Transcatheter Aortic Valve Implantation in Alkaptonuria-Аssociated Severe Aortic Stenosis: A 2.5-Year Follow-Up Case Report and Literature Review
Abstract
1. Introduction
2. Case Presentation
- There was dark staining of her underwear.
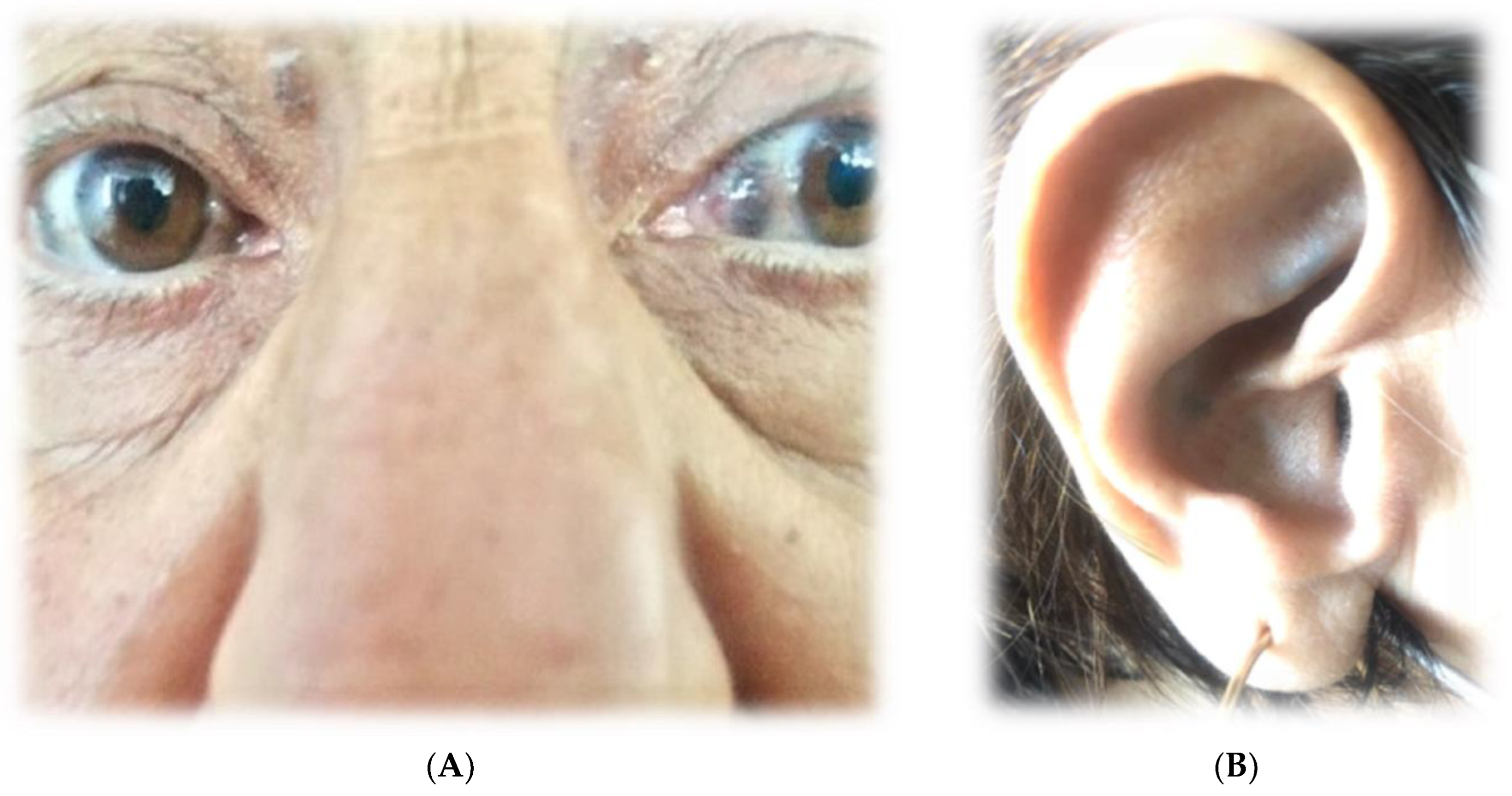
- Upon physical examination, there was bilateral dark-brown pigmentation of the sclera [Figure 1A] and bluish-black discoloration of both ear cartilages [Figure 1B]. She first noticed dark pigmentation in her eyes at the age of 30, but this had raised no concern since her father had similar changes at approximately the same age.
