Efficacy of Cannabis Oil in Improving Subjective Sleep Quality in Systemic Sclerosis: A Prospective Placebo-Controlled Study
Abstract
1. Introduction
2. Materials and Methods
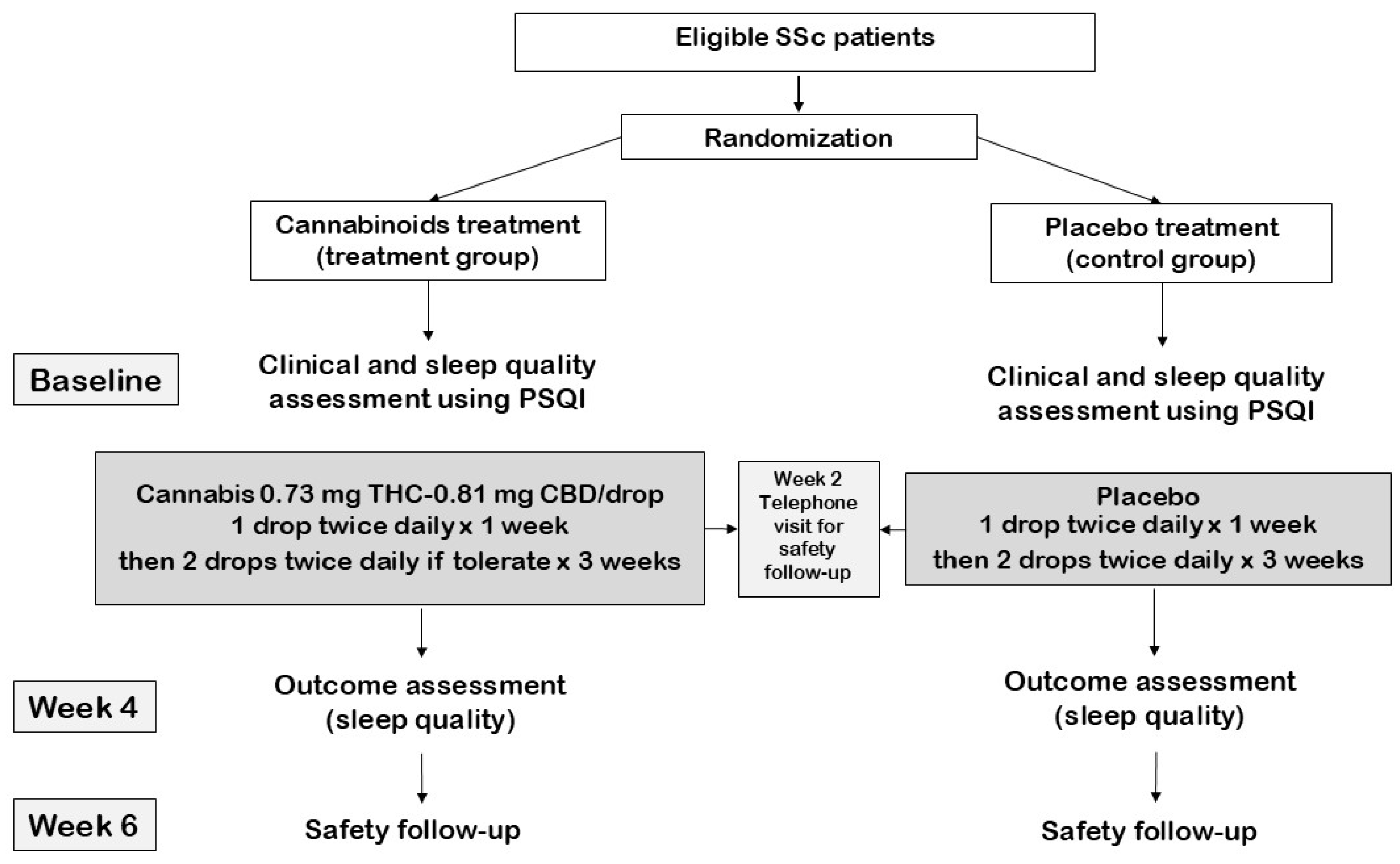
2.1. Study Design
2.2. Study Population
2.3. Intervention
2.4. Data Collection
2.5. Study Endpoint
2.6. Operational Definitions
2.7. Sample Size Calculation
2.8. Statistical Analysis
3. Results
3.1. Demographic Data
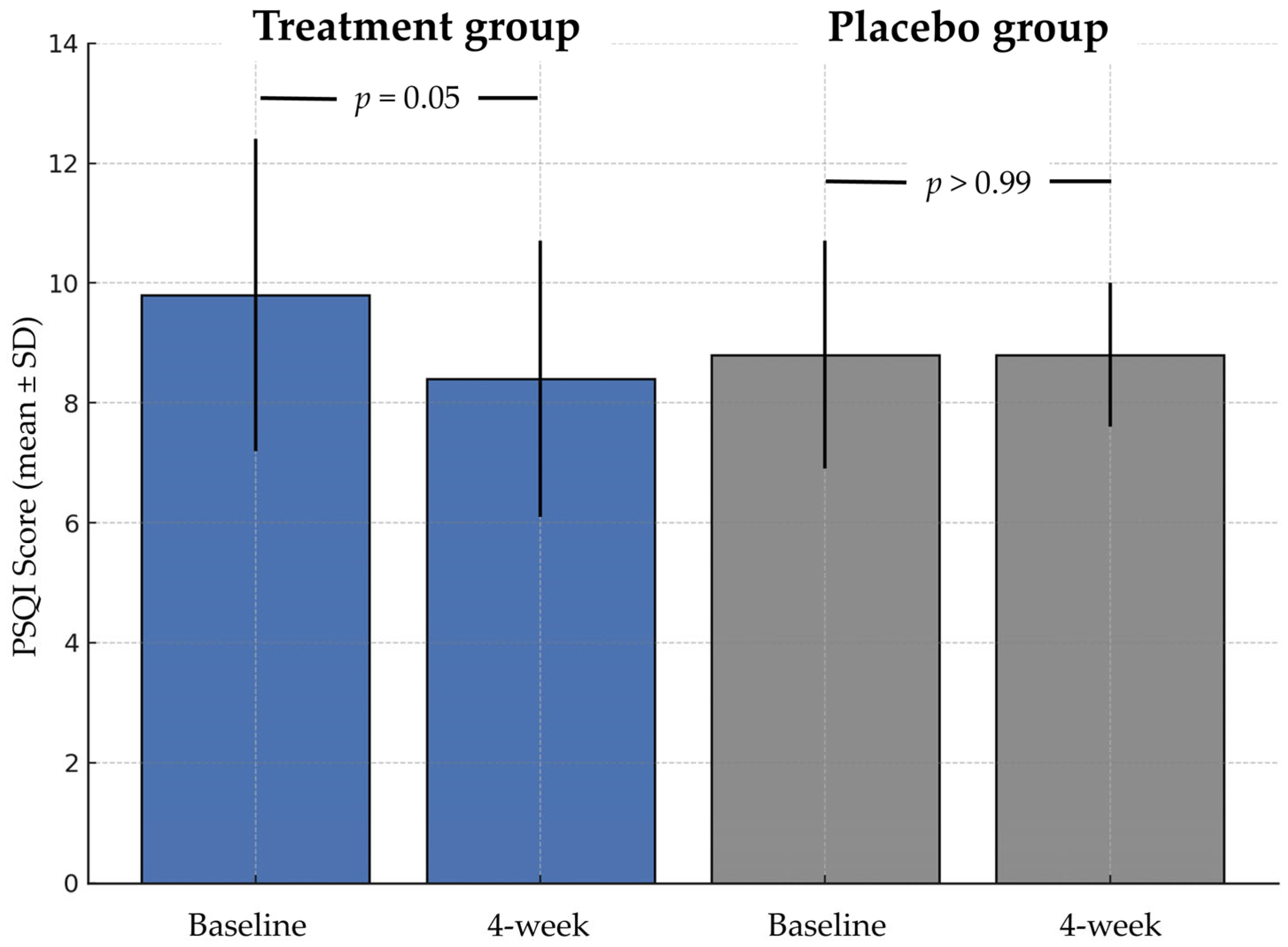
3.2. Primary Endpoint
3.3. Secondary Endpoint
3.4. Adverse Events
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MDPI | Multidisciplinary Digital Publishing Institute |
| DOAJ | Directory of open access journals |
| TLA | Three letter acronym |
| LD | Linear dichroism |
| CB1 | Cannabinoid receptor 1 |
| CB2 | Cannabinoid receptor 2 |
| CBD | Cannabidiol |
| CRP | C-reactive protein |
| ESR | Erythrocyte sedimentation rate |
| ILD | Interstitial lung disease |
| IQR | Interquartile range |
| PSQI | Pittsburgh Sleep Quality Index |
| QoL | Quality of life |
| REM | Rapid eye movement |
| SSc | Systemic sclerosis |
| SD | Standard deviation |
| THC | Tetrahydrocannabinol |
| WHO | World Health Organization |
Appendix A
Appendix A.1. Drug Information
Appendix A.2. Cannabinoids Preparation Process
Appendix B
| PSQI Parameters | Treatment Group | Placebo Group | p-Value |
|---|---|---|---|
| Age ≥ 60 years | n = 5 | n = 5 | |
| Mean difference in PSQI, mean (SD) | −0.8 (1.6) | 0.4 (1.8) | 0.31 |
| Sleep latency | 0.72 | ||
| Sleep duration | 0.42 | ||
| Sleep efficiency | NA | ||
| Sleep disturbances | 0.37 | ||
| Subjective sleep quality | 0.77 | ||
| Sleep medication used | NA | ||
| Daytime dysfunction | 0.06 | ||
| Female | n = 8 | n = 9 | |
| Mean difference in PSQI, mean (SD) | −1.13 (3.2) | 0.11 (2.2) | 0.36 |
| Sleep latency | 0.58 | ||
| Sleep duration | 0.49 | ||
| Sleep efficiency | NA | ||
| Sleep disturbances | 0.93 | ||
| Subjective sleep quality | 0.19 | ||
| Sleep medication used | 0.33 | ||
| Daytime dysfunction | 0.15 | ||
| dcSSc subset | n = 10 | n = 11 | |
| Mean difference in PSQI, mean (SD) | −1.4 (2.6) | −0.18 (1.4) | 0.19 |
| Sleep latency | 0.88 | ||
| Sleep duration | 0.59 | ||
| Sleep efficiency | 0.28 | ||
| Sleep disturbances | 0.38 | ||
| Subjective sleep quality | 0.62 | ||
| Sleep medication used | 0.30 | ||
| Daytime dysfunction | 0.62 | ||
| Disease duration ≥ 5 years | n = 10 | n = 8 | |
| Mean difference in PSQI, mean (SD) | −2.0 (2.5) | 0 (2.3) | 0.10 |
| Sleep latency | 0.98 | ||
| Sleep duration | 0.63 | ||
| Sleep efficiency | 0.36 | ||
| Sleep disturbances | 0.33 | ||
| Subjective sleep quality | 0.51 | ||
| Sleep medication used | 0.65 | ||
| Daytime dysfunction | 0.20 | ||
| WHO functional class II | n = 5 | n = 7 | |
| Mean difference in PSQI, mean (SD) | −1.8 (1.8) | 0 (1.7) | 0.11 |
