Integrative Strategies for Preventing and Managing Metabolic Syndrome: The Impact of Exercise and Diet on Oxidative Stress Reduction—A Review
Abstract
1. Introduction
2. State-of-the-Art
2.1. Autonomic Nervous System and Hypothalamic–Pituitary–Adrenal Axis Dysregulations in MetS
2.2. The Role of Adipocytokines in Hypertension and MetS
Paraventricular Nucleus–Sympathetic–Adipose Pathway Axis
2.3. Oxidative Stress and Inflammation in MetS
2.4. Hypertension and Its Links to Insulin Resistance
2.5. Genetic Variations in Metabolic Syndrome
3. Methods
4. Results
4.1. Physical Exercise
4.1.1. Low-Intensity Exercise
4.1.2. Moderate-Intensity Exercise
4.1.3. High-Intensity Interval Training
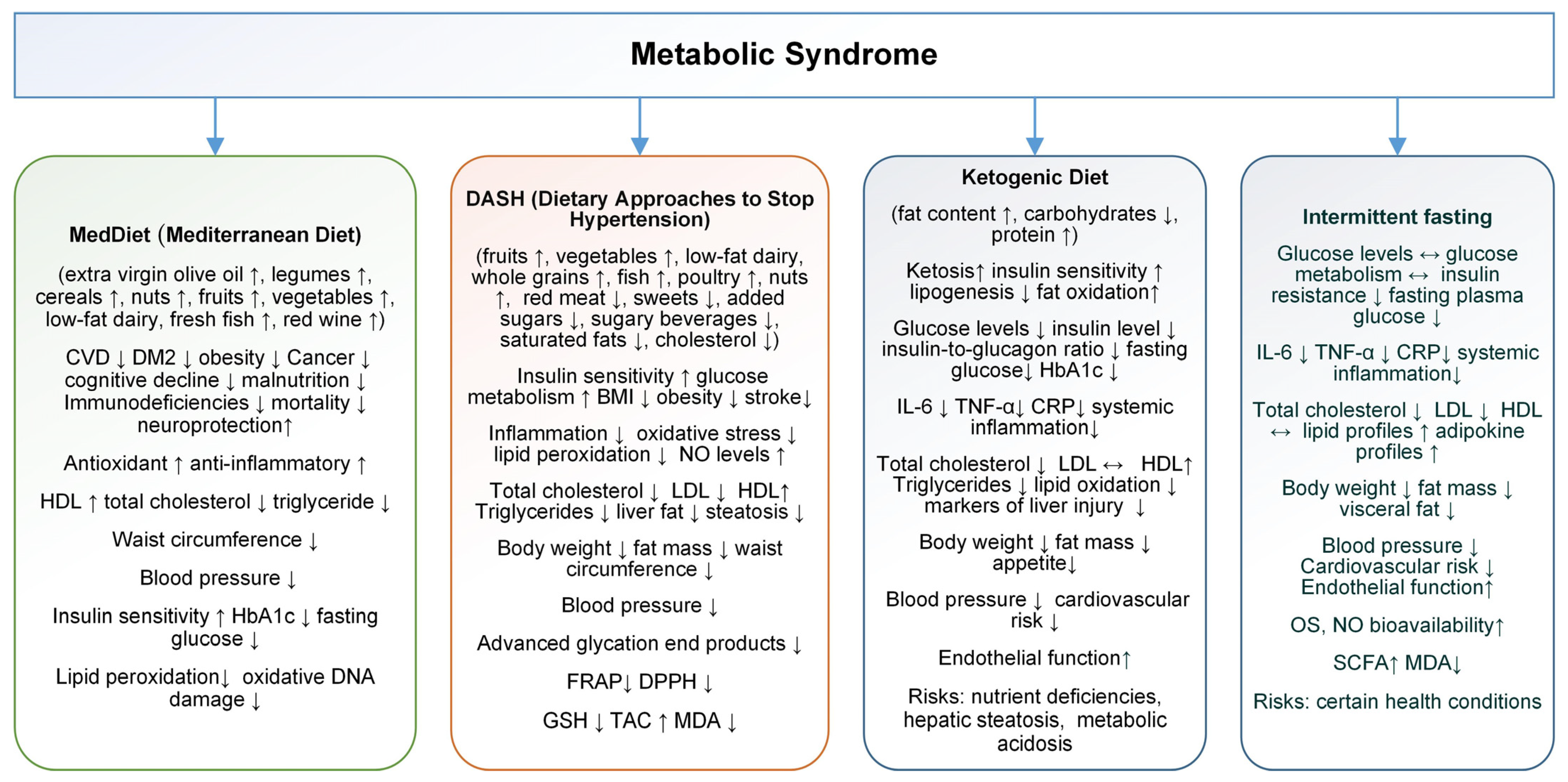
4.2. Dietary Intervention
4.2.1. Mediterranean Diet
4.2.2. Dietary Approaches to Stop Hypertension
4.2.3. Ketogenic Diet
4.2.4. Intermittent Fasting
5. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 2hPPG | 2 h postprandial glucose |
| 3-NT | 3-nitrotyrosine |
| 8-OHdG | 8-hydroxy-2′ -deoxyguanosine |
| AD | Alzheimer’s disease |
| AGE | advanced glycation end products |
| AIM | macrophage apoptosis inhibitor |
| AIT | aerobic interval training |
| AMPK | activated protein kinase |
| ANS | autonomic nervous system |
| AOPP | advanced oxidation protein products |
| ARC | arcuate nucleus |
| ATP | adenosine triphosphate |
| BAT | brown adipose tissue |
| BMI | body mass index |
| CAP1 | adenylyl cyclase-associated protein 1 |
| CAT | catalase |
| CCK | cholecystokinin |
| CG | control group |
| CK | creatine kinase |
| CME | continuous moderate exercise |
| CNS | central nervous system |
| COMB | combined exercise training |
| CRH | corticotropin-releasing hormone |
| CRP | C-reactive protein |
| CV | cardiovascular |
| CVD | cardiovascular disease |
| DASH-CD | DASH combined diet |
| DASH-NP | non-plant-based DASH diet |
| DASH-P | plant-based DASH diet |
| DBP | diastolic blood pressure |
| DDG | dash salt-restricted Group |
| DM2 | diabetes mellitus type 2 |
| DMH | dorsomedial hypothalamic nucleus |
| DPPH | α-diphenyl-β-picrylhydrazyl |
| EBRE | elastic band resistance exercise |
| EEC | enteroendocrine cells |
| ENS | enteric nervous systems |
| FAO | Food and Agriculture Organization |
| FeNO | fractionated nitric oxide |
| FEV1 | forced expiratory volume |
| FFQ | food frequency questionnaire |
| FLI | fatty liver index |
| FPG | fasting plasma glucose |
| FRAP | ferric-reducing antioxidant potential |
| FSH | follicle-stimulating hormone |
| FVC | forced vital capacity |
| GDM | gestational diabetes mellitus |
| GIP | glucose-dependent insulinotropic peptide |
| GLP-1 | glucagon-like peptide-1 |
| GPx | glutathione peroxidase |
| GRd | glutathione reductase |
| GSH | glutathione |
| HbA1c | glycosylated hemoglobin |
| HDL | high-density lipoprotein |
| HIIT | high-intensity interval training |
| HPA | hypothalamic–pituitary–adrenal |
| HRR | heart rate reserve |
| HRV | heart rate variability |
| HSI | hepatic steatosis index |
| ICKD | an isocaloric ketogenic diet |
| IDF | International Diabetes Federation |
| IR | insulin resistance |
| IF | intermittent fasting |
| ISAPP | International Scientific Association for Probiotics and Prebiotics |
| KD | ketogenic diet |
| LCDs | low-carbohydrate diets |
| LDL | low-density lipoprotein |
| MAPK | mitogen-activated kinase |
| MCP-1 | monocyte chemotactic protein-1 |
| MDA | malondialdehyde |
| MDG | medDiet salt-restricted Group |
| MetS | metabolic syndrome |
| MGBA | microbiota–gut–brain axis |
| MH-NO | metabolically healthy nonobese |
| MHO | metabolically healthy obese |
| MICT | moderate-intensity continuous training |
| MUFA | monounsaturated fatty acid |
| NO | nitric oxide |
| non-MHO | non-metabolically healthy obese |
| Nox | relationship between nitrite/nitrate |
| NRCTs | non-randomized controlled trials |
| NTS | neurotensin |
| OGTT | oral glucose tolerance tests |
| OS | oxidative stress |
| OSA | obstructive sleep apnea |
| OSI | oxidative stress index |
| OXM | oxyntomodulin |
| PBMC | peripheral blood mononuclear cells |
| PCOS | polycystic ovary syndrome |
| PD | Parkinson’s disease |
| PEF | peak expiratory flow |
| PI3K-PKB | phosphoinositide-3 kinase |
| PNS | parasympathetic nervous system |
| PPG | postprandial plasma glucose |
| PUFAs | polyunsaturated fatty acids |
| PVN | paraventricular nucleus |
| PYY | peptide YY |
| RCTs | randomized controlled trials |
| ROS | reactive oxygen species |
| SA | serum antioxidant activity |
| SBP | systolic blood pressure |
| SCFA | short-chain fatty acids |
| SCFAs | short-chain fatty acids |
| SFA | saturated fatty acids |
| SNS | sympathetic nervous system |
| SOD | superoxide dismutase |
| SRG | salt-restricted group |
| TAC | total antioxidant capacity |
| TAS | total antioxidant status |
| TBARS | thiobarbituric acid reactive substances |
| TRF | time-restricted feeding |
| TLR-4 | toll-like receptor 4 |
| TOS | total oxidative status |
| T-SH | total thiol content |
| UA | uric acid |
| VLCKD | very low-calorie ketogenic diet |
| VMH | ventromedial hypothalamus |
| VN | vagal nerve |
| VO2R | oxygen uptake reserve |
| WAT | white adipose tissue |
| WC | waist circumference |
| WHO | World Health Organization |
References
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C., Jr.; et al. A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef]
