Yeh, J.J.; et al. Relationship between Intrinsically Photosensitive Ganglion Cell Function and Circadian Regulation in Diabetic Retinopathy. Sci. Rep. 2020, 10, 1560. [Google Scholar] [CrossRef] [PubMed]
- CIE S 026/E:2018; CIE System for Metrology of Optical Radiation for IpRGC-Influenced Responses to Light. CIE Central Bureau: Vienna, Austria, 2018.
- Position Statement CIE Position Statement on Integrative Lighting Recommending Proper Light at the Proper Time, 3rd ed.; CIE: Vienna, Austria, 2024. [CrossRef]
- Brown, T.M. Melanopic Illuminance Defines the Magnitude of Human Circadian Light Responses under a Wide Range of Conditions. J. Pineal. Res. 2020, 69, e12655. [Google Scholar] [CrossRef] [PubMed]
- IWBI International Well Building Institute. WELL Building Standard v2 Pilot, Q3 2021 Version. Section L03: Circadian Lighting Design; IWBI International Well Building Institute: New York, NY, USA, 2021. [Google Scholar]
- Rea, M.S.; Nagare, R.; Figueiro, M.G. Modeling Circadian Phototransduction: Quantitative Predictions of Psychophysical Data. Front. Neurosci. 2021, 15, 615322. [Google Scholar] [CrossRef] [PubMed]
- Rea, M.S.; Nagare, R.; Figueiro, M.G. Modeling Circadian Phototransduction: Retinal Neurophysiology and Neuroanatomy. Front. Neurosci. 2021, 14, 615305. [Google Scholar] [CrossRef]
- Rea, M.S.; Figueiro, M.G.; Bullough, J.D.; Bierman, A. A Model of Phototransduction by the Human Circadian System. Brain Res. Rev. 2005, 50, 213–228. [Google Scholar] [CrossRef]
- Sánchez-cano, A.; Aporta, J. Optimization of Lighting Projects Including Photopic and Circadian Criteria: A Simplified Action Protocol. Appl. Sci. 2020, 10, 8068. [Google Scholar] [CrossRef]
- Schlangen, L.J.M.; Belgers, S.; Cuijpers, R.H.; Heynderickx, I.E.J. Invited Comment on “The Law of Reciprocity Holds (More or Less) for Circadian-Effective Lighting”, by M Rea, Accepted for Publication in LRT 2022. Light. Res. Technol. 2022, 54, 756–757. [Google Scholar]
