Proteome-Based Maternal Plasma and Serum Biomarkers for Preeclampsia: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search Strategy
2.2. Eligibility Criteria
2.3. Study Selection Process
2.4. Quality Assessment
2.5. Data Synthesis and Bioinformatics
3. Results
3.1. Quality Assessment, Methodological Trends, and Cohort Characteristics
3.2. PE-Specific Maternal Plasma/Serum Proteomic Profile
3.3. Bioinformatics Analysis
4. Discussion
4.1. First-Trimester Predictive Biomarkers of PE
4.2. Blood Proteome Alterations in Second-Trimester PE
4.3. Third-Trimester Protein Biomarkers of PE
4.4. Standardization of Blood Protein Analysis: Current Challenges and Future Directions for PE Diagnosis and Prediction
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 2-DE | Two-dimensional gel electrophoresis |
| 2D-DIGE | Two-dimensional difference gel electrophoresis with fluorescent labeling |
| AUC | Area-under-the-curve |
| CXCL10 | C-X-C motif chemokine ligand 10 |
| ECM | Extracellular matrix |
| ELISA | Enzyme-linked immunosorbent assay |
| FGR | Fetal growth restriction |
| FIGO | International Federation of Gynecology and Obstetrics |
| FMF | Fetal Medicine Foundation |
| HELLP | Hemolysis, Elevated Liver Enzymes, Low Platelet Count |
| HPLC-MS/MS | High-performance liquid chromatography–tandem mass spectrometry |
| IQR | Inter-quartile range |
| IS | Isotope-labeled internal standards (tryptic peptides) |
| iTRAQ | Isobaric tags for relative and absolute quantitation |
| LC-MS/MS | Liquid chromatography -tandem mass spectrometry |
| MALDI-MS/MS | Matrix-assisted laser desorption/ionization–tandem mass spectrometry |
| MRM | Multiple reaction monitoring |
| NGS | Next-generation sequencing |
| NMR | Nuclear magnetic resonance |
| PAPP-A | Pregnancy-associated plasma protein-A |
| PCR | Polymerase chain reaction |
| PE | Preeclampsia |
| PlGF | Placental growth factor |
| SD | Standard deviation |
| SDS-PAGE | Polyacrylamide gel electrophoresis with sodium dodecyl sulfate |
| sFlt-1 | Soluble fms-like tyrosine kinase-1 |
| SOMAmer | Slow off-rate modified DNA aptamers |
| SRM | Selected reaction monitoring |
| SWATH | Sequential window acquisition of all theoretical mass spectra |
| TMT | Tandem mass tag |
References
- Lo, J.O.; Mission, J.F.; Caughey, A.B. Hypertensive disease of pregnancy and maternal mortality. Curr. Opin. Obstet. Gynecol. 2013, 25, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Knight, M.; Bunch, K.; Felker, A.; Patel, R.; Kotnis, R.; Kenyon, S.; Kurinczuk, J.J. MBRRACE—UK: Care Saving Lives, Improving Mothers’ Care Lessons learned to Inform Maternity Care from the UK and Ireland Confidential Enquiries into Maternal Deaths and Morbidity State of the Nation Surveillance Report 20; National Perinatal Epidemiology Unit, University of Oxford: Oxford, UK, 2023; ISBN 978-1-7392619-4-8. [Google Scholar]
- Boghossian, N.S.; Yeung, E.; Mendola, P.; Hinkle, S.N.; Laughon, S.K.; Zhang, C.; Albert, P.S. Risk factors differ between recurrent and incident preeclampsia: A hospital-based cohort study. Ann. Epidemiol. 2014, 24, 871–877.e3. [Google Scholar] [CrossRef] [PubMed]
- NICE Guidelines Institute for Health and Care. Hypertension in Pregnancy: Diagnosis and Management; National Institute for Health and Care Excellence (NICE): London, UK, 2019; Volume 133, ISBN 1179-1411. [Google Scholar]
- Lisonkova, S.; Joseph, K.S. Incidence of preeclampsia: Risk factors and outcomes associated with early- versus late-onset disease. Am. J. Obstet. Gynecol. 2013, 209, 544.e1–544.e12. [Google Scholar] [CrossRef] [PubMed]
- Lisonkova, S.; Sabr, Y.; Mayer, C.; Young, C.; Skoll, A.; Joseph, K.S. Maternal morbidity associated with early-onset and late-onset Preeclampsia. Obstet. Gynecol. 2014, 124, 771–781. [Google Scholar] [CrossRef]
- Khan, S.; Siddique, A.B.; Jabeen, S.; Hossain, A.T.; Haider, M.M.; Zohora, F.T.; Rahman, M.M.; El Arifeen, S.; Rahman, A.E.; Jamil, K. Preeclampsia and eclampsia-specific maternal mortality in Bangladesh: Levels, trends, timing, and care-seeking practices. J. Glob. Health 2023, 13, 07003. [Google Scholar] [CrossRef]
- Melchiorre, K.; Sutherland, G.R.; Liberati, M.; Thilaganathan, B. Preeclampsia is associated with persistent postpartum cardiovascular impairment. Hypertension 2011, 58, 709–715. [Google Scholar] [CrossRef]
- Melchiorre, K.; Sutherland, G.R.; Liberati, M.; Thilaganathan, B. Maternal cardiovascular impairment in pregnancies complicated by severe fetal growth restriction. Hypertension 2012, 60, 437–443. [Google Scholar] [CrossRef]
- Jung, E.; Romero, R.; Yeo, L.; Gomez-Lopez, N.; Chaemsaithong, P.; Jaovisidha, A.; Gotsch, F.; Erez, O. The etiology of preeclampsia. Am. J. Obstet. Gynecol. 2022, 226, S844–S866. [Google Scholar] [CrossRef]
- Staff, A.C.; Fjeldstad, H.E.; Fosheim, I.K.; Moe, K.; Turowski, G.; Johnsen, G.M.; Alnaes-Katjavivi, P.; Sugulle, M. Failure of physiological transformation and spiral artery atherosis: Their roles in preeclampsia. Am. J. Obstet. Gynecol. 2022, 226, S895–S906. [Google Scholar] [CrossRef]
- Melchiorre, K.; Giorgione, V.; Thilaganathan, B. The placenta and preeclampsia: Villain or victim? Am. J. Obstet. Gynecol. 2022, 226, S954–S962. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists. Low-dose aspirin use during pregnancy. ACOG Committee Opinion No. 743. Obstet. Gynecol. 2018, 132, e44–e52. [Google Scholar] [CrossRef]
- Henderson, J.T.; Whitlock, E.P.; O’Connor, E.; Senger, C.A.; Thompson, J.H.; Rowland, M.G. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia: A systematic evidence review for the u.s. preventive services task force. Ann. Intern. Med. 2014, 160, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Youssef, L.; Testa, L.; Crovetto, F.; Crispi, F. 10. Role of high dimensional technology in preeclampsia (omics in preeclampsia). Best Pract. Res. Clin. Obstet. Gynaecol. 2024, 92, 102427. [Google Scholar] [CrossRef] [PubMed]
- Stepan, H.; Herraiz, I.; Schlembach, D.; Verlohren, S.; Brennecke, S.; Chantraine, F.; Klein, E.; Lapaire, O.; Llurba, E.; Ramoni, A.; et al. Implementation of the sFlt-1/PlGF ratio for prediction and diagnosis of pre-eclampsia in singleton pregnancy: Implications for clinical practice. Ultrasound Obstet. Gynecol. 2015, 45, 241–246. [Google Scholar] [CrossRef]
- Zeisler, H.; Llurba, E.; Chantraine, F.; Vatish, M.; Staff, A.C.; Sennström, M.; Olovsson, M.; Brennecke, S.P.; Stepan, H.; Allegranza, D.; et al. Predictive value of the sFlt-1:PlGF ratio in women with suspected preeclampsia. N. Engl. J. Med. 2016, 374, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Chaemsaithong, P.; Sahota, D.S.; Poon, L.C. First trimester preeclampsia screening and prediction. Am. J. Obstet. Gynecol. 2022, 226, S1071–S1097.e2. [Google Scholar] [CrossRef]
- Than, N.G.; Romero, R.; Posta, M.; Györffy, D.; Szalai, G.; Rossi, S.W.; Szilágyi, A.; Hupuczi, P.; Nagy, S.; Török, O.; et al. Classification of preeclampsia according to molecular clusters with the goal of achieving personalized prevention. J. Reprod. Immunol. 2024, 161, 104172. [Google Scholar] [CrossRef]
- Ontario Health Technology Assessment Series. First-Trimester Screening Program for the Risk of Pre-eclampsia Using a Multiple-Marker Algorithm: A Health Technology Assessment. Ont. Health Technol. Assess. Ser. 2022, 22, 1–118. [Google Scholar]
- Poon, L.C.; Shennan, A.; Hyett, J.A.; Kapur, A.; Hadar, E.; Divakar, H.; McAuliffe, F.; da Silva Costa, F.; von Dadelszen, P.; McIntyre, H.D.; et al. The International Federation of Gynecology and Obstetrics (FIGO) initiative on pre-eclampsia: A pragmatic guide for first-trimester screening and prevention. Int. J. Gynaecol. Obstet. Off. Organ. Int. Fed. Gynaecol. Obstet. 2019, 145, 1–33. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Wells, G.; Shea, B.; O’Connell, D.; Robertson, J.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. 2013, 21. Available online: https://www.ohri.ca/programs/clinical_epidemiology/nosgen.pdf (accessed on 11 May 2025).