- Suwała, S.; Junik, R. Assessment of the Liver Steatosis and Fibrosis Risk in Metabolic Syndrome and Its Individual Components, Considering the Varying Definitions Used in Clinical Practice Throughout Time: A Retrospective Cross-Sectional Study. Biomedicines 2024, 12, 1739. [Google Scholar] [CrossRef] [PubMed]
- Leon, B.M.; Maddox, T.M. Diabetes and cardiovascular disease: Epidemiology, biological mechanisms, treatment recommendations and future research. World J. Diabetes 2015, 6, 1246–1258. [Google Scholar] [CrossRef] [PubMed]
- Amadio, P.; Zarà, M.; Sandrini, L.; Ieraci, A.; Barbieri, S.S. Depression and cardiovascular disease: The viewpoint of platelets. Int. J. Mol. Sci. 2020, 21, 7560. [Google Scholar] [CrossRef] [PubMed]
- Branyan, K.W.; Devallance, E.R.; Lemaster, K.A.; Skinner, R.C.; Bryner, R.W.; Olfert, I.M.; Kelley, E.E.; Frisbee, J.C.; Chantler, P.D. Role of chronic stress and exercise on microvascular function in metabolic syndrome. Med. Sci. Sports Exerc. 2018, 50, 957–966. [Google Scholar] [CrossRef]
- Price, R.B.; Duman, R. Neuroplasticity in cognitive and psychological mechanisms of depression: An integrative model. Mol. Psychiatry 2020, 25, 530–543. [Google Scholar] [CrossRef]
- Daniela, M.; Catalina, L.; Ilie, O.; Paula, M.; Daniel-Andrei, I.; Ioana, B. Effects of exercise training on the autonomic nervous system with a focus on anti-inflammatory and antioxidants effects. Antioxidants 2022, 11, 350. [Google Scholar] [CrossRef]
- Morton, G.J.; Cummings, D.E.; Baskin, D.G.; Barsh, G.S.; Schwartz, M.W. Central nervous system control of food intake and body weight. Nature 2006, 443, 289–295. [Google Scholar] [CrossRef]
- Chu, B.; Marwaha, K.; Sanvictores, T.; Awosika, A.O.; Ayers, D. Physiology, Stress Reaction; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK541120/ (accessed on 6 May 2025).
- Koch, L.; Wunderlich, F.T.; Seibler, J.; Könner, A.C.; Hampel, B.; Irlenbusch, S.; Brabant, G.; Kahn, C.R.; Schwenk, F.; Brüning, J.C. Central insulin action regulates peripheral glucose and fat metabolism in mice. J. Clin. Investig. 2008, 118, 2132–2147. [Google Scholar] [CrossRef]
- Theander-Carrillo, C.; Wiedmer, P.; Cettour-Rose, P.; Nogueiras, R.; Perez-Tilve, D.; Pfluger, P.; Castaneda, T.R.; Muzzin, P.; Schürmann, A.; Szanto, I.; et al. Ghrelin action in the brain controls adipocyte metabolism. J. Clin. Investig. 2006, 116, 1983–1993. [Google Scholar] [CrossRef] [PubMed]
- Nogueiras, R.; Wiedmer, P.; Perez-Tilve, D.; Veyrat-Durebex, C.; Keogh, J.M.; Sutton, G.M.; Pfluger, P.T.; Castaneda, T.R.; Neschen, S.; Hofmann, S.M.; et al. The central melanocortin system directly controls peripheral lipid metabolism. J. Clin. Investig. 2007, 117, 3475–3488. [Google Scholar] [CrossRef]
- Wang, M. The role of glucocorticoid action in the pathophysiology of the Metabolic Syndrome. Nutr. Metab. 2005, 2, 3. [Google Scholar] [CrossRef] [PubMed]
- Fagius, J. Sympathetic nerve activity in metabolic control—Some basic concepts. Acta Physiol. Scand. 2003, 177, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Aronne, L.J.; Mackintosh, R.; Rosenbaum, M.; Leibel, R.L.; Hirsch, J. Autonomic nervous system activity in weight gain and weight loss. Am. J. Physiol. Integr. Comp. Physiol. 1995, 269, R222–R225. [Google Scholar] [CrossRef]
- Landsberg, L. Insulin-mediated sympathetic stimulation: Role in the pathogenesis of obesity-related hypertension (or, how insulin affects blood pressure, and why). J. Hypertens. 2001, 19, 523–528. [Google Scholar] [CrossRef]
- Brunner, E.J.; Hemingway, H.; Walker, B.R.; Page, M.; Clarke, P.; Juneja, M.; Shipley, M.J.; Kumari, M.; Andrew, R.; Seckl, J.R.; et al. Adrenocortical, autonomic, andinflammatory causes of the metabolic syndrome: Nested case-control study. Circulation 2002, 106, 2659–2665. [Google Scholar] [CrossRef]
- Lopes, H.F.; Egan, B.M. Desequilíbrio autonômico e síndrome metabólica: Parceiros patológicos e muma pandemia global emergente. Arq. Bras. Cardiol. 2006, 87, 538–547. [Google Scholar] [CrossRef]
- Guarino, D.; Nannipieri, M.; Iervasi, G.; Taddei, S.; Bruno, R.M. The Role of the Autonomic Nervous System in the Pathophysiology of Obesity. Front. Physiol. 2017, 8, 665. [Google Scholar] [CrossRef]
- Kalil, G.Z.; Haynes, W.G. Sympathetic nervous system in obesity-related hypertension: Mechanisms and clinical implications. Hypertens. Res. 2012, 35, 4–16. [Google Scholar] [CrossRef]
- Mano-Otagiri, A.; Ohata, H.; Iwasaki-Sekino, A.; Nemoto, T.; Shibasaki, T. Ghrelin suppresses noradrenaline release in the brown adipose tissue of rats. J. Endocrinol. 2009, 201, 341–349. [Google Scholar] [CrossRef]
- Yanai, H.; Tomono, Y.; Ito, K.; Furutani, N.; Yoshida, H.; Tada, N. The Underlying Mechanisms for Development of Hypertension in the Metabolic Syndrome. Nutr. J. 2008, 7, 10. [Google Scholar] [CrossRef]
- Lent-Schochet, D.; McLaughlin, M.; Ramakrishnan, N.; Jialal, I. Exploratory Metabolomics of Metabolic Syndrome: A Status Report. World J. Diabetes 2019, 10, 23–36. [Google Scholar] [CrossRef]
- Balistreri, C.R.; Caruso, C.; Candore, G. The role of adipose tissue and adipokines in obesity-related inflammatory disease. Mediat. Inflamm. 2010, 2010, 802078. [Google Scholar] [CrossRef]
- Wang, J.; Sun, L.; You, J.; Peng, H.; Yan, H.; Wang, J.; Sun, F.; Cui, M.; Wang, S.; Zhang, Z.; et al. Role and mechanism of PVN-sympathetic-adipose circuit in depression and insulin resistance induced by chronic stress. EMBO Rep. 2023, 24, e57176. [Google Scholar] [CrossRef] [PubMed]
