Active Substances from the Micro-Immunotherapy Medicine 2LMIREG Display Antioxidative Properties In Vitro in Two Colorectal Cancer Cell Lines
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Investigational Product
2.3. Human Cell Lines
2.4. Cell Treatment with the Tested Item MIM-10
2.5. Cell Viability Assay
2.6. ROS Production
2.7. Index of Mitochondrial Mass
2.8. mtDNA Quantification
2.9. Enzymatic Activities
2.10. Statistics
3. Results
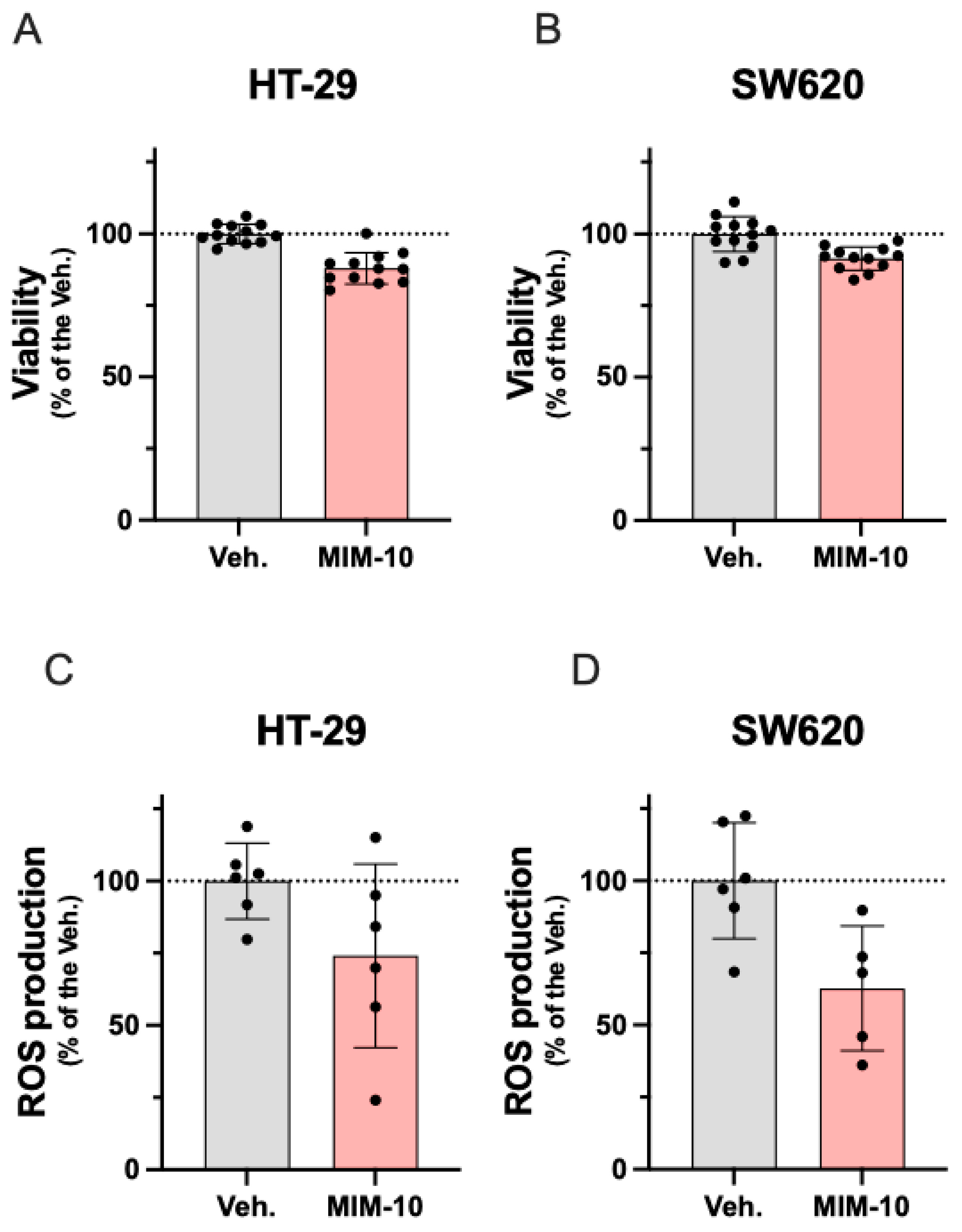
3.1. MIM-10 Slightly Reduced the Viability and Diminished the Reactive Oxygen Species Production Within the HT-29 Cells and the Metastatic Colon Cancer SW620 Cells
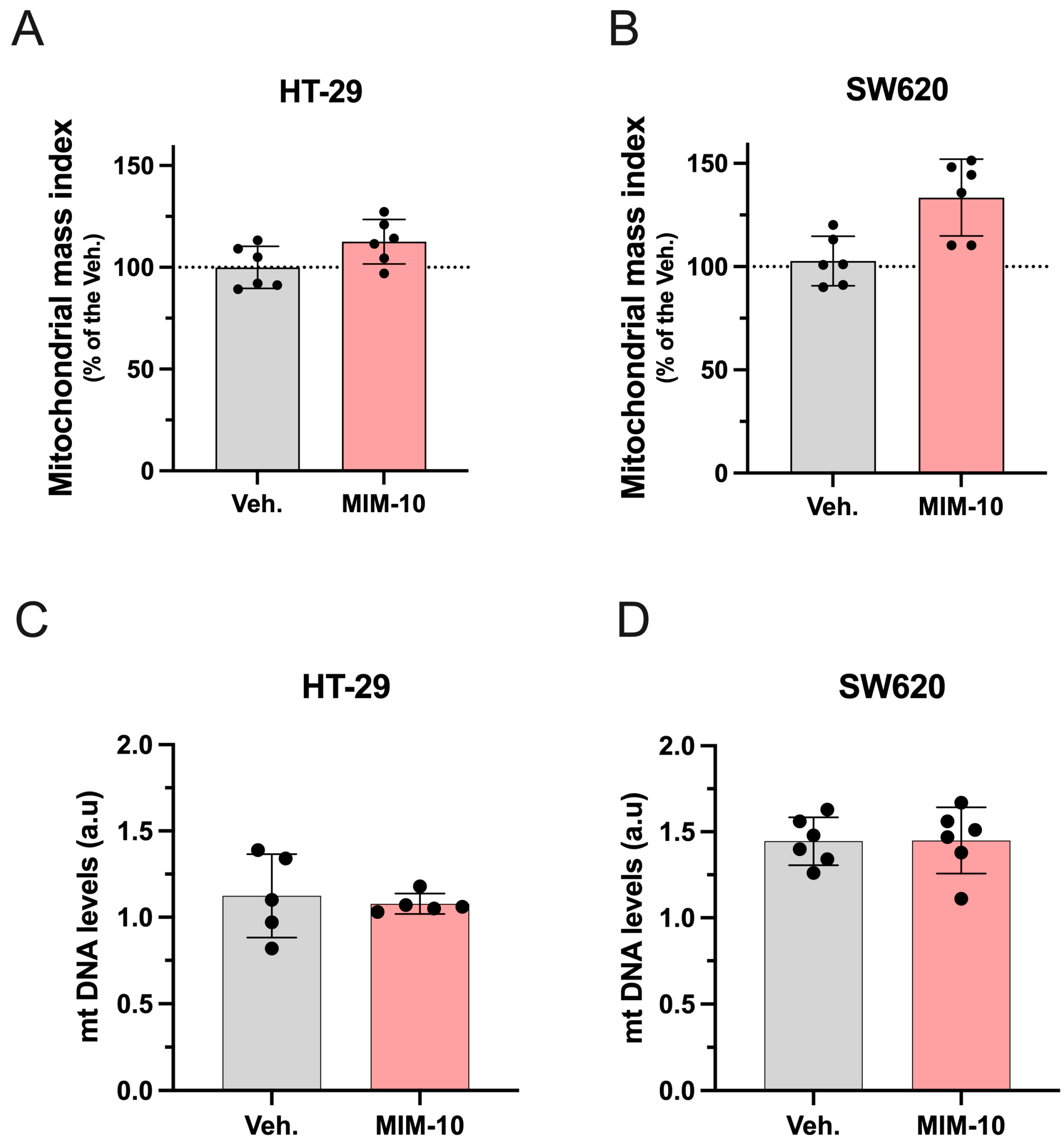
3.2. MIM-10 Slightly Increased the Mitochondrial Mass Index Without Affecting the Mitochondrial DNA Level Within the HT-29 Cells and the Metastatic SW620 Cells
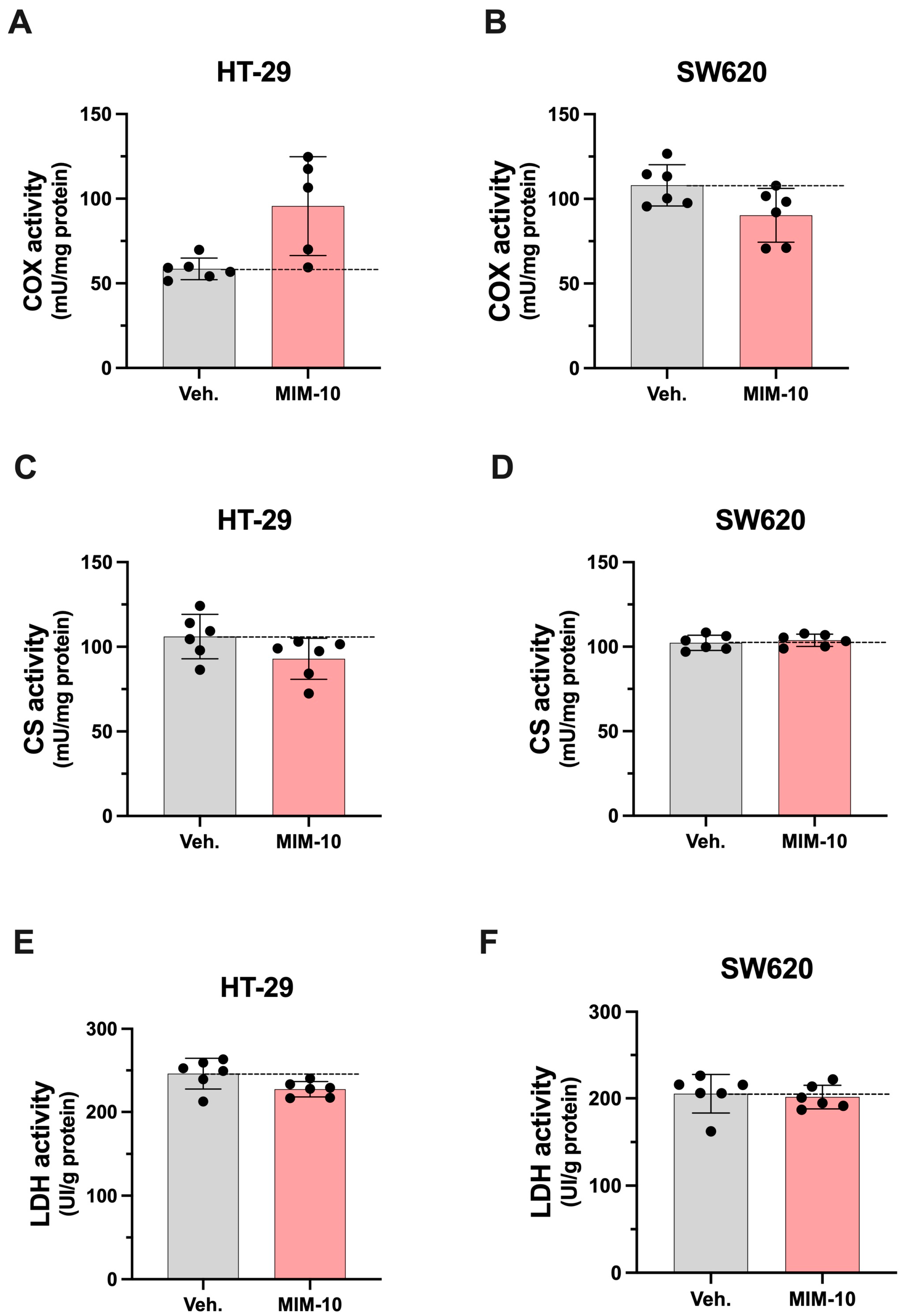
3.3. MIM-10 Affects the Activity of Enzymes Related to Mitochondrial Function Within HT-29 and SW620 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Xi, Y.; Xu, P. Global Colorectal Cancer Burden in 2020 and Projections to 2040. Transl. Oncol. 2021, 14, 101174. [Google Scholar] [CrossRef]
- Lei, Y.; Huang, K.; Gao, C.; Lau, Q.C.; Pan, H.; Xie, K.; Li, J.; Liu, R.; Zhang, T.; Xie, N.; et al. Proteomics Identification of ITGB3 as a Key Regulator in Reactive Oxygen Species-Induced Migration and Invasion of Colorectal Cancer Cells. Mol. Cell. Proteom. 2011, 10, M110.005397. [Google Scholar] [CrossRef]
- Bardelčíková, A.; Šoltys, J.; Mojžiš, J. Oxidative Stress, Inflammation and Colorectal Cancer: An Overview. Antioxidants 2023, 12, 901. [Google Scholar] [CrossRef]
- Mena, S.; Ortega, A.; Estrela, J.M. Oxidative Stress in Environmental-Induced Carcinogenesis. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2009, 674, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Porporato, P.E.; Filigheddu, N.; Pedro, J.M.B.S.; Kroemer, G.; Galluzzi, L. Mitochondrial Metabolism and Cancer. Cell Res. 2018, 28, 265–280. [Google Scholar] [CrossRef]