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING database in 2023: Protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.D.; Ebert, D.; Muruganujan, A.; Mushayahama, T.; Albou, L.P.; Mi, H. PANTHER: Making genome-scale phylogenetics accessible to all. Protein Sci. 2022, 31, 8–22. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, K.R.; Blumenstein, M.; Black, M.A.; Wu, S.H.; Kasabov, N.; Taylor, R.S.; Cooper, G.J.S.; North, R.A. An altered pattern of circulating apolipoprotein E3 isoforms is implicated in preeclampsia. J. Lipid Res. 2009, 50, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Beernink, R.H.J.; Zwertbroek, E.F.; Schuitemaker, J.H.N.; Cremers, T.I.F.H.; Scherjon, S.A. First trimester serum biomarker discovery study for early onset, preterm onset and preeclampsia at term. Placenta 2022, 128, 39–48. [Google Scholar] [CrossRef]
- Chen, H.; Aneman, I.; Nikolic, V.; Karadzov Orlic, N.; Mikovic, Z.; Stefanovic, M.; Cakic, Z.; Jovanovic, H.; Town, S.E.L.; Padula, M.P.; et al. Maternal plasma proteome profiling of biomarkers and pathogenic mechanisms of early-onset and late-onset preeclampsia. Sci. Rep. 2022, 12, 19099. [Google Scholar] [CrossRef]
- Degnes, M.H.L.; Westerberg, A.C.; Andresen, I.J.; Henriksen, T.; Roland, M.C.P.; Zucknick, M.; Michelsen, T.M. Protein biomarker signatures of preeclampsia—A longitudinal 5000-multiplex proteomics study. Sci. Rep. 2024, 14, 23654. [Google Scholar] [CrossRef]
- Erez, O.; Romero, R.; Maymon, E.; Chaemsaithong, P.; Done, B.; Pacora, P.; Panaitescu, B.; Chaiworapongsa, T.; Hassan, S.S.; Tarca, A.L. The prediction of late-onset preeclampsia: Results from a longitudinal proteomics study. PLoS ONE 2017, 12, e0181468. [Google Scholar] [CrossRef]
- Kolialexi, A.; Tsangaris, G.T.; Sifakis, S.; Gourgiotis, D.; Katsafadou, A.; Lykoudi, A.; Marmarinos, A.; Mavreli, D.; Pergialiotis, V.; Fexi, D.; et al. Plasma biomarkers for the identification of women at risk for early-onset preeclampsia. Expert Rev. Proteom. 2017, 14, 269–276. [Google Scholar] [CrossRef]
- Lim, J.H.; Lim, J.M.; Lee, H.M.; Lee, H.J.; Kwak, D.W.; Han, Y.J.; Kim, M.Y.; Jung, S.H.; Kim, Y.R.; Ryu, H.M.; et al. Systematic Proteome Profiling of Maternal Plasma for Development of Preeclampsia Biomarkers. Mol. Cell Proteom. 2024, 23, 100826. [Google Scholar] [CrossRef]
- Liu, L.Y.; Yang, T.; Ji, J.; Wen, Q.; Morgan, A.A.; Jin, B.; Chen, G.; Lyell, D.J.; Stevenson, D.K.; Ling, X.B.; et al. Integrating multiple “omics” analyses identifies serological protein biomarkers for preeclampsia. BMC Med. 2013, 11, 236. [Google Scholar] [CrossRef]
- Lu, Q.; Liu, C.; Liu, Y.; Zhang, N.; Deng, H.; Zhang, Z. Serum markers of pre-eclampsia identified on proteomics. J. Obstet. Gynaecol. Res. 2016, 42, 1111–1118. [Google Scholar] [CrossRef] [PubMed]
- Odenkirk, M.T.; Stratton, K.G.; Gritsenko, M.A.; Bramer, L.M.; Webb-Robertson, B.J.M.; Bloodsworth, K.J.; Weitz, K.K.; Lipton, A.K.; Monroe, M.E.; Ash, J.R.; et al. Unveiling molecular signatures of preeclampsia and gestational diabetes mellitus with multi-omics and innovative cheminformatics visualization tools. Mol. Omi. 2020, 16, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.-F.; Zhang, J.-L.; Liu, K.; Wang, L.; Wang, H.-P.; Wu, H.-Y. Detection of serum major histocompatibility complex I (HLA-1) and β2-microglobulin (β2M) in pre-eclampsia using isobaric tags for relative and absolute quantitation (iTRAQ). Int. J. Gynaecol. Obstet. Off. Organ. Int. Fed. Gynaecol. Obstet. 2024, 165, 1072–1084. [Google Scholar] [CrossRef]
- Starodubtseva, N.; Tokareva, A.; Kononikhin, A.; Brzhozovskiy, A.; Bugrova, A.; Kukaev, E.; Muminova, K.; Nakhabina, A.; Frankevich, V.E.; Nikolaev, E.; et al. First-Trimester Preeclampsia-Induced Disturbance in Maternal Blood Serum Proteome: A Pilot Study. Int. J. Mol. Sci. 2024, 25, 10653. [Google Scholar] [CrossRef]
- Than, N.G.; Romero, R.; Tarca, A.L.; Kekesi, K.A.; Xu, Y.; Xu, Z.; Juhasz, K.; Bhatti, G.; Leavitt, R.J.; Gelencser, Z.; et al. Integrated systems biology approach identifies novel maternal and placental pathways of preeclampsia. Front. Immunol. 2018, 9, 1661. [Google Scholar] [CrossRef]