- The physical examination found: the patient’s range of motion in the spine, shoulders and knees was limited. In addition, muffled heart sounds and a rough systolic murmur propagating towards the carotid arteries with a punctum maximum on the aortic valve were auscultated.
- Based on the history, physical examination and instrumental studies, ochronosis was considered in the differential diagnostic plan. The patient was referred by a rheumatologist to HGA concentration testing in her blood and urine, and it was significantly elevated—it was above the upper limit of the reference range—1.54 g/24 h. Therefore, the diagnosis of alkaptonuria was confirmed.
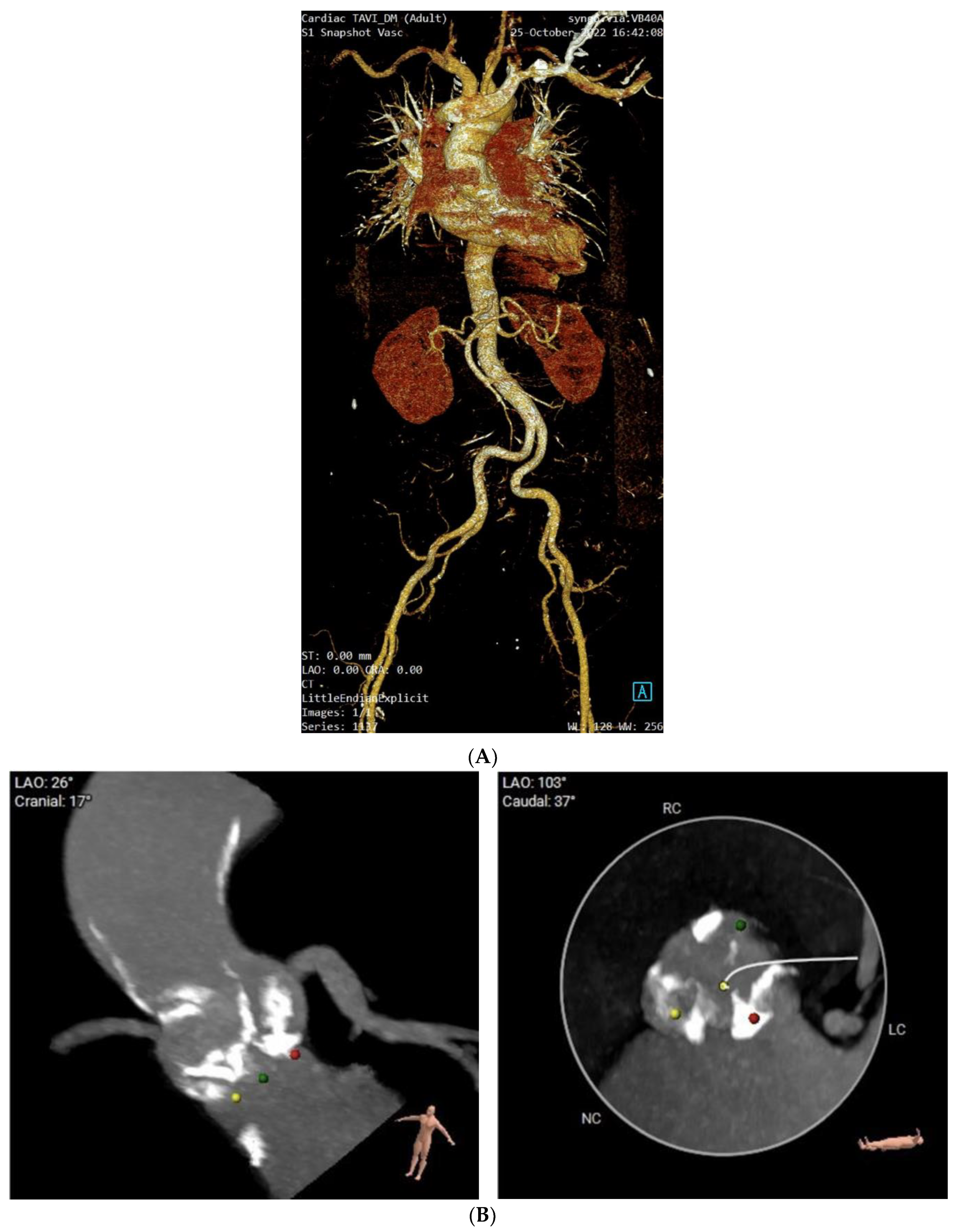
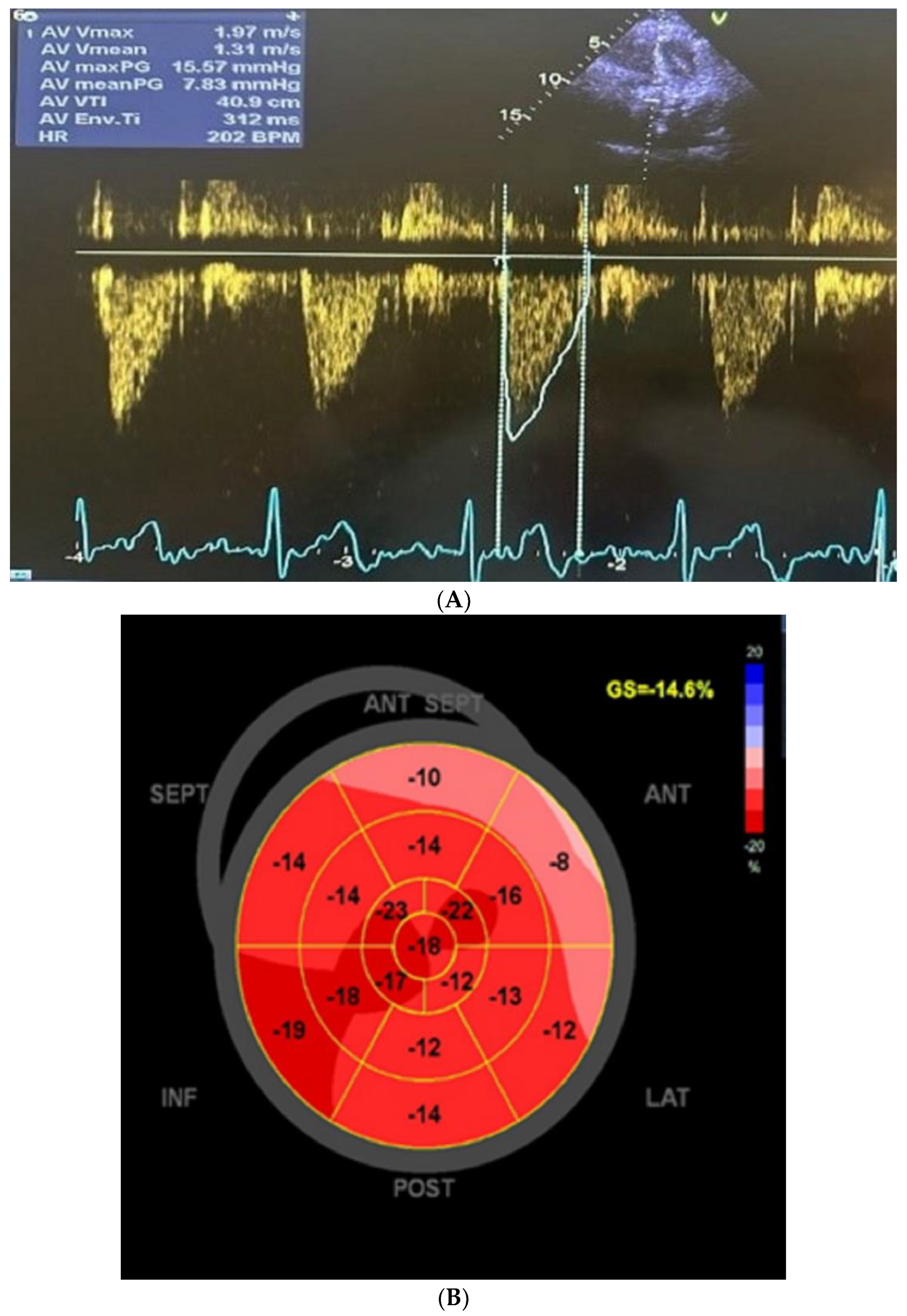
3. Cardiac Findings
4. Patient Management
5. Discussion and Conclusions
- The need for inclusion of RAAS inhibitors in hypertensive patients with severe aortic stenosis (before and after TAVI) is important for myocardial remodelling and clinical course.