- CIE Position Statement on Non-Visual Effects of Light Recommending Proper Light at the Proper Time, 2nd ed.; CIE: Vienna, Austria, 2019; Volume 1.
- Wyszecki, G.; Stiles, W.S. Color Science: Concepts and Methods, Quantitative Data and Formulae; John Wiley & Sons: Hoboken, NJ, USA, 2000; Volume 40, ISBN 0471399183. [Google Scholar]
- Govardovskii, V.I.; Fyhrquist, N.; Reuter, T.O.M.; Kuzmin, D.G.; Donner, K. In Search of the Visual Pigment Template. Vis. Neurosci. 2000, 17, 509–528. [Google Scholar] [CrossRef]
- Smith, V.C.; Pokorny, J. Spectral Sensitivity of the Foveal Cone Photopigments between 400 and 500 Nm. Vis. Res. 1975, 15, 161–171. [Google Scholar] [CrossRef]
- Commission International de l’Eclairage (CIE). Light as a True Visual Quantity: Principles of Measurement; CIE Central Bureau CIE: Vienna, Austria, 1978; Volume 41. [Google Scholar]
- Sanchez-Cano, A.; Orduna-Hospital, E.; Fernández-Espinosa, G.; Aporta, J. Method to Calculate Melanopic Light Reaching the Retina Depending on the Optical Density of an Aging Crystalline Lens. Appl. Sci. 2023, 13, 2569. [Google Scholar] [CrossRef]
- Phillips, A.J.K.; Vidafar, P.; Burns, A.C.; McGlashan, E.M.; Anderson, C.; Rajaratnam, S.M.W.; Lockley, S.W.; Cain, S.W. High Sensitivity and Interindividual Variability in the Response of the Human Circadian System to Evening Light. Proc. Natl. Acad. Sci. USA 2019, 116, 12019–12024. [Google Scholar] [CrossRef] [PubMed]
- Prayag, A.S.; Jost, S.; Avouac, P.; Dumortier, D.; Gronfier, C. Dynamics of Non-Visual Responses in Humans: As Fast as Lightning? Front. Neurosci. 2019, 13, 126. [Google Scholar] [CrossRef]
- Morioka, H.; Ozawa, H.; Kato, T. Physiological Study of Visual and Non-Visual Effects of Light Exposure. Appl. Sci. 2023, 13, 5785. [Google Scholar] [CrossRef]
- Lee, S.; Kakitsuba, N.; Katsuura, T. Do Green-Blocking Glasses Enhance the Nonvisual Effects of White Polychromatic Light? J. Physiol. Anthropol. 2018, 37, 29. [Google Scholar] [CrossRef]
