The Relationship Between Obesity, Bariatric Surgery, and Infertility: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Quality Assessment
- Selection of research participants, including representativeness of the exposed group, selection of the non-exposed population, and exposure assessment.
- Study groups should be comparable, with research controlling for confounding variables like as age, BMI, comorbidities, or pre-existing infertility issues.
- Outcome evaluation, including adequate follow-up, clarity of outcome definitions, and possible loss due to follow-up biases.
- Low risk of bias (7–9 points): Studies in this category were deemed methodologically sound, with clearly specified participant selection, extensive result evaluation, and adequate comparison procedures. These studies demonstrated substantial evidence for a link between bariatric surgery and reproductive results, with few complicating variables.
- Modest risk of bias (5–6 points): This group includes studies with modest constraints, such as small sample numbers, retrospective design, or minor methodological problems. While these studies nevertheless offered useful information, their findings were susceptible to potential biases or confounding variables that might have impacted outcomes.
- High risk of bias (0–4 points): Studies in this category have severe methodological flaws, such as selection bias, a lack of control groups, short follow-up periods, or insufficient data reporting. Their findings should be viewed with caution since there is a risk of significant bias influencing the stated results.
- Study design (prospective, retrospective, or randomized controlled trial), with prospective and RCT studies being regarded as better quality owing to their capacity to account for variables.
- Larger samples were preferred because they provided more statistical power and generalizability.
- Selection bias was evaluated based on how participants were recruited, whether rigorous inclusion criteria were followed, and if adequate control groups were employed.
- Outcome evaluation ensured that fertility, pregnancy, and neonatal outcomes were well-defined and regularly measured.
- Follow-up time, as studies with longer follow-up durations gave more information about long-term reproductive results following bariatric surgery.
- Risk of bias, which took into account concerns including recollection bias, inadequate data, loss to follow-up, and potential conflicts of interest.
- Larger, more representative sample sizes will improve statistical power.
- Long-term follow-up periods are used to investigate the long-term impact of bariatric surgery on reproductive health.
- Standardized outcome measurements include fertility, pregnancy problems, and newborn health markers.
- Improved control of confounding variables such age, BMI, metabolic health, and pre-existing reproductive diseases.
- Large sample size studies (e.g., Gosman et al., 2010, Sheiner et al., 2011, Neto et al., 2012) were evaluated as high-quality because their findings were more generalizable to the larger population of women undergoing bariatric surgery for infertility therapy.
- Studies with a smaller sample size (e.g., Bilenka et al., 1995, Rochester et al., 2009, and Musella et al., 2011) had a higher risk of bias and were ranked worse in quality due to less statistical power and generalizability.
- Studies on PCOS populations (e.g., Eid et al., 2005, Jamal et al., 2012, Ezzat et al., 2021) had moderate quality ratings due to limited sample sizes, but they gave particular insights into how bariatric surgery impacts fertility in PCOS individuals.
- High-quality research (e.g., Gosman et al., 2010, Sheiner et al., 2011, Karadag et al., 2020) enrolled a varied variety of individuals with various levels of obesity and reproductive health concerns, making their findings more generalizable to the general population.
- Studies that focused primarily on PCOS patients (e.g., Eid et al., 2005, Jamal et al., 2012, and Ezzat et al., 2021) showed lower external validity since their findings only applied to a subset of obese women experiencing infertility.
- Studies with relatively small sample sizes (e.g., Bilenka et al., 1995; Rochester et al., 2009) were more prone to selection bias, as their findings may not be typical of the larger population.
- Long-term follow-up studies (e.g., Marceau et al., 2004, Neto et al., 2012, Karadag et al., 2020) have offered important insights into the long-term durability of fertility improvements and pregnancy outcomes following bariatric surgery.
- Studies with short follow-up periods (e.g., Rochester et al., 2009, Sahab Al Kabbi et al., 2018, and Jamal et al., 2012) received lower ratings due to a lack of data on long-term reproductive outcomes.
2.2. Eligibility Criteria
2.2.1. Inclusion Criteria
- Eligible studies met the following criteria:
2.2.2. Exclusion Criteria
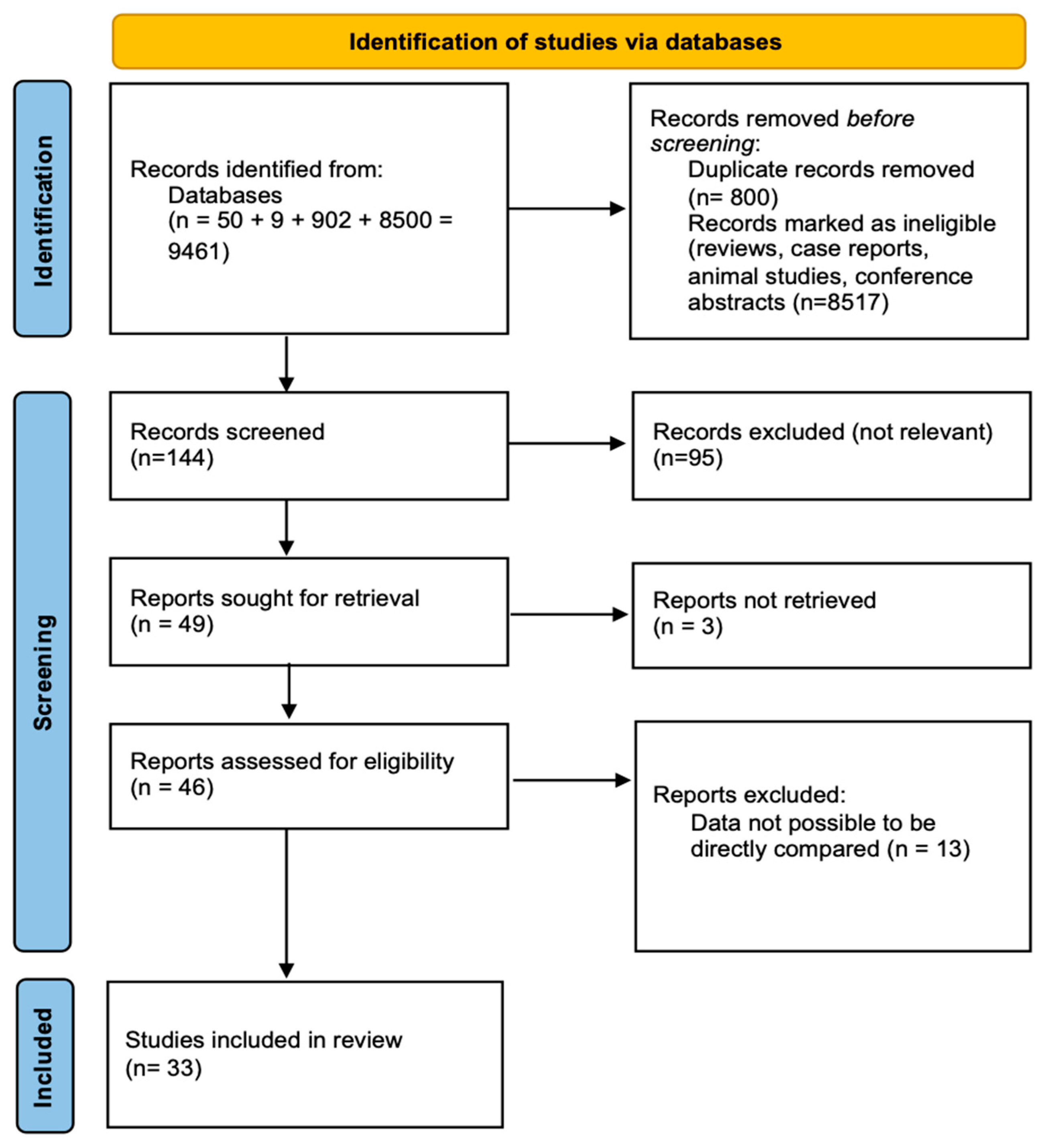
3. Results
3.1. Study Selection and Characteristics
- Weight reduction therapies have been studied without regard for reproductive results.
- Research on male fertility after bariatric surgery.
- Studies that describe metabolic or endocrine results without specifically assessing monthly regularity, ovulation, pregnancy rates, or newborn health.
- Population-based research in which reproductive results were not divided according to bariatric surgery status.
3.2. Study Designs and Methodological Features
3.3. Fertility and Neonatal Outcomes
3.4. Patient Characteristics
3.5. Fertility, Endocrine Outcomes, and Vitamin Deficiencies
3.6. Pregnancy Complications Following Bariatric Surgery
4. Discussion
4.1. Bariatric Surgery and Fertility: The Restoration of Ovulatory Function and Enhanced ART Success
4.2. Maternal Outcomes: Metabolic Benefits and Nutritional Risks
4.3. Neonatal Outcomes: The Challenge of Fetal Growth Restriction and Developmental Health
4.4. Preterm Birth and Increased NICU Admissions
4.5. Comparing Roux-en-Y Gastric Bypass (RYGB) and Sleeve Gastrectomy (SG): Which Procedure Is Optimal?
4.6. Fetal Growth Outcomes: The Risk of Small-for-Gestational-Age (SGA) Infant
- Reduced placental nutrition transfer causes intrauterine growth restriction (IUGR).
- Maternal anemia and iron deficiency might impede fetal oxygenation and growth.
- Vitamin B12 and folate deficits raise the risk of placental insufficiency and fetal malnutrition.
