Decreased Serum Decorin Levels Are Correlated with Aortic Stiffness as Assessed Using Carotid–Femoral Pulse Wave Velocity in Patients with Peritoneal Dialysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection and Data Collection
2.2. Anthropometric and Biochemical Assessments
2.3. Assessment of Carotid–Femoral Pulse Wave Velocity as an Indicator of Arterial Stiffness
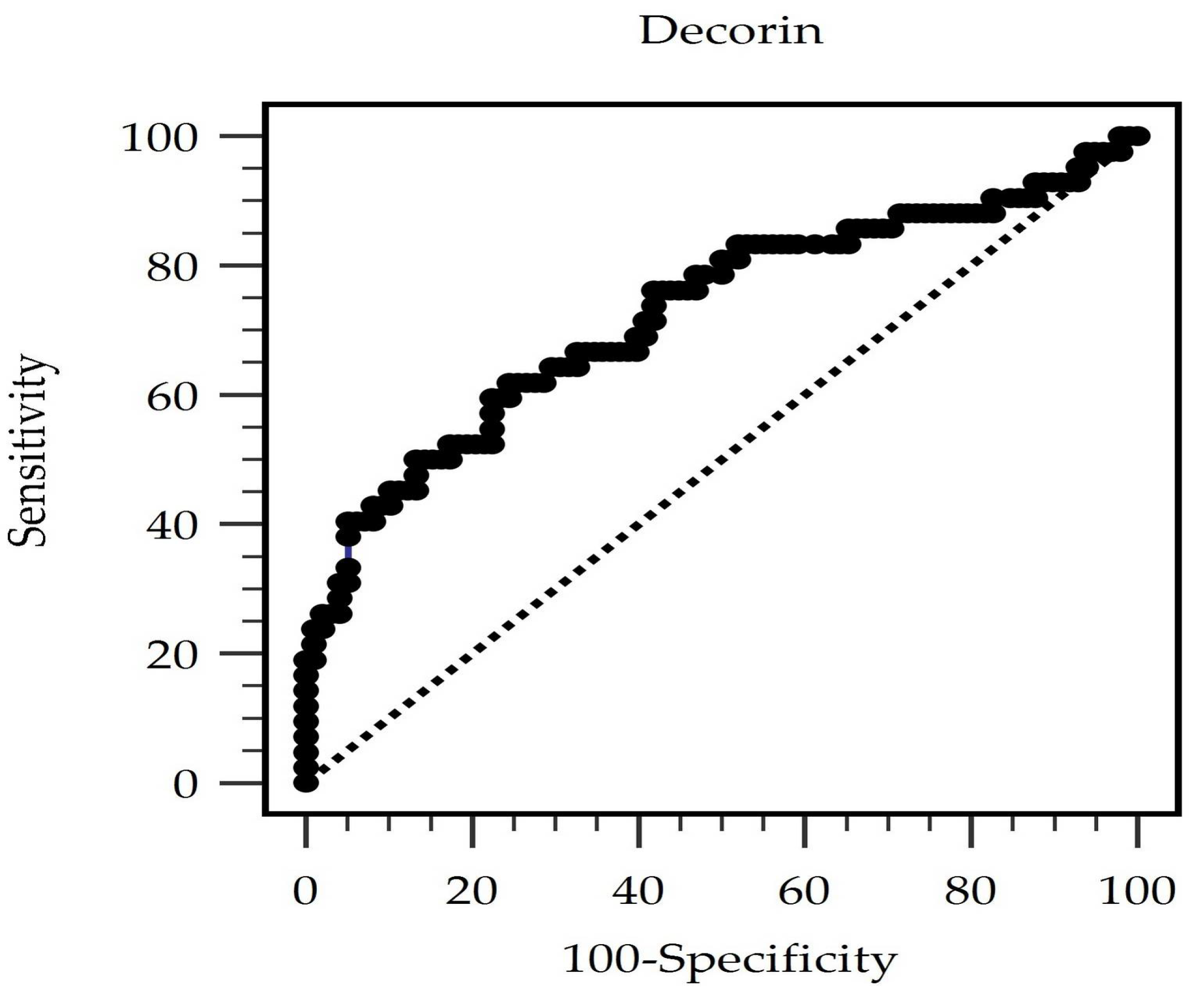
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, A.Y.; Brimble, K.S.; Brunier, G.; Holt, S.G.; Jha, V.; Johnson, D.W.; Kang, S.W.; Kooman, J.P.; Lambie, M.; McIntyre, C.; et al. ISPD cardiovascular and metabolic guidelines in adult peritoneal dialysis patients Part II—Management of various cardiovascular complications. Perit. Dial. Int. 2015, 35, 388–396. [Google Scholar]
- Bansal, N. Evolution of cardiovascular disease during the transition to end-stage renal disease. Semin. Nephrol. 2017, 37, 120–131. [Google Scholar]
- Vlachopoulos, C.; Aznaouridis, K.; Stefanadis, C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness. A systematic review and meta-analysis. J. Am. Coll. Cardiol. 2010, 55, 1318–1327. [Google Scholar]
- Georgianos, P.I.; Sarafidis, P.A.; Lasaridis, A.N. Arterial stiffness: A novel cardiovascular risk factor in kidney disease patients. Curr. Vasc. Pharmacol. 2015, 13, 229–238. [Google Scholar]
- Taal, M.W. Arterial stiffness in chronic kidney disease: An update. Curr. Opin. Nephrol. Hypertens. 2014, 23, 169–173. [Google Scholar]
- Sipahioglu, M.H.; Kucuk, H.; Unal, A.; Kaya, M.G.; Oguz, F.; Tokgoz, B.; Oymak, O.; Utas, C. Impact of arterial stiffness on adverse cardiovascular outcomes and mortality in peritoneal dialysis patients. Perit. Dial. Int. 2012, 32, 73–80. [Google Scholar]
- Briet, M.; Boutouyrie, P.; Laurent, S.; London, G.M. Arterial stiffness and pulse pressure in CKD and ESRD. Kidney Int. 2012, 82, 388–400. [Google Scholar]
- Townsend, R.R.; Wilkinson, I.B.; Schiffrin, E.L.; Avolio, A.P.; Chirinos, J.A.; Cockcroft, J.R.; Heffernan, K.S.; Lakatta, E.G.; McEniery, C.M.; Mitchell, G.F.; et al. Recommendations for improving and standardizing vascular research on arterial stiffness: A scientific statement from the American Heart Association. Hypertension 2015, 66, 698–722. [Google Scholar]