- Alorda-Clara, M.; Torrens-Mas, M.; Morla-Barcelo, P.M.; Roca, P.; Sastre-Serra, J.; Pons, D.G.; Oliver, J. High Concentrations of Genistein Decrease Cell Viability Depending on Oxidative Stress and Inflammation in Colon Cancer Cell Lines. Int. J. Mol. Sci. 2022, 23, 7526. [Google Scholar] [CrossRef] [PubMed]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial Reactive Oxygen Species (ROS) and ROS-Induced ROS Release. Physiol. Rev. 2014, 94, 909. [Google Scholar] [CrossRef] [PubMed]
- Okon, I.S.; Zou, M.-H. Mitochondrial ROS and Cancer Drug Resistance: Implications for Therapy. Pharmacol. Res. 2015, 100, 170–174. [Google Scholar] [CrossRef]
- Liou, G.-Y.; Storz, P. Reactive Oxygen Species in Cancer. Free Radic. Res. 2010, 44, 479–496. [Google Scholar] [CrossRef]
- Wu, F.; Shao, R.; Zheng, P.; Zhang, T.; Qiu, C.; Sui, H.; Li, S.; Jin, L.; Pan, H.; Jin, X.; et al. Isoalantolactone Enhances the Antitumor Activity of Doxorubicin by Inducing Reactive Oxygen Species and DNA Damage. Front. Oncol. 2022, 12, 813854. [Google Scholar] [CrossRef]
- Gorrini, C.; Harris, I.S.; Mak, T.W. Modulation of Oxidative Stress as an Anticancer Strategy. Nat. Rev. Drug Discov. 2013, 12, 931–947. [Google Scholar] [CrossRef] [PubMed]
- Reczek, C.R.; Chandel, N.S. ROS Promotes Cancer Cell Survival through Calcium Signaling. Cancer Cell. 2018, 33, 949–951. [Google Scholar] [CrossRef]
- Rahim, N.F.C.; Hussin, Y.; Aziz, M.N.M.; Mohamad, N.E.; Yeap, S.K.; Masarudin, M.J.; Abdullah, R.; Akhtar, M.N.; Alitheen, N.B. Cytotoxicity and Apoptosis Effects of Curcumin Analogue (2E,6E)-2,6-Bis(2,3-Dimethoxybenzylidine) Cyclohexanone (DMCH) on Human Colon Cancer Cells HT29 and SW620 In Vitro. Molecules 2021, 26, 1261. [Google Scholar] [CrossRef]
- Sorolla, M.A.; Hidalgo, I.; Sorolla, A.; Montal, R.; Pallisé, O.; Salud, A.; Parisi, E. Microenvironmental Reactive Oxygen Species in Colorectal Cancer: Involved Processes and Therapeutic Opportunities. Cancers 2021, 13, 5037. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Zuo, J.; Li, B.; Chen, R.; Luo, K.; Xiang, X.; Lu, S.; Huang, C.; Liu, L.; Tang, J.; et al. Drug-Induced Oxidative Stress in Cancer Treatments: Angel or Devil? Redox Biol. 2023, 63, 102754. [Google Scholar] [CrossRef]
- Jacques, C.; Chatelais, M.; Fekir, K.; Fauconnier, L.; Mellier, M.; Togbe, D.; Floris, I. The Micro-Immunotherapy Medicine 2LEID Exhibits an Immunostimulant Effect by Boosting Both Innate and Adaptive Immune Responses. Int. J. Mol. Sci. 2021, 23, 110. [Google Scholar] [CrossRef] [PubMed]