- Than, N.G.; Posta, M.; Györffy, D.; Orosz, L.; Orosz, G.; Rossi, S.W.; Ambrus-Aikelin, G.; Szilágyi, A.; Nagy, S.; Hupuczi, P.; et al. Early pathways, biomarkers, and four distinct molecular subclasses of preeclampsia: The intersection of clinical, pathological, and high-dimensional biology studies. Placenta 2022, 125, 10–19. [Google Scholar] [CrossRef]
- Uchida, Y.; Higuchi, T.; Shirota, M.; Kagami, S.; Saigusa, D.; Koshiba, S.; Yasuda, J.; Tamiya, G.; Kuriyama, S.; Kinoshita, K.; et al. Identification and validation of combination plasma biomarker of afamin, fibronectin and sex hormone-binding globulin to predict pre-eclampsia. Biol. Pharm. Bull. 2021, 44, 804–815. [Google Scholar] [CrossRef]
- Wang, X.; Yip, K.C.; He, A.; Tang, J.; Liu, S.; Yan, R.; Zhang, Q.; Li, R. Plasma Olink Proteomics Identifies CCL20 as a Novel Predictive and Diagnostic Inflammatory Marker for Preeclampsia. J. Proteome Res. 2022, 21, 2998–3006. [Google Scholar] [CrossRef]
- Youssef, L.; Miranda, J.; Blasco, M.; Paules, C.; Crovetto, F.; Palomo, M.; Torramade-Moix, S.; García-Calderó, H.; Tura-Ceide, O.; Dantas, A.P.; et al. Complement and coagulation cascades activation is the main pathophysiological pathway in early-onset severe preeclampsia revealed by maternal proteomics. Sci. Rep. 2021, 11, 3048. [Google Scholar] [CrossRef]
- Dimitriadis, E.; Rolnik, D.L.; Zhou, W.; Estrada-Gutierrez, G.; Koga, K.; Francisco, R.P.V.; Whitehead, C.; Hyett, J.; da Silva Costa, F.; Nicolaides, K.; et al. Pre-eclampsia. Nat. Rev. Dis. Prim. 2023, 9, 8. [Google Scholar] [CrossRef]
- Burwick, R.M.; Feinberg, B.B. Complement activation and regulation in preeclampsia and hemolysis, elevated liver enzymes, and low platelet count syndrome. Am. J. Obstet. Gynecol. 2022, 226, S1059–S1070. [Google Scholar] [CrossRef] [PubMed]
- Girardi, G.; Lingo, J.J.; Fleming, S.D.; Regal, J.F. Essential Role of Complement in Pregnancy: From Implantation to Parturition and Beyond. Front. Immunol. 2020, 11, 1681. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, T.; Tomimatsu, T.; Mimura, K.; Yagi, K.; Kawanishi, Y.; Kakigano, A.; Nakamura, H.; Endo, M.; Kimura, T. Complement activation by an angiogenic imbalance leads to systemic vascular endothelial dysfunction: A new proposal for the pathophysiology of preeclampsia. J. Reprod. Immunol. 2021, 145, 103322. [Google Scholar] [CrossRef] [PubMed]
- Fischetti, F.; Tedesco, F. Cross-talk between the complement system and endothelial cells in physiologic conditions and in vascular diseases. Autoimmunity 2006, 39, 417–428. [Google Scholar] [CrossRef]
- Than, N.G.; Romero, R.; Györffy, D.; Posta, M.; Bhatti, G.; Done, B.; Chaemsaithong, P.; Jung, E.; Suksai, M.; Gotsch, F.; et al. Molecular subclasses of preeclampsia characterized by a longitudinal maternal proteomics study: Distinct biomarkers, disease pathways and options for prevention. J. Perinat. Med. 2023, 51, 51–68. [Google Scholar] [CrossRef]
- Balduit, A.; Agostinis, C.; Mangogna, A.; Zito, G.; Stampalija, T.; Ricci, G.; Bulla, R. Systematic review of the complement components as potential biomarkers of pre-eclampsia: Pitfalls and opportunities. Front. Immunol. 2024, 15, 1419540. [Google Scholar] [CrossRef]
- Regal, J.F.; Burwick, R.M.; Fleming, S.D. The Complement System and Preeclampsia. Curr. Hypertens. Rep. 2017, 19, 87. [Google Scholar] [CrossRef]
- Pierik, E.; Prins, J.R.; van Goor, H.; Dekker, G.A.; Daha, M.R.; Seelen, M.A.J.; Scherjon, S.A. Dysregulation of Complement Activation and Placental Dysfunction: A Potential Target to Treat Preeclampsia? Front. Immunol. 2020, 10, 3098. [Google Scholar] [CrossRef]
- National Collaborating Centre for Women’s and Children’s Health. Hypertension in Pregnancy: The Management of Hypertensive Disorders During Pregnancy Hypertension in Pregnancy the Management of Hypertensive Disorders; Welsh, A., Ed.; RCOG Press: London, UK, 2010. [Google Scholar]