5. Future Research Directions and Clinical Recommendations
6. Limitations
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Yang, M.; Liu, S.; Zhang, C. The Related Metabolic Diseases and Treatments of Obesity. Healthcare 2022, 10, 1616. [Google Scholar] [CrossRef]
- Zheng, L.; Yang, L.; Guo, Z.; Yao, N.; Zhang, S.; Pu, P. Obesity and its impact on female reproductive health: Unraveling the connections. Front. Endocrinol. 2024, 14, 1326546. [Google Scholar] [CrossRef]
- Ozcan Dag, Z.; Dilbaz, B. Impact of obesity on infertility in women. J. Turk. Ger. Gynecol. Assoc. 2015, 16, 111–117. [Google Scholar] [CrossRef]
- Carson, S.A.; Kallen, A.N. Diagnosis and Management of Infertility: A Review. JAMA 2021, 326, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Silvestris, E.; De Pergola, G.; Rosania, R.; Loverro, G. Obesity as disruptor of the female fertility. Reprod. Biol. Endocrinol. 2018, 16, 22. [Google Scholar] [CrossRef] [PubMed]
- Westerman, R.; Kuhnt, A.-K. Metabolic risk factors and fertility disorders: A narrative review of the female perspective. Reprod. Biomed. Soc. Online 2022, 14, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Lonardo, M.S.; Cacciapuoti, N.; Guida, B.; Di Lorenzo, M.; Chiurazzi, M.; Damiano, S.; Menale, C. Hypothalamic-Ovarian axis and Adiposity Relationship in Polycystic Ovary Syndrome: Physiopathology and Therapeutic Options for the Management of Metabolic and Inflammatory Aspects. Curr. Obes. Rep. 2024, 13, 51–70. [Google Scholar] [CrossRef]
- Baraskar, K.; Thakur, P.; Shrivastava, R.; Shrivastava, V.K. Female obesity: Association with endocrine disruption and reproductive dysfunction. Obes. Med. 2021, 28, 100375. [Google Scholar] [CrossRef]
- Purwar, A.; Nagpure, S. Insulin Resistance in Polycystic Ovarian Syndrome. Cureus 2022, 14, e30351. [Google Scholar] [CrossRef]
- Ding, H.; Zhang, J.; Zhang, F.; Zhang, S.; Chen, X.; Liang, W.; Xie, Q. Resistance to the Insulin and Elevated Level of Androgen: A Major Cause of Polycystic Ovary Syndrome. Front. Endocrinol. 2021, 12, 741764. [Google Scholar] [CrossRef]
- Codner, E.; Escobar-Morreale, H.F. Hyperandrogenism and Polycystic Ovary Syndrome in Women with Type 1 Diabetes Mellitus. J. Clin. Endocrinol. Metab. 2007, 92, 1209–1216. [Google Scholar] [CrossRef] [PubMed]
- Cena, H.; Chiovato, L.; Nappi, R.E. Obesity, Polycystic Ovary Syndrome, and Infertility: A New Avenue for GLP-1 Receptor Agonists. J. Clin. Endocrinol. Metab. 2020, 105, e2695–e2709. [Google Scholar] [CrossRef] [PubMed]
- Manna, P.; Jain, S.K. Obesity, Oxidative Stress, Adipose Tissue Dysfunction, and the Associated Health Risks: Causes and Therapeutic Strategies. Metab. Syndr. Relat. Disord. 2015, 13, 423–444. [Google Scholar] [CrossRef] [PubMed]
- Moustakli, E.; Zikopoulos, A.; Skentou, C.; Bouba, I.; Dafopoulos, K.; Georgiou, I. Evolution of Minimally Invasive and Non-Invasive Preimplantation Genetic Testing: An Overview. J. Clin. Med. 2024, 13, 2160. [Google Scholar] [CrossRef]
- Almutairi, H.; Aldhalea, M.S.; Almaaz, M.A.; Aljuhani, S.A.; Aloraini, R.I.; Alamoudi, A.A.; Alkhalifah, W.F.; Alrushaid, L.A.; Alanzy, H.W.; Alzuwayyid, M.; et al. The Effectiveness of Bariatric Surgery on Treating Infertility in Women—A Systematic Review and Meta-Analysis. J. Clin. Med. 2024, 13, 5569. [Google Scholar] [CrossRef]
- Santos-Sousa, H.; Nogueiro, J.; Lindeza, L.; Carmona, M.N.; Amorim-Cruz, F.; Resende, F.; Costa-Pinho, A.; Preto, J.; Sousa-Pinto, B.; Carneiro, S.; et al. Roux-en-Y gastric bypass and sleeve gastrectomy as revisional bariatric procedures after adjustable gastric banding: A retrospective cohort study. Langenbecks Arch. Surg. 2023, 408, 441. [Google Scholar] [CrossRef]
- Huluță, I.; Apostol, L.-M.; Botezatu, R.; Panaitescu, A.M.; Gică, C.; Sima, R.-M.; Gică, N.; Nedelea, F.M. Beyond Weight Loss: A Comprehensive Review of Pregnancy Management following Bariatric Procedures. Medicina 2024, 60, 635. [Google Scholar] [CrossRef]
- Wang, H.-Q.; Zhang, W.-D.; Yuan, B.; Zhang, J.-B. Advances in the Regulation of Mammalian Follicle-Stimulating Hormone Secretion. Animals 2021, 11, 1134. [Google Scholar] [CrossRef]
- Hezelgrave, N.L.; Oteng-Ntim, E. Pregnancy after Bariatric Surgery: A Review. J. Obes. 2011, 2011, 501939. [Google Scholar] [CrossRef]
- Pg Baharuddin, D.M.; Payus, A.O.; Abdel Malek Fahmy, E.H.; Sawatan, W.; Than, W.W.; Abdelhafez, M.M.; Oo Leik, N.K.; Ag Daud, D.M.; Mohd Daud, M.N.; Ahmad, Z.N.S. Bariatric surgery and its impact on fertility, pregnancy and its outcome: A narrative review. Ann. Med. Surg. 2021, 72, 103038. [Google Scholar] [CrossRef]
- Akhter, Z.; Heslehurst, N.; Ceulemans, D.; Rankin, J.; Ackroyd, R.; Devlieger, R. Pregnancy after Bariatric Surgery: A Nested Case-Control Study of Risk Factors for Small for Gestational Age Babies in AURORA. Nutrients 2021, 13, 1699. [Google Scholar] [CrossRef] [PubMed]
- Deitel, M.; Stone, E.; Kassam, H.A.; Wilk, E.J.; Sutherland, D.J. Gynecologic-obstetric changes after loss of massive excess weight following bariatric surgery. J. Am. Coll. Nutr. 1988, 7, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Bilenka, B.; Ben-Shlomo, I.; Cozacov, C.; Gold, C.H.; Zohar, S. Fertility, miscarriage and pregnancy after vertical banded gastroplasty operation for morbid obesity. Acta Obstet. Gynecol. Scand. 1995, 74, 42–44. [Google Scholar] [CrossRef] [PubMed]
- Sheiner, E.; Levy, A.; Silverberg, D.; Menes, T.S.; Levy, I.; Katz, M.; Mazor, M. Pregnancy after bariatric surgery is not associated with adverse perinatal outcome. Am. J. Obstet. Gynecol. 2004, 190, 1335–1340. [Google Scholar] [CrossRef]
- Marceau, P.; Kaufman, D.; Biron, S.; Hould, F.-S.; Lebel, S.; Marceau, S.; Kral, J.G. Outcome of Pregnancies after Biliopancreatic Diversion. Obes. Surg. 2004, 14, 318–324. [Google Scholar] [CrossRef]
- Eid, G.M.; Cottam, D.R.; Velcu, L.M.; Mattar, S.G.; Korytkowski, M.T.; Gosman, G.; Hindi, P.; Schauer, P.R. Effective treatment of polycystic ovarian syndrome with Roux-en-Y gastric bypass. Surg. Obes. Relat. Dis. 2005, 1, 77–80. [Google Scholar] [CrossRef]
- Rochester, D.; Jain, A.; Polotsky, A.J.; Polotsky, H.; Gibbs, K.; Isaac, B.; Zeitlian, G.; Hickmon, C.; Feng, S.; Santoro, N. Partial recovery of luteal function after bariatric surgery in obese women. Fertil. Steril. 2009, 92, 1410–1415. [Google Scholar] [CrossRef]
- Gosman, G.G.; King, W.C.; Schrope, B.; Steffen, K.J.; Strain, G.W.; Courcoulas, A.P.; Flum, D.R.; Pender, J.R.; Simhan, H.N. Reproductive health of women electing bariatric surgery. Fertil. Steril. 2010, 94, 1426–1431. [Google Scholar] [CrossRef]
- Sheiner, E.; Edri, A.; Balaban, E.; Levi, I.; Aricha-Tamir, B. Pregnancy outcome of patients who conceive during or after the first year following bariatric surgery. Am. J. Obstet. Gynecol. 2011, 204, 50.E1–50.E6. [Google Scholar] [CrossRef]
- Musella, M.; Milone, M.; Bellini, M.; Sosa Fernandez, M.E.; Sosa Fernandez, L.M.; Leongito, M.; Milone, F. The Potential Role of Intragastric Balloon in the Treatment of Obese-Related Infertility: Personal Experience. Obes. Surg. 2011, 21, 426–430. [Google Scholar] [CrossRef]
- Facchiano, E.; Iannelli, A.; Santulli, P.; Mandelbrot, L.; Msika, S. Pregnancy after laparoscopic bariatric surgery: Comparative study of adjustable gastric banding and Roux-en-Y gastric bypass. Surg. Obes. Relat. Dis. 2012, 8, 429–433. [Google Scholar] [CrossRef]
- Jamal, M.; Gunay, Y.; Capper, A.; Eid, A.; Heitshusen, D.; Samuel, I. Roux-en-Y gastric bypass ameliorates polycystic ovary syndrome and dramatically improves conception rates: A 9-year analysis. Surg. Obes. Relat. Dis. 2012, 8, 440–444. [Google Scholar] [CrossRef] [PubMed]
- Legro, R.S.; Dodson, W.C.; Gnatuk, C.L.; Estes, S.J.; Kunselman, A.R.; Meadows, J.W.; Kesner, J.S.; Krieg, E.F.; Rogers, A.M.; Haluck, R.S.; et al. Effects of Gastric Bypass Surgery on Female Reproductive Function. J. Clin. Endocrinol. Metab. 2012, 97, 4540–4548. [Google Scholar] [CrossRef] [PubMed]
- Neto, R.M.L.; Herbella, F.A.M.; Tauil, R.M.; Silva, F.S.; De Lima, S.E. Comorbidities Remission After Roux-en-Y Gastric Bypass for Morbid Obesity is Sustained in a Long-Term Follow-up and Correlates with Weight Regain. Obes. Surg. 2012, 22, 1580–1585. [Google Scholar] [CrossRef] [PubMed]
- Alatishe, A.; Ammori, B.J.; New, J.P.; Syed, A.A. Bariatric surgery in women of childbearing age. QJM 2013, 106, 717–720. [Google Scholar] [CrossRef]