- Jacques, C.; Floris, I.; Lejeune, B. Ultra-Low Dose Cytokines in Rheumatoid Arthritis, Three Birds with One Stone as the Rationale of the 2LARTH® Micro-Immunotherapy Treatment. Int. J. Mol. Sci. 2021, 22, 6717. [Google Scholar] [CrossRef]
- Floris, I.; García-González, V.; Palomares, B.; Appel, K.; Lejeune, B. The Micro-Immunotherapy Medicine 2LARTH® Reduces Inflammation and Symptoms of Rheumatoid Arthritis In Vivo. Int. J. Rheumatol. 2020, 2020, 1594573. [Google Scholar] [CrossRef]
- Floris, I.; Rose, T.; Rojas, J.A.C.; Appel, K.; Roesch, C.; Lejeune, B. Pro-Inflammatory Cytokines at Ultra-Low Dose Exert Anti-Inflammatory Effect In Vitro: A Possible Mode of Action Involving Sub-Micron Particles? Dose-Response 2020, 18, 1–11. [Google Scholar] [CrossRef]
- Ferrà-Cañellas, M.D.M.; Munar-Bestard, M.; Floris, I.; Ramis, J.M.; Monjo, M.; Garcia-Sureda, L. A Sequential Micro-Immunotherapy Medicine Increases Collagen Deposition in Human Gingival Fibroblasts and in an Engineered 3D Gingival Model under Inflammatory Conditions. Int. J. Mol. Sci. 2023, 24, 10484. [Google Scholar] [CrossRef]
- Ferrà-Cañellas, M.D.M.; Munar-Bestard, M.; Garcia-Sureda, L.; Lejeune, B.; Ramis, J.M.; Monjo, M. BMP4 Micro-Immunotherapy Increases Collagen Deposition and Reduces PGE2 Release in Human Gingival Fibroblasts and Increases Tissue Viability of Engineered 3D Gingiva under Inflammatory Conditions. J. Periodontol. 2021, 92, 1448–1459. [Google Scholar] [CrossRef]
- Ferrà-Cañellas, M.D.M.; Garcia-Sureda, L. Exploring the Potential of Micro-Immunotherapy in the Treatment of Periodontitis. Life 2024, 14, 552. [Google Scholar] [CrossRef]
- Jacques, C.; Floris, I. How an Immune-Factor-Based Formulation of Micro-Immunotherapy Could Interfere with the Physiological Processes Involved in the Atopic March. Int. J. Mol. Sci. 2023, 24, 1483. [Google Scholar] [CrossRef] [PubMed]
- Floris, I.; Chenuet, P.; Togbe, D.; Volteau, C.; Lejeune, B. Potential Role of the Micro-Immunotherapy Medicine 2LALERG in the Treatment of Pollen-Induced Allergic Inflammation. Dose-Response 2020, 18, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jacques, C.; Floris, I. Special Focus on the Cellular Anti-Inflammatory Effects of Several Micro-Immunotherapy Formulations: Considerations Regarding Intestinal-, Immune-Axis-Related- and Neuronal-Inflammation Contexts. J. Inflamm. Res. 2022, 15, 6695–6717. [Google Scholar] [CrossRef]