- Milosavljevic, N. How Does Light Regulate Mood and Behavioral State? Clocks Sleep 2019, 1, 319–331. [Google Scholar] [CrossRef] [PubMed]
- van Duijnhoven, J.; Aarts, M.P.J.; van den Heuvel, E.R.; Kort, H.S.M. Exploring the Relationship between Light and Subjective Alertness Using Personal Lighting Conditions. J. Phys. Conf. Ser. 2021, 2042, 012119. [Google Scholar] [CrossRef]
- Fazlali, F.; Lazar, R.; Yahya, F.; Epple, C.; Spitschan, M.; Stefani, O.; Cajochen, C. Lack of Evidence for the Contribution of Cone Photoreceptors to Human Melatonin Suppression and Alerting Response to Light at Night. bioRxiv 2024. [Google Scholar] [CrossRef]
- Aguilar-Carrasco, M.T.; Acosta, I.; Domínguez-Amarillo, S. CircaLight, a New Circadian Light Assessment Tool for Grasshopper Environment: Development and Reliability Testing. J. Build. Eng. 2023, 71, 106527. [Google Scholar] [CrossRef]
- Gubin, D.G.; Borisenkov, M.F.; Kolomeichuk, S.N.; Markov, A.A.; Weinert, D.; Cornelissen, G.; Stefani, O. Evaluating Circadian Light Hygiene: Methodology and Health Implications. Russ. Open Med. J. 2024, 13, e0415. [Google Scholar] [CrossRef]
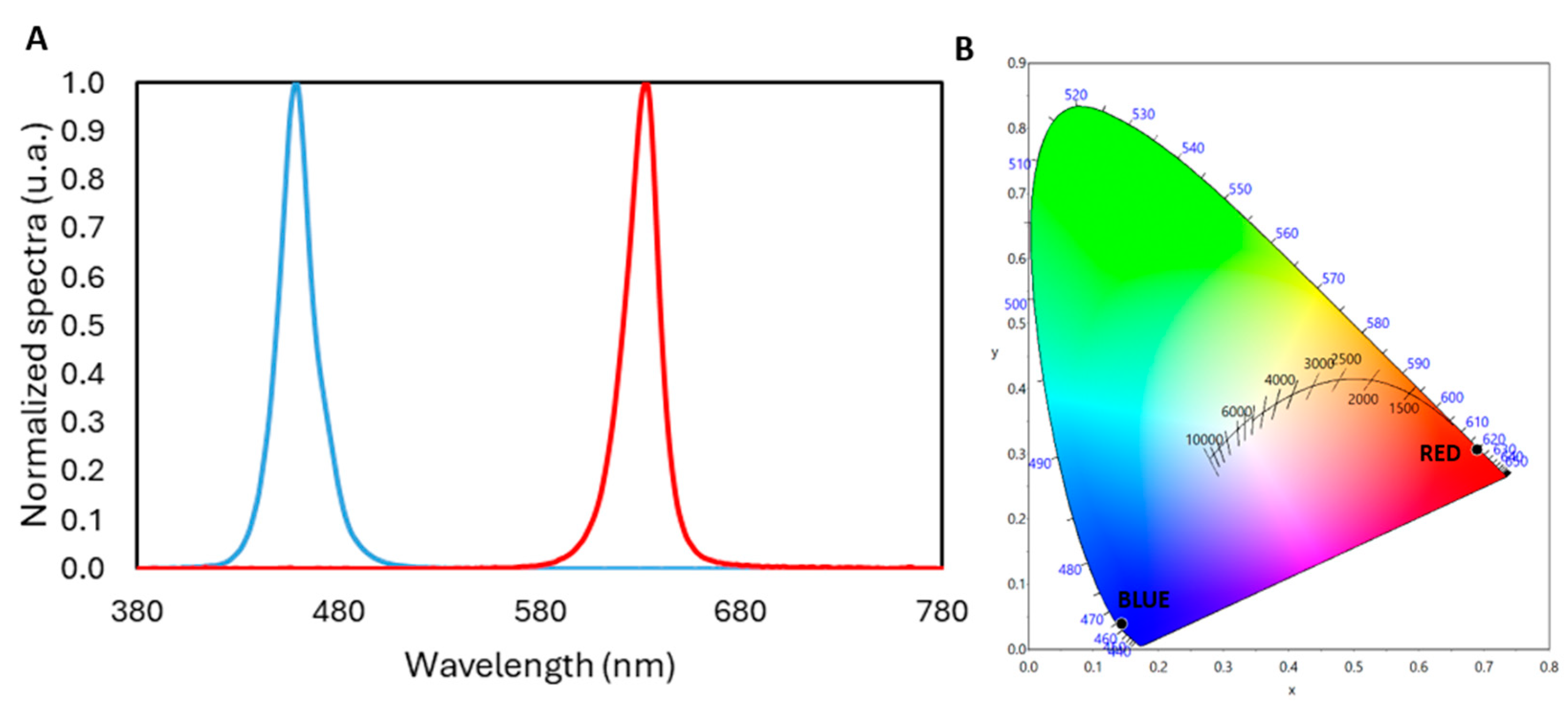

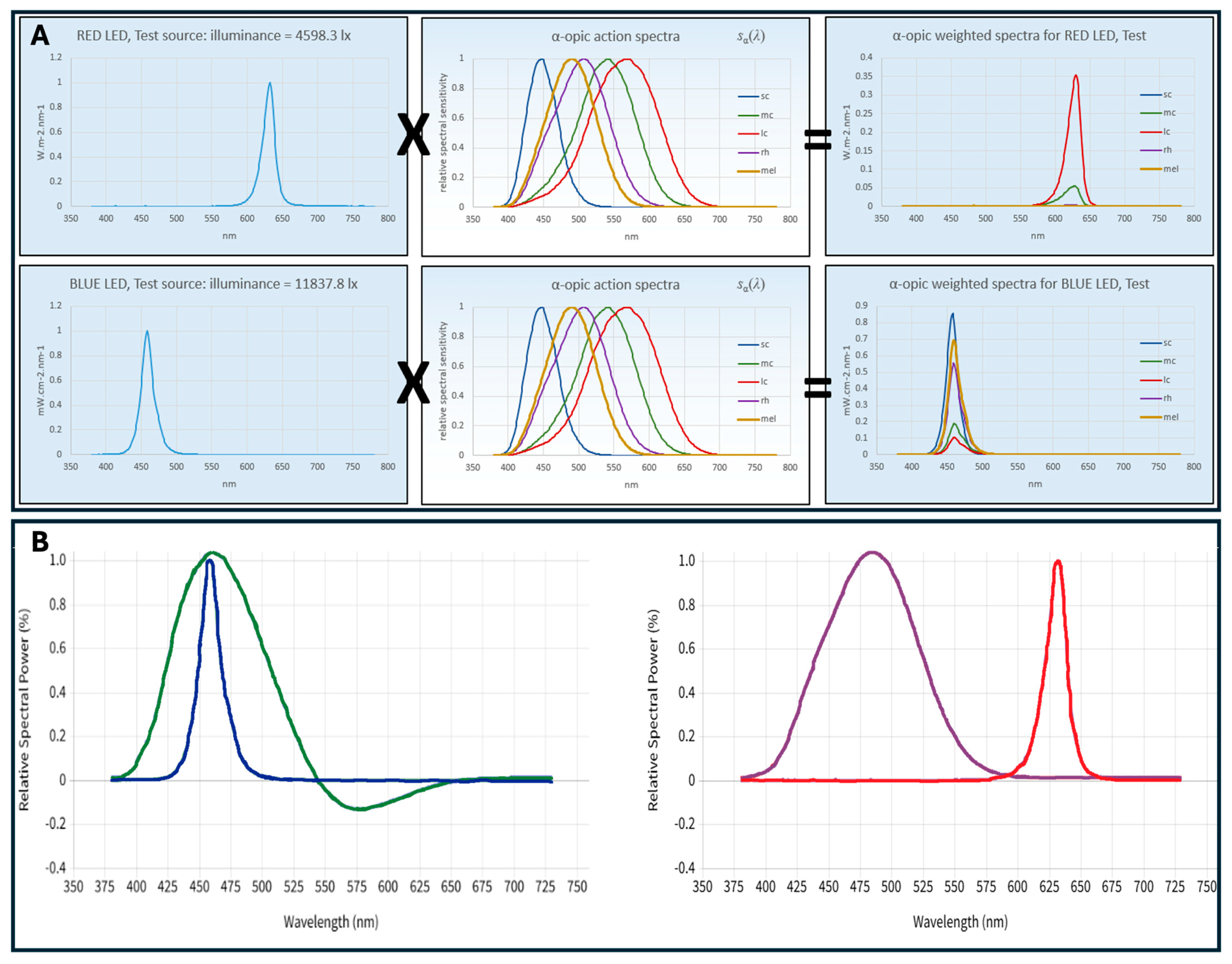
| Blue | Red | |
|---|---|---|
| Irradiance (W·m−2) | 1.628 | 0.407 |
| Chromaticity Coordinates (x, y) | (0.1438, 0.0389) | (0.6898, 0.3068) |
| Illuminance 80 lx | Blue | Red | ||
|---|---|---|---|---|
| CIES026 [22,23,31] | Irradiance (W·m−2) | S-cone | 1.256 | 6.71 × 10−4 |
| M-cone | 0.339 | 3.23 × 10−2 | ||
| L-cone | 0.196 | 1.59 × 10−1 | ||
| Rhodopic | 0.913 | 3.76 × 10−3 | ||
| Melanopic | 1.123 | 1.42 × 10−3 | ||
| α-opic DER for (α-opic daylight (D65) efficacy ratio is 1.000) | S-cone-opic | 19.2131 | 0.0103 | |
| M-cone-opic | 2.9101 | 0.2775 | ||
| L-cone-opic | 1.5070 | 1.2199 | ||
| Rhodopic | 7.8763 | 0.0324 | ||