- Smekal, A.; Vaclavik, J. Adipokines and cardiovascular disease: A comprehensive review. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2017, 161, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Suárez, V.J.; Redondo-Flórez, L.; Beltrán-Velasco, A.I.; Martín-Rodríguez, A.; Martínez-Guardado, I.; Navarro-Jiménez, E.; Laborde-Cárdenas, C.C.; Tornero-Aguilera, J.F. The Role of Adipokines in Health and Disease. Biomedicines 2023, 11, 1290. [Google Scholar] [CrossRef]
- Ilyas, M.N.; Atif, A.B.; Al-Hatamleh, M.A.I.; Al-Shajrawi, O.M.; Ariff, T.M.; Simbak, N. Rising trend of obesity in Malaysia; Role of inflammation and inflammatory marker’s in obesity related insulin resistance: A nuclear Factor Kappa B (Nfkb) Perspective. Curr. Trends Biomed. Eng. Biosci. 2017, 10, 1–3. [Google Scholar]
- Lee, S.; Kwak, H.B. Role of adiponectin in metabolic and cardiovascular disease. J. Exerc. Rehabil. 2014, 10, 54–59. [Google Scholar] [CrossRef]
- Reis, C.E.G.; Bressan, J.; Alfenas, R.C.G. Effect of the diet components on adiponectin levels. Nutr. Hosp. 2010, 25, 881–888. [Google Scholar]
- Lin, Z.; Tian, H.; Lam, K.S.; Lin, S.; Hoo, R.C.; Konishi, M.; Itoh, N.; Wang, Y.; Bornstein, S.R.; Xu, A.; et al. Adiponectin mediates the metabolic effects of FGF21 on glucose homeostasis and insulin sensitivity in mice. Cell Metab. 2013, 17, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Myers, M.G.; Cowley, M.A.; M¨unzberg, H. Mechanisms of leptin action and leptin resistance. Annu. Rev. Physiol. 2008, 70, 537–556. [Google Scholar] [CrossRef]
- Ouchi, N.; Higuchi, A.; Ohashi, K.; Oshima, Y.; Gokce, N.; Shibata, R.; Akasaki, Y.; Shimono, A.; Walsh, K. Sfrp5 is an anti-inflammatory adipokine that modulates metabolic dysfunction in obesity. Science 2010, 329, 454–457. [Google Scholar] [CrossRef]
- Masenga, S.K.; Kabwe, L.S.; Chakulya, M.; Kirabo, A. Mechanisms of Oxidative Stress in Metabolic Syndrome. Int. J. Mol. Sci. 2023, 24, 7898. [Google Scholar] [CrossRef] [PubMed]
- Savaş, E.M.; Oğuz, S.H.; Samadi, A.; Yılmaz Işıkhan, S.; Ünlütürk, U.; Lay, İ.; Gürlek, A. Apoptosis Inhibitor of Macrophage, Monocyte Chemotactic Protein-1, and C-Reactive Protein Levels Are Increased in Patients with Metabolic Syndrome: A Pilot Study. Metab. Syndr. Relat. Disord. 2020, 18, 197–205. [Google Scholar] [CrossRef]
- Monserrat-Mesquida, M.; Quetglas-Llabrés, M.; Capó, X.; Bouzas, C.; Mateos, D.; Pons, A.; Tur, J.A.; Sureda, A. Metabolic Syndrome Is Associated with Oxidative Stress and Proinflammatory State. Antioxidants 2020, 9, 236. [Google Scholar] [CrossRef] [PubMed]
- Tahapary, D.L.; Pratisthita, L.B.; Fitri, N.A.; Marcella, C.; Wafa, S.; Kurniawan, F.; Rizka, A.; Tarigan, T.J.E.; Harbuwono, D.S.; Purnamasari, D.; et al. Challenges in the diagnosis of insulin resistance: Focusing on the role of HOMA-IR and Tryglyceride/glucose index. Diabetes Metab. Syndr. 2022, 16, 102581. [Google Scholar] [CrossRef]
- Lebovitz, H.E. Insulin resistance: Definition and consequences. Exp. Clin. Endocrinol. Diabetes 2001, 109, S135–S148. [Google Scholar] [CrossRef]
- Tripathy, D.; Mohanty, P.; Dhindsa, S.; Syed, T.; Ghanim, H.; Aliada, A.; Dandona, P. Elevation of Free Fatty Acids Induces Inflammation and Impairs Vascular Reactivity in Healthy Subjects. Diabetes 2003, 52, 2882–2887. [Google Scholar] [CrossRef]
- Oliveira, J.S.; Boery, R.N.S.O. An integrative review of associations between polymorphic variants and the metabolic syndrome. J. Vasc. Bras. 2018, 17, 141–147. [Google Scholar] [CrossRef]
- Rao, D.S.; Kumar, A.; Agarwal, S.; Elshaikh, R.H.; Choudhary, A.; Choudhary, R.K.; Babker, A.M.A.; Rathore, R.; Nurmakhanova, Z.; Nurgaliyeva, Z.; et al. The role of genetic variations in metabolic syndrome: Insights into etiology, diagnosis, and management. Ital. J. Med. 2025, 19, 1. [Google Scholar] [CrossRef]
- Barati, E.; Ghazizadeh, H.; Sadabadi, F.; Kazemi, E.; Ferns, G.A.; Avan, A.; Ghayour-Mobarhan, M. Association of the IL6 gene polymorphism with component features of metabolic syndrome in obese subjects. Biochem. Genet. 2019, 57, 695–708. [Google Scholar] [CrossRef] [PubMed]
- Khella, M.S.; Hamdy, N.M.; Amin, A.I.; El-Mesallamy, H.O. The (FTO) gene polymorphism is associated with metabolic syndrome risk in Egyptian females: A case-control study. BMC Med. Genet. 2017, 18, 101. [Google Scholar] [CrossRef]
- Ko, E.J.; Kim, E.J.; Cho, H.J.; Oh, J.; Park, H.S.; Ryu, C.S.; Kim, J.O.; Jun, H.H.; Chong, S.Y.; Kim, J.W.; et al. Prognostic significance of three endothelial nitric oxide synthase (eNOS) polymorphisms and metabolic syndrome (MetS) in patients with colorectal cancer. Genes Genom. 2022, 44, 659–670. [Google Scholar] [CrossRef] [PubMed]
- Aghajani, R.; Saeidi, M.; Amiriani, T.; Marjani, M.; Amiriani, A.H.; Akhavan Tabib, A.; Marjani, A. Genetic polymorphisms -137 (G > C) (rs187238) and -607 (C > A) (rs1946518) and serum level of interleukin 18 in Fars ethnic groups with metabolic syndrome in Northern Iran. Arch. Physiol. Biochem. 2022, 28, 1596–1602. [Google Scholar] [CrossRef]
- Sidabutar, L.M.G.B.; Pongcharoen, T.; Suttisansanee, U.; On-Nom, N.; Luealai, P.; Khemthong, P.; Chupeerach, C. The association of cholesterol transport ABCG1 polymorphism towards the susceptibility of metabolic syndrome risk factor in thai adolescents. Curr. Res. Nutr. Food Sci. J. 2022, 10, 512–520. [Google Scholar] [CrossRef]
- Ghareeb, D.; Abdelazem, A.S.; Hussein, E.M.; Al-Karamany, A.S. Association of TNF-α-308 G>A (rs1800629) polymorphism with susceptibility of metabolic syndrome. J. Diabetes Metab. Disord. 2021, 20, 209–215. [Google Scholar] [CrossRef]