- Ducat, A.; Vargas, A.; Doridot, L.; Bagattin, A.; Lerner, J.; Vilotte, J.L.; Buffat, C.; Pontoglio, M.; Miralles, F.; Vaiman, D. Low-dose aspirin protective effects are correlated with deregulation of HNF factor expression in the preeclamptic placentas from mice and humans. Cell Death Discov. 2019, 5, 94. [Google Scholar] [CrossRef]
- Nguyen, T.P.H.; Patrick, C.J.; Parry, L.J.; Familari, M. Using proteomics to advance the search for potential biomarkers for preeclampsia: A systematic review and meta-analysis. PLoS ONE 2019, 14, e0214671. [Google Scholar] [CrossRef]
- Ricklin, D.; Lambris, J.D. Complement in Immune and Inflammatory Disorders: Pathophysiological Mechanisms. J. Immunol. 2013, 190, 3831–3838. [Google Scholar] [CrossRef] [PubMed]
- Levi, M.; Van Der Poll, T. Inflammation and coagulation. Crit. Care Med. 2010, 38, S26–S34. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Li, X.; Shi, X.; Zhu, M.; Wang, J.; Huang, S.; Huang, X.; Wang, H.; Li, L.; Deng, H.; et al. Platelet integrin αiIbβ3: Signal transduction, regulation, and its therapeutic targeting. J. Hematol. Oncol. 2019, 12, 26. [Google Scholar] [CrossRef] [PubMed]
- Tarca, A.L.; Romero, R.; Benshalom-Tirosh, N.; Than, N.G.; Gudicha, D.W.; Done, B.; Pacora, P.; Chaiworapongsa, T.; Panaitescu, B.; Tirosh, D.; et al. The prediction of early preeclampsia: Results from a longitudinal proteomics study. PLoS ONE 2019, 14, e0217273. [Google Scholar] [CrossRef]
- Bahabayi, A.; Yang, N.; Xu, T.; Xue, Y.; Ma, L.; Gu, X.; Wang, Y.; Jia, K. Expression of Matrix Metalloproteinase-2,-7,-9 in Serum during Pregnancy in Patients with Pre-Eclampsia: A Prospective Study. Int. J. Environ. Res. Public Health 2022, 19, 14500. [Google Scholar] [CrossRef]
- Jia, Y.; Lu, W.; Xie, H.; Sheng, Y.; Wang, L.; Lv, W.; Ling, L.; Dong, J.; Jia, X.; Wu, S.; et al. Upregulation of Siglec-6 induces mitochondrial dysfunction by promoting GPR20 expression in early-onset preeclampsia. J. Transl. Med. 2024, 22, 674. [Google Scholar] [CrossRef]
- Rumer, K.K.; Uyenishi, J.; Hoffman, M.C.; Fisher, B.M.; Winn, V.D. Siglec-6 expression is increased in placentas from pregnancies complicated by preterm preeclampsia. Reprod. Sci. 2013, 20, 646–653. [Google Scholar] [CrossRef]
- Maynard, S.E.; Min, J.; Merchan, J.; Lim, K.; Li, J.; Mondal, S.; Libermann, T.A.; Morgan, J.P.; Sellke, F.W.; Stillman, I.E.; et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J. Clin. Investig. 2003, 111, 649–658. [Google Scholar] [CrossRef]
- Maragoudakis, M.E. Angiogenesis in health and disease. Nat. Med. 2003, 9, 653–660. [Google Scholar] [CrossRef]
- Bellamy, L.; Casas, J.P.; Hingorani, A.D.; Williams, D.J. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: Systematic review and meta-analysis. Br. Med. J. 2007, 335, 974–977. [Google Scholar] [CrossRef]
- Wu, P.; Haththotuwa, R.; Kwok, C.S.; Babu, A.; Kotronias, R.A.; Rushton, C.; Zaman, A.; Fryer, A.A.; Kadam, U.; Chew-Graham, C.A.; et al. Preeclampsia and future cardiovascular health. Circ. Cardiovasc. Qual. Outcomes 2017, 10, e003497. [Google Scholar] [CrossRef] [PubMed]
- Lisowska, M.; Pietrucha, T.; Sakowicz, A. Preeclampsia and Related Cardiovascular Risk: Common Genetic Background. Curr. Hypertens. Rep. 2018, 20, 71. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, D.P.; Røysland, R.; Strand, H.; Moe, K.; Sugulle, M.; Omland, T.; Staff, A.C. Circulating cardiovascular biomarkers during and after preeclampsia: Crosstalk with placental function? Pregnancy Hypertens. 2022, 30, 103–109. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, T.E.; Ray, J.G.; Chan, W.-S. Maternal Body Mass Index and The Risk of Preeclampsia. A Systematic overview. Epidemiology 2003, 14, 368–374. [Google Scholar] [CrossRef]
- Sierra-Laguado, J.; García, R.G.; Celedón, J.; Arenas-Mantilla, M.; Pradilla, L.P.; Camacho, P.A.; López-Jaramillo, P. Determination of Insulin Resistance Using the Homeostatic Model Assessment (HOMA) and its Relation With the Risk of Developing Pregnancy-Induced Hypertension. Am. J. Hypertens. 2007, 20, 437–442. [Google Scholar] [CrossRef]