- Christofolini, J.; Bianco, B.; Santos, G.; Adami, F.; Christofolini, D.; Barbosa, C.P. Bariatric surgery influences the number and quality of oocytes in patients submitted to assisted reproduction techniques. Obesity 2014, 22, 939–942. [Google Scholar] [CrossRef]
- Eid, G.M.; McCloskey, C.; Titchner, R.; Korytkowski, M.; Gross, D.; Grabowski, C.; Wilson, M. Changes in hormones and biomarkers in polycystic ovarian syndrome treated with gastric bypass. Surg. Obes. Relat. Dis. 2014, 10, 787–791. [Google Scholar] [CrossRef]
- Goldman, R.H.; Missmer, S.A.; Robinson, M.K.; Farland, L.V.; Ginsburg, E.S. Reproductive Outcomes Differ Following Roux-en-Y Gastric Bypass and Adjustable Gastric Band Compared with Those of an Obese Non-Surgical Group. Obes. Surg. 2016, 26, 2581–2589. [Google Scholar] [CrossRef]
- Milone, M.; Sosa Fernandez, L.M.; Sosa Fernandez, L.V.; Manigrasso, M.; Elmore, U.; De Palma, G.D.; Musella, M.; Milone, F. Does Bariatric Surgery Improve Assisted Reproductive Technology Outcomes in Obese Infertile Women? Obes. Surg. 2017, 27, 2106–2112. [Google Scholar] [CrossRef]
- Yau, P.O.; Parikh, M.; Saunders, J.K.; Chui, P.; Zablocki, T.; Welcome, A.U. Pregnancy after bariatric surgery: The effect of time-to-conception on pregnancy outcomes. Surg. Obes. Relat. Dis. Off. J. Am. Soc. Bariatr. Surg. 2017, 13, 1899–1905. [Google Scholar] [CrossRef]
- Nilsson-Condori, E.; Hedenbro, J.L.; Thurin-Kjellberg, A.; Giwercman, A.; Friberg, B. Impact of diet and bariatric surgery on anti-Müllerian hormone levels. Hum. Reprod. 2018, 33, 690–693. [Google Scholar] [CrossRef]
- Kabbi, M.S.N.; Al-Taee, H.A.; Kareem Al Hussaini, S. Impact of Bariatric surgery on antimularian hormone in reproductive age women. Middle East Fertil. Soc. J. 2018, 23, 273–277. [Google Scholar] [CrossRef]
- Vincentelli, C.; Maraninchi, M.; Valéro, R.; Béliard, S.; Maurice, F.; Emungania, O.; Berthet, B.; Lombard, E.; Dutour, A.; Gaborit, B.; et al. One-year impact of bariatric surgery on serum anti-Mullerian-hormone levels in severely obese women. J. Assist. Reprod. Genet. 2018, 35, 1317–1324. [Google Scholar] [CrossRef] [PubMed]
- Cruz, S.; Matos, A.; Cruz, S.; Pereira, S.; Saboya, C.; Ramalho, A. Pregnancy after 24 Postoperative Months of Roux-En-Y Gastric Bypass Presents Risk of Pregnancy Complications Similar to Pregnancy within the First Postoperative Year. Ann. Nutr. Metab. 2019, 75, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Early pregnancy after bariatric surgery: A single-institute preliminary experience. Turk. J. Med. Sci. 2020, 50, 171–176. [CrossRef]
- Menke, M.N.; King, W.C.; White, G.E.; Gosman, G.G.; Courcoulas, A.P.; Dakin, G.F.; Flum, D.R.; Orcutt, M.J.; Pomp, A.; Pories, W.J.; et al. Conception rates and contraceptive use after bariatric surgery among women with infertility: Evidence from a prospective multicenter cohort study. Surg. Obes. Relat. Dis. 2019, 15, 777–785. [Google Scholar] [CrossRef]
- Grzegorczyk-Martin, V.; Fréour, T.; De Bantel Finet, A.; Bonnet, E.; Merzouk, M.; Roset, J.; Roger, V.; Cédrin-Durnerin, I.; Wainer, R.; Avril, C.; et al. IVF outcomes in patients with a history of bariatric surgery: A multicenter retrospective cohort study. Hum. Reprod. 2020, 35, 2755–2762. [Google Scholar] [CrossRef]
- Jacamon, A.-S.; Merviel, P.; Herrmann, S.; Pan-Petesch, B.; Lacut, K.; Thereaux, J. Outcomes of pregnancy after bariatric surgery: Results of a French matched-cohort study. Surg. Obes. Relat. Dis. Off. J. Am. Soc. Bariatr. Surg. 2020, 16, 1275–1282. [Google Scholar] [CrossRef]
- Karadağ, C.; Demircan, S.; Çalışkan, E. Effects of laparoscopic sleeve gastrectomy on obstetric outcomes within 12 months after surgery. J. Obstet. Gynaecol. Res. 2020, 46, 266–271. [Google Scholar] [CrossRef]
- Ezzat, R.S.; Abdallah, W.; Elsayed, M.; Saleh, H.S.; Abdalla, W. Impact of bariatric surgery on androgen profile and ovarian volume in obese polycystic ovary syndrome patients with infertility. Saudi J. Biol. Sci. 2021, 28, 5048–5052. [Google Scholar] [CrossRef]
- Thornton, O.; Daggett, E.; Zia, L.; Quian, A.; Close, E.; Khaitan, L.; El-Nashar, S.A.; Shaker, M. Counseling, contraception, and conception rates in patients undergoing bariatric surgery: A retrospective review. Contraception 2021, 104, 202–205. [Google Scholar] [CrossRef]
- Snoek, K.M.; Van De Woestijne, N.; Ritfeld, V.E.E.G.; Klaassen, R.A.; Versendaal, H.; Galjaard, S.; Willemsen, S.P.; Laven, J.S.E.; Steegers-Theunissen, R.P.M.; Schoenmakers, S. Preconception maternal gastric bypass surgery and the impact on fetal growth parameters. Surg. Obes. Relat. Dis. 2024, 20, 128–137. [Google Scholar] [CrossRef]
- Joly, M.-A.; Peyronnet, V.; Coupaye, M.; Ledoux, S.; Pourtier, N.; Pencole, L.; Mandelbrot, L. Comparison of pregnancy outcomes after bariatric surgery by sleeve gastrectomy versus gastric bypass. Eur. J. Obstet. Gynecol. Reprod. Biol. X 2024, 22, 100309. [Google Scholar] [CrossRef]
- Samarasinghe, S.N.S.; Leca, B.; Alabdulkader, S.; Dimitriadis, G.K.; Davasgaium, A.; Thadani, P.; Parry, K.; Luli, M.; O’Donnell, K.; Johnson, B.; et al. Bariatric surgery for spontaneous ovulation in women living with polycystic ovary syndrome: The BAMBINI multicentre, open-label, randomised controlled trial. Lancet 2024, 403, 2489–2503. [Google Scholar] [CrossRef]
- Musella, M.; Milone, M.; Bellini, M.; Sosa Fernandez, L.M.; Leongito, M.; Milone, F. Effect of bariatric surgery on obesity-related infertility. Surg. Obes. Relat. Dis. 2012, 8, 445–449. [Google Scholar] [CrossRef]
- Giviziez, C.R.; Sanchez, E.G.M.; Approbato, M.S.; Maia, M.C.S.; Fleury, E.A.B.; Sasaki, R.S.A. Obesity and anovulatory infertility: A review. JBRA Assist. Reprod. 2016, 20, 240–245. [Google Scholar] [CrossRef]
- Morgan, H.D.; Morrison, A.E.; Hamza, M.; Jones, C.; Cassar, C.B.; Meek, C.L. The approach to a pregnancy after bariatric surgery. Clin. Med. 2025, 25, 100275. [Google Scholar] [CrossRef]
- Snoek, K.M.; Steegers-Theunissen, R.P.M.; Hazebroek, E.J.; Willemsen, S.P.; Galjaard, S.; Laven, J.S.E.; Schoenmakers, S. The effects of bariatric surgery on periconception maternal health: A systematic review and meta-analysis. Hum. Reprod. Update 2021, 27, 1030–1055. [Google Scholar] [CrossRef]
- Vieira De Sousa, J.P.; Santos-Sousa, H.; Vieira, S.; Nunes, R.; Nogueiro, J.; Pereira, A.; Resende, F.; Costa-Pinho, A.; Preto, J.; Sousa-Pinto, B.; et al. Assessing Nutritional Deficiencies in Bariatric Surgery Patients: A Comparative Study of Roux-en-Y Gastric Bypass versus Sleeve Gastrectomy. J. Pers. Med. 2024, 14, 650. [Google Scholar] [CrossRef]
- Catalano, P.M. The impact of gestational diabetes and maternal obesity on the mother and her offspring. J. Dev. Orig. Health Dis. 2010, 1, 208–215. [Google Scholar] [CrossRef]
- Johansen, J.E.; Broberger, C.; Lavebratt, C.; Johansson, C.; Kuhar, M.J.; Hökfelt, T.; Schalling, M. Hypothalamic CART and serum leptin levels are reduced in the anorectic (anx/anx) mouse. Brain Res. Mol. Brain Res. 2000, 84, 97–105. [Google Scholar] [CrossRef]
- Mustafa, H.J.; Javinani, A.; Seif, K.; Aghajani, F.; Makar, E.J.; Selhorst, S.; Crimmins, S. Prepregnancy Roux-en-Y gastric bypass vs sleeve gastrectomy: A systematic review, pairwise, and network meta-analysis of obstetrical and neonatal outcomes. Am. J. Obstet. Gynecol. 2023, 5, 100914. [Google Scholar] [CrossRef]
- Kjær, M.M.; Nilas, L. Pregnancy after bariatric surgery–a review of benefits and risks. Acta Obstet. Gynecol. Scand. 2013, 92, 264–271. [Google Scholar] [CrossRef]
- Al Mansoori, A.; Shakoor, H.; Ali, H.I.; Feehan, J.; Al Dhaheri, A.S.; Cheikh Ismail, L.; Bosevski, M.; Apostolopoulos, V.; Stojanovska, L. The Effects of Bariatric Surgery on Vitamin B Status and Mental Health. Nutrients 2021, 13, 1383. [Google Scholar] [CrossRef]
- Han, Y.; Jia, Y.; Wang, H.; Cao, L.; Zhao, Y. Comparative analysis of weight loss and resolution of comorbidities between laparoscopic sleeve gastrectomy and Roux-en-Y gastric bypass: A systematic review and meta-analysis based on 18 studies. Int. J. Surg. 2020, 76, 101–110. [Google Scholar] [CrossRef]
- Al-Dewik, N.I.; Samara, M.; Mahmah, A.; Al-Dewik, A.; Abou Nahia, S.; Abukhadijah, H.J.; Samara, Y.; Hammuda, S.; Razzaq, A.; Al-Dweik, M.R.; et al. Maternal and neonatal risks and outcomes after bariatric surgery: A comparative population based study across BMI categories in Qatar. Sci. Rep. 2024, 14, 27107. [Google Scholar] [CrossRef]
- Adams, T.D.; Hammoud, A.O.; Davidson, L.E.; Laferrère, B.; Fraser, A.; Stanford, J.B.; Hashibe, M.; Greenwood, J.L.J.; Kim, J.; Taylor, D.; et al. Maternal and neonatal outcomes for pregnancies before and after gastric bypass surgery. Int. J. Obes. 2015, 39, 686–694. [Google Scholar] [CrossRef]