- Mancia, G.; De Backer, G.; Dominiczak, A.; Cifkova, R.; Fagard, R.; Germano, G.; Grassi, G.; Heagerty, A.M.; Kjeldsen, S.E.; Laurent, S.; et al. 2007 Guidelines for the management of arterial hypertension: The task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J. Hypertens. 2007, 25, 1105–1187. [Google Scholar]
- Sequí-Domínguez, I.; Cavero-Redondo, I.; Álvarez-Bueno, C.; Pozuelo-Carrascosa, D.P.; de Arenas-Arroyo, S.N.; Martínez-Vizcaíno, V. Accuracy of pulse wave velocity predicting cardiovascular and all-cause mortality. A systematic review and meta-analysis. J. Clin. Med. 2020, 9, 2080. [Google Scholar] [CrossRef]
- Dong, Y.; Zhong, J.; Dong, L. The role of decorin in autoimmune and inflammatory diseases. J. Immunol. Res. 2022, 2022, 1283383. [Google Scholar]
- Wight, T.N. A role for proteoglycans in vascular disease. Matrix Biol. 2018, 71–72, 396–420. [Google Scholar]
- Iozzo, R.V.; Schaefer, L. Proteoglycan form and function: A comprehensive nomenclature of proteoglycans. Matrix Biol. 2015, 42, 11–55. [Google Scholar]
- Kubo, E.; Shibata, S.; Shibata, T.; Sasaki, H.; Singh, D.P. Role of decorin in the lens and ocular diseases. Cells 2022, 12, 74. [Google Scholar] [CrossRef]
- Sofeu Feugaing, D.D.; Götte, M.; Viola, M. More than matrix: The multifaceted role of decorin in cancer. Eur. J. Cell Biol. 2013, 92, 1–11. [Google Scholar]
- Vu, T.T.; Marquez, J.; Le, L.T.; Nguyen, A.T.T.; Kim, H.K.; Han, J. The role of decorin in cardiovascular diseases: More than just a decoration. Free Radic. Res. 2018, 52, 1210–1219. [Google Scholar]
- Valera, G.; Figuer, A.; Caro, J.; Yuste, C.; Morales, E.; Ceprián, N.; Bodega, G.; Ramírez, R.; Alique, M.; Carracedo, J. Plasma glycocalyx pattern: A mirror of endothelial damage in chronic kidney disease. Clin. Kidney J. 2023, 16, 1278–1287. [Google Scholar]
- Jiang, N.; Zhang, Q.; Chau, M.K.; Yip, M.S.; Lui, S.L.; Liu, S.; Chu, K.M.; Ngan, H.Y.; Chan, T.M.; Yung, S. Anti-fibrotic effect of decorin in peritoneal dialysis and PD-associated peritonitis. EBioMedicine 2020, 52, 102661. [Google Scholar]