- García, R.G.; Celedón, J.; Sierra-Laguado, J.; Alarcón, M.A.; Luengas, C.; Silva, F.; Arenas-Mantilla, M.; López-Jaramillo, P. Raised C-Reactive Protein and Impaired Flow-Mediated Vasodilation Precede the Development of Preeclampsia. Am. J. Hypertens. 2007, 20, 98–103. [Google Scholar] [CrossRef][Green Version]
- Lopez-Jaramillo, P.; Barajas, J.; Rueda-Quijano, S.M.; Lopez-Lopez, C.; Felix, C. Obesity and Preeclampsia: Common Pathophysiological Mechanisms. Front. Physiol. 2018, 9, 1838. [Google Scholar] [CrossRef]
- López-Jaramillo, P.; Terán, E.; Ringqvist, A.; Moya, W.; Rivera, J.; Berrazueta, J.R. P-375—Oxidised low-density lipoproteins and nitric oxide during normal pregnancy and preeclampsia. Jpn. J. Pharmacol. 1997, 75, 116. [Google Scholar] [CrossRef]
- Reyes, L.M.; García, R.G.; Ruiz, S.L.; Broadhurst, D.; Aroca, G.; Davidge, S.T.; López-Jaramillo, P. Angiogenic imbalance and plasma lipid alterations in women with preeclampsia from a developing country. Growth Factors 2012, 30, 158–166. [Google Scholar] [CrossRef]
- Reyes, L.M.; García, R.G.; Ruiz, S.L.; Camacho, P.A.; Ospina, M.B.; Aroca, G.; Accini, J.L.; López-Jaramillo, P. Risk factors for preeclampsia in women from Colombia: A case-control study. PLoS ONE 2012, 7, e41622. [Google Scholar] [CrossRef]
- Hubel, C.A.; McLaughlin, M.K.; Evans, R.W.; Hauth, B.A.; Sims, C.J.; Roberts, J.M. Fasting serum triglycerides, free fatty acids, and malondialdehyde are increased in preeclampsia, are positively correlated, and decrease within 48 hours post partum. Am. J. Obstet. Gynecol. 1996, 174, 975–982. [Google Scholar] [CrossRef] [PubMed]
- Staff, A.C.; Dechend, R.; Pijnenborg, R. Learning from the placenta: Acute atherosis and vascular remodeling in preeclampsia-novel aspects for atherosclerosis and future cardiovascular health. Hypertension 2010, 56, 1026–1034. [Google Scholar] [CrossRef] [PubMed]
- Von Eckardstein, A.; Nordestgaard, B.G.; Catapano, A.L.; Remaley, A.T. High-density lipoprotein revisited: Biological functions and clinical relevance. Eur. Heart J. 2023, 44, 1394–1407. [Google Scholar] [CrossRef] [PubMed]
- Brites, F.; Martin, M.; Guillas, I.; Kontush, A. Antioxidative activity of high-density lipoprotein (HDL): Mechanistic insights into potential clinical bene fi t. BBA Clin. 2017, 8, 66–77. [Google Scholar] [CrossRef]
- Shah, A.S.; Tan, L.; Long, J.L.; Davidson, W.S. Proteomic diversity of high density lipoproteins: Our emerging understanding of its importance in lipid transport and beyond. J. Lipid Res. 2013, 54, 2575–2585. [Google Scholar] [CrossRef]
- Stadler, J.T.; Scharnagl, H.; Wadsack, C.; Marsche, G. Preeclampsia Affects Lipid Metabolism and HDL Function in Mothers and Their Offspring. Antioxidants 2023, 12, 795. [Google Scholar] [CrossRef]
- Bellos, I.; Papantoniou, N.; Pergialiotis, V. Serum ceruloplasmin levels in preeclampsia: A meta-analysis. J. Matern. Neonatal Med. 2018, 31, 2342–2348. [Google Scholar] [CrossRef]
- Sak, S.; Barut, M.; Çelik, H.; Incebiyik, A.; Ağaçayak, E.; Uyanikoglu, H.; Kirmit, A.; Sak, M. Copper and ceruloplasmin levels are closely related to the severity of preeclampsia. J. Matern. Neonatal Med. 2020, 33, 96–102. [Google Scholar] [CrossRef]
- Gaillard, R.; Arends, L.R.; Steegers, E.A.P.; Hofman, A.; Jaddoe, V.W.V. Second-and third-trimester placental hemodynamics and the risks of pregnancy complications. Am. J. Epidemiol. 2013, 177, 743–754. [Google Scholar] [CrossRef]
- Myatt, L.; Cui, X. Oxidative stress in the placenta. Histochem. Cell Biol. 2004, 122, 369–382. [Google Scholar] [CrossRef]
- Raijmakers, M.T.M.; Dechend, R.; Poston, L. Oxidative stress and preeclampsia: Rationale for antioxidant clinical trials. Hypertension 2004, 44, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Schwarzbauer, J.E. Fibronectin fibrillogenesis, a cell-mediated matrix assembly process. Matrix Biol. 2005, 24, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Hao, M.; Tong, H.; Yang, H.; Huang, B.; Zhang, Z.; Luo, K.Q. The interactions between integrin α5β1 of liver cancer cells and fibronectin of fibroblasts promote tumor growth and angiogenesis. Int. J. Biol. Sci. 2022, 18, 5019–5037. [Google Scholar] [CrossRef]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef]