- In alkaptonuria, the choice of intervention for severe aortic stenosis should be based on the individual specifics of the patient and follow the standard criteria for risk assessment by the Heart Team.
- TAVI is a reliable choice in alkaptonuria-associated severe aortic stenosis.
6. Patient Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AKU | Alkaptonuria |
| PCR | Polymerase Chain Reaction |
| EchoCG | Echocardiography |
| AS | Aortic Stenosis |
| SAVR | Surgical Aortic Valve Replacemen |
| TAVI | Transcatheter Aortic Valve Implantation |
References
- Phornphutkul, C.; Introne, W.J.; Perry, M.B.; Bernardini, I.; Murphey, M.D.; Fitzpatrick, D.L.; Anderson, P.D.; Huizing, M.; Anikster, Y.; Gerber, L.H.; et al. Natural history of alkaptonuria. N. Engl. J. Med. 2002, 347, 2111–2121. [Google Scholar] [CrossRef] [PubMed]
- Zatkova, A.; Ranganath, L.; Kadasi, L. Alkaptonuria: Current Perspectives. Appl. Clin. Genet. 2020, 13, 37–47. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Van Offel, J.F.; De Clerck, L.S.; Francx, L.M.; Stevens, W.J. The clinical manifestations of ochronosis: A review. Acta Clin. Belg. 1995, 50, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Pettit, S.J.; Fisher, M.; Gallagher, J.A.; Ranganath, L.R. Cardiovascular manifestations of Alkaptonuria. J. Inherit. Metab. Dis. 2011, 34, 1177–1181. [Google Scholar] [CrossRef] [PubMed]
- Selvakumar, D.; Sian, K.; Sugito, S.; Singh, T. Ochronosis of the aortic valve. J. Thorac. Dis. 2018, 10, E332–E334. [Google Scholar] [CrossRef]
- Kostova, T.; Batalov, Z.; Karalilova, R.; Batalov, A. Ochronotic arthropathy in the context of spondyloarthritis differential diagnosis: A case-based review. Rheumatol. Int. 2022, 42, 2277–2282. [Google Scholar] [CrossRef] [PubMed]
- Ather, N.; Roberts, W.C. Cardiovascular ochronosis. Cardiovasc. Pathol. 2020, 48, 107219. [Google Scholar] [CrossRef] [PubMed]
- Planinc, M.; Unic, D.; Baric, D.; Blazekovic, R.; Sribar, A.; Sutlic, Z.; Rudez, I. The Dark Side of the Heart: Cardiovascular Manifestation of Ochronosis. Ann. Thorac. Surg. 2019, 108, e257–e259. [Google Scholar] [CrossRef] [PubMed]
- Ffolkes, L.V.; Brull, D.; Krywawych, S.; Hayward, M.; Hughes, S.E. Aortic stenosis in cardiovascular ochronosis. J. Clin. Pathol. 2007, 60, 92–93. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. ESC/EACTS Scientific Document Group. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef]
- Olive, J.K.; Alnajar, A.; Gnanashanmugam, S.; Lamelas, J. Transcatheter Aortic Valve Replacement for Alkaptonuria-Associated Aortic Stenosis. Ann. Thorac. Surg. 2019, 108, e377–e379. [Google Scholar] [CrossRef] [PubMed]
- Vaz Ferreira, V.; Pedro, P.G.; Fiarresga, A. Alkaptonuria: A rare cause of severe aortic stenosis treated by transcatheter aortic valve replacement. Rev. Port. de Cardiol. 2023, 42, 191–193. [Google Scholar] [CrossRef]