- Qasem, A.; Ramesh, S.; Naser, S.A. Genetic polymorphisms in tumour necrosis factor receptors (TNFRSF1A/1B) illustrate differential treatment response to TNFα inhibitors in patients with Crohn’s disease. BMJ Open Gastroenterol. 2019, 6, e000246. [Google Scholar] [CrossRef]
- Kochetova, O.V.; Avzaletdinova, D.S.; Morugova, T.V.; Mustafina, O.E. Chemokine gene polymorphisms association with increased risk of type 2 diabetes mellitus in Tatar ethnic group, Russia. Mol. Biol. Rep. 2019, 46, 887–896. [Google Scholar] [CrossRef]
- Masood, S.H.; Khan, T.A.; Baloch, A.A.; Hasan, S.M.; Naqvi, A.M.; Iqbal, M.U.N. Association of Visfatin gene polymorphism with obesity related metabolic disorders among Pakistani population: A case control study. Sci. Rep. 2023, 13, 23002. [Google Scholar] [CrossRef]
- Dasso, N.A. How is exercise different from physical activity? A concept analysis. Nurs. Forum 2019, 54, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Frese, E.M.; Albert, S.G.; Villareal, D.T. Effects of weight loss on lean mass, strength, bone, and aerobic capacity. Med. Sci. Sports Exerc. 2017, 49, 206–217. [Google Scholar]
- Blaha, M.J.; Hung, R.K.; Dardari, Z.; I Feldman, D.; Whelton, S.P.; Nasir, K.; Blumenthal, R.S.; A Brawner, C.; Ehrman, J.K.; Keteyian, S.J.; et al. Age-dependent prognostic value of exercise capacity and derivation of fitness-associated biologic age. Heart 2016, 102, 431–437. [Google Scholar] [CrossRef]
- Gleeson, M.; Bishopm, N.C.; Stensel, D.J.; Lindley, M.R.; Mastana, S.S.; Nimmo, M.A. The anti-inflammatory effects of exercise: Mechanisms and implications for the prevention and treatment of disease. Nat. Rev. Immunol. 2011, 11, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.; Sun, Y.; Woods, J.A. Exercise and the regulation of inflammatory responses. Prog. Mol. Biol. Transl. Sci. 2015, 135, 337–354. [Google Scholar]
- Nishii, K.; Aizu, N.; Yamada, K. Review of the health-promoting effects of exercise and the involvement of myokines. Fujita Med. J. 2023, 9, 171–178. [Google Scholar]
- Scheele, C.; Nielsen, S.; Pedersen, B.K. ROS and myokines promote muscle adaptation to exercise. Trends Endocrinol. Metab. 2009, 20, 95–99. [Google Scholar] [CrossRef]
- Monserrat-Mesquida, M.; Quetglas-Llabrés, M.M.; Bouzas, C.; García, S.; Mateos, D.; Ugarriza, L.; Gómez, C.; Tur, J.A.; Sureda, A. Effects of Regular Exercise on the Biochemical, Oxidative, and Inflammatory Profiles and Quality of Life in Older Spaniards with Metabolic Syndrome. Antioxidants 2024, 13, 450. [Google Scholar] [CrossRef]
- Armstrong, L. ACSM’s Guidelines for Exercise Testing and Prescription; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2006. [Google Scholar]
- Piercy, K.L.; Troiano, R.P.; Ballard, R.M.; Carlson, S.A.; Fulton, J.E.; Galuska, D.A.; George, S.M.; Olson, R.D. The Physical Activity Guidelines for Americans. JAMA 2018, 320, 2020–2028. [Google Scholar] [CrossRef]
- Cramer, H.; Langhorst, J.; Dobos, G.; Lauche, R. Yoga for metabolic syndrome: A systematic review and meta-analysis. Eur. J. Prev. Cardiol. 2016, 23, 1982–1993. [Google Scholar] [CrossRef]
- Khoshnaw, D.M.; Ghadge, A.A. Yoga as a complementary therapy for metabolic syndrome: A narrative review. J. Integr. Med. 2021, 19, 6–12. [Google Scholar] [CrossRef]
- Patil, S.G.; Dhanakshirur, G.B.; Aithala, M.R.; Naregal, G.; Das, K.K. Effect of yoga on oxidative stress in elderly with grade-I hypertension: A randomized controlled study. J. Clin. Diagn. Res. 2014, 8, BC04–BC07. [Google Scholar] [CrossRef]
- Venugopal, V.; Geethanjali, S.; Poonguzhali, S.; Padmavathi, R.; Mahadevan, S.; Silambanan, S.; Maheshkumar, K. Effect of Yoga on Oxidative Stress in Type 2 Diabetes Mellitus: A Systematic Review and Meta-analysis. Curr. Diabetes Rev. 2022, 18, e050421192663. [Google Scholar]
- Promsrisuk, T.; Kongsui, R.; Sriraksa, N.; Boonla, O.; Srithawong, A. Elastic band resistance combined with modified Thai yoga exercise to alleviate oxidative stress and airway inflammation in type 2 diabetes mellitus. J. Exerc. Rehabil. 2023, 19, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Rosado-Pérez, J.; Castelán-Martínez, O.D.; Mújica-Calderón, A.J.; Sánchez-Rodríguez, M.A.; Mendoza-Núñez, V.M. Effect of Tai Chi on Markers of Oxidative Stress: Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2021, 18, 3458. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Núñez, V.M.; Arista-Ugalde, T.L.; Rosado-Pérez, J.; Ruiz-Ramos, M.; Santiago-Osorio, E. Hypoglycemic and antioxidant effect of Tai chi exercise training in older adults with metabolic syndrome. Clin. Interv. Aging 2018, 13, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Liu, H. Effects of combined resistance training and Tai Chi on oxidative stress, blood glucose and lipid metabolism and quality of life in elderly patients with type 2 diabetes mellitus. Res. Sports Med. 2024, 32, 871–884. [Google Scholar] [CrossRef]
- Rosado-Pérez, J.; Ortiz, R.; Santiago-Osorio, E.; Mendoza-Núñez, V.M. Effect of Tai Chi versus Walking on Oxidative Stress in Mexican Older Adults. Oxidative Med. Cell. Longev. 2013, 2013, 298590. [Google Scholar] [CrossRef]
- American Heart Association Recommendations for Physical Activity in Adults and Kids. Available online: https://www.heart.org/en/healthy-living/fitness/fitness-basics/aha-recs-for-physical-activity-in-adults (accessed on 30 March 2025).