- Van Bortel, L.M.; Laurent, S.; Boutouyrie, P.; Chowienczyk, P.; Cruickshank, J.K.; De Backer, T.; Filipovsky, J.; Huybrechts, S.; Mattace-Raso, F.U.S.; Protogerou, A.D.; et al. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J. Hypertens. 2012, 30, 445–448. [Google Scholar]
- Brunner-La Rocca, H.P. Towards applicability of measures of arterial stiffness in clinical routine. Eur. Heart J. 2010, 31, 2320–2322. [Google Scholar]
- O’Rourke, M.F.; Nichols, W.W. Aortic diameter, aortic stiffness, and wave reflection increase with age and isolated systolic hypertension. Hypertension 2005, 45, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Logan, J.G.; Engler, M.B.; Kim, H. Genetic determinants of arterial stiffness. J. Cardiovasc. Transl. Res. 2015, 8, 23–43. [Google Scholar] [CrossRef] [PubMed]
- Meaney, E.; Samaniego, V.; Alva, F.; Valdovinos, R.A.; Marrufo, R.; Vela, A.; Allen, T.; Misra, A.; Madsen, R. Increased arterial stiffness in children with a parental history of hypertension. Pediatr. Cardiol. 1999, 20, 203–205. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, A.; Kosger, P.; Ozdemir, G.; Sahin, F.M.; Ucar, B.; Kilic, Z. Carotid intima-media thickness and elastic properties of aortas in normotensive children of hypertensive parents. Hypertens. Res. 2015, 38, 621–626. [Google Scholar] [CrossRef]
- Vallée, A.; Cinaud, A.; Protogerou, A.; Zhang, Y.; Topouchian, J.; Safar, M.E.; Blacher, J. Arterial stiffness and coronary ischemia: New aspects and paradigms. Curr. Hypertens. Rep. 2020, 22, 5. [Google Scholar]
- Boutouyrie, P.; Tropeano, A.I.; Asmar, R.; Gautier, I.; Benetos, A.; Lacolley, P.; Laurent, S. Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: A longitudinal study. Hypertension 2002, 39, 10–15. [Google Scholar] [CrossRef]
- L Beros, A.; Sluyter, J.D.; Scragg, R.K. Evidence of a bi-directional relationship between arterial stiffness and diabetes: A systematic review and meta-analysis of cohort studies. Curr. Diabetes Rev. 2025, in press.