- Kjær, M.M.; Lauenborg, J.; Breum, B.M.; Nilas, L. The risk of adverse pregnancy outcome after bariatric surgery: A nationwide register-based matched cohort study. Am. J. Obstet. Gynecol. 2013, 208, 464.E1–464.E5. [Google Scholar] [CrossRef]
- Johansson, K.; Cnattingius, S.; Näslund, I.; Roos, N.; Trolle Lagerros, Y.; Granath, F.; Stephansson, O.; Neovius, M. Outcomes of Pregnancy after Bariatric Surgery. N. Engl. J. Med. 2015, 372, 814–824. [Google Scholar] [CrossRef]
- Yu, Y.; Ma, Q.; Groth, S.W. Risk factors for preterm birth in pregnancies following bariatric surgery: An analysis of the Longitudinal Assessment of Bariatric Surgery-2. Surg. Obes. Relat. Dis. Off. J. Am. Soc. Bariatr. Surg. 2022, 18, 1304–1312. [Google Scholar] [CrossRef]
- Pathak, M.M.; Shekhar, K.V.; Meshram, R.J. A Narrative Review on Effects of Maternal Bariatric Surgery on Offspring. Cureus 2023, 15, e48513. [Google Scholar] [CrossRef]
- Cristian, A.; Tarry-Adkins, J.L.; Aiken, C.E. The Uterine Environment and Childhood Obesity Risk: Mechanisms and Predictions. Curr. Nutr. Rep. 2023, 12, 416–425. [Google Scholar] [CrossRef]
- Grandfils, S.; Demondion, D.; Kyheng, M.; Duhamel, A.; Lorio, E.; Pattou, F.; Deruelle, P. Impact of gestational weight gain on perinatal outcomes after a bariatric surgery. J. Gynecol. Obstet. Hum. Reprod. 2019, 48, 401–405. [Google Scholar] [CrossRef]
- Maffazioli, G.D.; Stanford, F.C.; Campoverde Reyes, K.J.; Stanley, T.L.; Singhal, V.; Corey, K.E.; Pratt, J.S.; Bredella, M.A.; Misra, M. Comparing Outcomes of Two Types of Bariatric Surgery in an Adolescent Obese Population: Roux-en-Y Gastric Bypass vs. Sleeve Gastrectomy. Front. Pediatr. 2016, 4, 78. [Google Scholar] [CrossRef]
- Parrettini, S.; Caroli, A.; Torlone, E. Nutrition and Metabolic Adaptations in Physiological and Complicated Pregnancy: Focus on Obesity and Gestational Diabetes. Front. Endocrinol. 2020, 11, 611929. [Google Scholar] [CrossRef]
- Yin, B.; Zhang, H.; Xie, J.; Wei, Y.; Zhang, C.; Meng, L. Validation of preimplantation genetic tests for aneuploidy (PGT-A) with DNA from spent culture media (SCM): Concordance assessment and implication. Reprod. Biol. Endocrinol. 2021, 19, 41. [Google Scholar] [CrossRef]
- Mohamed, A.M.; Aljabal, H.; Alalawi, A.S.; Al-Nooh, N. The Impact of Bariatric Surgery on Type 2 Diabetes Mellitus Remission: A Systematic Review. Cureus 2024, 16, e74755. [Google Scholar] [CrossRef]
- Makhsosi, B.R.; Ghobadi, P.; Otaghi, M.; Tardeh, Z. Impact of bariatric surgery on infertility in obese women: A systematic review and meta-analysis. Ann. Med. Surg. 2024, 86, 7042–7048. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Al Qurashi, A.A.; Qadri, S.H.; Lund, S.; Ansari, U.S.; Arif, A.; Durdana, A.R.; Maryam, R.; Saadi, M.; Zohaib, M.; Khan, M.K.; et al. The effects of bariatric surgery on male and female fertility: A systematic review and meta-analysis. Ann. Med. Surg. 2022, 80, 103881. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Author | Study Design | Sample Size | Selection Bias | Outcome Assessment | Follow-Up Duration | Risk of Bias | Overall Quality |
|---|---|---|---|---|---|---|---|
| Deitel et al., 1988 [22] | Retrospective | 138 | Moderate (strict inclusion criteria) | Well-defined, clear pregnancy outcomes | 2–5 years | Moderate (retrospective design) | Moderate |
| Bilenka et al., 1995 [23] | Retrospective | 9 | High (small sample, limited data) | Well-defined fertility outcomes | Not reported | High (small cohort, potential recall bias) | Low |
| Sheiner et al., 2004 [24] | Retrospective | 298 | Low (large sample size) | Pregnancy outcomes well reported | Not reported | Moderate (retrospective nature) | Moderate |
| Marceau et al., 2004 [25] | Retrospective | 132 | Moderate (high response rate) | Well-defined fertility outcomes | 2–18 years | Moderate (potential recall bias) | Moderate |
| Eid et al., 2005 [26] | Retrospective | 24 | Moderate (PCOS-focused) | Clear reproductive outcomes | Not reported | Moderate (small cohort) | Moderate |
| Rochester et al., 2009 [27] | Prospective | 9 | High (small sample, selection bias) | Clear eligibility criteria, but limited follow-up | 6–12 months | High (small sample) | Low |
| Gosman et al., 2010 [28] | Retrospective | 1538 | Low (large sample) | Clear pregnancy outcomes | Not reported | Moderate | High |
| Sheiner et al., 2011 [29] | Retrospective | 489 | Low (large sample) | Well-defined pregnancy outcomes | Not reported | Moderate | High |
| Musella et al., 2011 [30] | Retrospective | 23 | Moderate (strict inclusion criteria) | Well-reported fertility outcomes | 1–11 months | Moderate (small sample) | Moderate |
| Facchiano et al., 2012 [31] | Retrospective | 36 | Moderate | Clear reproductive outcomes | Not reported | Moderate | Moderate |
| Jamal et al., 2012 [32] | Retrospective | 20 | Moderate (PCOS focus) | Clear fertility and pregnancy outcomes | Not reported | Moderate | Moderate |
| Legro et al., 2012 [33] | Prospective | 29 | Moderate | Well-reported pregnancy rates | Up to 24 months | Moderate | Moderate |
| Neto et al., 2012 [34] | Retrospective | 140 | Low (good follow-up) | Well-defined infertility remission outcomes | 5+ years | Moderate | High |
| Alatishe et al., 2013 [35] | Retrospective | 232 | Low | Well-reported pregnancy outcomes | Median 11 months | Moderate | High |
| Christofolini et al., 2014 [36] | Retrospective | 94 | Moderate (bariatric surgery group small) | No significant differences between groups | Not reported | Moderate | Moderate |
| Eid et al., 2014 [37] | Prospective | 14 | Moderate (small PCOS cohort) | Clear hormonal and menstrual outcomes | 12 months | Moderate | Moderate |
| Goldman et al., 2016 [38] | Retrospective | 219 | Low | Well-defined pregnancy outcomes | <18 months | Moderate | High |
| Milone et al., 2017 [39] | Retrospective | 40 | Moderate | Well-reported pregnancy rates | ≤24 months | Moderate | Moderate |
| Yau et al., 2017 [40] | Retrospective | 54 | Moderate | Well-defined pregnancy outcomes | Not reported | Moderate | Moderate |
| Nilsson-Condori et al., 2018 [41] | Prospective | 48 | Moderate | Clear AMH, FSH, and hormone level changes | 12 months | Moderate | Moderate |
| Sahab Al Kabbi et al., 2018 [42] | Prospective | 60 | Moderate | Well-reported menstrual improvement | 65 ± 5 days | Moderate | Moderate |
| Vincentelli et al., 2018 [43] | Retrospective | 39 | Moderate | AMH and hormone assessment well documented | 12 months | Moderate | Moderate |
| Cruz et al., 2019 [44] | Retrospective | 42 | Moderate | Well-defined neonatal outcomes | Not reported | Moderate | Moderate |
| Gunakan et al., 2019 [45] | Retrospective | 23 | Moderate | Well-defined pregnancy outcomes | Not reported | Moderate | Moderate |
| Menke et al., 2019 [46] | Prospective | 650 | Low | High pregnancy rates post-surgery | <7 years | Low | High |
| Grzegorczyk-Martin et al., 2020 [47] | Retrospective | 83 | Moderate | IVF-related pregnancy rates | Mean 2.98 years | Moderate | Moderate |
| Jacamon et al., 2020 [48] | Retrospective | 52 | Moderate | Well-defined pregnancy outcomes | Median 3 years | Moderate | Moderate |
| Karadag et al., 2020 [49] | Retrospective | 144 | Low | Well-defined pregnancy and neonatal outcomes | 25.8 months | Moderate | High |
| Ezzat et al., 2021 [50] | Prospective | 36 | Moderate | Clear PCOS infertility assessment | 1 year | Moderate | Moderate |
| Thornton et al., 2021 [51] | Retrospective | 460 | Low | Well-defined pregnancy rates | 18 months | Moderate | High |
| Snoek et al., 2024 [52] | Prospective | 97 | Moderate | Neonatal and maternal outcomes well documented | Median 18.3 months | Moderate | High |
| Joly et al., 2024 [53] | Retrospective | 244 | Low | Clear pregnancy outcomes | 33.7 months (sleeve), 40.9 months (bypass) | Moderate | High |
| Samarasinghe et al., 2024 [54] | RCT | 80 | Low | Strong PCOS and fertility outcomes | 12–18 months | Low | High |
| Author | Type of Study | Sample Size | Inclusion Criteria | Exclusion Criteria | Type of Bariatric Surgery | Time Interval Between Bariatric Surgery and Pregnancy | Pregnancy Outcome |
|---|---|---|---|---|---|---|---|