- Carroll, S.; Dudfield, M. What is the Relationship Between Exercise and Metabolic Abnormalities? Sports Med. 2004, 34, 371–418. [Google Scholar] [CrossRef]
- Tjønna, A.E.; Lee, S.J.; Rognmo, Ø.; Stølen, T.O.; Bye, A.; Haram, P.M.; Loennechen, J.P.; Al-Share, Q.Y.; Skogvoll, E.; Slørdahl, S.A.; et al. Aerobic interval training versus continuous moderate exercise as a treatment for the metabolic syndrome: A pilot study. Circulation 2008, 118, 346–354. [Google Scholar] [CrossRef]
- Ostman, C.; Smart, N.A.; Morcos, D.; Duller, A.; Ridley, W.; Jewiss, D. The effect of exercise training on clinical outcomes in patients with the metabolic syndrome: A systematic review and meta-analysis. Cardiovasc. Diabetol. 2017, 16, 110. [Google Scholar] [CrossRef] [PubMed]
- Lwow, F.; Dunajska, K.; Milewicz, A.; Jedrzejuk, D.; Kik, K.; Szmigiero, L. Effect of moderate-intensity exercise on oxidative stress indices in metabolically healthy obese and metabolically unhealthy obese phenotypes in postmenopausal women: A pilot study. Menopause 2011, 18, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Farinha, J.B.; Steckling, F.M.; Stefanello, S.T.; Cardoso, M.S.; Nunes, L.S.; Barcelos, R.P.; Duarte, T.; Kretzmann, N.A.; Mota, C.B.; Bresciani, G.; et al. Response of oxidative stress and inflammatory biomarkers to a 12-week aerobic exercise training in women with metabolic syndrome. Sports Med. 2015, 1, 19. [Google Scholar] [CrossRef] [PubMed]
- Poblete Aro, C.E.; Russell Guzmán, J.A.; Soto Muñoz, M.E.; Villegas González, B.E. Effects of high intensity interval training versus moderate intensity continuous training on the reduction of oxidative stress in type 2 diabetic adult patients: CAT. Medwave 2015, 15, e6212. [Google Scholar] [CrossRef]
- Rytz, C.L.; Pialoux, V.; Mura, M.; Martin, A.; Hogan, D.B.; Hill, M.D.; Poulin, M.J. Impact of aerobic exercise, sex, and metabolic syndrome on markers of oxidative stress: Results from the Brain in Motion study. J. Appl. Physiol. 2020, 128, 748–756. [Google Scholar] [CrossRef]
- Nojima, H.; Watanabe, H.; Yamane, K.; Kitahara, Y.; Sekikawa, K.; Yamamoto, H.; Yokoyama, A.; Inamizu, T.; Asahara, T.; Kohno, N. Hiroshima University Health Promotion Study Group. Effect of aerobic exercise training on oxidative stress in patients with type 2 diabetes mellitus. Metabolism 2008, 57, 170–176. [Google Scholar] [CrossRef]
- Dekleva, M.; Lazic, J.S.; Arandjelovic, A.; Mazic, S. Beneficial and harmful effects of exercise in hypertensive patients: The role of oxidative stress. Hypertens. Res. 2017, 40, 15–20. [Google Scholar] [CrossRef]
- Buchheit, M.; Laursen, P.B. High-intensity interval training, solutions to the programming puzzle. Part I: Cardiopulmonary emphasis. Sports Med. 2013, 43, 313–338. [Google Scholar] [CrossRef]
- Gibala, M.J.; McGee, S.L. Metabolic adaptations to short-term high intensity interval training: A little pain for a lot of gain? Exerc. Sport. Sci. Rev. 2008, 36, 58–63. [Google Scholar] [CrossRef]
- Dun, Y.; Thomas, R.J.; Smith, J.R.; Medina-Inojosa, J.R.; Squires, R.W.; Bonikowske, A.R.; Huang, H.; Liu, S.; Olson, T.P. High-intensity interval training improves metabolic syndrome and body composition in outpatient cardiac rehabilitation patients with myocardial infarction. Cardiovasc. Diabetol. 2019, 18, 104. [Google Scholar] [CrossRef]
- Liu, J.X.; Zhu, L.; Li, P.J.; Li, N.; Xu, Y.B. Effectiveness of high-intensity interval training on glycemic control and cardiorespiratory fitness in patients with type 2 diabetes: A systematic review and meta-analysis. Aging Clin. Exp. Res. 2019, 31, 575–593. [Google Scholar] [CrossRef]
- De Araujo, G.G.; Papoti, M.; dos Reis, I.G.M.; de Mello, M.A.R.; Gobatto, C.A. Short- and long-term effects of high-intensity interval training on hormones, metabolites, antioxidant system, glycogen concentration and aerobic performance adaptations in rats. Front. Physiol. 2016, 7, 505. [Google Scholar] [CrossRef]
- De Sousa, C.V.; Sales, M.M.; Rosa, T.S.; Lewis, J.E.; de Andrade, R.V.; Simões, H.G. The Antioxidant Effect of Exercise: A Systematic Review and Meta-Analysis. Sports Med. 2017, 47, 277–293. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Debnatha, M.; Dasa, M.; Bandyopadhyayc, A.; Kr Deyd, S.; Dattaa, G. Effect of high intensity interval training on antioxidant status, inflammatory response and muscle damage indices in endurance team male players. Apunt. Sports Med. 2021, 56, 100352. [Google Scholar] [CrossRef]
- D’Alleva, M.; Vaccari, F.; Graniero, F.; Giovanelli, N.; Floreani, M.; Fiori, F.; Marinoni, M.; Parpinel, M.; Lazzer, S. Effects of 12-week combined training versus high intensity interval training on cardiorespiratory fitness, body composition and fat metabolism in obese male adults. J. Exerc. Sci. Fit. 2023, 21, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, X.; Shen, F.; Xu, N.; Li, Y.; Xu, K.; Li, J.; Liu, Y. Effects of high-intensity interval training versus moderate-intensity continuous training on blood pressure in patients with hypertension: A meta-analysis. Medicine 2022, 101, e32246. [Google Scholar] [CrossRef] [PubMed]
- Romero-Vera, L.; Ulloa-Díaz, D.; Araya-Sierralta, S.; Guede-Rojas, F.; Andrades-Ramírez, O.; Carvajal-Parodi, C.; Muñoz-Bustos, G.; Matamala-Aguilera, M.; Martínez-García, D. Effects of High-Intensity Interval Training on Blood Pressure Levels in Hypertensive Patients: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Life 2024, 14, 1661. [Google Scholar] [CrossRef]
- Coretti, M.; Nahas Donatello, N.; Bianco, G.; Cidral-Filho, F.J. An integrative review of the effects of high-intensity interval training on the autonomic nervous system. Sports Med. Health Sci. 2025, 7, 77–84. [Google Scholar] [CrossRef]
- Swarup, S.; Ahmed, I.; Grigorova, Y.; Zeltser, R. Metabolic Syndrome. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Hsu, C.N.; Hou, C.Y.; Hsu, W.H.; Tain, Y.L. Early-Life Origins of Metabolic Syndrome: Mechanisms and Preventive Aspects. Int. J. Mol. Sci. 2021, 22, 11872. [Google Scholar] [CrossRef]
- Angelico, F.; Baratta, F.; Coronati, M.; Ferro, D.; Del Ben, M. Diet and metabolic syndrome: A narrative review. Intern. Emerg. Med. 2023, 18, 1007–1017. [Google Scholar] [CrossRef]