- Goldman-Wohl, D.; Yagel, S. Regulation of trophoblast invasion: From normal implantation to pre-eclampsia. Mol. Cell. Endocrinol. 2002, 187, 233–238. [Google Scholar] [CrossRef]
- Chavarría, M.E.; Lara-González, L.; González-Gleason, A.; Sojo, I.; Reyes, A. Maternal plasma cellular fibronectin concentrations in normal and preeclamptic pregnancies: A longitudinal study for early prediction of preeclampsia. Am. J. Obstet. Gynecol. 2002, 187, 595–601. [Google Scholar] [CrossRef]
- Rasanen, J.; Girsen, A.; Lu, X.; Lapidus, J.A.; Standley, M.; Reddy, A.; Dasari, S.; Thomas, A.; Jacob, T.; Pouta, A.; et al. Comprehensive maternal serum proteomic profiles of preclinical and clinical preeclampsia. J. Proteome Res. 2010, 9, 4274–4281. [Google Scholar] [CrossRef]
- Rasanen, J.; Quinn, M.J.; Laurie, A.; Bean, E.; Roberts, C.T.; Nagalla, S.R.; Gravett, M.G. Maternal serum glycosylated fibronectin as a point-of-care biomarker for assessment of preeclampsia. Am. J. Obstet. Gynecol. 2015, 212, 82.e1–82.e9. [Google Scholar] [CrossRef]
- Moungmaithong, S.; Wang, X.; Lau, C.S.L.; Tse, A.W.T.; Lee, N.M.W.; Leung, H.H.Y.; Poon, L.C.; Sahota, D.S. Glycosylated fibronectin improves first-trimester prediction of pre-eclampsia. Ultrasound Obstet. Gynecol. 2023, 62, 512–521. [Google Scholar] [CrossRef]
- Rifai, N.; Gillette, M.A.; Carr, S.A. Protein biomarker discovery and validation: The long and uncertain path to clinical utility. Nat. Biotechnol. 2006, 24, 971–983. [Google Scholar] [CrossRef]
- Brandwijk, R.J.M.G.E.; Michels, M.A.H.M.; van Rossum, M.; de Nooijer, A.H.; Nilsson, P.H.; de Bruin, W.C.C.; Toonen, E.J.M. Pitfalls in complement analysis: A systematic literature review of assessing complement activation. Front. Immunol. 2022, 13, 1007102. [Google Scholar] [CrossRef] [PubMed]
- Smejkal, G.B. Proteomics Sample Preparation, Preservation, and Fractionation. Int. J. Proteom. 2012, 2012, 701230. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Klont, F.; Bras, L.; Wolters, J.C.; Ongay, S.; Bischoff, R.; Halmos, G.B.; Horvatovich, P. Assessment of Sample Preparation Bias in Mass Spectrometry-Based Proteomics. Anal. Chem. 2018, 90, 5405–5413. [Google Scholar] [CrossRef] [PubMed]
- Guryča, V.; Roeder, D.; Piraino, P.; Lamerz, J.; Ducret, A.; Langen, H.; Cutler, P. Automated sample preparation platform for mass spectrometry-based plasma proteomics and biomarker discovery. Biology 2014, 3, 205–219. [Google Scholar] [CrossRef]
- Yang, S.; McGookey, M.; Wang, Y.; Cataland, S.R.; Wu, H.M. Effect of blood sampling, processing, and storage on the measurement of complement activation biomarkers. Am. J. Clin. Pathol. 2015, 143, 558–565. [Google Scholar] [CrossRef]
- Hassis, M.E.; Niles, R.K.; Braten, M.N.; Albertolle, M.E.; Ewa Witkowska, H.; Hubel, C.A.; Fisher, S.J.; Williams, K.E. Evaluating the effects of preanalytical variables on the stability of the human plasma proteome. Anal. Biochem. 2015, 478, 14–22. [Google Scholar] [CrossRef]
- Han, L.; Holland, O.J.; Da Silva Costa, F.; Perkins, A. V Potential biomarkers for late-onset and term preeclampsia: A scoping review. Front. Physiol. 2023, 14, 1143543. [Google Scholar] [CrossRef]
- Leavey, K.; Grynspan, D.; Cox, B.J. Both “canonical” and “immunological” preeclampsia subtypes demonstrate changes in placental immune cell composition. Placenta 2019, 83, 53–56. [Google Scholar] [CrossRef]
- Vasapollo, B.; Zullino, S.; Novelli, G.P.; Farsetti, D.; Ottanelli, S.; Clemenza, S.; Micaglio, M.; Ferrazzi, E.; Di Martino, D.D.; Ghi, T.; et al. Maternal Hemodynamics from Preconception to Delivery: Research and Potential Diagnostic and Therapeutic Implications: Position Statement by Italian Association of Preeclampsia and Italian Society of Perinatal Medicine. Am. J. Perinatol. 2024, 41, 1999–2013. [Google Scholar] [CrossRef]