- Jacques, C.; Marchesi, I.; Fiorentino, F.P.; Chatelais, M.; Lilli, N.L.; Appel, K.; Lejeune, B.; Floris, I. A Micro-Immunotherapy Sequential Medicine MIM-Seq Displays Immunomodulatory Effects on Human Macrophages and Anti-Tumor Properties towards In Vitro 2D and 3D Models of Colon Carcinoma and in an In Vivo Subcutaneous Xenograft Colon Carcinoma Model. Int. J. Mol. Sci. 2022, 23, 6059. [Google Scholar] [CrossRef] [PubMed]
- Jacques, C.; Marchand, F.; Chatelais, M.; Floris, I. Actives from the Micro-Immunotherapy Medicine 2LMIREG® Reduce the Expression of Cytokines and Immune-Related Markers Including Interleukin-2 and HLA-II While Modulating Oxidative Stress and Mitochondrial Function. J. Inflamm. Res. 2024, 17, 1161–1181. [Google Scholar] [CrossRef]
- Weagel, E.G.; Townsend, M.H.; Anderson, M.D.; Velazquez, E.J.; Weber, K.S.; Robison, R.A.; O’Neill, K.L. Abstract 2149: Unusual Expression of HPRT on the Surface of the Colorectal Cancer Cell Lines HT29 and SW620. Cancer Res. 2017, 77, 2149. [Google Scholar] [CrossRef]
- Banskota, S.; Regmi, S.C.; Kim, J.-A. NOX1 to NOX2 Switch Deactivates AMPK and Induces Invasive Phenotype in Colon Cancer Cells through Overexpression of MMP-7. Mol. Cancer 2015, 14, 123. [Google Scholar] [CrossRef]
- Santandreu, F.M.; Valle, A.; Fernández De Mattos, S.; Roca, P.; Oliver, J. Hydrogen Peroxide Regulates the Mitochondrial Content of Uncoupling Protein 5 in Colon Cancer Cells. Cell. Physiol. Biochem. 2009, 24, 379–390. [Google Scholar] [CrossRef]
- Jacques, C.; Chatelais, M.; Fekir, K.; Brulefert, A.; Floris, I. The Unitary Micro-Immunotherapy Medicine Interferon-γ (4 CH) Displays Similar Immunostimulatory and Immunomodulatory Effects than Those of Biologically Active Human Interferon-γ on Various Cell Types. Int. J. Mol. Sci. 2022, 23, 2314. [Google Scholar] [CrossRef]
- Sastre-Serra, J.; Ahmiane, Y.; Roca, P.; Oliver, J.; Pons, D.G. Xanthohumol, a Hop-Derived Prenylflavonoid Present in Beer, Impairs Mitochondrial Functionality of SW620 Colon Cancer Cells. Int. J. Food Sci. Nutr. 2019, 70, 396–404. [Google Scholar] [CrossRef]