| Sleep latency | 0.10 | ||
| Sleep duration | 0.40 | ||
| Sleep efficiency | 0.22 | ||
| Sleep disturbances | 0.24 | ||
| Subjective sleep quality | 0.64 | ||
| Sleep medication used | 0.19 | ||
| Daytime dysfunction | 0.64 | ||
| Interstitial lung disease | n = 9 | n = 10 | |
| Mean difference in PSQI, mean (SD) | −1.0 (2.2) | −0.2 (1.8) | 0.39 |
| Sleep latency | 0.85 | ||
| Sleep duration | 0.47 | ||
| Sleep efficiency | 0.28 | ||
| Sleep disturbances | 0.20 | ||
| Subjective sleep quality | 0.51 | ||
| Sleep medication used | 0.55 | ||
| Daytime dysfunction | 0.36 | ||
| Heartburn | n = 2 | n = 2 | |
| Mean difference in PSQI, mean (SD) | −5.0 (1.4) | −1.0 (1.4) | 0.11 |
| Sleep latency | 0.37 | ||
| Sleep duration | 0.37 | ||
| Sleep efficiency | NA | ||
| Sleep disturbances | 0.25 | ||
| Subjective sleep quality | 0.25 | ||
| Sleep medication used | NA | ||
| Daytime dysfunction | 0.25 |
References
- Volkmann, E.R.; Andréasson, K.; Smith, V. Systemic Sclerosis. Lancet 2023, 401, 304–318. [Google Scholar] [CrossRef] [PubMed]
- Prado, G.F.; Allen, R.P.; Trevisani, V.M.F.; Toscano, V.G.; Earley, C.J. Sleep Disruption in Systemic Sclerosis (Scleroderma) Patients: Clinical and Polysomnographic Findings. Sleep Med. 2002, 3, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Pihtili, A.; Bingol, Z.; Kiyan, E.; Cuhadaroglu, C.; Issever, H.; Gulbaran, Z. Obstructive Sleep Apnea Is Common in Patients with Interstitial Lung Disease. Sleep Breath. 2013, 17, 1281–1288. [Google Scholar] [CrossRef] [PubMed]
- Valencia-Flores, M.; Resendiz, M.; Castaño, V.A.; Santiago, V.; Campos, R.M.; Sandino, S.; Valencia, X.; Alcocer, J.; Ramos, G.G.; Bliwise, D.L. Objective and Subjective Sleep Disturbances in Patients with Systemic Lupus Erythematosus. Arthritis Rheum. 1999, 42, 2189–2193. [Google Scholar] [CrossRef]
- Bassel, M.; Hudson, M.; Taillefer, S.S.; Schieir, O.; Baron, M.; Thombs, B.D. Frequency and Impact of Symptoms Experienced by Patients with Systemic Sclerosis: Results from a Canadian National Survey. Rheumatology 2011, 50, 762–767. [Google Scholar] [CrossRef]
- Çakır Edis, E.; Mutlucan Eraslan, R.; Hatipoğlu, O. Polysomnography Findings and Risk Factors for Sleep-Disordered Breathing in Patients with Systemic Sclerosis. Arch. Rheumatol. 2021, 36, 360–365. [Google Scholar] [CrossRef]
- Nokes, B.T.; Raza, H.A.; Cartin-Ceba, R.; Lyng, P.J.; Krahn, L.E.; Wesselius, L.; Jokerst, C.E.; Umar, S.B.; Griffing, W.L.; Neville, M.R.; et al. Individuals With Scleroderma May Have Increased Risk of Sleep-Disordered Breathing. J. Clin. Sleep Med. 2019, 15, 1665–1669. [Google Scholar] [CrossRef]
- Wongthawa, N.; So-Gnern, A.; Mahakkanukrauh, A.; Suwannaroj, S.; Foocharoen, C. Sleep Quality and Clinical Association with Sleep Disturbance in Systemic Sclerosis. BMC Rheumatol. 2023, 7, 21. [Google Scholar] [CrossRef]