- Eed, R.; Helal, H.; Afefy, T.; Shehabeldin, W.; Ismail, M. The Adequacy of Nutrients Intakes Among Persons with Metabolic Syndrome, Case-Control Study. J. Home Econ.—Menofia Univ. 2021, 31, 1–19. [Google Scholar]
- Castro-Barquero, S.; Ruiz-León, A.M.; Sierra-Pérez, M.; Estruch, R.; Casas, R. Dietary Strategies for Metabolic Syndrome: A Comprehensive Review. Nutrients 2020, 12, 2983. [Google Scholar] [CrossRef]
- Hoyas, I.; Leon-Sanz, M. Nutritional Challenges in Metabolic Syndrome. J. Clin. Med. 2019, 8, 1301. [Google Scholar] [CrossRef] [PubMed]
- Rathor, P.; Ch, R. The Impacts of Dietary Intervention on Brain Metabolism and Neurological Disorders: A Narrative Review. Dietetics 2024, 3, 289–307. [Google Scholar] [CrossRef]
- Song, P.; Zhang, X.; Li, Y.; Man, Q.; Jia, S.; Zhang, J.; Ding, G. MetS Prevalence and Its Association with Dietary Patterns among Chinese Middle-Aged and Elderly Population: Results from a National Cross-Sectional Study. Nutrients 2022, 14, 5301. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, Y.M.; Shin, M.H.; Koh, S.B.; Chang Kim, H.; Kim, M.K. Empirically identified dietary patterns and metabolic syndrome risk in a prospective cohort study: The Cardiovascular Disease Association Study. Clin. Nutr. 2022, 41, 2156–2162. [Google Scholar] [CrossRef]
- Tsygankova, D.P.; Bazdyrev, E.D.; Agienko, A.S.; Nakhratova, O.V.; Indukaeva, E.V.; Artamonova, G.V.; Barbarash, O.L. Cardioprotective dietary pattern of Siberian population. Russ. Open Med. J. 2023, 12, e0302. [Google Scholar] [CrossRef]
- Guasch-Ferre, M.; Willett, W.C. The Mediterranean diet and health: A comprehensive overview. J. Intern. Med. 2021, 290, 549–566. [Google Scholar] [CrossRef]
- Martini, D. Health benefits of Mediterranean diet. Nutrients 2019, 11, 1802. [Google Scholar] [CrossRef]
- Román, G.C.; Jackson, R.E.; Reis, J.; Román, A.N.; Toledo, J.B.; Toledo, E. Extra-virgin olive oil for potential prevention of Alzheimer disease. Rev. Neurol. 2019, 175, 705–723. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Martínez-González, M.A.; Tong, T.Y.; Forouhi, N.G.; Khandelwal, S.; Prabhakaran, D.; Mozaffarian, D.; de Lorgeril, M. Definitions and potential health benefits of the Mediterranean diet: Views from experts around the world. BMC Med. 2014, 12, 112. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Wang, Y.; Ren, Z.; Liu, X.; Zhao, J.; Yuan, Y.; Fei, X.; Song, X.; Wang, F.; Liang, B. Mediterranean diet lowers all-cause and cardiovascular mortality for patients with metabolic syndrome. Diabetol. Metab. Syndr. 2023, 15, 107. [Google Scholar] [CrossRef]
- Menotti, A.; Kromhout, D.; Blackburn, H.; Fidanza, F.; Buzina, R.; Nissinen, A.; Seven Countries Study Research Group. Food Intake Patterns and 25-Year Mortality from Coronary Heart Disease: Cross-Cultural Correlations in the Seven Countries Study. Eur. J. Epidemiol. 1999, 15, 507–515. [Google Scholar] [CrossRef]
- Merra, G.; Noce, A.; Marrone, G.; Cintoni, M.; Tarsitano, M.G.; Capacci, A.; De Lorenzo, A. Influence of Mediterranean Diet on Human Gut Microbiota. Nutrients 2020, 13, 7. [Google Scholar] [CrossRef] [PubMed]
- Rees, K.; Takeda, A.; Martin, N.; Ellis, L.; Wijesekara, D.; Vepa, A.; Das, A.; Hartley, L.; Stranges, S. Mediterranean-style diet for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2019, 3, CD009825. [Google Scholar] [CrossRef]
- Dominguez, L.J.; Veronese, N.; Di Bella, G.; Cusumano, C.; Parisi, A.; Tagliaferri, F.; Ciriminna, S.; Barbagallo, M. Mediterranean diet in the management and prevention of obesity. Exp. Gerontol. 2023, 174, 112121. [Google Scholar] [CrossRef] [PubMed]
- Zúnica-García, S.; Blanquer-Gregori, J.J.; Sánchez-Ortiga, R.; Jiménez-Trujillo, M.I.; Chicharro-Luna, E. Relationship between diabetic peripheral neuropathy and adherence to the Mediterranean diet in patients with type 2 diabetes mellitus: An observational study. J. Endocrinol. Investig. 2024, 47, 2603–2613. [Google Scholar] [CrossRef]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef]
- Gómez-Sánchez, L.; Gómez-Sánchez, M.; Tamayo-Morales, O.; Lugones-Sánchez, C.; González-Sánchez, S.; Martí-Lluch, R.; Rodríguez-Sánchez, E.; García-Ortiz, L.; Gómez-Marcos, M.A. Relationship Between the Mediterranean Diet and Metabolic Syndrome and Each of the Components That Form It in Caucasian Subjects: A Cross-Sectional Trial. Nutrients 2024, 16, 1948. [Google Scholar] [CrossRef]
- Milano, A.; Kabbaha, S.; Thorlund, K. Effects of the mediterranean diet versus low-fat diet on metabolic syndrome outcomes: A systematic review and meta-analysis of randomized controlled trials. Hum. Nutr. Metab. 2022, 30, 200175. [Google Scholar] [CrossRef]
- Bakaloudi, D.R.; Chrysoula, L.; Kotzakioulafi, E.; Theodoridis, X.; Chourdakis, M. Impact of the Level of Adherence to Mediterranean Diet on the Parameters of Metabolic Syndrome: A Systematic Review and Meta-Analysis of Observational Studies. Nutrients 2021, 13, 1514. [Google Scholar] [CrossRef]
- Saneei, P.; Salehi-Abargouei, A.; Esmaillzadeh, A.; Azadbakht, L. Influence of Dietary Approaches to Stop Hypertension (DASH) diet on blood pressure: A systematic review and meta-analysis on randomized controlled trials. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 1253–1261. [Google Scholar] [CrossRef]
- Shirani, F.; Salehi-Abargouei, A.; Azadbakht, L. Effects of Dietary Approaches to Stop Hypertension (DASH) diet on some risk for developing type 2 diabetes: A systematic review and meta-analysis on controlled clinical trials. Nutrition 2013, 29, 939–947. [Google Scholar] [CrossRef]
- Soltani, S.; Chitsazi, M.J.; Salehi-Abargouei, A. The effect of dietary approaches to stop hypertension (DASH) on serum inflammatory markers: A systematic review and meta-analysis of randomized trials. Clin. Nutr. 2018, 37, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Aihemaiti, G.; Guo, H. Effect of Dietary Approaches to Stop Hypertension (DASH) on Patients with Metabolic Syndrome and Its Potential Mechanisms. Diabetes Metab. Syndr. Obes. 2024, 17, 3103–3110. [Google Scholar] [CrossRef] [PubMed]
- Sangouni, A.A.; Hosseinzadeh, M.; Parastouei, K. The effect of dietary approaches to stop hypertension (DASH) diet on fatty liver and cardiovascular risk factors in subjects with metabolic syndrome: A randomized controlled trial. BMC Endocr. Disord. 2024, 24, 126. [Google Scholar] [CrossRef] [PubMed]