- Torrens-Mas, M.; Hernández-López, R.; Pons, D.G.; Roca, P.; Oliver, J.; Sastre-Serra, J. Sirtuin 3 Silencing Impairs Mitochondrial Biogenesis and Metabolism in Colon Cancer Cells. Am. J. Physiol. Cell Physiol. 2019, 317, C398–C404. [Google Scholar] [CrossRef] [PubMed]
- Del Mar Blanquer-Rosselló, M.; Hernández-López, R.; Roca, P.; Oliver, J.; Valle, A. Resveratrol Induces Mitochondrial Respiration and Apoptosis in SW620 Colon Cancer Cells. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Colom, B.; Oliver, J.; Garcia-Palmer, F.J. Sexual Dimorphism in the Alterations of Cardiac Muscle Mitochondrial Bioenergetics Associated to the Ageing Process. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 70, 1360–1369. [Google Scholar] [CrossRef] [PubMed]
- Vaux, D.L. Know When Your Numbers Are Significant. Nature 2012, 492, 180–181. [Google Scholar] [CrossRef]
- Pagano, G.; Aiello Talamanca, A.; Castello, G.; Cordero, M.D.; D’Ischia, M.; Gadaleta, M.N.; Pallardó, F.V.; Petrović, S.; Tiano, L.; Zatterale, A. Oxidative Stress and Mitochondrial Dysfunction across Broad-Ranging Pathologies: Toward Mitochondria-Targeted Clinical Strategies. Oxid. Med. Cell. Longev. 2014, 2014, 541230. [Google Scholar] [CrossRef]
- Wallace, D.C. A Mitochondrial Paradigm of Metabolic and Degenerative Diseases, Aging, and Cancer: A Dawn for Evolutionary Medicine. Annu. Rev. Genet. 2005, 39, 359–407. [Google Scholar] [CrossRef]
- Icard, P.; Shulman, S.; Farhat, D.; Steyaert, J.-M.; Alifano, M.; Lincet, H. How the Warburg Effect Supports Aggressiveness and Drug Resistance of Cancer Cells? Drug Resist. Updates 2018, 38, 1–11. [Google Scholar] [CrossRef]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does It Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef]
- Vyas, S.; Zaganjor, E.; Haigis, M.C. Mitochondria and Cancer. Cell 2016, 166, 555–566. [Google Scholar] [CrossRef]
- Jia, D.; Park, J.H.; Jung, K.H.; Levine, H.; Kaipparettu, B.A. Elucidating the Metabolic Plasticity of Cancer: Mitochondrial Reprogramming and Hybrid Metabolic States. Cells 2018, 7, 21. [Google Scholar] [CrossRef] [PubMed]