| Melanopic | 10.5823 | 0.0134 | ||
| Melanopic Equivalent Daylight (D65) Illuminance [lux]; mEDI | 847 | 1 | ||
| WELL [25] | Equivalent Melanopic Lux [m-lux]; EML | 934 | 1 | |
| Melanopic Ratio, R (M/P) | 11.68 | 0.01 | ||
| Rea et al. [26,27] | CS | 0.588 | 0.003 | |
| CLA 2.0 | 1600 | 2 | ||
| Group | Blue Light 1 h vs. | Red Light 1 h vs. | ||||
|---|---|---|---|---|---|---|
| Baseline | 2 h | 3 h | Baseline | 2 h | 3 h | |
| All (n = 12) Figure 4A | 0.182 | 0.583 | 0.386 | 0.041 * | 0.028 * | 0.012 * |
| Younger (n = 8) Figure 4B | 0.063 | 1.000 | 0.735 | 0.025 * | 0.093 | 0.093 |
| Older (n = 4) Figure 4C | 0.068 | 0.273 | 0.109 | 0.465 | 0.068 | 0.068 |
| Female (n = 7) Figure 4D | 0.917 | 0.398 | 0.249 | 0.237 | 0.018 * | 0.028 * |
| Male (n = 5) Figure 4E | 0.225 | 0.686 | 0.715 | 0.138 | 0.500 | 0.225 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanchez-Cano, A.; Luesma-Bartolomé, M.J.; Solanas, E.; Orduna-Hospital, E. Comparative Effects of Red and Blue LED Light on Melatonin Levels During Three-Hour Exposure in Healthy Adults. Life 2025, 15, 715. https://doi.org/10.3390/life15050715
Sanchez-Cano A, Luesma-Bartolomé MJ, Solanas E, Orduna-Hospital E. Comparative Effects of Red and Blue LED Light on Melatonin Levels During Three-Hour Exposure in Healthy Adults. Life. 2025; 15(5):715. https://doi.org/10.3390/life15050715
Chicago/Turabian StyleSanchez-Cano, Ana, María José Luesma-Bartolomé, Estela Solanas, and Elvira Orduna-Hospital. 2025. "Comparative Effects of Red and Blue LED Light on Melatonin Levels During Three-Hour Exposure in Healthy Adults" Life 15, no. 5: 715. https://doi.org/10.3390/life15050715
APA StyleSanchez-Cano, A., Luesma-Bartolomé, M. J., Solanas, E., & Orduna-Hospital, E. (2025). Comparative Effects of Red and Blue LED Light on Melatonin Levels During Three-Hour Exposure in Healthy Adults. Life, 15(5), 715. https://doi.org/10.3390/life15050715