- Santos, G.d.S.; Barros, M.F.; Matta, D.N.d.; Tenório, A.d.S.; Gonçalves, R.S.G.; Duarte, A.L.B.P.; Dantas, A.T. Quality of Sleep in Individuals with Systemic Sclerosis and Its Correlation with Functional Disability and Quality of Life: A Cross-Sectional Study. Rev. Assoc. Med. Bras. 2024, 70, e20231254. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A New Instrument for Psychiatric Practice and Research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Sitasuwan, T.; Bussaratid, S.; Ruttanaumpawan, P.; Chotinaiwattarakul, W. Reliability and Validity of the Thai Version of the Pittsburgh Sleep Quality Index. J. Med. Assoc. Thail. 2014, 97 (Suppl. 3), S57–S67. [Google Scholar]
- Babson, K.A.; Sottile, J.; Morabito, D. Cannabis, Cannabinoids, and Sleep: A Review of the Literature. Curr. Psychiatry Rep. 2017, 19, 23. [Google Scholar] [CrossRef] [PubMed]
- Low, Z.X.B.; Lee, X.R.; Soga, T.; Goh, B.H.; Alex, D.; Kumari, Y. Cannabinoids: Emerging Sleep Modulator. Biomed. Pharmacother. 2023, 165, 115102. [Google Scholar] [CrossRef] [PubMed]
- Brisbois, T.D.; de Kock, I.H.; Watanabe, S.M.; Mirhosseini, M.; Lamoureux, D.C.; Chasen, M.; MacDonald, N.; Baracos, V.E.; Wismer, W.V. Delta-9-Tetrahydrocannabinol May Palliate Altered Chemosensory Perception in Cancer Patients: Results of a Randomized, Double-Blind, Placebo-Controlled Pilot Trial. Ann. Oncol. 2011, 22, 2086–2093. [Google Scholar] [CrossRef] [PubMed]
- Ranum, R.M.; Whipple, M.O.; Croghan, I.; Bauer, B.; Toussaint, L.L.; Vincent, A. Use of Cannabidiol in the Management of Insomnia: A Systematic Review. Cannabis Cannabinoid Res. 2023, 8, 213–229. [Google Scholar] [CrossRef]
- Vadivelu, N.; Kai, A.M.; Kodumudi, G.; Sramcik, J.; Kaye, A.D. Medical Marijuana: Current Concepts, Pharmacological Actions of Cannabinoid Receptor Mediated Activation, and Societal Implications. Curr. Pain. Headache Rep. 2018, 22, 3. [Google Scholar] [CrossRef]
- Allen, J.H.; de Moore, G.M.; Heddle, R.; Twartz, J.C. Cannabinoid Hyperemesis: Cyclical Hyperemesis in Association with Chronic Cannabis Abuse. Gut 2004, 53, 1566–1570. [Google Scholar] [CrossRef]
- LeRoy, E.C.; Black, C.; Fleischmajer, R.; Jablonska, S.; Krieg, T.; Medsger, T.A.; Rowell, N.; Wollheim, F. Scleroderma (Systemic Sclerosis): Classification, Subsets and Pathogenesis. J. Rheumatol. 1988, 15, 202–205. [Google Scholar]
- Omachi, T.A. Measures of Sleep in Rheumatologic Diseases: Epworth Sleepiness Scale (ESS), Functional Outcome of Sleep Questionnaire (FOSQ), Insomnia Severity Index (ISI), and Pittsburgh Sleep Quality Index (PSQI). Arthritis Care Res. 2011, 63 (Suppl. 11), S287–S296. [Google Scholar] [CrossRef]