- Mateus, C.; Carvalho, A.F.; Fonte Boa, A. The use of near infrared spectroscopy in alkaptonuria—A case report and literature review. Port. J. Card. Thorac. Vasc. Surg. 2023, 28, 47–49. [Google Scholar]
- Nagare, K.; Idhrees, M.; Ibrahim, M.; Jacob, A.; Velayudhan, B. Narrowing of the dark valve: Aortic stenosis in alkaptonuria. Indian J. Thorac. Cardiovasc. Surg. 2021, 37, 320–322. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kenny, D.; Ptacin, M.J.; Bamrah, V.S.; Almagro, U. Cardiovascular ochronosis: A case report and review of the medical literature. Cardiology 1990, 77, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, S.; Shimada, T.; Miyanaga, T. Minimally invasive endoscopic aortic valve replacement for alkaptonuria-associated aortic stenosis: A case report and literature review. Gen. Thorac. Cardiovasc. Surg. 2020, 69, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Vo, T.; Edwards, J.R.M. A black heart: Aortic valve ochronosis secondary to alkaptonuria causing aortic stenosis. J. Card. Surg. 2021, 36, 758–760. [Google Scholar] [CrossRef]
- Thakur, S.; Markman, P.; Cullen, H. Choice of valve prosthesis in a rare clinical condition: Aortic stenosis due to alkaptonuria. Heart Lung Circ. 2013, 22, 870–872. [Google Scholar] [CrossRef]
- Capuano, F.; Angeloni, E.; Roscitano, A.; Bianchini, R.; Refice, S.; Lechiancole, A.; Melina, G.; Comito, C.; Sinatra, R. Blackish pigmentation of the aorta in patient with alkaptonuria and Heyde’s syndrome. AORTA 2014, 2, 74–76. [Google Scholar] [CrossRef]
- Hannoush, H.; Introne, W.J.; Chen, M.Y.; Lee, S.-J.; O’Brien, K.; Suwannarat, P.; Kayser, M.A.; Gahl, W.A.; Sachdev, V. Aortic stenosis and vascular calcification in alkaptonuria. Mol. Genet. Metab. 2011, 105, 198–202. [Google Scholar] [CrossRef]
- Putz, C.; Putz, F.J.; Keyser, A.; Schmid, C. Black Aortic Valve: Incidental Finding of Alkaptonuria. Thorac. Cardiovasc. Surg. Rep. 2021, 10, E39–E41. [Google Scholar] [CrossRef] [PubMed]
- Yoshikai, M.; Murayama, J.; Yamada, N. Aortic valve regurgitation in alkaptonuria. J. Heart Valve Dis. 2004, 13, 863–865. [Google Scholar] [PubMed]
- Basile, C.; Mancusi, C.; Franzone, A.; Avvedimento, M.; Bardi, L.; Angellotti, D.; Castiello, D.S.; Mariani, A.; Manzo, R.; De Luca, N.; et al. Renin-angiotensin system inhibitors reduce cardiovascular mortality in hypertensive patients with severe aortic stenosis undergoing transcatheter aortic valve implantation: Insights from the EffecTAVI registry. Front. Cardiovasc. Med. 2023, 10, 1234368. [Google Scholar] [CrossRef]
- Vinayak, M.; Leone, P.P.; Tanner, R.; Dhulipala, V.; Camaj, A.; Makhija, R.R.K.; Hooda, A.; Kini, A.S.; Sharma, S.K.; Khera, S. Transcatheter Aortic Valve Replacement: Current Status and Future Indications. J. Clin. Med. 2024, 13, 373. [Google Scholar] [CrossRef] [PubMed]
| In Favour of TAVI | In Favour of SAVR | |
|---|---|---|
| Clinical Characteristics | ||