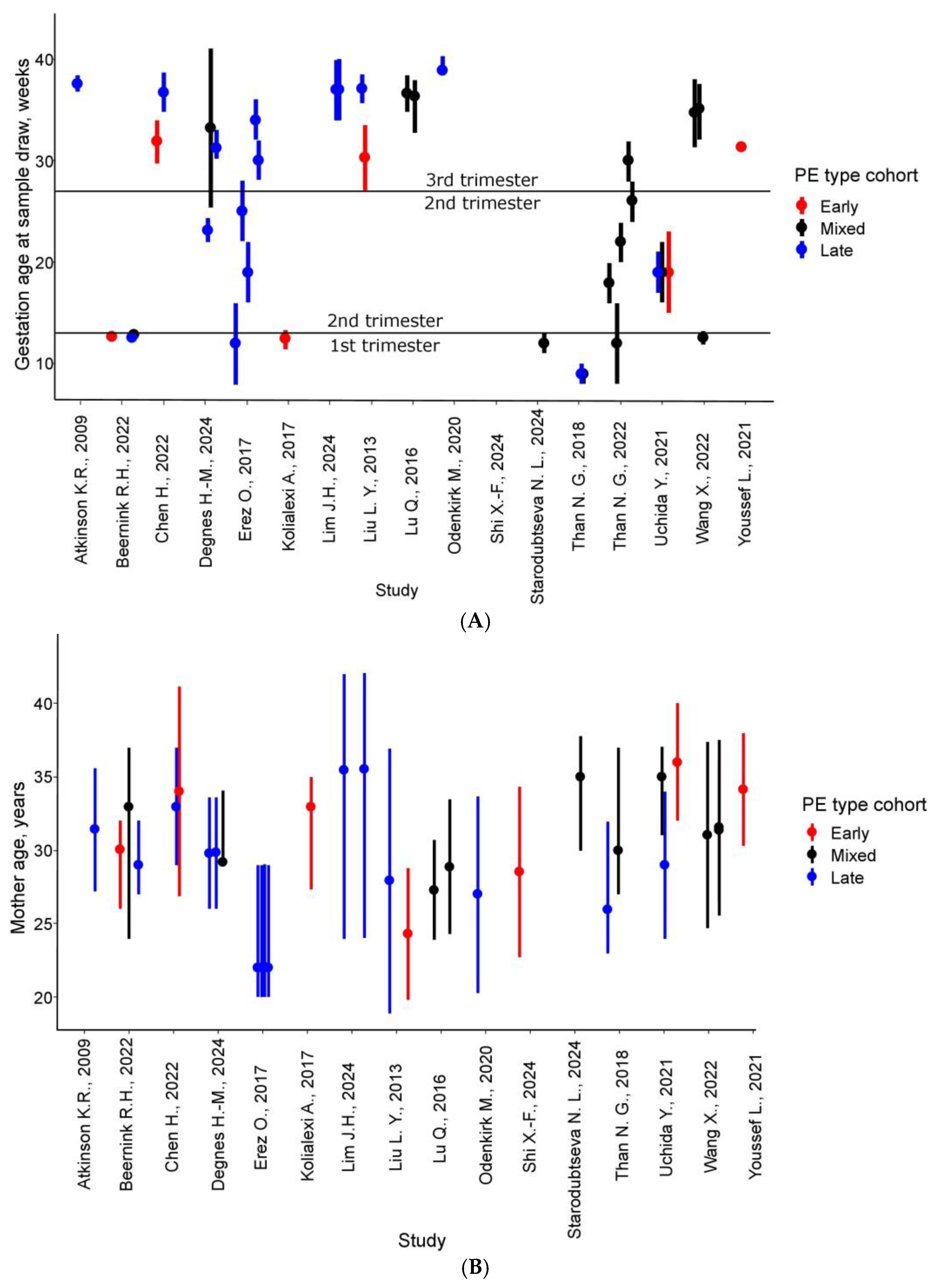
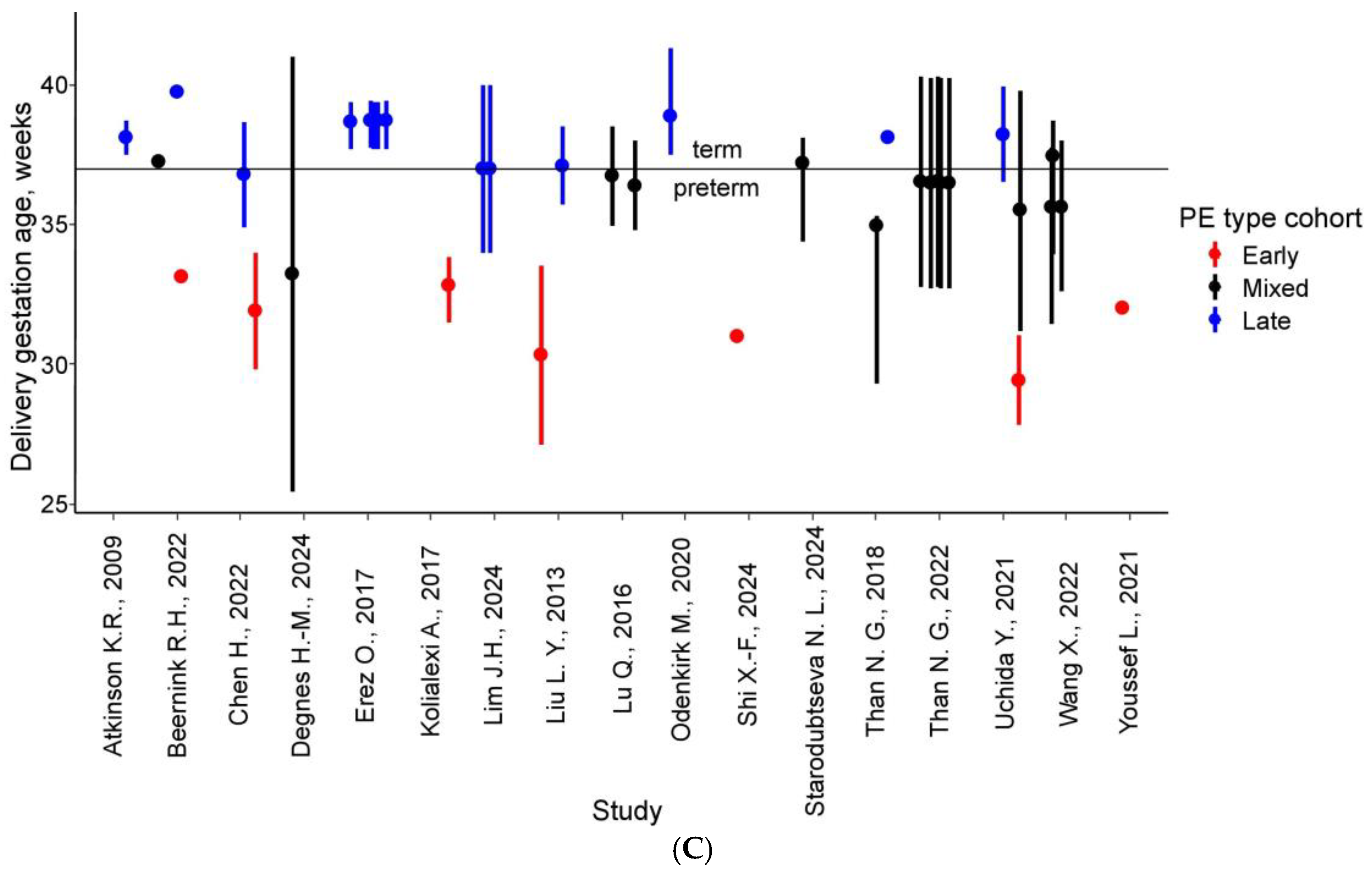


| Article | Sample Collection, Weeks | PE (n) | Control (n) | Delivery, Weeks ** | Sample Type | Proteins * | Matched Proteins ** | Method |
|---|---|---|---|---|---|---|---|---|
| Atkinson, K.R., 2009 [26] | 37.6 ± 0.8 | 24 | 24 | 38.1 ± 0.6 | plasma, serum | 7 | 6 | Depletion of 6 major plasma proteins, 2-DE, 2D-DIGE, LC-MS/MS |
| Beernink, R.H., 2022 [27] | 12.7 | 9 | 9 | 33.1 | serum | 6 | 2 | SDS-PAGE, LC-MS/MS |
| 12.8 | 8 | 8 | 37.2 | serum | 4 | 2 | ||
| 12.5 | 6 | 6 | 39.7 | serum | 8 | 2 | ||
| Chen, H., 2022 [28] | 31.9 ± 2.1 | 17 | 18 | 31.9 ± 2.1 | plasma | 26 | 18 | LC-MS/MS |
| 36.8 ± 1.9 | 11 | 18 | 36.8 ± 1.9 | plasma | 20 | 14 | ||
| 31.9 ± 2.1 | 17 | 18 | 31.9 ± 2.1 | plasma | 9 | 7 | ||
| 36.8 ± 1.9 | 11 | 18 | 36.8 ± 1.9 | plasma | 13 | 10 | ||
| Degnes, H.-M., 2024 [29] | 23.2 ± 1.2 | 35 | 70 | ≥34 | plasma | 2 | 0 | SOMAmer-4979 proteins |
| 31.2 ± 1.0 | 35 | 70 | ≥34 | plasma | 60 | 13 | ||
| 25.4–41 | 37 | 75 | 25.4–41 | plasma | 35 | 10 | ||
| Erez, O., 2017 [30] | 28.1–32 | 76 | 90 | 38.7 (37.7–39.4) | plasma | 15 | 9 | SOMAmer-1125 proteins |
| 8.0–16.0 | 76 | 90 | 38.7 (37.7–39.4) | plasma | 24 | 16 | ||
| 16.1–22 | 76 | 90 | 38.7 (37.7–39.4) | plasma | 26 | 17 | ||
| 22.1–28 | 76 | 90 | 38.7 (37.7–39.4) | plasma | 18 | 12 | ||
| 32.1–36 | 76 | 90 | 38.7 (37.7–39.4) | plasma | 15 | 8 | ||
| Kolialexi, A., 2017 [31] | 12.5 (11.4–13.3) | 10 | 40 | 32.2 (28.8–37.3) | plasma | 11 | 11 | 2-DE, MALDI-MS/MS |
| 12.5 (11.4–13.3) | 10 | 40 | 32.2 (28.8–37.3) | plasma | 4 | 2 | ELISA | |
| Lim, J.H., 2024 [32] | 37 (34–40) | 26 | 29 | 37 (34–40) | plasma | 21 | 15 | Depletion of 14 major plasma proteins, LC-MRM-MS with IS (41 proteins) |
| 37 (35–40) | 10 | 30 | 37 (35–40) | plasma | 3 | 2 | ELISA (3 proteins) | |
| Liu, L. Y., 2013 [33] | 30.3 ± 3.2 | 15 | 16 | 30.3 ± 3.2 | serum | 11 | 8 | ELISA (22 proteins) |
| 37.1 ± 1.4 | 17 | 16 | 37.1 ± 1.4 | serum | 11 | 8 | ||
| Lu, Q., 2016 [34] | 36.4 ± 1.6 | 10 | 10 | 36.4 ± 1.6 | serum | 26 | 15 | Depletion of major plasma proteins, SDS-PAGE, LC-MS/MS |
| 36.7 ± 1.8 | 69 | 60 | 36.7 ± 1.8 | serum | 1 | 1 | ELISA (1 protein) | |
| Odenkirk, M., 2020 [35] | 38.9 ± 1.4 | 48 | 98 | 38.9 ± 1.4 | plasma | 160 | 56 | Depletion of 14 major plasma proteins, LC-IMS-MS |
| Shi, X.-F., 2024 [36] | 31 | 42 | 58 | 31 | serum | 63 | 8 | Depletion of major plasma proteins, iTRAQ, high pH RPLC, LC-MS/MS |
| Starodubtseva, N.L., 2024 [37] | 11.0–13.0 | 30 | 13 | 37.2 [34.4; 38.1] | serum | 6 | 3 | LC-MRM-MS/MS with IS (125 proteins) |
| Than, N.G., 2018 [38] | 9 (8–9) | 5 | 5 | 34.9 (29.3–35.3) | serum | 14 | 8 | Depletion of 14 major plasma proteins, 2D-DIGE, LC-MS/MS |
| 9 (8–10) | 5 | 5 | 38.1 (38.0–38.1) | serum | 8 | 5 | ||
| Than, N.G., 2022 [39] | 8–15.9 | 109 | 90 | 39.6 ± 1.17 | plasma | 28 | 14 | SOMAmer-1125 proteins |
| 16–19.9 | 109 | 90 | 39.6 ± 1.17 | plasma | 60 | 19 | ||
| 20–23.9 | 109 | 90 | 39.6 ± 1.17 | plasma | 43 | 17 | ||
| 24–27.9 | 109 | 90 | 39.6 ± 1.17 | plasma | 19 | 8 | ||
| 28–31.9 | 109 | 90 | 39.6 ± 1.17 | plasma | 38 | 14 | ||
| Uchida, Y., 2021 [40] | 19 ± 4 | 7 | 14 | 29.4 ± 1.6 | plasma | 105 | 47 | LC-SWATH-MS/MS |
| 19 ± 3 | 36 | 120 | 35.5 ± 4.3 | plasma | 4 | 2 | LC-SRM-MS/MS with IS (4 proteins) | |
| 19 ± 2 | 36 | 54 | 38.2 ± 1.7 | plasma | 2 | 1 | LC-SRM-MS/MS with IS (2 proteins) | |
| Wang, X., 2022 [41] | 34.7 ± 3.3 | 15 | 15 | 35.6 [31.4; 37.7] | plasma | 25 | 3 | Olink Inflammation panel (92 proteins) |
| 35.1 [32.1; 37.6] | 43 | 44 | 35.6 [32.6; 38.0] | plasma | 1 | 0 | ELISA (1 protein) | |
| 12.5 ± 0.7 | 37 | 37 | 37.4 [33.9; 38.7] | plasma | 1 | 0 | ELISA (1 protein) | |
| Youssef, L., 2021 [42] | 31.3 | 14 | 6 | 32 | serum | 17 | 14 | Depletion of 7 major plasma proteins, TMT, LC-MS/MS |
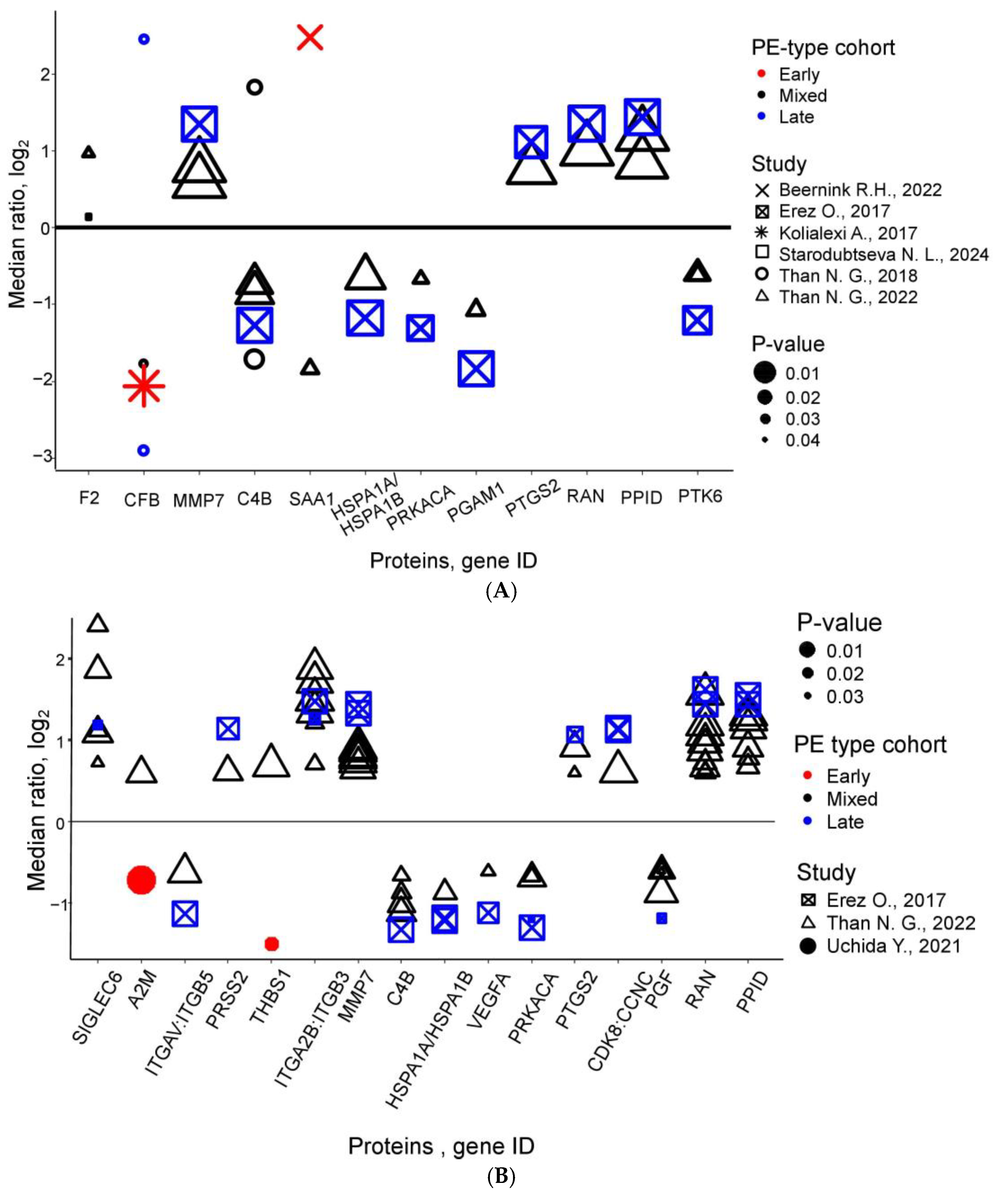
| Protein Name | Uniprot ID | Gene ID | Trimester (PE Type) | Number of Comparisons | Fold Change (Min–Max) | References |
|---|---|---|---|---|---|---|