- Ried, K.; Tamanna, T.; Matthews, S.; Sali, A. Medicinal Cannabis Improves Sleep in Adults with Insomnia: A Randomised Double-Blind Placebo-Controlled Crossover Study. J. Sleep Res. 2023, 32, e13793. [Google Scholar] [CrossRef]
- Walsh, J.H.; Maddison, K.J.; Rankin, T.; Murray, K.; McArdle, N.; Ree, M.J.; Hillman, D.R.; Eastwood, P.R. Treating Insomnia Symptoms with Medicinal Cannabis: A Randomized, Crossover Trial of the Efficacy of a Cannabinoid Medicine Compared with Placebo. Sleep 2021, 44, zsab149. [Google Scholar] [CrossRef]
- Saleska, J.L.; Bryant, C.; Kolobaric, A.; D’Adamo, C.R.; Colwell, C.S.; Loewy, D.; Chen, J.; Pauli, E.K. The Safety and Comparative Effectiveness of Non-Psychoactive Cannabinoid Formulations for the Improvement of Sleep: A Double-Blinded, Randomized Controlled Trial. J. Am. Nutr. Assoc. 2024, 43, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bell, A.D.; MacCallum, C.; Margolese, S.; Walsh, Z.; Wright, P.; Daeninck, P.J.; Mandarino, E.; Lacasse, G.; Kaur Deol, J.; de Freitas, L.; et al. Clinical Practice Guidelines for Cannabis and Cannabinoid-Based Medicines in the Management of Chronic Pain and Co-Occurring Conditions. Cannabis Cannabinoid Res. 2024, 9, 669–687. [Google Scholar] [CrossRef] [PubMed]
- May, M.B.; Glode, A.E. Dronabinol for Chemotherapy-Induced Nausea and Vomiting Unresponsive to Antiemetics. Cancer Manag. Res. 2016, 8, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Nagarkatti, M.; Nagarkatti, P.; Pandey, R.; Rieder, S.A.; Hegde, V.L.; Nagarkatti, M. Cannabinoids as Novel Anti-Inflammatory Drugs. Future Med. Chem. 2009, 1, 1333–1349. [Google Scholar] [CrossRef]
- Zurier, R.B.; Burstein, S.H. Cannabinoids, Inflammation, and Fibrosis. FASEB J. 2016, 30, 3682–3689. [Google Scholar] [CrossRef]
- Sumariwalla, P.F.; Gallily, R.; Tchilibon, S.; Fride, E.; Mechoulam, R.; Feldmann, M. A Novel Synthetic, Nonpsychoactive Cannabinoid Acid (HU-320) with Antiinflammatory Properties in Murine Collagen-Induced Arthritis. Arthritis Rheum. 2004, 50, 985–998. [Google Scholar] [CrossRef]
- Martin, E.L.; Strickland, J.C.; Schlienz, N.J.; Munson, J.; Jackson, H.; Bonn-Miller, M.O.; Vandrey, R. Antidepressant and Anxiolytic Effects of Medicinal Cannabis Use in an Observational Trial. Front. Psychiatry 2021, 12, 729800. [Google Scholar] [CrossRef]
- Finsterer, J. The Quality of Sleep in Systemic Sclerosis Patients Is Determined Not Only by the Underlying Disease but Also by Many Internal and External Factors. Rev. Assoc. Med. Bras. 2024, 70, e20240946. [Google Scholar] [CrossRef]
- Whiting, P.F.; Wolff, R.F.; Deshpande, S.; Di Nisio, M.; Duffy, S.; Hernandez, A.V.; Keurentjes, J.C.; Lang, S.; Misso, K.; Ryder, S.; et al. Cannabinoids for Medical Use: A Systematic Review and Meta-Analysis. JAMA 2015, 313, 2456–2473. [Google Scholar] [CrossRef]
| Clinical Characteristics | Overall N = 27 | Treatment Group N = 14 | Placebo Group N = 13 | p-Value # |