- Jin, P.; Jiang, J.; Zhou, L.; Huang, Z.; Nice, E.C.; Huang, C.; Fu, L. Mitochondrial Adaptation in Cancer Drug Resistance: Prevalence, Mechanisms, and Management. J. Hematol. Oncol. 2022, 15, 97. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, A.B.; Pour, P.M. Cell Lines. In Brenner’s Encyclopedia of Genetics; Elsevier: Amsterdam, The Netherlands, 2001; pp. 481–482. [Google Scholar]
- Hernández-López, R.; Torrens-Mas, M.; Pons, D.G.; Company, M.M.; Falcó, E.; Fernández, T.; Ibarra de la Rosa, J.M.; Roca, P.; Oliver, J.; Sastre-Serra, J. Mitochondrial Function Differences between Tumor Tissue of Human Metastatic and Premetastatic CRC. Biology 2022, 11, 293. [Google Scholar] [CrossRef]
- Selivanov, V.A.; Votyakova, T.V.; Pivtoraiko, V.N.; Zeak, J.; Sukhomlin, T.; Trucco, M.; Roca, J.; Cascante, M. Reactive Oxygen Species Production by Forward and Reverse Electron Fluxes in the Mitochondrial Respiratory Chain. PLoS Comput. Biol. 2011, 7, e1001115. [Google Scholar] [CrossRef] [PubMed]
- Aaronson, N.K.; Ahmedzai, S.; Bergman, B.; Bullinger, M.; Cull, A.; Duez, N.J.; Filiberti, A.; Flechtner, H.; Fleishman, S.B.; de Haes, J.C. The European Organization for Research and Treatment of Cancer QLQ-C30: A Quality-of-Life Instrument for Use in International Clinical Trials in Oncology. J. Natl. Cancer Inst. 1993, 85, 365–376. [Google Scholar] [CrossRef]
- Liu, R.M.; Desai, L.P. Reciprocal Regulation of TGF-β and Reactive Oxygen Species: A Perverse Cycle for Fibrosis. Redox Biol. 2015, 6, 565–577. [Google Scholar] [CrossRef]
- Chang, C.H.; Pauklin, S. ROS and TGFβ: From Pancreatic Tumour Growth to Metastasis. J. Exp. Clin. Cancer Res. 2021, 40, 152. [Google Scholar] [CrossRef]
- Ramundo, V.; Giribaldi, G.; Aldieri, E. Transforming Growth Factor-β and Oxidative Stress in Cancer: A Crosstalk in Driving Tumor Transformation. Cancers 2021, 13, 3093. [Google Scholar] [CrossRef]
- Muhammad, S.; Fan, T.; Hai, Y.; Gao, Y.; He, J. Reigniting Hope in Cancer Treatment: The Promise and Pitfalls of IL-2 and IL-2R Targeting Strategies. Mol. Cancer 2023, 22, 121. [Google Scholar] [CrossRef]
- Annesley, S.J.; Fisher, P.R. Mitochondria in Health and Disease. Cells 2019, 8, 680. [Google Scholar] [CrossRef] [PubMed]
- McBride, H.M.; Neuspiel, M.; Wasiak, S. Mitochondria: More Than Just a Powerhouse. Curr. Biol. 2006, 16, R551–R560. [Google Scholar] [CrossRef]