| Deitel et al., 1988 [22] | Retrospective | 138 | - Morbidly obese women (≥45 kg over ideal weight, ≥2 × ideal weight) - Underwent bariatric surgery (jejunoileal bypass, horizontal/vertical banded gastroplasty) - Lost ≥50% of excess weight post-surgery - Euthyroid preoperatively (normal thyroid function) - Complete gynecologic-obstetric data pre- and post-surgery (2–5 years) | - Weight loss < 50% post-surgery - Untreated thyroid disorders - Missing postoperative follow-up data | Jejunoileal bypass (6), horizontal gastroplasty (23), vertical banded gastroplasty (109) | - ≥18 months after jejunoileal bypass - ≥12 months after gastroplasty (horizontal or vertical banded) | - Total Pregnancies After Surgery: 9-all resulted in live births - Infertility resolved in 8/9 patients |
| Bilenka et al., 1995 [23] | Retrospective | 9 | - Women who underwent VBG for morbid obesity - available pre- and post-surgery reproductive history - Age at the time of surgery: 24–36 years (mean: 31.9 years) - Mean weight loss post-surgery: 36 kg (range: 19–55 kg) - At least 1 pregnancy before or after surgery | Women - Who did not undergo VBG - Without available reproductive history - Who did not attempt pregnancy before or after VBG - Other major medical conditions interfering with pregnancy | Vertical banded gastroplasty (VBG) | N/A | - Improved fertility. After VBG: 14 pregnancies in 9 women. All conceived spontaneously - Miscarriage rates decreased significantly (7.1% after VBG vs. 38.9% before) - Pregnancy complications were lower post-surgery. - Infant outcomes were generally favorable |
| Sheiner et al., 2004 [24] | Retrospective | 298 | Pregnancies with history of bariatric surgery | N/A | Restrictive and malabsorptive (open/laparoscopic) | N/A | No significant adverse outcomes; Higher CS rates |
| Marceau et al., 2004 [25] | Retrospective | 132 | Women who had successfully undergone biliopancreatic diversion (BPD) | - Who did not successfully undergo BPD surgery - Women who did not respond to the questionnaire (although response rate was high at 85.5%). - Women who had BPD less than two years prior to the study may have been excluded from certain analyses | Biliopancreatic diversion (BPD) | Varied; pregnancies occurred 2–18 years after surgery, with some within 2 years | Increased fertility post-surgery (47% of previously infertile women conceived) |
| Eid et al., 2005 [26] | Retrospective | 24 | Women with PCOS undergoing bariatric surgery | Incomplete follow-up data or medication use | Laparoscopic Roux-en-Y gastric bypass | N/A | All five infertile women conceived post-surgery without medication |
| Rochester et al., 2009 [27] | Prospective | 9 | Age 18–48, regular menstrual cycles (21–40 days), BMI ≥ 35 kg/m2, uterus and at least one ovary | Renal, hepatic, or systemic disease, exogenous hormone use in last 3 months, diabetes, alcoholism, PCOS | Gastric bypass (10), Lap-band (6) | N/A | N/A |
| Gosman et al., 2010 [28] | Retrospective | 1538 | Women undergoing bariatric surgery, age ≥ 18, no prior weight loss surgery | Women reporting tubal sterilization, or a husband/live-in partner who had a vasectomy, missing pregnancy history data, prior bariatric surgery | Gastric bypass (74.1%), adjustable gastric banding (23.2%), other (2.7%) | N/A | 72.5% had at least one live birth, 25.2% had at least one miscarriage, 2.0% had at least one stillbirth |
| Sheiner et al., 2011 [29] | Retrospective | 489 | Women who conceived after bariatric surgery | Multiple gestations | Laparoscopic gastric banding (LAGB), silastic ring vertical gastroplasty (SRVG), vertical-banded gastroplasty (VBG), Roux-en-Y gastric bypass (RGB) | <12 months (mean 7.0 ± 3.5 months), >12 months (mean 56.7 ± 49.1 months) | 104 conceived < 12 months post-op, 385 conceived > 12 months post-op; Comparable perinatal outcomes between groups, 1.9% congenital malformations in early group vs. 1.3% in late group |
| Musella et al., 2011 [30] | Retrospective | 23 | Obese women with infertility, undergoing intragastric balloon treatment | Infertile male partner (4 women excluded) | Intragastric balloon (BIB® Bioenterics Intragastric Balloon) | 1–11 months after weight loss | 15/18 (83.3%) women who lost weight became pregnant; all pregnancies ended with live births and no complications |
| Facchiano et al., 2012 [31] | Retrospective | 36 (19 with LAGB, 17 with LRYGB); 42 pregnancies | Women who underwent LAGB or LRYGB and later became pregnant | None | Laparoscopic adjustable gastric banding (LAGB) (19), laparoscopic Roux-en-Y gastric gypass (LRYGB) (17) | LAGB: 30 months LRYGB: 25 months | 42 pregnancies. No significant difference between LAGB and LRYGB in obstetric or neonatal outcomes |
| Jamal et al., 2012 [32] | Retrospective | 20 | Morbidly obese women undergoing Roux-en-Y gastric bypass (RYGB), PCOS diagnosis | Postmenopausal women (6), lost to follow-up (5) | Roux-en-Y gastric bypass (RYGB) | N/A | 100% conception rate in infertile PCOS patients post-surgery, no reported complications |
| Legro et al., 2012 [33] | Prospective | 29 | Women aged 18–40, BMI > 40 or BMI 35–39.9 with comorbidities, premenopausal, intact ovaries and uterus, no hormonal contraceptive use | Smoking, alcohol/substance abuse, pregnancy, unwillingness to use barrier contraception, metabolic disorders (hypothyroidism, Cushing’s syndrome, genetic predisposition) | Roux-en-Y gastric bypass (RYGB) | Up to 24 months | 5 pregnancies post-surgery |
| Musella et al., 2012 [55] | Retrospective | 110 | Obese women with infertility, undergoing bariatric surgery | Infertile male partner, lost to follow-up, biliopancreatic diversion patients who had not reached maximal weight loss | Intragastric balloon, adjustable gastric banding (LAGB), sleeve gastrectomy (LSG), gastric bypass (GB) | Mean 2.5 years | 69/110 (62.7%) pregnant, all pregnancies ended with live births, no reported complications; Weight loss ≥ 5 BMI kg/m2 was a significant predictor of pregnancy (p < 0.001) |
| Neto et al., 2012 [34] | Retrospective | 140 | Morbidly obese patients undergoing Roux-en-Y gastric bypass (RYGB) with at least 5 years of follow-up | Lost to follow-up, mortality cases | Roux-en-Y gastric bypass (RYGB) | N/A | Infertility remission was sustained long-term, infertility not affected by weight regain |
| Alatishe et al., 2013 [35] | Retrospective | 232 | Women aged 18–45 who underwent bariatric surgery | N/A | Roux-en-Y gastric bypass (RYGB) (84.9%), laparoscopic adjustable gastric banding (LAGB) (8.2%), sleeve gastrectomy (3.4%) | Median 11 months (1.5–36 months) | 24 live births (85.7%), 3 terminations (10.7%), 1 miscarriage (3.6%) |
| Christofolini et al., 2014 [36] | Retrospective | GI: 29 patients who had bariatric surgery; GII: 57 obese patients (BMI > 30 kg/m2); GIII: 94 patients | Women who underwent at least one cycle of controlled ovarian hyperstimulation (COH) | Previous weight reduction surgery for GII and GIII groups | Restrictive and/or malabsorptive bariatric surgery | Median 4.81 years (0.4–10 years) | No difference in pregnancy rates between post-bariatric surgery and control groups |
| Eid et al., 2014 [37] | Prospective | 14 | Premenopausal women aged 18–45 with PCOS diagnosis confirmed by an endocrinologist | Cushing syndrome, thyroid dysfunction, hyperprolactinemia, extreme androgen excess, pregnancy, immune diseases, or receiving other PCOS treatments | Laparoscopic Roux-en-Y gastric bypass (RYGB) | 12 months | Not reported, but menstrual regularity restored in all patients by 6 months |
| Goldman et al., 2016 [38] | Retrospective | 219 | Women aged 18–45 who had a consultation for bariatric surgery | Patients who underwent sleeve gastrectomy or had an unidentified procedure | Roux-en-Y gastric bypass (RYGB) (111), Adjustable gastric banding (AGB) (66), controls (42) | <18 months | Lower odds of live birth post-AGB (OR = 0.19), increased miscarriage rate post-RYGB (OR = 9.81) |
| Milone et al., 2017 [39] | Retrospective | 40 | Obese women (BMI > 40 or BMI 35–39.9 with comorbidities) with previous ART failure | Male infertility, age > 38 years, AMH < 2 ng/mL, poor ovarian response, bariatric procedures other than sleeve gastrectomy | Sleeve gastrectomy | ≤24 months | Pregnancy rate increased to 37.5% (15/40), live birth rate increased to 35% (14/40) |
| Yau et al., 2017 [40] | Retrospective | 54 | Women who became pregnant after bariatric surgery | 15 pregnancies lost to follow-up, 4 ongoing pregnancies at data collection | Roux-en-Y gastric bypass (RYGB), sleeve gastrectomy (SG), laparoscopic adjustable gastric banding (LAGB) | <2 years (26 pregnancies), >2 years (15 pregnancies) | 73 pregnancies, 96% healthy births in <2 years group, 93% in >2 years group; miscarriage rate higher in shorter interval group |