|---|---|---|---|---|
| Age (years), mean (SD) | 55.9 (10.4) | 57.6 (9.0) | 54.0 (11.7) | 0.38 |
| Duration of disease (years), mean (SD) | 7.8 (6.1) | 6.8 (3.7) | 8.9 (7.9) | 0.37 |
| Male, n (%) | 9 (33.3) | 5 (35.7) | 4 (30.8) | 0.99 |
| Diffuse cutaneous SSc subset, n (%) | 21 (77.8) | 10 (71.4) | 11 (84.6) | 0.65 |
| SSc clinical characteristics | ||||
| WHO functional class | 0.57 | |||
| I, n (%) | 14 (51.9) | 8 (57.1) | 6 (46.2) | |
| II, n (%) | 13 (48.1) | 6 (42.9) | 7 (53.9) | |
| Raynaud’s phenomenon, n (%) | 14 (51.9) | 7 (50.0) | 7 (53.9) | 0.84 |
| Digital ulcer, n (%) | 5 (18.5) | 4 (28.6) | 1 (7.7) | 0.34 |
| Telangiectasia, n (%) | 17 (63.0) | 9 (64.3) | 8 (61.5) | 0.99 |
| Salt and pepper skin appearance, n (%) | 19 (70.4) | 11 (78.6) | 8 (61.5) | 0.42 |
| Edematous skin, n (%) | 2 (7.4) | 0 | 2 (15.4) | 0.22 |
| Tendon friction rub, n (%) | 4 (14.8) | 2 (14.3) | 2 (15.4) | 0.99 |
| Hand deformity, n (%) | 17 (63.0) | 8 (57.1) | 9 (69.2) | 0.70 |
| Synovitis, n (%) | 0 | 0 | 0 | NA |
| Dysphagia, n (%) | 11 (40.7) | 3 (21.4) | 8 (61.5) | 0.054 |
| Heartburn, n (%) | 4 (14.8) | 3 (21.4) | 1 (15.4) | 0.99 |
| Stomach involvement, n (%) | 5 (18.5) | 2 (14.3) | 3 (23.1) | 0.65 |
| Intestinal involvement, n (%) | 5 (18.5) | 1 (7.1) | 4 (30.8) | 0.17 |
| Interstitial lung disease, n (%) | 19 (70.4) | 9 (64.3) | 10 (76.9) | 0.68 |
| Pulmonary hypertension, n (%) | 1 (3.7) | 0 | 1 (7.7) | 0.48 |
| mRSS (points), median (IQR) | 9 (2–19) | 11.5 (2–19) | 6 (2–23) | 0.95 |
| Investigational results | ||||
| Anti-topoisomerase I positive, n (%) | 22 (88) | 10 (76.9) | 12 (100) | 0.22 |
| ESR (mm/h), mean (SD) | 72.0 (30.5) | 70.4 (28.0) | 74 (34.4) | 0.77 |
| CRP (mg/L), median (IQR) | 3.0 (1.1–6.3) | 3.2 (1.1–10.3) | 2.4 (1.8–5.6) | 0.55 |
| Creatine kinase (U/L), median (IQR) | 127 (88–197) | 99.5 (66–170) | 140 (118–235) | 0.22 |
| Forced vital capacity (%predicted), mean (SD) | 63.3 (16.5) | 66.5 (14.4) | 59.9 (18.5) | 0.31 |
| Treatment | ||||
| Prednisolone (mg/d), median (IQR) | 3.8 (0–10) | 1.9 (0–5) | 5 (0–10) | 0.31 |
| Cyclophosphamide, n (%) | 2 (7.4) | 1 (7.1) | 1 (7.7) | >0.99 |
| Mycophenolate, n (%) | 8 (29.6) | 3 (21.4) | 5 (38.5) | 0.33 |
| Methotrexate, n (%) | 5 (18.5) | 2 (14.3) | 3 (23.1) | 0.56 |
| PSQI, mean (SD) | 9.3 (2.3) | 9.8 (2.6) | 8.8 (1.9) | 0.31 |
| Poor sleep quality, n (%) | 26 (96.3) | 13 (92.9) | 13 (100) | 0.99 |
| PSQI parameters | ||||
| Sleep latency | 0.75 | |||
| ≤15 min, n (%) | 6 (22.2) | 3 (21.4) | 3 (23.1) | |
| 16–30 min, n (%) | 6 (22.2) | 2 (14.3) | 4 (30.8) | |
| 31–60 min, n (%) | 10 (37.0) | 6 (42.9) | 4 (30.8) | |
| >60 min, n (%) | 5 (18.5) | 3 (21.4) | 2 (15.3) | |
| Sleep duration | 0.20 | |||
| >7 h, n (%) | 4 (14.8) | 1 (7.1) | 3 (23.1) | |
| 6–7 h, n (%) | 6 (22.2) | 4 (28.6) | 2 (15.3) | |