- Rizos, E.C.; Ntzani, E.E.; Rangraze, I.R.; El-Tanani, M.; Rizzo, M. The importance of arterial stiffness in pediatric patients with type 1 diabetes mellitus: What’s new? J. Diabetes Complicat. 2024, 38, 108877. [Google Scholar]
- Vallée, A. Association between lipids and arterial stiffness for primary cardiovascular prevention in a general middle-aged European population. Front. Cardiovasc. Med. 2022, 9, 899841. [Google Scholar]
- Baba, M.; Maris, M.; Jianu, D.; Luca, C.T.; Stoian, D.; Mozos, I. The impact of the blood lipids levels on arterial stiffness. J. Cardiovasc. Dev. Dis. 2023, 10, 127. [Google Scholar] [CrossRef]
- Ferreira, I.; Henry, R.M.; Twisk, J.W.; van Mechelen, W.; Kemper, H.C.; Stehouwer, C.D. The metabolic syndrome, cardiopulmonary fitness, and subcutaneous trunk fat as independent determinants of arterial stiffness: The Amsterdam growth and health longitudinal study. Arch. Intern Med. 2005, 165, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Li, G.; Ren, P.; He, Q.; Pan, Y.; Chen, S.; Zhang, X. Associations between objectively measured patterns of sedentary behaviour and arterial stiffness in Chinese community-dwelling older women. Eur. J. Cardiovasc. Nurs. 2023, 22, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Kool, M.J.; Hoeks, A.P.; Struijker Boudier, H.A.; Reneman, R.S.; Van Bortel, L.M. Short and long-term effects of smoking on arterial wall properties in habitual smokers. J. Am. Coll. Cardiol. 1993, 22, 1881–1886. [Google Scholar] [CrossRef] [PubMed]
- Boutouyrie, P.; Chowienczyk, P.; Humphrey, J.D.; Mitchell, G.F. Arterial stiffness and cardiovascular risk in hypertension. Circ. Res. 2021, 128, 864–886. [Google Scholar]
- Radić, J.; Vučković, M.; Đogaš, H.; Gelemanović, A.; Belančić, A.; Radić, M. Is arterial stiffness interconnected with cardiovascular drug prescription patterns, body composition parameters, and the quality of blood pressure regulation in hypertensive patients? Biomedicines 2024, 12, 2062. [Google Scholar] [CrossRef]
- Jia, G.; Aroor, A.R.; Jia, C.; Sowers, J.R. Endothelial cell senescence in aging-related vascular dysfunction. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 1802–1809. [Google Scholar] [CrossRef]
- Zhong, H.; Shao, Y.; Guo, G.; Zhan, Y.; Liu, B.; Shao, M.; Li, L. Association between the triglyceride-glucose index and arterial stiffness: A meta-analysis. Medicine 2023, 102, e33194. [Google Scholar] [CrossRef]
- Hultgårdh-Nilsson, A.; Borén, J.; Chakravarti, S. The small leucine-rich repeat proteoglycans in tissue repair and atherosclerosis. J. Intern Med. 2015, 278, 447–461. [Google Scholar]
- Allawadhi, P.; Singh, V.; Khurana, I.; Rawat, P.S.; Renushe, A.P.; Khurana, A.; Navik, U.; Allwadhi, S.; Kumar Karlapudi, S.; Banothu, A.K.; et al. Decorin as a possible strategy for the amelioration of COVID-19. Med. Hypotheses 2021, 152, 110612. [Google Scholar]
- Xie, X.; Li, D.; Cui, Y.; Xie, T.; Cai, J.; Yao, Y. Decorin protects retinal pigment epithelium cells from oxidative stress and apoptosis via AMPK-MTOR-regulated autophagy. Oxid. Med. Cell Longev. 2022, 2022, 3955748. [Google Scholar]
- Xie, C.; Mondal, D.K.; Ulas, M.; Neill, T.; Iozzo, R.V. Oncosuppressive roles of decorin through regulation of multiple receptors and diverse signaling pathways. Am. J. Physiol. Cell Physiol. 2022, 322, C554–C566. [Google Scholar] [PubMed]