| Complement C4b | P0C0L5 | C4B | 1 (m, l) | 4 | 0.30–0.61 | [30,38,39] |
| 2 (m, l) | 5 | 0.39–0.63 | [30,39] | |||
| Complement factor B | P00751 | CFB | 1 (e, m, l) | 3 | 0.13–0.29 | [31,38] |
| Matrilysin | P09237 | MMP7 | 1 (m, l) | 3 | 1.50–2.55 | [30,39] |
| 2 (m, l) | 9 | 1.58–2.70 | [30,39] | |||
| Peptidyl-prolyl cis-trans isomerase D | Q08752 | PPID | 1 (m, l) | 3 | 1.78–2.71 | [30,39] |
| 2 (m, l) | 8 | 1.60–2.93 | [30,39] | |||
| Protein-tyrosine kinase 6 | Q13882 | PTK6 | 1 (m, l) | 3 | 0.43–0.66 | [30,39] |
| GTP-binding nuclear protein Ran | P62826 | RAN | 2 (m, l) | 11 | 1.52–3.07 | [30,39] |
| 3 (m, l) | 4 | 1.75–2.74 | [30,39] | |||
| Integrin alpha-IIb: beta-3 complex | P08514:P05106 | ITGA2B:ITGB3 | 2 (m, l) | 8 | 1.64–3.68 | [30,39] |
| 3 (m, l) | 4 | 1.66–2.56 | [30,39] | |||
| Sialic acid-binding Ig-like lectin 6 | O43699 | SIGLEC6 | 2 (m, l) | 6 | 1.65–5.33 | [30,39] |
| 3 (m, l) | 6 | 1.94–6.04 | [29,30,39] | |||
| Placenta growth factor | P49763 | PGF | 2 (m, l) | 6 | 0.44–0.66 | [30,39] |
| 3 (m, l) | 6 | 0.41–0.63 | [30,39] | |||
| cAMP-dependent protein kinase catalytic subunit alpha | P17612 | PRKACA | 2 (m, l) | 4 | 0.41–0.63 | [30,39] |
| Heat shock 70 kDa protein 1A/1B | P0DMV8/P0DMV9 | HSPA1A/HSPA1B | 2 (m, l) | 3 | 0.43–0.55 | [30,39] |
| Prostaglandin G/H synthase 2 | P35354 | PTGS2 | 2 (m, l) | 3 | 1.52–2.10 | [30,39] |
| Cyclin-dependent kinase 8:Cyclin-C complex | P49336:P24863 | CDK8:CCNC | 2 (m, l) | 3 | 1.53–2.19 | [30,39] |
| Inter-alpha-trypsin inhibitor heavy chain H2 | P19823 | ITIH2 | 3 (e, l) | 3 | 1.22–1.31 | [28,42] |
| Inter-alpha-trypsin inhibitor heavy chain H3 | Q06033 | ITIH3 | 3 (e, l) | 6 | 1.22–1.60 | [28,35,42] |
| Vascular endothelial growth factor receptor 1 | P17948 | FLT1 | 3 (e, m, l) | 6 | 1.60–6.15 | [29,32,33] |
| Apolipoprotein A-I | P02647 | APOA1 | 3 (e, l) | 3 | 0.56–0.86 | [32,33] |
| Apolipoprotein C-III | P02656 | APOC3 | 3 (e, l) | 5 | 1.33–1.92 | [28,33,35] |
| Apolipoprotein E | P02649 | APOE | 3 (e, l) | 6 | 1.36–26.0 | [26,28,36] |
| Carboxypeptidase N subunit 2 | P22792 | CPN2 | 3 (e, l) | 5 | 1.13–1.39 | [28,35] |
| Hemopexin | P02790 | HPX | 3 (e, l) | 5 | 0.76–0.91 | [28,35] |
| Inhibin beta A chain | P08476 | INHBA | 3 (m, l) | 5 | 1.31–2.04 | [29,39] |
| N-acylethanolamine-hydrolyzing acid amidase | Q02083 | NAAA | 3 (m, l) | 5 | 1.53–2.68 | [30,39] |
| Complement factor D | P00746 | CFD | 3 (e, l) | 3 | 1.19–2.81 | [28,35] |
| Fibronectin | P02751 | FN1 | 3 (e, m, l) | 6 | 1.40–2.19 | [28,32,34,35] |
| Insulin-like growth factor-binding protein 4 | P22692 | IGFBP4 | 3 (e, l) | 3 | 1.19–30.1 | [28,35] |
| Nidogen-1 | P14543 | NID1 | 3 (e, l) | 3 | 1.36–2.18 | [29,30] |
| Protein AMBP | P02760 | AMBP | 3 (e, l) | 3 | 1.32–1.44 | [28,42] |
| von Willebrand factor | P04275 | VWF | 3 (e, l) | 3 | 1.52–1.60 | [28,35,42] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Starodubtseva, N.; Poluektova, A.; Tokareva, A.; Kukaev, E.; Avdeeva, A.; Rimskaya, E.; Khodzayeva, Z. Proteome-Based Maternal Plasma and Serum Biomarkers for Preeclampsia: A Systematic Review and Meta-Analysis. Life 2025, 15, 776. https://doi.org/10.3390/life15050776
Starodubtseva N, Poluektova A, Tokareva A, Kukaev E, Avdeeva A, Rimskaya E, Khodzayeva Z. Proteome-Based Maternal Plasma and Serum Biomarkers for Preeclampsia: A Systematic Review and Meta-Analysis. Life. 2025; 15(5):776. https://doi.org/10.3390/life15050776
Chicago/Turabian StyleStarodubtseva, Natalia, Alina Poluektova, Alisa Tokareva, Evgenii Kukaev, Anna Avdeeva, Elena Rimskaya, and Zulfiya Khodzayeva. 2025. "Proteome-Based Maternal Plasma and Serum Biomarkers for Preeclampsia: A Systematic Review and Meta-Analysis" Life 15, no. 5: 776. https://doi.org/10.3390/life15050776
APA StyleStarodubtseva, N., Poluektova, A., Tokareva, A., Kukaev, E., Avdeeva, A., Rimskaya, E., & Khodzayeva, Z. (2025). Proteome-Based Maternal Plasma and Serum Biomarkers for Preeclampsia: A Systematic Review and Meta-Analysis. Life, 15(5), 776. https://doi.org/10.3390/life15050776