- Sastre-Serra, J.; Nadal-Serrano, M.; Pons, D.G.; Roca, P.; Oliver, J. Mitochondrial Dynamics Is Affected by 17β-Estradiol in the MCF-7 Breast Cancer Cell Line. Effects on Fusion and Fission Related Genes. Int. J. Biochem. Cell Biol. 2012, 44, 1901–1905. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-C.; Tseng, L.-M.; Lee, H.-C. Role of Mitochondrial Dysfunction in Cancer Progression. Exp. Biol. Med. 2016, 241, 1281–1295. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chan, D.C. Mitochondrial Dynamics in Regulating the Unique Phenotypes of Cancer and Stem Cells. Cell Metab. 2017, 26, 39–48. [Google Scholar] [CrossRef]
- Smith, A.L.M.; Whitehall, J.C.; Bradshaw, C.; Gay, D.; Robertson, F.; Blain, A.P.; Hudson, G.; Pyle, A.; Houghton, D.; Hunt, M.; et al. Age-Associated Mitochondrial DNA Mutations Cause Metabolic Remodeling That Contributes to Accelerated Intestinal Tumorigenesis. Nat. Cancer 2020, 1, 976–989. [Google Scholar] [CrossRef]
- Sun, X.; Zhan, L.; Chen, Y.; Wang, G.; He, L.; Wang, Q.; Zhou, F.; Yang, F.; Wu, J.; Wu, Y.; et al. Increased MtDNA Copy Number Promotes Cancer Progression by Enhancing Mitochondrial Oxidative Phosphorylation in Microsatellite-Stable Colorectal Cancer. Signal Transduct. Target. Ther. 2018, 3, 8. [Google Scholar] [CrossRef]
- Guo, W.; Liu, Y.; Ji, X.; Guo, S.; Xie, F.; Chen, Y.; Zhou, K.; Zhang, H.; Peng, F.; Wu, D.; et al. Mutational Signature of MtDNA Confers Mechanistic Insight into Oxidative Metabolism Remodeling in Colorectal Cancer. Theranostics 2023, 13, 324–338. [Google Scholar] [CrossRef]
- Abdelmaksoud, N.M.; Abulsoud, A.I.; Abdelghany, T.M.; Elshaer, S.S.; Rizk, S.M.; Senousy, M.A. Mitochondrial Remodeling in Colorectal Cancer Initiation, Progression, Metastasis, and Therapy: A Review. Pathol. Res. Pract. 2023, 246, 154509. [Google Scholar] [CrossRef]
- Gilliam, L.A.A.; St. Clair, D.K. Chemotherapy-Induced Weakness and Fatigue in Skeletal Muscle: The Role of Oxidative Stress. Antioxid. Redox Signal 2011, 15, 2543–2563. [Google Scholar] [CrossRef]