- Cui, J.; Zhang, S.; Acharya, K.; Xu, Y.; Guo, H.; Li, T.; Fu, D.; Yang, Z.; Hou, L.; Xing, X.; et al. Decorin attenuates hypertrophic scar fibrosis via TGFβ/Smad signaling. Exp. Dermatol. 2024, 33, e15133. [Google Scholar] [PubMed]
- Singla, S.; Hu, C.; Mizeracki, A.; Mehta, J.L. Decorin in atherosclerosis. Ther. Adv. Cardiovasc. Dis. 2011, 5, 305–314. [Google Scholar] [PubMed]
- Coban, M.; Inci, A.; Yılmaz, Ü.; Asilturk, E. The Association of Fibroblast Growth Factor 23 with Arterial Stiffness and Atherosclerosis in Patients with Autosomal Dominant Polycystic Kidney Disease. Kidney Blood Press Res. 2018, 43, 1160–1173. [Google Scholar]
- Krishnasamy, R.; Tan, S.-J.; Tan, S.-J.; Hawley, C.M.; Hawley, C.M.; Hawley, C.M.; Johnson, D.W.; Johnson, D.W.; Johnson, D.W.; Stanton, T.; et al. Progression of Arterial Stiffness Is Associated with Changes in Bone Mineral Markers in Advanced CKD. BMC Nephrol. 2017, 18, 281. [Google Scholar]
- Ibrahim, W.H.; Ahmad, A.B.; Sayed, N.G. Association of Serum Fibroblast Growth Factor-23 with Doppler Pulse Wave Velocity in Hemodialysis Patients. Saudi J. Kidney Dis. Transpl. 2018, 29, 95–100. [Google Scholar]
- Marques, G.L.; Hayashi, S.Y.; Bjällmark, A.; Larsson, M.; Riella, M.C.; Olandoski, M.; Lindholm, B.; Nascimento, M.M.; Nascimento, M.M. Osteoprotegerin Is a Marker of Cardiovascular Mortality in Patients with Chronic Kidney Disease Stages 3–5. Sci Rep. 2021, 11, 2473. [Google Scholar]
- Kamińska, J.; Stopiński, M.; Mucha, K.; Pac, M.; Gołębiowski, M.; Niewczas, M.A.; Pączek, L.; Pączek, L.; Foroncewicz, B. Circulating Osteoprotegerin in Chronic Kidney Disease and All-Cause Mortality. Int. J. Gen. Med. 2021, 14, 2413–2420. [Google Scholar]
- Shanahan, C.M.; Crouthamel, M.H.; Kapustin, A.N.; Giachelli, C.M. Arterial Calcification in Chronic Kidney Disease: Key Roles for Calcium and Phosphate. Circ. Res. 2011, 109, 697–711. [Google Scholar]
- Ogata, H.; Sugawara, H.; Yamamoto, M.; Ito, H. Phosphate and Coronary Artery Disease in Patients with Chronic Kidney Disease. J. Atheroscler. Thromb. 2024, 31, 1–14. [Google Scholar]
- Yamada, S.; Nakano, T. Role of Chronic Kidney Disease (CKD)-Mineral and Bone Disorder (MBD) in the Pathogenesis of Cardiovascular Disease in CKD. J. Atheroscler. Thromb. 2023, 30, 835–850. [Google Scholar] [PubMed]
- Arefin, S.; Löfgren, L.; Stenvinkel, P.; Granqvist, A.; Kublickiene, K. Associations of Biopterins and ADMA with Vascular Function in Peripheral Microcirculation from Patients with Chronic Kidney Disease. Int. J. Mol. Sci. 2023, 24, 5582. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Xiong, R.; Feng, S.; Lu, X.; Li, H.; Wang, S. Association of Circulating Levels of ADMA with Carotid Intima-Media Thickness in Patients with CKD: A Systematic Review and Meta-Analysis. Kidney Blood Press. Res. 2018, 43, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.-H.; Chen, M.-S.; Lin, Y.L.; Hsu, B.-G. Resistin: A Potential Indicator of Aortic Stiffness in Non-Dialysis Chronic Kidney Disease Patients. Medicina 2023, 59, 1652. [Google Scholar] [CrossRef]
- Lo Cicero, L.; Lentini, P.; Sessa, C.; Castellino, N.; Dapos Anca, A.; Torrisi, I.; Marcantoni, C.; Castellino, P.; Santoro, D.; Zanoli, L. Inflammation and Arterial Stiffness as Drivers of Cardiovascular Risk in Kidney Disease. Cardiorenal Med. 2025, 15, 29–40. [Google Scholar]