| Nilsson-Condori et al., 2018 [41] | Prospective | 48 | Women aged 18–35 with BMI 40.9 ± 3.6 kg/m2 undergoing RYGB | Cushing syndrome, thyroid dysfunction, hyperprolactinemia, extreme androgen excess | Laparoscopic Roux-en-Y gastric bypass (RYGB) | 12 months | N/A |
| Sahab Al kabbi et al., 2018 [42] | Prospective | 60 | Women aged 18–40 years, BMI ≥ 36 kg/m2, without medical diseases or hormonal abnormalities, seeking bariatric surgery | Patients with PCOS, pregnancy, recent hormonal contraception use, endocrine disorders | Gastric sleeve (15), Gastric bypass (45) | 65 ± 5 days | Improvement in menstrual cycle regularity post-op (from 58.3% to 86.7%) |
| Vincentelli et al., 2018 [43] | Retrospective | 39 | Women aged 18–45 years, BMI ≥ 40 or ≥35 kg/m2 with obesity-related comorbidities, failure of conservative treatment for at least 6–12 months | Women with ovarian surgery, postmenopausal women | Sleeve gastrectomy (23 women), Roux-en-Y gastric bypass (16 women) | 12 months | AMH levels significantly decreased post-op |
| Cruz et al., 2019 [44] | Retrospective | 42 | Adult pregnant women who previously underwent RYGB, single-fetus pregnancy, gestational age ≤ 13 weeks, followed up by routine prenatal care | Other bariatric procedures, malabsorptive syndromes, neoplasias, liver/kidney diseases, failure to comply with vitamin supplementation, lack of prenatal follow-up | Roux-en-Y gastric bypass (RYGB) | Groups: ≤12 months (G1), >12 to <24 months (G2), ≥24 months (G3) | Higher neonatal complications and inadequate birth weight in G1, G3 |
| Gunakan et al., 2019 [45] | Retrospective | 23 (16 conceived ≤ 12 months, 7 conceived > 12 months post-surgery) | Women who conceived after laparoscopic sleeve gastrectomy | N/A | Laparoscopic sleeve gastrectomy | ≤12 months (n = 16), >12 months (n = 7) | No significant differences in obstetric outcomes between early and late conception groups |
| Menke et al., 2019 [46] | Prospective | 650 | Women aged 18–44, no history of menopause, hysterectomy, or hormone replacement therapy | Women with missing reproductive history data | Various bariatric procedures, mostly Roux-en-Y gastric bypass (RYGB) | ≤7 years | Higher conception rates in women with preoperative infertility, miscarriage rate 25% |
| Grzegorczyk-Martin et al., 2020 [47] | Retrospective | 83 women with bariatric surgery vs. 166 BMI-matched controls vs. 83 non-operated severely obese women | Women undergoing their first IVF cycle, aged 18–43 years | Donor cycles, missing reproductive history data | Sleeve gastrectomy (60), adjustable gastric banding (13), Roux-en-Y gastric bypass (10) | Mean 2.98 ± 1.9 years before IVF | No significant difference in cumulative live birth rate (CLBR) between operated and non-operated BMI-matched women |
| Jacamon et al., 2020 [48] | Retrospective | 52 women with bariatric surgery vs. 104 controls matched on pre-surgery BMI (Group A) vs. 104 controls matched on pre-pregnancy BMI (Group B) | Pregnant women with a history of bariatric surgery between April 2015 and January 2019 | Twin pregnancies, pregnancies after gastric band removal, pregnancies occurring before bariatric surgery, refusal to participate, lack of matched control | Sleeve gastrectomy (58%), Roux-en-Y gastric bypass (37%), Adjustable gastric banding (5%) | Median 3 years, 35% of pregnancies occurred within 1-year post-op | 71.2% pregnancies; Reduced gestational diabetes and large-for-gestational-age infants, but higher risk of small-for-gestational-age infants and neonatal intensive care unit admission |
| Karadag et al., 2020 [49] | Retrospective | 144 pregnant women: 48 (Group A, <12 months post-surgery), 42 (Group B, >12 months post-surgery), 54 (Group C, obese controls) | Pregnant women who underwent laparoscopic sleeve gastrectomy (LSG) | Multiple pregnancies, miscarriages, intrauterine fetal demise | Laparoscopic sleeve gastrectomy (LSG) | 7.8 ± 3.4 months (Group A), 25.8 ± 13.4 months (Group B) | Increased risk of small-for-gestational-age (SGA) infants in Group A, lower gestational diabetes mellitus (GDM) risk in both surgery groups vs. controls |
| Ezzat et al., 2021 [50] | Prospective | 36 | Obese women suffering from infertility due to PCOS (after excluding other causes), age 22–40 years, post-surgery BMI ≤ 35 kg/m2 | Age > 40 years, post-surgery BMI > 35 kg/m2, medical disorders, other infertility factors, use of oral contraceptives | Laparoscopic sleeve gastrectomy (22 patients), laparoscopic gastric bypass (14 patients) | N/A | No pregnancies reported during the 1-year follow-up period |
| Thornton et al., 2021 [51] | Retrospective | 460 | Female patients aged 18 to 45 who underwent bariatric surgery from 2013 to 2018 | History of permanent contraception, hysterectomy, tubal ligation | N/A | 18 months | 6% pregnancy rate within 18 months post-surgery |
| Snoek et al., 2024 [52] | Prospective | 97 post-gastric bypass (pGB) pregnancies vs. 440 non-bariatric pregnancies | Singleton pregnancies after gastric bypass surgery with available fetal growth and birthweight data | Multiple pregnancies, pregnancies with missing growth data | Roux-en-Y gastric bypass (RYGB) | Median 18.3 months (IQR 9.4–28.5) | Increased risk of small-for-gestational-age (SGA) infants, reduced birthweight, but no significant impact on maternal pregnancy complications |
| Joly et al., 2024 [53] | Retrospective | 244 | Patients with a history of sleeve or bypass who delivered between 2004 and 2021 after their first pregnancy post-bariatric surgery | Subsequent pregnancies post-bariatric surgery, gastric band procedures, deliveries in another center, multiple pregnancies, terminations of pregnancy, miscarriages | Sleeve gastrectomy (145), Roux-en-Y gastric bypass (99) | 33.7 months (sleeve), 40.9 months (bypass) | Higher incidence of SGA (26.2% sleeve vs. 22.22% bypass), lower preterm birth in sleeve (3.45%) vs. bypass (12.12%) |
| Samarasinghe et al., 2024 [54] | RCT | 80 (40 bariatric surgery, 40 medical care) | - ≥18 years. - PCOS - BMI ≥ 35 kg/m2. - Presence of oligomenorrhoea or amenorrhea, defined as: cycle length < 21 days or >35 days, <8 cycles in the past 12 months or absence of menstruation | - Diabetes - Inability of effective non-hormonal contraception - Current pregnancy or breastfeeding - Gastro-esophageal reflux disease - Avoid conception 12–18 months post-bariatric surgery | Vertical sleeve gastrectomy (VSG) | 12–18 months post-bariatric surgery | 2.5 times more spontaneous ovulations 93% women with menstrual dysfunction pre-op complete resolution within 6 months post-op 1 pregnancy in the surgical group 2 pregnancies in the medical group |
| Author | Number of Patients | Age | BMI Before Surgery | BMI After Surgery | Follow-Up Time | PCOS (Number of Patients) | Cause of Infertility |
|---|---|---|---|---|---|---|---|
| Deitel et al., 1988 [22] | 138 | 34.8 ± 8.7 (17–56) | 45.5 ± 6.3 | 29.1 ± 4.7 | 2–5 years | N/A | Menstrual irregularities (40.4%) Hyperandrogenism (31.9%) Obesity-related endocrine dysfunction Stress urinary incontinence and pelvic floor dysfunction (61.2%) |
| Bilenka et al., 1995 [23] | 9 | 24 to 36 years (mean 31.9) | 35–50 kg/m2. | 27–38 kg/m2 | N/A | N/A | Morbid obesity |
| Sheiner et al., 2004 [24] | 298 | 29.1 ± 5.7 | N/A | 10.7% remained obese (BMI ≥ 30) | N/A | N/A | Obesity |
| Marceau et al., 2004 [25] | 132 | Mean 37.3 ± 10.3 years at surgery | Mean 47.1 ± 8.3 | Mean 30.9 ± 6.4 | Mean 7.9 ± 4.2 years (range 2–18 years) | N/A | Obesity-related anovulation |
| Eid et al., 2005 [37] | 24 | 34 ± 9.7 years | 50 ± 7.5 | 30 ± 4.5 | 27.5 ± 16 months | 24 | PCOS-related anovulation |
| Rochester et al., 2009 [27] | 9 | 38.0 ± 8.0 years | 47.3 ± 5.2 kg/m2 | 32.0 ± 2.9 kg/m2 | 6–12 months post-op | N/A | Obesity |
| Gosman et al., 2010 [28] | 1538 | 44.8 ± 11.2 years (18–78) | 47.2 ± 7.5 kg/m2 (33.8–87.3) | N/A | N/A | 201 (13.1%) | Obesity |
| Sheiner et al., 2011 [29] | 489 | 30.5 ± 4.7 years (<12 months), 31.3 ± 5.1 years (>12 months) | 42.2 ± 5.1 kg/m2 (<12 months), 42.6 ± 5.0 kg/m2 (>12 months) | N/A | N/A | N/A | N/A |
| Musella et al., 2011 [30] | 19 | 31 ± 4.8 years (22–39) | 41 ± 2.7 kg/m2 (36–45) | Decreased by 7.5 ± 1.1 BMI units in 18 patients | At least 1 year | N/A | Obesity |
| Facchiano et al., 2012 [31] | 36 | 30.4 ± 4.7 years (LAGB), 31.2 ± 3.9 years (LRYGB) | 42.7 ± 3.8 kg/m2 (LAGB), 50.5 ± 4.9 kg/m2 (LRYGB) | N/A | N/A | N/A | Obesity |
| Jamal et al., 2012 [32] | 20 | 32 ± 5.8 years (22–42) | 52.8 ± 9.08 kg/m2 (37–76) | 34.3 ± 5.7 kg/m2 | Mean 46.7 months (15–123 months) | 20 | PCOS-related anovulation, menstrual irregularity, hyperandrogenism |