- Icard, P.; Coquerel, A.; Wu, Z.; Gligorov, J.; Fuks, D.; Fournel, L.; Lincet, H.; Simula, L. Understanding the Central Role of Citrate in the Metabolism of Cancer Cells and Tumors: An Update. Int. J. Mol. Sci. 2021, 22, 6587. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Park, J.S.; Deng, J.H.; Bai, Y. Cytochrome c Oxidase Subunit IV Is Essential for Assembly and Respiratory Function of the Enzyme Complex. J. Bioenerg. Biomembr. 2006, 38, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, S.; Avadhani, N.G. Cytochrome c Oxidase Dysfunction in Oxidative Stress. Free Radic. Biol. Med. 2012, 53, 1252–1263. [Google Scholar] [CrossRef]
- Xu, Y.; Dang, H.; Teng, C.; Yin, D.; Yan, L. ATP Inhibition for Starvation/Mild Photothermal/Photodynamic Synergy Therapy Using Polypeptide Nanoparticles Conjugating 2-Deoxy-D-Glucose and Dye under NIR Phototheranostic Strategy. Adv. Healthc. Mater. 2024, 13, e2401219. [Google Scholar] [CrossRef]
- Floris, I.; Appel, K.; Rose, T.; Lejeune, B. 2LARTH®, a Micro-Immunotherapy Medicine, Exerts Anti-Inflammatory Effects in Vitro and Reduces TNF-α and IL-1β Secretion. J. Inflamm. Res. 2018, 11, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and Tumor Progression: Signaling Pathways and Targeted Intervention. Signal Transduct. Target. Ther. 2021, 6, 263. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia-Sureda, L.; Jacques, C.; Pons, D.G.; Sastre-Serra, J.; Oliver, J.; Floris, I. Active Substances from the Micro-Immunotherapy Medicine 2LMIREG Display Antioxidative Properties In Vitro in Two Colorectal Cancer Cell Lines. Life 2025, 15, 743. https://doi.org/10.3390/life15050743
Garcia-Sureda L, Jacques C, Pons DG, Sastre-Serra J, Oliver J, Floris I. Active Substances from the Micro-Immunotherapy Medicine 2LMIREG Display Antioxidative Properties In Vitro in Two Colorectal Cancer Cell Lines. Life. 2025; 15(5):743. https://doi.org/10.3390/life15050743
Chicago/Turabian StyleGarcia-Sureda, Laura, Camille Jacques, Daniel G. Pons, Jorge Sastre-Serra, Jordi Oliver, and Ilaria Floris. 2025. "Active Substances from the Micro-Immunotherapy Medicine 2LMIREG Display Antioxidative Properties In Vitro in Two Colorectal Cancer Cell Lines" Life 15, no. 5: 743. https://doi.org/10.3390/life15050743
APA StyleGarcia-Sureda, L., Jacques, C., Pons, D. G., Sastre-Serra, J., Oliver, J., & Floris, I. (2025). Active Substances from the Micro-Immunotherapy Medicine 2LMIREG Display Antioxidative Properties In Vitro in Two Colorectal Cancer Cell Lines. Life, 15(5), 743. https://doi.org/10.3390/life15050743