| 5–6 h, n (%) | 9 (33.3) | 3 (21.4) | 6 (46.2) | |
| <5 h, n (%) | 8 (29.6) | 6 (42.9) | 2 (15.4) | |
| Sleep efficiency | 0.30 | |||
| >85%, n (%) | 1 (3.7) | 1 (7.1) | 0 (0) | |
| 75–84%, n (%) | 0 | 0 (0) | 0 (0) | |
| 65–74%, n (%) | 0 | 0 (0) | 0 (0) | |
| <65%, n (%) | 0 | 13 (92.9) | 13 (100) | |
| Sleep disturbance | 0.13 | |||
| Score of 0, n (%) | 0 | 0 (0) | 0 (0) | |
| Score of 1–9, n (%) | 21 (77.8) | 11 (78.6) | 10 (77.0) | |
| Score of 10–18, n (%) | 5 (18.5) | 2 (14.3) | 3 (23.0) | |
| Score of 19–27, n (%) | 1 (3.7) | 1 (7.1) | 0 (0) | |
| Subjective sleep quality | 1.00 | |||
| Very good, n (%) | 2 (7.4) | 1 (7.1) | 1 (7.7) | |
| Fairly good, n (%) | 19 (70.4) | 9 (64.3) | 10 (77.0) | |
| Fairly bad, n (%) | 5 (18.5) | 3 (21.4) | 2 (15.3) | |
| Very bad, n (%) | 1 (3.7) | 1 (7.1) | 0 (0) | |
| Sleep medication used | 0.29 | |||
| Not during the past month, n (%) | 25 (92.6) | 12 (85.7) | 13 (100) | |
| Less than once a week, n (%) | 2 (7.4) | 2 (14.3) | 0 (0) | |
| Once or twice a week, n (%) | 0 | 0 (0) | 0 (0) | |
| Three or more times a week, n (%) | 0 | 0 (0) | 0 (0) | |
| Daytime dysfunction | 0.31 | |||
| Score of 0, n (%) | 12 (44.4) | 6 (42.9) | 6 (46.2) | |
| Score of 1–2, n (%) | 13 (48.2) | 7 (50.0) | 6 (46.2) | |
| Score of 3–4, n (%) | 2 (7.4) | 1 (7.1) | 1 (7.7) | |
| Score of 5–6, n (%) | 0 | 0 (0) | 0 (0) |
| PSQI Parameters | Treatment Group N =13 | Placebo Group N = 13 | p-Value |
|---|---|---|---|
| PSQI, mean (SD) | 8.4 (2.3) | 8.8 (1.2) | 0.53 |
| Mean difference in PSQI, mean (SD) | −1.5 (2.5) | 0 | 0.09 |
| Poor sleep quality, n (%) | 11 (84.6) | 13 (100) | 0.14 |
| Sleep latency | 0.81 | ||
| ≤15 min, n (%) | 3 (23.1) | 3 (23.1) | |
| 16–30 min, n (%) | 2 (15.4) | 4 (30.8) | |
| 31–60 min, n (%) | 5 (38.5) | 4 (30.8) | |
| >60 min, n (%) | 3 (23.1) | 2 (15.4) | |
| Sleep duration | 0.21 | ||
| >7 h, n (%) | 3 (23.1) | 2 (15.3) | |
| 6–7 h, n (%) | 5 (38.5) | 3 (23.1) | |
| 5–6 h, n (%) | 2 (15.4) | 7 (53.8) | |
| <5 h, n (%) | 3 (23.1) | 1 (7.7) | |
| Sleep efficiency | 0.31 | ||
| >85%, n (%) | 1 (7.7) | 0 (0) | |
| 75–84%, n (%) | 0 (0) | 0 (0) | |
| 65–74%, n (%) | 0 (0) | 0 (0) | |
| <65%, n (%) | 12 (92.3) | 13 (100) | |
| Sleep disturbance | 0.22 | ||
| Score of 0, n (%) | 2 (15.4) | 0 (0) | |
| Score of 1–9, n (%) | 10 (76.9) | 10 (76.9) | |
| Score of 10–18, n (%) | 1 (7.7) | 3 (23.1) | |
| Score of 19–27, n (%) | 0 (0) | 0 (0) | |
| Subjective sleep quality | >0.99 | ||
| Very good, n (%) | 4 (30.8) | 4 (30.8) | |
| Fairly good, n (%) | 8 (61.5) | 8 (61.5) | |
| Fairly bad, n (%) | 1 (7.7) | 1 (7.7) | |
| Very bad, n (%) | 0 (0) | 0 (0) | |
| Sleep medication used | 0.59 | ||
| Not during past month, n (%) | 11 (84.6) | 12 (92.3) | |
| Less than once a week, n (%) | 1 (7.7) | 0 (0) | |
| Once or twice a week, n (%) | 1 (7.7) | 1 (7.7) | |