| Proposed Role | Description | References |
|---|---|---|
| Interacting With Extracellular Matrix | Involved in collagen formation and interactions with extracellular matrix components | [11,12] |
| Signaling Networks | Participates in signaling networks and interacts with growth factors and receptor tyrosine kinases | [12,13] |
| Immune Regulation | Plays a role in regulating immune responses | [11,14] |
| Vascular Health | Contributes to vascular health through anti-inflammatory, anti-oxidative, and anti-fibrotic effects | [11,16] |
| Suppression of Cancer | Exhibits cancer suppression properties by inhibiting metastasis and angiogenesis | [11,15] |
| Ocular Health | Involved in eye diseases caused by ischemic or fibrotic mechanisms | [11,14] |
| Linked to Vascular Diseases | Related to cardiovascular diseases by involvement in inflammation and changes in endothelial phenotypes | [12,16,17] |
| Decrease in Peritoneal Fibrosis | Associated with reduced peritoneal fibrosis in peritoneal dialysis patients | [18] |
| Clinical Variables | All Enrolled Patients (n = 140) | Control Group (n = 98) | Arterial Stiffness Group (n = 42) | p-Value |
|---|---|---|---|---|
| Age (years) | 58.80 ± 13.27 | 57.28 ± 14.30 | 62.36 ± 9.74 | 0.037 * |
| Peritoneal dialysis vintage (months) | 50.82 (25.00–87.12) | 48.96 (23.76–84.87) | 51.72 (26.23–103.00) | 0.481 |
| Body mass index (kg/m2) | 25.10 ± 4.15 | 24.85 ± 4.33 | 25.67 ± 3.68 | 0.287 |
| Carotid–femoral PWV (m/s) | 9.34 ± 1.66 | 8.51 ± 1.01 | 11.28 ± 1.17 | <0.001 * |
| Systolic blood pressure (mmHg) | 149.24 ± 21.94 | 146.50 ± 21.15 | 155.64 ± 20.26 | 0.023 * |
| Diastolic blood pressure (mmHg) | 84.59 ± 14.81 | 83.41 ± 15.60 | 87.36 ± 12.52 | 0.149 |
| Hemoglobin (g/dL) | 9.54 ± 1.44 | 9.61 ± 1.29 | 9.39 ± 1.74 | 0.407 |
| Total cholesterol (mg/dL) | 164.36 ± 41.53 | 163.90 ± 44.01 | 165.45 ± 35.54 | 0.840 |
| Triglyceride (mg/dL) | 128.50 (82.25–183.75) | 122.00 (74.00–171.75) | 138.50 (110.25–225.25) | 0.003 * |
| Fasting glucose (mg/dL) | 103.00 (92.25–123.00) | 101.00 (91.00–116.50) | 111.00 (94.75–155.75) | 0.017 * |
| Albumin (mg/dL) | 3.56 ± 0.35 | 3.56 ± 0.32 | 3.56 ± 0.41 | 0.941 |
| Blood urea nitrogen (mg/dL) | 65.17 ± 22.04 | 66.07 ± 22.50 | 63.07 ± 21.02 | 0.462 |
| Creatinine (mg/dL) | 10.97 ± 3.03 | 10.94 ± 3.17 | 11.04 ± 2.72 | 0.863 |
| Total calcium (mg/dL) | 9.43 ± 0.78 | 9.38 ± 0.74 | 9.53 ± 0.87 | 0.312 |
| Phosphorus (mg/dL) | 5.09 ± 0.85 | 5.12 ± 0.85 | 5.03 ± 0.84 | 0.562 |
| Intact parathyroid hormone (pg/mL) | 237.80 (93.35–482.03) | 237.80 (104.04–462.80) | 232.21 (80.43–526.48) | 0.757 |
| Decorin (ng/mL) | 18.57 (16.03–23.30) | 20.34 (1694–23.74) | 16.10 (13.98–19.55) | <0.001 * |
| Weekly Kt/V | 2.05 ± 0.41 | 2.05 ± 0.43 | 2.03 ± 0.38 | 0.826 |
| Peritoneal Kt/V | 1.86 ± 0.46 | 1.85 ± 0.49 | 1.88 ± 0.39 | 0.774 |
| Total clearance of creatinine (L/week) | 57.78 ± 16.77 | 56.85 ± 17.01 | 59.96 ± 16.19 | 0.316 |
| Peritoneal clearance of creatinine (L/week) | 47.65 ± 13.26 | 46.73 ± 14.30 | 49.79 ± 10.27 | 0.211 |
| Female, n (%) | 71 (50.7) | 53 (54.1) | 18 (42.9) | 0.223 |
| Diabetes, n (%) | 57 (40.7) | 32 (32.7) | 25 (29.5) | 0.003 * |
| Hypertension, n (%) | 105 (75.0) | 68 (69.4) | 37 (88.1) | 0.019 * |
| CAPD, n (%) | 56 (40.0) | 42 (42.9) | 14 (33.3) | 0.292 |