- Filippou, C.D.; Thomopoulos, C.G.; Konstantinidis, D.G.; Dimitriadis, K.S.; Chrysochoou, C.A.; Tatakis, F.A.; Siafi, E.P.; Tousoulis, D.M.; Nihoyannopoulos, P.I.; Panagiotakos, D.B.; et al. Effect of DASH vs. mediterranean diet accompanied by a salt restriction on metabolic syndrome and cardiometabolic risk factors in adults with high normal blood pressure or grade 1 hypertension: Secondary analyses of a randomized controlled trial. Hell. J. Cardiol 2024, S1109-9666(24)00110-6. [Google Scholar] [CrossRef]
- Valenzuela-Fuenzalida, J.J.; Bravo, V.S.; Valarezo, L.M.; Delgado Retamal, M.F.; Leiva, J.M.; Bruna-Mejías, A.; Nova-Baeza, P.; Orellana-Donoso, M.; Suazo-Santibañez, A.; Oyanedel-Amaro, G.; et al. Effectiveness of DASH Diet Versus Other Diet Modalities in Patients with Metabolic Syndrome: A Systematic Review and Meta-Analysis. Nutrients 2024, 16, 3054. [Google Scholar] [CrossRef]
- Daneshzad, E.; Heshmati, J.; Basirat, V.; Keshavarz, S.-A.; Qorbani, M.; Larijani, B.; Bellissimo, N.; Azadbakht, L. The Effect of the Dietary Approaches to Stop Hypertension (DASH) Diet on Sleep, Mental Health, and Hormonal Changes: A Randomized Clinical Trial in Women with Type 2 Diabetes. Front. Nutr. 2022, 9, 775543. [Google Scholar] [CrossRef]
- Valipur, G.; Asemi, Z.; Samimi, M.; Tabassi, Z.; Sabihi, S.S.; Saneei, P.; Esmaillzadeh, A. The effect of DASH diet on insulin resistance, inflammation, and oxidative stress in gestational diabetes: A randomized controlled clinical trial. ijdld 2014, 13, 352–361. [Google Scholar]
- Lopes, H.F.; Martin, K.L.; Nashar, K.; Morrow, J.D.; Goodfriend, T.L.; Egan, B.M. DASH diet lowers blood pressure and lipid-induced oxidative stress in obesity. Hypertension 2003, 41, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Asemi, Z.; Samimi, M.; Tabassi, Z.; Shakeri, H.; Sabihi, S.S.; Esmaillzadeh, A. Effects of DASH diet on lipid profiles and biomarkers of oxidative stress in overweight and obese women with polycystic ovary syndrome: A randomized clinical trial. Nutrition 2014, 30, 1287–1293. [Google Scholar] [CrossRef] [PubMed]
- Pirouzeh, R.; Heidarzadeh-Esfahani, N.; Morvaridzadeh, M.; Izadi, A.; Yosaee, S.; Potter, E.; Heshmati, J.; Pizarro, A.B.; Omidi, A.; Heshmati, S. Effect of DASH diet on oxidative stress parameters: A systematic review and meta-analysis of randomized clinical trials. Diabetes Metab. Syndr. 2020, 14, 2131–2138. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, A.; Zarban, A.; Dehghani, H.; Mohamadifard, M.; Tavallaie, S.; Ferns, G.A. The Relationship Between Adherence to a Dietary Approach to Stop Hypertension Diet with Oxidative Stress and Antioxidant Capacity in Young Women. Endocrinol. Res. Pract. 2022, 26, 141–147. [Google Scholar] [CrossRef]
- Arab, A.; Khorvash, F.; Karimi, E.; Heidari, Z.; Askari, G. The effects of the dietary approaches to stop hypertension (DASH) diet on oxidative stress and clinical indices of migraine patients: A randomized controlled trial. Nutr. Neurosci. 2022, 25, 2259–2268. [Google Scholar] [CrossRef]
- Larsson, S.C.; Wallin, A.; and Wolk, A. Dietary Approaches to Stop Hypertension Diet and Incidence of Stroke: Results from 2 Prospective Cohorts. Stroke 2016, 47, 4. [Google Scholar] [CrossRef]
- Niknam, M.; Saadatnia, M.; Shakeri, F.; Keshteli, A.H.; Saneei, P.; Esmaillzadeh, A. Adherence to a DASH-Style Diet in Relation to Stroke: A Case-Control Study. J. Am. Coll. Nutr. 2015, 34, 408–415. [Google Scholar] [CrossRef]
- Agarwal, P.; Leurgans, S.E.; Agrawal, S.; Aggarwal, N.T.; Cherian, L.J.; James, B.D.; Dhana, K.; Barnes, L.L.; Bennett, D.A.; Schneider, J.A. Association of Mediterranean-DASH Intervention for Neurodegenerative Delay and Mediterranean Diets With Alzheimer Disease Pathology. Neurology 2023, 100, e2259–e2268. [Google Scholar] [CrossRef]
- Aleksandrova, K.; Koelman, L.; Rodrigues, C.E. Dietary patterns and biomarkers of oxidative stress and inflammation: A systematic review of observational and intervention studies. Redox Biol. 2021, 42, 101869. [Google Scholar] [CrossRef]
- Peterman, M. The ketogenic diet in the treatment of epilepsy: A preliminary report. J. Nerv. Ment. Dis. 1926, 64, 96–97. [Google Scholar] [CrossRef]
- Ebbert, J.O.; Jensen, M.D. Fat depots, free fatty acids, and dyslipidemia. Nutrients 2013, 5, 498–508. [Google Scholar] [CrossRef]
- Zhu, H.; Bi, D.; Zhang, Y.; Kong, C.; Du, J.; Wu, X.; Wei, Q.; Qin, H. Ketogenic diet for human diseases: The underlying mechanisms and potential for clinical implementations. Signal Transduct. Target. Ther. 2022, 7, 11. [Google Scholar] [CrossRef]
- Yuan, X.; Wang, J.; Yang, S.; Gao, M.; Cao, L.; Li, X.; Hong, D.; Tian, S.; Sun, C. Effect of the ketogenic diet on glycemic control, insulin resistance, and lipid metabolism in patients with T2DM: A systematic review and meta-analysis. Nutr. Diabetes 2020, 10, 38. [Google Scholar] [CrossRef] [PubMed]
- Battezzati, A.; Foppiani, A.; Leone, A.; De Amicis, R.; Spadafranca, A.; Mari, A.; Bertoli, S. Acute Insulin Secretory Effects of a Classic Ketogenic Meal in Healthy Subjects: A Randomized Cross-Over Study. Nutrients 2023, 15, 1119. [Google Scholar] [CrossRef] [PubMed]
- Rafiullah, M.; Musambil, M.; David, S.K. Effect of a very low-carbohydrate ketogenic diet vs recommended diets in patients with type 2 diabetes: A meta-analysis. Nutr. Rev. 2022, 80, 488–502. [Google Scholar] [CrossRef] [PubMed]
- Brinkworth, G.D.; Noakes, M.; Buckley, J.D.; Keogh, J.B.; Clifton, P.M. Long-term effects of a very-low-carbohydrate weight loss diet compared with an isocaloric low-fat diet after 12 mo. Am. J. Clin. Nutr. 2009, 90, 23–32. [Google Scholar] [CrossRef]
- Nordmann, A.J.; Nordmann, A.; Briel, M.; Keller, U.; Yancy, W.S., Jr.; Brehm, B.J.; Bucher, H.C. Effects of low-carbohydrate vs low-fat diets on weight loss and cardiovascular risk factors: A meta-analysis of randomized controlled trials. Arch. Intern. Med. 2006, 166, 285–293. [Google Scholar] [CrossRef]
- Tay, J.; Brinkworth, G.D.; Noakes, M.; Keogh, J.; Clifton, P.M. Metabolic effects of weight loss on a very-low-carbohydrate diet compared with an isocaloric high-carbohydrate diet in abdominally obese subjects. J. Am. Coll. Cardiol. 2008, 51, 59–67. [Google Scholar] [CrossRef]