| Three or more times a week, n (%) | 0 (0) | 0 (0) | |
| Daytime dysfunction | 0.32 | ||
| Score of 0, n (%) | 5 (38.5) | 5 (38.5) | |
| Score of 1–2, n (%) | 8 (61.5) | 6 (46.2) | |
| Score of 3–4, n (%) | 0 (0) | 2 (15.2) | |
| Score of 5–6, n (%) | 0 (0) | 0 (0) | |
| PSQI, mean (SD) | 8.4 (2.3) | 8.8 (1.2) | 0.53 |
| Mean difference in PSQI, mean (SD) | −1.5 (2.5) | 0 | 0.09 |
| Poor sleep quality, n (%) | 11 (84.6) | 13 (100) | 0.14 |
| Sleep latency | 0.81 | ||
| ≤15 min, n (%) | 3 (23.1) | 3 (23.1) | |
| 16–30 min, n (%) | 2 (15.4) | 4 (30.8) | |
| 31–60 min, n (%) | 5 (38.5) | 4 (30.8) | |
| >60 min, n (%) | 3 (23.1) | 2 (15.4) | |
| Sleep duration | 0.21 | ||
| >7 h, n (%) | 3 (23.1) | 2 (15.3) | |
| 6–7 h, n (%) | 5 (38.5) | 3 (23.1) | |
| 5–6 h, n (%) | 2 (15.4) | 7 (53.8) | |
| <5 h, n (%) | 3 (23.1) | 1 (7.7) | |
| Sleep efficiency | 0.31 | ||
| >85%, n (%) | 1 (7.7) | 0 (0) | |
| 75–84%, n (%) | 0 (0) | 0 (0) | |
| 65–74%, n (%) | 0 (0) | 0 (0) | |
| <65%, n (%) | 12 (92.3) | 13 (100) | |
| Sleep disturbance | 0.22 | ||
| Score of 0, n (%) | 2 (15.4) | 0 (0) | |
| Score of 1–9, n (%) | 10 (76.9) | 10 (76.9) | |
| Score of 10–18, n (%) | 1 (7.7) | 3 (23.1) | |
| Score of 19–27, n (%) | 0 (0) | 0 (0) | |
| Subjective sleep quality | >0.99 | ||
| Very good, n (%) | 4 (30.8) | 4 (30.8) | |
| Fairly good, n (%) | 8 (61.5) | 8 (61.5) | |
| Fairly bad, n (%) | 1 (7.7) | 1 (7.7) | |
| Very bad, n (%) | 0 (0) | 0 (0) | |
| Sleep medication used | 0.59 | ||
| Not during past month, n (%) | 11 (84.6) | 12 (92.3) | |
| Less than once a week, n (%) | 1 (7.7) | 0 (0) | |
| Once or twice a week, n (%) | 1 (7.7) | 1 (7.7) | |
| Three or more times a week, n (%) | 0 (0) | 0 (0) |
| PSQI Parameters | Treatment Group | Placebo Group | ||||
|---|---|---|---|---|---|---|
| Before Treatment N = 14 | After Treatment N = 13 | p-Value | Before Treatment N = 13 | After Treatment N = 13 | p-Value | |
| PSQI, mean (SD) | 9.8 (2.6) | 8.4 (2.3) | 0.05 | 8.8 (1.9) | 8.8 (1.2) | >0.99 |
| Poor sleep quality, n (%) | 13 (92.9) | 11 (84.6) | 0.56 | 13 (100) | 13 (100) | >0.99 |
| Sleep latency | >0.99 | >0.99 | ||||
| ≤15 min, n (%) | 3 (21.4) | 3 (23.1) | 3 (23.1) | 3 (23.1) | ||
| 16–30 min, n (%) | 2 (14.3) | 2 (15.4) | 4 (30.8) | 4 (30.8) | ||
| 31–60 min, n (%) | 6 (42.9) | 5 (38.5) | 4 (30.8) | 4 (30.8) | ||
| >60 min, n (%) | 3 (21.4) | 3 (23.1) | 2 (15.3) | 2 (15.4) | ||
| Sleep duration | 0.16 | 0.56 | ||||
| >7 h, n (%) | 1 (7.1) | 3 (23.1) | 3 (23.1) | 2 (15.3) | ||
| 6–7 h, n (%) | 4 (28.6) | 5 (38.5) | 2 (15.3) | 3 (23.1) | ||
| 5–6 h, n (%) | 3 (21.4) | 2 (15.4) | 6 (46.2) | 7 (53.8) | ||
| <5 h, n (%) | 6 (42.9) | 3 (23.1) | 2 (15.4) | 1 (7.7) | ||
| Sleep efficiency | 0.32 | NA | ||||
| >85%, n (%) | 1 (7.1) | 1 (7.7) | 0 (0) | 0 (0) | ||