| ARB use, n (%) | 90 (64.3) | 60 (61.2) | 30 (71.4) | 0.248 |
| β-blocker use, n (%) | 47 (52.9) | 53 (54.1) | 21 (50.0) | 0.658 |
| CCB use, n (%) | 86 (61.4) | 61 (62.2) | 25 (59.5) | 0.762 |
| Statin use, n (%) | 45 (32.1) | 30 (30.6) | 15 (35.7) | 0.554 |
| Clinical Variables | Odds Ratio | 95% Confidence interval | p-Value |
|---|---|---|---|
| Decorin, 1 ng/mL | 0.863 | 0.784–0.949 | 0.002 * |
| Age, 1 year | 1.053 | 1.013–1.095 | 0.009 * |
| Triglyceride (mg/dL) | 1.008 | 1.002–1.014 | 0.009 * |
| Systolic blood pressure, 1 mmHg | 1.010 | 0.987–1.033 | 0.417 |
| Glucose, 1 mg/dL | 1.003 | 0.991–1.016 | 0.592 |
| Diabetes, present | 1.763 | 0.639–4.864 | 0.274 |
| Hypertension, present | 3.453 | 0.949–12.559 | 0.060 |
| Clinical Variables | Carotid–Femoral Pulse Wave Velocity (m/s) | ||||
|---|---|---|---|---|---|
| Univariable Regression | Multivariable Regression | ||||
| r | p-Value | β | Adjusted R2 Change | p-Value | |
| Age (years) | 0.248 | 0.001 * | 0.330 | 0.074 | <0.001 * |
| Body mass index (kg/m2) | 0.154 | 0.069 | – | – | – |
| Log-PD duration (months) | 0.040 | 0.639 | – | – | – |
| SBP (mmHg) | 0.254 | 0.002 * | 0.259 | 0.065 | 0.001 * |
| DBP (mmHg) | 0.144 | 0.090 | – | – | – |
| Hb (g/dL) | 0.013 | 0.877 | – | – | – |
| Total cholesterol (mg/dl) | −0.050 | 0.559 | – | – | – |
| Log-triglyceride (mg/dL) | 0.233 | 0.006 * | 0.215 | 0.042 | 0.004 * |
| Log-glucose (mg/dL) | 0.192 | 0.023 * | – | – | – |
| BUN (mg/dL) | −0.119 | 0.161 | – | – | – |
| Cr (mg/dL) | −0.062 | 0.467 | – | – | – |
| Albumin (mg/dL) | −0.075 | 0.379 | – | – | – |
| Log-iPTH (pg/mL) | −0.073 | 0.389 | – | – | – |
| Total calcium (mg/dL) | 0.078 | 0.362 | – | – | – |
| Phosphorus (mg/dL) | −0.041 | 0.632 | – | – | – |
| Log-decorin (ng/mL) | −0.264 | 0.002 * | −0.289 | 0.084 | <0.001 * |
| Total creatinine clearance (L/week) | 0.074 | 0.384 | – | – | – |
| Peritoneal creatinine clearance (L/week) | 0.117 | 0.167 | – | – | – |
| Weekly Kt/V | −0.040 | 0.636 | – | – | – |
| Peritoneal Kt/V | 0.020 | 0.811 | – | – | – |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chern, Y.-B.; Huang, P.-Y.; Lin, Y.-L.; Wang, C.-H.; Tsai, J.-P.; Hsu, B.-G. Decreased Serum Decorin Levels Are Correlated with Aortic Stiffness as Assessed Using Carotid–Femoral Pulse Wave Velocity in Patients with Peritoneal Dialysis. Life 2025, 15, 541. https://doi.org/10.3390/life15040541
Chern Y-B, Huang P-Y, Lin Y-L, Wang C-H, Tsai J-P, Hsu B-G. Decreased Serum Decorin Levels Are Correlated with Aortic Stiffness as Assessed Using Carotid–Femoral Pulse Wave Velocity in Patients with Peritoneal Dialysis. Life. 2025; 15(4):541. https://doi.org/10.3390/life15040541
Chicago/Turabian StyleChern, Yahn-Bor, Po-Yu Huang, Yu-Li Lin, Chih-Hsien Wang, Jen-Pi Tsai, and Bang-Gee Hsu. 2025. "Decreased Serum Decorin Levels Are Correlated with Aortic Stiffness as Assessed Using Carotid–Femoral Pulse Wave Velocity in Patients with Peritoneal Dialysis" Life 15, no. 4: 541. https://doi.org/10.3390/life15040541
APA StyleChern, Y.-B., Huang, P.-Y., Lin, Y.-L., Wang, C.-H., Tsai, J.-P., & Hsu, B.-G. (2025). Decreased Serum Decorin Levels Are Correlated with Aortic Stiffness as Assessed Using Carotid–Femoral Pulse Wave Velocity in Patients with Peritoneal Dialysis. Life, 15(4), 541. https://doi.org/10.3390/life15040541