| Legro et al., 2012 [33] | 29 | 34.5 ± 4.3 years | 49 ± 7 kg/m2 | 34.3 ± 5.7 kg/m2 at 12 months | Up to 24 months | N/A | Obesity, ovulatory irregularities |
| Musella et al., 2012 [55] | 110 | 29.3 ± 3.9 years (pregnant group), 28.6 ± 3.2 years (non-pregnant group) | 43.9 ± 4.1 kg/m2 (pregnant group), 45.1 ± 3.7 kg/m2 (non-pregnant group) | 34.2 ± 2.4 kg/m2 (pregnant group), 41.5 ± 2.8 kg/m2 (non-pregnant group) | >2.5 years | N/A | Obesity |
| Neto et al., 2012 [34] | 140 | 41.4 ± 10.6 years (range 19–62) | 52.5 ± 7.9 kg/m2 | 29.3 ± 5.2 kg/m2 (nadir), 33.7 ± 6.3 kg/m2 (final follow-up) | Mean 90 months (60–155 months) | N/A | Amenorrhea, irregular menstrual cycles, inability to conceive after 6 months |
| Alatishe et al., 2013 [35] | 232 | 34.0 ± 5.9 years | 50.6 ± 7.2 kg/m2 | N/A | Median 30 months | N/A | Obesity |
| Christofolini et al., 2014 [36] | GI: 29 patients undergone bariatric surgery; GII: 57 obese patients (BMI > 30 kg/m2); GIII: 94 patients | 35.0 ± 3.6 years | N/A | 26.6 (22.8–35.8) kg/m2 | N/A | 1/29 | Idiopathic (24.1%), male factor (34.4%), endometriosis (13.7%), tubal factor (20.6%) |
| Eid et al., 2014 [37] | 14 | 36.3 ± 8.4 years (range 19–49) | 44.8 ± 5.9 kg/m2 (range 37.0–56.6) | 29.2 ± 5.9 kg/m2 at 12 months | 12 months | 14 | PCOS-related anovulation, menstrual irregularities, and hyperandrogenism |
| Goldman et al., 2016 [38] | 219 | Mean 39.4 years (RYGB), 40.4 years (AGB) | N/A | Decrease in BMI: 14.71 ± 6.35 (RYGB), 9.17 ± 6.14 (AGB) | <18 months | 28 (25.5%) in RYGB group, 16 (24.6%) in AGB group | PCOS, obesity |
| Milone et al., 2017 [39] | 40 | 31.4 ± 4.7 years (pre-surgery), 32.4 ± 4.4 years (post-surgery) | 40.7 ± 2 kg/m2 | 35 ± 2.6 kg/m2 | ≤24 months | N/A | Idiopathic infertility |
| Yau et al., 2017 [40] | 54 | 32.1 years (<2 years), 31.1 years (>2 years), p = 0.539 | 46.7 kg/m2 (<2 years), 44.5 kg/m2 (>2 years), p = 0.366 | 29.8 kg/m2 (<2 years), 32.6 kg/m2 (>2 years), p = 0.205 | N/A | N/A | N/A |
| Nilsson-Condori et al., 2018 [41] | 48 | 26.5 ± 4.3 years | 40.9 ± 3.6 kg/m2 | 25.4 ± 6.4 kg/m2 at 12 months | 12 months | 10 | N/A |
| Sahab Al kabbi et al., 2018 [42] | 60 | 32.77 ± 6.64 years (18–40) | 48.95 ± 7.46 kg/m2 | 41.18 ± 6.76 kg/m2 | Mean 65 ± 5 days | Excluded from study | N/A |
| Vincentelli et al., 2018 [43] | 39 | 34.6 ± 1.1 years | 45.4 ± 1.0 kg/m2 | 31.4 ± 0.9 kg/m2 at 12 months | 12 months | 6 (15%) | PCOS, obesity, ART failures in 4 patients (endometriosis, impaired ovarian reserve, male factor infertility) |
| Cruz et al., 2019 [44] | 42 | 22–39 years | 46.35 ± 7.51 kg/m2 (G1), 44.62 ± 6.64 kg/m2 (G2), 40.83 ± 3.05 kg/m2 (G3) | 27.80 ± 3.98 kg/m2 (G1), 27.74 ± 3.34 kg/m2 (G2), 25.73 ± 2.58 kg/m2 (G3) | N/A | N/A | N/A |
| Gunakan et al., 2019 [45] | 23 | 32.4 ± 4.2 years | 46.6 ± 4.4 kg/m2 | 29.7 ± 3.8 kg/m2 | N/A | N/A | 9 patients (39.1%) history of infertility |
| Menke et al., 2019 [46] | 650 | Median 34 years (30–39) | N/A | N/A | <7 years | N/A | PCOS, obesity-related infertility, prior ART failure |
| Grzegorczyk-Martin et al., 2020 [47] | 83 | 33.1 ± 4.4 years | 43.6 kg/m2 (39–54) | 28.9 ± 4.7 kg/m2 | Mean 2.98 years before IVF | 25.3% | Ovulatory (16.7%), tubal (15.4%), male (32.1%), idiopathic (16.7%), endometriosis (2.6%), mixed (16.7%) |
| Jacamon et al., 2020 [48] | 52 | 31.1 ± 5.0 years | 46.0 ± 4.6 kg/m2 | 29.4 ± 6.1 kg/m2 | <3 years | N/A | N/A |
| Karadag et al., 2020 [49] | 144 | 30.3 ± 5.1 years (Group A), 28.8 ± 4.7 years (Group B), 27.5 ± 3.9 years (Group C) | 44.37 ± 3.66 kg/m2 (Group A), 42.71 ± 3.71 kg/m2 (Group B) | 32.83 ± 3.63 kg/m2 (Group A), 28.90 ± 2.84 kg/m2 (Group B) | <25.8 months | N/A | N/A |
| Ezzat et al., 2021 [50] | 36 | 27.2 ± 4.2 years (21–36) | 43.6 ± 1.76 kg/m2 | 33.5 ± 1.36 kg/m2 (6 months), 29.1 ± 1.17 kg/m2 (1 year) | 1 year | 36 | PCOS |
| Thornton et al., 2021 [51] | 460 | N/A | N/A | N/A | 18 months | N/A | N/A |
| Snoek et al., 2024 [52] | 97 | Median 29.2 years (IQR 26.0–32.2) | Median 43.6 kg/m2 | Median 29.8 kg/m2 at conception | N/A | N/A | N/A |
| Joly et al., 2024 [53] | 244 | 32.1 years (sleeve), 32.3 years (bypass) | 43.5 kg/m2 (sleeve), 46.5 kg/m2 (bypass) | 30.4 kg/m2 (sleeve), 31.1 kg/m2 (bypass) | N/A | N/A | N/A |
| Samarasinghe et al., 2024 [54] | 80 (40 bariatric surgery, 40 medical care) | Mean 32 ± 6 years (median 31 y.o) | - Surgical group: 45.0 kg/m2 (41.4–50.8 kg/m2) - Medical group: 41.9 kg/m2 (38.1–45.1 kg/m2) | - Surgical group: 32.8 kg/m2 (29.9–37.1 kg/m2) - Medical group: 41.6 kg/m2 (37.7–44.9 kg/m2) | 52 weeks (1 year) | 80 | PCOS (79% oligomenorrhoea or amenorrhea) Obesity |
| Author | Type of Bariatric Surgery | Fertility | Vitamin Status Post-op | Endocrine Changes (AMH, LH, FSH, Estradiol, Testosterone, Androstenedione) | Miscarriage Rate |
|---|---|---|---|---|---|
| Deitel et al., 1988 [22] | Various procedures | Improved | N/A | N/A | N/A |
| Bilenka et al., 1995 [23] | Vertical banded gastroplasty (VBG) | Improved | N/A | N/A | 7.1% after VBG vs. 38.9% before |
| Sheiner et al., 2004 [24] | Restrictive and malabsorptive (open/laparoscopic) | N/A | Risk of anemia (iron, folate, B12 deficiency) | N/A | N/A |
| Marceau et al., 2004 [25] | Biliopancreatic diversion (BPD) | Improved | Serum albumin decreased from 40.4 ± 2.7 to 35.7 ± 5.5 g/L; 4 cases of severe hypoalbuminemia (<26 g/L) requiring parenteral nutrition | N/A | Pre-op: 21.6% (341/1577 pregnancies); post-op: 26% (57/219 pregnancies) |
| Eid et al., 2005 [26] | Laparoscopic Roux-en-Y gastric bypass | Improved | N/A | N/A | N/A |
| Rochester et al., 2009 [27] | Gastric bypass (10), Lap-band (6) | Improved | N/A | LH increased, FSH stable, estradiol decreased, progesterone increased but lower than controls | N/A |
| Gosman et al., 2010 [28] | Gastric bypass (74.1%), adjustable gastric banding (23.2%) | N/A | N/A | N/A | 25.2% had at least one miscarriage, miscarriage in 17.4% of pregnancies |
| Sheiner et al., 2011 [29] | LAGB (61.5% < 12 months, 41.0% > 12 months), SRVG, VBG, RGB | N/A | N/A | N/A | N/A |
| Musella et al., 2011 [55] | Intragastric balloon (BIB® Bioenterics Intragastric Balloon) | Improved | N/A | N/A | None |
| Facchiano et al., 2012 [31] | LAGB and LRYGB | Improved; no significant difference between LAGB and LRYGB | N/A | N/A | 2 miscarriages (LRYGB group) at 16 and 22 weeks |
| Jamal et al., 2012 [32] | Roux-en-Y gastric bypass (RYGB) | Improved | N/A | Menstrual cycle improved (82%), hirsutism resolved (29%), T2DM resolved (77.8%) | N/A |
| Legro et al., 2012 [33] | Roux-en-Y gastric bypass (RYGB) | Not improved | N/A | Increased SHBG, decreased testosterone and estradiol, shortened follicular phase | N/A |
| Musella et al., 2012 [55] | Intragastric balloon, LAGB, LSG, GB | Improved | N/A | N/A | None |
| Neto et al., 2012 [34] | Roux-en-Y gastric bypass (RYGB) | Not improved | N/A | N/A | N/A |
| Alatishe et al., 2013 [35] | Roux-en-Y gastric bypass (RYGB), LAGB, sleeve gastrectomy | Improved | N/A | N/A | Pre-surgery: 20.7%; post-surgery: 3.6% |
| Christofolini et al., 2014 [36] | Restrictive and/or malabsorptive bariatric surgery | Not improved | N/A | N/A | N/A |
| Eid et al., 2014 [37] | Laparoscopic Roux-en-Y gastric bypass (RYGB) | Menstrual regularity restored in all patients by 6 months | N/A | Testosterone decreased, fasting insulin decreased, LH increased, FSH stable | N/A |
| Goldman et al., 2016 [38] | Roux-en-Y gastric bypass (RYGB), adjustable gastric banding (AGB) | Menstrual cycle regularity improved post-RYGB (OR = 0.21), no significant improvement post-AGB | N/A | N/A | Higher post-RYGB compared to controls (OR = 9.81) |
| Milone et al., 2017 [39] | Sleeve gastrectomy | Improved | N/A | FSH stable, AMH stable | 2.5% (1/40) |
| Yau et al., 2017 [40] | Roux-en-Y gastric bypass (RYGB), sleeve gastrectomy (SG), laparoscopic adjustable gastric banding (LAGB) | Improved | Vitamin B1 deficiency: 46% (<2 years), 20% (>2 years), p = 0.177; vitamin D deficiency: 65% (<2 years), 87% (>2 years), p = 0.168 | N/A | Higher in <2 years group (7/33, 21%) vs. >2 years group (1/16, 6%) |
| Nilsson-Condori et al., 2018 [41] | Laparoscopic Roux-en-Y gastric bypass (RYGB) | N/A | N/A | AMH decreased, FAI decreased, SHBG increased, testosterone decreased, estradiol increased | N/A |
| Sahab Al kabbi et al., 2018 [42] | Gastric sleeve, gastric bypass | N/A | N/A | AMH decreased significantly post-op | N/A |