| 75–84%, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| 65–74%, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| <65%, n (%) | 13 (92.9) | 12 (92.3) | 13 (100) | 13 (100) | ||
| Sleep disturbance | 0.16 | >0.99 | ||||
| Score of 0, n (%) | 0 (0) | 2 (15.4) | 0 (0) | 0 (0) | ||
| Score of 1–9, n (%) | 11 (78.6) | 10 (76.9) | 10 (77.0) | 10 (76.9) | ||
| Score of 10–18, n (%) | 2 (14.3) | 1 (7.7) | 3 (23.0) | 3 (23.1) | ||
| Score of 19–27, n (%) | 1 (7.1) | 0 (0) | 0 (0) | 0 (0) | ||
| Subjective sleep quality | 0.19 | 0.08 | ||||
| Very good, n (%) | 1 (7.1) | 4 (30.8) | 1 (7.7) | 4 (30.8) | ||
| Fairly good, n (%) | 9 (64.3) | 8 (61.5) | 10 (77.0) | 8 (61.5) | ||
| Fairly bad, n (%) | 3 (21.4) | 1 (7.7) | 2 (15.3) | 1 (7.7) | ||
| Very bad, n (%) | 1 (7.1) | 0 (0) | 0 (0) | 0 (0) | ||
| Sleep medication used | 0.56 | 0.32 | ||||
| Not during past month, n (%) | 12 (85.7) | 11 (84.6) | 13 (100) | 12 (92.3) | ||
| Less than once a week, n (%) | 2 (14.3) | 1 (7.7) | 0 (0) | 0 (0) | ||
| Once or twice a week, n (%) | 0 (0) | 1 (7.7) | 0 (0) | 1 (7.7) | ||
| Three or more times a week, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| Daytime dysfunction | >0.99 | 0.65 | ||||
| Score of 0, n (%) | 6 (42.9) | 5 (38.5) | 6 (46.2) | 5 (38.5) | ||
| Score of 1–2, n (%) | 7 (50.0) | 8 (61.5) | 6 (46.2) | 6 (46.2) | ||
| Score of 3–4, n (%) | 1 (7.1) | 0 (0) | 1 (7.7) | 2 (15.2) | ||
| Score of 5–6, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
So-ngern, A.; Sripanichkulchai, B.; Mahakkanukrauh, A.; Suwannaroj, S.; Pongkulkiat, P.; Onchan, T.; Kanokmedhakul, S.; Foocharoen, C. Efficacy of Cannabis Oil in Improving Subjective Sleep Quality in Systemic Sclerosis: A Prospective Placebo-Controlled Study. Life 2025, 15, 727. https://doi.org/10.3390/life15050727
So-ngern A, Sripanichkulchai B, Mahakkanukrauh A, Suwannaroj S, Pongkulkiat P, Onchan T, Kanokmedhakul S, Foocharoen C. Efficacy of Cannabis Oil in Improving Subjective Sleep Quality in Systemic Sclerosis: A Prospective Placebo-Controlled Study. Life. 2025; 15(5):727. https://doi.org/10.3390/life15050727
Chicago/Turabian StyleSo-ngern, Apichart, Bungon Sripanichkulchai, Ajanee Mahakkanukrauh, Siraphop Suwannaroj, Patnarin Pongkulkiat, Tippawan Onchan, Somdej Kanokmedhakul, and Chingching Foocharoen. 2025. "Efficacy of Cannabis Oil in Improving Subjective Sleep Quality in Systemic Sclerosis: A Prospective Placebo-Controlled Study" Life 15, no. 5: 727. https://doi.org/10.3390/life15050727
APA StyleSo-ngern, A., Sripanichkulchai, B., Mahakkanukrauh, A., Suwannaroj, S., Pongkulkiat, P., Onchan, T., Kanokmedhakul, S., & Foocharoen, C. (2025). Efficacy of Cannabis Oil in Improving Subjective Sleep Quality in Systemic Sclerosis: A Prospective Placebo-Controlled Study. Life, 15(5), 727. https://doi.org/10.3390/life15050727