| STS-PROM/EuroSCORE II < 4% [logistic EuroSCORE I < 10%] | ||
| STS-PROM/EuroSCORE II > 4% 4.68% [logistic EuroSCORE I > 10%] | 1 8.9/1.9% | |
| Presence of significant comorbidity [Inadequately recorded in the scores] | 1 | |
| Age below 75 years | 1 | |
| Age above 75 years | ||
| Previous cardiac surgery | 1 | |
| Frailty | 1 | |
| Limited mobility and conditions that may affect the rehabilitation process following the procedure | 1 | |
| Suspected endocarditis | 1 | |
| Total | 2 | 5 |
| Anatomical and technical aspects | In favour of TAVI | In favour of SAVR |
| Appropriate transfemoral access for TAVI | 1 | |
| Inappropriate [different] access for TAVI | 1 | |
| Thoracal radiotherapy consequences | 1 | |
| Porcelain aorta | 1 | |
| Presence of operating by-pass with sternotomy risk | 1 | |
| Expected prosthesis–patient mismatch | 1 | |
| Pronounced thoracic deformity or scoliosis | 1 | |
| Short distance between coronary ostia and aortic annulus | 1 | |
| Aortic annulus size out of range for TAVI | 1 | |
| Aortic root morphology unfavourable for TAVI | 1 | |
| Morphology [bicuspidia, calcification type and degree unfavourable for TAVI] | 1 | |
| Thrombus presence in the aorta or LC | 1 | |
| Total | 6 | 6 |
| Cardiac conditions apart from aortic stenosis requiring concomitant intervention | In favour of TAVI | In favour of SAVR |
| Significant coronary disease requiring revascularization through CAB | 1 | |
| Significant primary mitral valve disease requiring surgical treatment | 1 | |
| Significant tricuspid valve disease | 1 | |
| Ascending aortic aneurism | 1 | |
| Septal hypertrophy requiring myomectomy | 1 | |
| Total | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kitov, S.; Kitova, M.-F.; Goranov, G.; Kraev, K.; Kraeva, M.; Kitova, L. Transcatheter Aortic Valve Implantation in Alkaptonuria-Аssociated Severe Aortic Stenosis: A 2.5-Year Follow-Up Case Report and Literature Review. Life 2025, 15, 737. https://doi.org/10.3390/life15050737
Kitov S, Kitova M-F, Goranov G, Kraev K, Kraeva M, Kitova L. Transcatheter Aortic Valve Implantation in Alkaptonuria-Аssociated Severe Aortic Stenosis: A 2.5-Year Follow-Up Case Report and Literature Review. Life. 2025; 15(5):737. https://doi.org/10.3390/life15050737
Chicago/Turabian StyleKitov, Spas, Maria-Florance Kitova, George Goranov, Krasimir Kraev, Maria Kraeva, and Lyudmila Kitova. 2025. "Transcatheter Aortic Valve Implantation in Alkaptonuria-Аssociated Severe Aortic Stenosis: A 2.5-Year Follow-Up Case Report and Literature Review" Life 15, no. 5: 737. https://doi.org/10.3390/life15050737
APA StyleKitov, S., Kitova, M.-F., Goranov, G., Kraev, K., Kraeva, M., & Kitova, L. (2025). Transcatheter Aortic Valve Implantation in Alkaptonuria-Аssociated Severe Aortic Stenosis: A 2.5-Year Follow-Up Case Report and Literature Review. Life, 15(5), 737. https://doi.org/10.3390/life15050737