| Vincentelli et al., 2018 [43] | Sleeve gastrectomy, Roux-en-Y gastric bypass | N/A | N/A | AMH decreased significantly | N/A |
| Cruz et al., 2019 [44] | Roux-en-Y gastric bypass (RYGB) | N/A | N/A | N/A | 23.8% (G1), 47.6% (G2), 28.6% (G3) |
| Gunakan et al., 2019 [45] | Laparoscopic sleeve gastrectomy | Improved | N/A | N/A | 3 cases (13%) |
| Menke et al., 2019 [46] | Various procedures, predominantly RYGB | Improved | N/A | N/A | 25% |
| Grzegorczyk-Martin et al., 2020 [47] | Sleeve gastrectomy, adjustable gastric banding, Roux-en-Y gastric bypass | Stable | N/A | N/A | 38.7% (operated group), 35.8% (BMI-matched non-operated), 56.5% (severely obese non-operated) |
| Jacamon et al., 2020 [48] | Sleeve gastrectomy, Roux-en-Y gastric bypass, adjustable gastric banding | Improved | N/A | N/A | 29% post-surgery, compared to 32% (Group A) and 22% (Group B) |
| Karadag et al., 2020 [49] | Laparoscopic sleeve gastrectomy (LSG) | N/A | N/A | N/A | N/A |
| Ezzat et al., 2021 [50] | Laparoscopic sleeve gastrectomy, laparoscopic gastric bypass | N/A | N/A | Free and total testosterone decreased, SHBG increased, Free androgen index decreased | N/A |
| Thornton et al., 2021 [51] | N/A | Improved | N/A | N/A | N/A |
| Snoek et al., 2024 [52] | Roux-en-Y gastric bypass (RYGB) | N/A | Lower vitamin serum levels post-op | N/A | N/A |
| Joly et al., 2024 [53] | Sleeve gastrectomy, Roux-en-Y gastric bypass | N/A | Higher vitamin deficiencies in bypass group | N/A | N/A |
| Samarasinghe et al., 2024 [54] | Vertical sleeve gastrectomy (VSG) | Improved | - Iron deficiency: 3 patients (8%). - Vitamin B12 deficiency: 0 patients (0%) - Folic acid deficiency: 7 patients (19%) - Vitamin D deficiency: 5 patients (14%) | AMH decreased in the surgical group - Baseline: 46.4 pmol/L (medical) vs. 31.2 pmol/L (surgical). - 52 weeks: 40.5 pmol/L (medical) vs. 23.7 pmol/L (surgical) (p = 0.0061). LH non-significant decrease - Baseline: 8.3 U/L (medical) vs. 9.2 U/L (surgical) - 52 weeks: 8.7 U/L (medical) vs. 6.4 U/L (surgical) (p = 0.080) FSH stable - Baseline: 5.0 U/L (medical) vs. 5.3 U/L (surgical) - 52 weeks: 5.5 U/L (medical) vs. 5.3 U/L (surgical) (p = 0.65) E2 no significant difference - Baseline: 267.4 pmol/L (medical) vs. 356.7 pmol/L (surgical) - 52 weeks: 290.3 pmol/L (medical) vs. 303.8 pmol/L (surgical) (p = 0.82) Testosterone significant decrease - Baseline: 2.0 nmol/L (medical) vs. 1.8 nmol/L (surgical) - 52 weeks: 1.8 nmol/L (medical) vs. 1.2 nmol/L (surgical) (p = 0.0065) Androstenedione significant decrease - Baseline: 7.0 nmol/L (medical) vs. 6.4 nmol/L (surgical) - 52 weeks: 5.7 nmol/L (medical) vs. 4.5 nmol/L (surgical) (p = 0.039) | N/A |
| Author | IUGR | GDM | Preeclampsia | Congenital Malformation | Other Complications |
|---|---|---|---|---|---|
| Deitel et al., 1988 [22] | Decreased | Decreased | Decreased | N/A | Decreased |
| Bilenka et al., 1995 [23] | None | Decreased | Decreased | None | Decreased |
| Sheiner et al., 2004 [24] | 5% | 9.4% | 5.7% | 5% | Higher rates of previous CS, labor induction, PROM |
| Marceau et al., 2004 [25] | Increased Pre-op 3.1% (20/638) Post-op: 9.6% (15/156) | N/A | Increased Pre-op 0 Post-op 1 | Increased Pre-op 2.6% (33/1245) Post-op 4.2% (7/166) | Pre-op miscarriage rate: 21.6% (341/1577); cesarean section rate: not reported Post-op: miscarriage rate: 26% (57/219 non-terminated pregnancies); cesarean section rate: 20.3%; 4 severe hypoalbuminemia requiring parenteral nutrition; 1 placental detachment; 1 intestinal obstruction |
| Eid et al., 2005 [26] | N/A | N/A | N/A | N/A | N/A |
| Rochester et al., 2009 [27] | N/A | N/A | N/A | N/A | N/A |
| Gosman et al., 2010 [28] | N/A | N/A | N/A | N/A | Higher stillbirth rate (13.2 per 1000 live births) compared to U.S. general population (6.2 per 1000 live births) |
| Sheiner et al., 2011 [29] | 3.8% (<12 months), 2.3% (>12 months), p = 0.396 | 10.5% (<12 months), 7.3% (>12 months), p = 0.159 | 15.4% (<12 months), 11.2% (>12 months), p = 0.392 | 1.9% (<12 months), 1.3% (>12 months), p = 0.458 | Higher rate of LAGB in early group (61.5%) vs. late group (41.0%) |
| Musella et al., 2011 [30] | None | None | None | None | None |
| Facchiano et al., 2012 [31] | 1 case (LAGB), 2 cases (LRYGB) | 3 cases (LAGB), 2 cases (LRYGB) | 3 cases (LAGB), 0 cases (LRYGB) | None | 7 cesarean deliveries (LAGB), 8 cesarean deliveries (LRYGB); 2 postpartum hemorrhages (LAGB); minor complications secondary to bariatric surgery in LRYGB group |
| Jamal et al., 2012 [32] | N/A | N/A | N/A | N/A | N/A |
| Legro et al., 2012 [33] | N/A | N/A | N/A | N/A | N/A |
| Musella et al., 2012 [55] | N/A | N/A | N/A | N/A | N/A |
| Neto et al., 2012 [34] | N/A | N/A | N/A | N/A | N/A |
| Alatishe et al., 2013 [35] | N/A | N/A | N/A | N/A | None |
| Christofolini et al., 2014 [36] | N/A | N/A | N/A | N/A | N/A |
| Eid et al., 2014 [37] | N/A | N/A | N/A | N/A | N/A |
| Goldman et al., 2016 [38] | N/A | N/A | N/A | N/A | Lower neonatal birth weight post-RYGB (2983 g vs. 3583 g in controls) |
| Milone et al., 2017 [39] | N/A | N/A | N/A | N/A | N/A |
| Yau et al., 2017 [40] | N/A | 0% (<2 years), 6% (>2 years), p = 0.984 | 4% (<2 years), 6% (>2 years), p = 0.995 | N/A | Gestational hypertension: 12% in both groups, hyperemesis gravidarum: 31% (<2 years), 40% (>2 years) |
| Nilsson-Condori et al., 2018 [41] | N/A | N/A | N/A | N/A | N/A |
| Sahab Al kabbi et al., 2018 [42] | N/A | N/A | N/A | N/A | N/A |
| Vincentelli et al., 2018 [43] | N/A | N/A | N/A | N/A | N/A |
| Cruz et al., 2019 [44] | N/A | N/A | N/A | N/A | Neonatal complications: 30% (G1), 5% (G2), 0% (G3); Weight inadequacy at birth: 20% (G1), 0% (G2, G3) |
| Gunakan et al., 2019 [45] | 2 cases (10%) | 2 cases (10%) | 2 cases (10%) | Not reported | Preterm delivery in 1 case (5%), no significant difference between groups |
| Menke et al., 2019 [46] | N/A | N/A | N/A | N/A | N/A |
| Grzegorczyk-Martin et al., 2020 [47] | N/A | N/A | N/A | N/A | N/A |
| Jacamon et al., 2020 [48] | N/A | Reduced from 44% (controls) to 12% (post-bariatric surgery) | Reduced from 13% (controls) to 2% (post-bariatric surgery) | N/A | Higher risk of small-for-gestational-age infants and NICU admission in post-bariatric surgery group |
| Karadag et al., 2020 [49] | N/A | Lower in surgery groups vs. control (4.3% Group A, 9.5% Group B, 29.6% Group C, p = 0.004) | 8.3% (Group A), 9.5% (Group B), 7.4% (Group C), p = 0.695 | N/A | SGA risk higher in Group A (22.9%) vs. Group B (11.9%) and Group C (7.4%), p = 0.025 |
| Ezzat et al., 2021 [50] | N/A | N/A | N/A | N/A | N/A |
| Thornton et al., 2021 [51] | N/A | N/A | N/A | N/A | N/A |
| Snoek et al., 2024 [52] | N/A | No significant difference | No significant difference | N/A | Significantly higher risk of small-for-gestational-age (SGA) infants |
| Joly et al., 2024 [53] | N/A | No significant difference | No significant difference | N/A | Higher risk of SGA in sleeve (26.2%) vs. bypass (22.22%), higher preterm birth in bypass group (12.12%) vs. sleeve (3.45%) |
| Samarasinghe et al., 2024 [54] | N/A | N/A | N/A | N/A | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Voros, C.; Varthaliti, A.; Bananis, K.; Mavrogianni, D.; Athanasiou, D.; Athanasiou, A.; Athanasiou, A.; Papahliou, A.-M.; Zografos, C.G.; Kondili, P.; et al. The Relationship Between Obesity, Bariatric Surgery, and Infertility: A Systematic Review. Life 2025, 15, 758. https://doi.org/10.3390/life15050758
Voros C, Varthaliti A, Bananis K, Mavrogianni D, Athanasiou D, Athanasiou A, Athanasiou A, Papahliou A-M, Zografos CG, Kondili P, et al. The Relationship Between Obesity, Bariatric Surgery, and Infertility: A Systematic Review. Life. 2025; 15(5):758. https://doi.org/10.3390/life15050758
Chicago/Turabian StyleVoros, Charalampos, Antonia Varthaliti, Kyriakos Bananis, Despoina Mavrogianni, Diamantis Athanasiou, Antonia Athanasiou, Aikaterini Athanasiou, Anthi-Maria Papahliou, Constantinos G. Zografos, Panagiota Kondili, and et al. 2025. "The Relationship Between Obesity, Bariatric Surgery, and Infertility: A Systematic Review" Life 15, no. 5: 758. https://doi.org/10.3390/life15050758
APA StyleVoros, C., Varthaliti, A., Bananis, K., Mavrogianni, D., Athanasiou, D., Athanasiou, A., Athanasiou, A., Papahliou, A.-M., Zografos, C. G., Kondili, P., Darlas, M., Papapanagiotou, I., Daskalaki, M. A., Theodora, M., Antsaklis, P., Daskalakis, G., & Loutradis, D. (2025). The Relationship Between Obesity, Bariatric Surgery, and Infertility: A Systematic Review. Life, 15(5), 758. https://doi.org/10.3390/life15050758