- Suarez, R.; Chapela, S.; Llobera, N.D.; Montalván, M.; Vásquez, C.A.; Martinuzzi, A.L.N.; Katsanos, C.S.; Verde, L.; Frias-Toral, E.; Barrea, L.; et al. Very Low Calorie Ketogenic Diet: What Effects on Lipid Metabolism? Curr. Nutr. Rep. 2024, 13, 516–526. [Google Scholar] [CrossRef]
- Yancy, W.S., Jr.; Olsen, M.K.; Guyton, J.R.; Bakst, R.P.; Westman, E.C. A low-carbohydrate, ketogenic diet versus a low-fat diet to treat obesity and hyperlipidemia: A randomized, controlled trial. Ann. Intern. Med. 2004, 140, 769–777. [Google Scholar] [CrossRef]
- Bueno, N.B.; de Melo, I.S.V.; de Oliveira, S.L.; da Rocha Ataide, T. Very-low-carbohydrate ketogenic diet v low-fat diet for long-term weight loss: A meta-analysis of randomised controlled trials. Br. J. Nutr. 2013, 110, 1178–1187. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Mi, J.; Wang, Y.; Xue, L.; Liu, J.; Fan, M.; Zhang, D.; Wang, L.; Qian, H.; Li, Y. Effects of low-carbohydrate diet and ketogenic diet on glucose and lipid metabolism in type 2 diabetic mice. Nutrition 2021, 89, 111230. [Google Scholar] [CrossRef] [PubMed]
- López-Espinoza, M.Á.; Chacón-Moscoso, S.; Sanduvete-Chaves, S.; Ortega-Maureira, M.J.; Barrientos-Bravo, T. Effect of a Ketogenic Diet on the Nutritional Parameters of Obese Patients: A Systematic Review and Meta-Analysis. Nutrients 2021, 13, 2946. [Google Scholar] [CrossRef]
- Luo, W.; Zhang, J.; Xu, D.; Zhou, Y.; Qu, Z.; Yang, Q.; Lv, Q. Low carbohydrate ketogenic diets reduce cardiovascular risk factor levels in obese or overweight patients with T2DM: A meta-analysis of randomized controlled trials. Front. Nutr. 2022, 9, 1092031. [Google Scholar] [CrossRef]
- Paoli, A.; Mancin, L.; Giacona, M.C.; Bianco, A.; Caprio, M. Effects of a ketogenic diet in overweight women with polycystic ovary syndrome. J. Transl. Med. 2020, 18, 104. [Google Scholar] [CrossRef]
- Di Raimondo, D.; Buscemi, S.; Musiari, G.; Rizzo, G.; Pirera, E.; Corleo, D.; Pinto, A.; Tuttolomondo, A. Ketogenic Diet, Physical Activity, and Hypertension—A Narrative Review. Nutrients 2021, 13, 2567. [Google Scholar] [CrossRef] [PubMed]
- Forsythe, C.E.; Phinney, S.D.; Fernandez, M.L.; Quann, E.E.; Wood, R.J.; Bibus, D.M.; Kraemer, W.J.; Feinman, R.D.; Volek, J.S. Comparison of low fat and low carbohydrate diets on circulating fatty acid composition and markers of inflammation. Lipids 2008, 43, 65–77. [Google Scholar] [CrossRef]
- Hallberg, S.J.; McKenzie, A.L.; Williams, P.T.; Bhanpuri, N.H.; Peters, A.L.; Campbell, W.W.; Hazbun, T.L.; Volk, B.M.; McCarter, J.P.; Phinney, S.D.; et al. Effectiveness and Safety of a Novel Care Model for the Management of Type 2 Diabetes at 1 Year: An Open-Label, Non-Randomized, Controlled Study. Diabetes Ther. 2018, 9, 583–612. [Google Scholar] [CrossRef]
- Almabruk, B.A.; Alharbi, S.H.; Alsaqer, F.S.; Al Essa, A.; Eid, H.; Alqahtani, O.; Badawood, M.A.; Alzahrani, E.M.; Alzahrani, E.M.; Alshaikh, F.K.; et al. The Role of Intermittent Fasting on Metabolic Syndrome: A Systematic Review and Meta-Analysis. Cureus 2024, 16, e71623. [Google Scholar] [CrossRef]
- Vrdoljak, J.; Kumric, M.; Vilovic, M.; Martinovic, D.; Rogosic, V.; Borovac, J.A.; Ticinovic Kurir, T.; Bozic, J. Can Fasting Curb the Metabolic Syndrome Epidemic? Nutrients 2022, 14, 456. [Google Scholar] [CrossRef]
- Vasim, I.; Majeed, C.N.; DeBoer, M.D. Intermittent Fasting and Metabolic Health. Nutrients 2022, 14, 631. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Luo, S.; Ye, Y.; Yin, S.; Fan, J.; Xia, M. Intermittent Fasting Improves Cardiometabolic Risk Factors and Alters Gut Microbiota in Metabolic Syndrome Patients. J. Clin. Endocrinol. Metab. 2021, 106, 64–79. [Google Scholar] [CrossRef] [PubMed]
- Manoogian, E.N.C.; Wilkinson, M.J.; O’Neal, M.; Laing, K.; Nguyen, J.; Van, D.; Rosander, A.; Pazargadi, A.; Gutierrez, N.R.; Fleischer, J.G.; et al. Time-Restricted Eating in Adults with Metabolic Syndrome: A Randomized Controlled Trial. Ann. Intern. Med. 2024, 177, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- Mezhal, F.; Ahmad, A.; Abdulle, A.; Leinberger-Jabari, A.; Oulhaj, A.; AlJunaibi, A.; Alnaeemi, A.; Al Dhaheri, A.S.; AlZaabi, E.; Al-Maskari, F.; et al. Metabolic Syndrome in Fasting and Non-Fasting Participants: The UAE Healthy Future Study. Int. J. Environ. Res. Public Health 2022, 19, 13757. [Google Scholar] [CrossRef]
- Parvaresh, A.; Razavi, R.; Abbasi, B.; Yaghoobloo, K.; Hassanzadeh, A.; Mohammadifard, N.; Safavi, S.M.; Hadi, A.; Clark, C.C.T. Modified alternate-day fasting vs. calorie restriction in the treatment of patients with metabolic syndrome: A randomized clinical trial. Complement. Ther. Med. 2019, 47, 102187. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onu, A.; Trofin, D.-M.; Tutu, A.; Onu, I.; Galaction, A.-I.; Sardaru, D.-P.; Trofin, D.; Onita, C.A.; Iordan, D.-A.; Matei, D.-V. Integrative Strategies for Preventing and Managing Metabolic Syndrome: The Impact of Exercise and Diet on Oxidative Stress Reduction—A Review. Life 2025, 15, 757. https://doi.org/10.3390/life15050757
Onu A, Trofin D-M, Tutu A, Onu I, Galaction A-I, Sardaru D-P, Trofin D, Onita CA, Iordan D-A, Matei D-V. Integrative Strategies for Preventing and Managing Metabolic Syndrome: The Impact of Exercise and Diet on Oxidative Stress Reduction—A Review. Life. 2025; 15(5):757. https://doi.org/10.3390/life15050757
Chicago/Turabian StyleOnu, Ana, Daniela-Marilena Trofin, Andrei Tutu, Ilie Onu, Anca-Irina Galaction, Dragos-Petrica Sardaru, Dan Trofin, Cristiana Amalia Onita, Daniel-Andrei Iordan, and Daniela-Viorelia Matei. 2025. "Integrative Strategies for Preventing and Managing Metabolic Syndrome: The Impact of Exercise and Diet on Oxidative Stress Reduction—A Review" Life 15, no. 5: 757. https://doi.org/10.3390/life15050757
APA StyleOnu, A., Trofin, D.-M., Tutu, A., Onu, I., Galaction, A.-I., Sardaru, D.-P., Trofin, D., Onita, C. A., Iordan, D.-A., & Matei, D.-V. (2025). Integrative Strategies for Preventing and Managing Metabolic Syndrome: The Impact of Exercise and Diet on Oxidative Stress Reduction—A Review. Life, 15(5), 757. https://doi.org/10.3390/life15050757







