Evaluation of a Four Week Interdisciplinary Multimodal Pain Therapy on Chronic Pain Patients—A Comprehensive Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Cohort
2.2. Inclusion and Exclusion Criteria
2.3. Ethics and Data Privacy
2.4. Statistical Analysis
2.5. HUBER® 360
2.6. Outcome Measures
2.7. Questionnaires
- German Pain Questionnaire (DSF) and Depression, Anxiety, and Stress Scale (DASS): The DSF is widely used in pain therapy settings to collect baseline data and develop tailored therapy concepts. It includes demographic data (age, gender, weight, height), subjective pain descriptions (occurrence, frequency, intensity, location), questions about daily life, medical history, psychological factors (depression, stress, anxiety, quality of life), and social data (education, insurance, employment, family, retirement) [29,30]. In this study, DSF version 2015.2, which includes the DASS, was used. Comorbidities were recorded and the Charlson Comorbidity Index calculated [31]. To assess the development of pain intensity and the success of therapy for patients in the outpatient pain clinic and beyond, various follow-up questionnaires of the DSF were used [32], such as the progress questionnaire, which was sent to the patients 3 months after intervention.
- Numerical Rating Scale (NRS): The NRS asks patients to rate their pain on an 11-point scale from 0 (no pain) to 10 (maximum pain) [33]. During the therapy, pain intensity was recorded every morning using the NRS for a total of 20 days, to tailor the therapy and evaluate its success.
- Mainz Pain Staging System (MPSS) by Gerbershagen: The MPSS is a coding system used to stage chronic pain based on temporal and spatial aspects, medication behavior, and patient history [34,35]. This classification facilitates the comparison of chronic pain patients and the provision of appropriate therapy. The MPSS is widely used in Germany, as it allows quick and simple evaluation from patients’ data, showing a strong correlation between pain chronification, psychological condition, and daily life impairment. High chronic pain stages often correlate with significant work disability [36].
- Graded Chronic Pain Status (GCPS) by von Korff: The GCPS is an internationally accepted method for assessing chronic pain based on pain intensity and disability. The classification by von Korff uses pain intensity, disability, persistence, and frequency to categorize chronic pain in a straightforward manner [37].
- European Quality of Life 5 Dimensions 3 Level Version (EQ-5D-3L): Developed by the EuroQol Group, this questionnaire assesses health-related quality of life across five dimensions: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression [38]. An extra question about current health status was added for this study. A visual analog scale (EQ-VAS) is included, where patients rate their health on a scale from 0 (worst imaginable health) to 100 (best imaginable health).
- Short-Form 36 Health Survey (SF-36): The SF-36 is widely used to measure health-related quality of life (HRQOL) across eight subcategories, with an additional question on health changes over the past year. Subcategories include physical functioning, physical role, emotional role, social functioning, bodily pain, vitality, mental health, and general health perception. It includes two summary scores: physical health component (PCS) and mental health component (MCS) [39,40,41]. Higher scores indicate less impairment and better quality of life. Chronic pain significantly impacts these scores, particularly with increased pain severity, leading to lower HRQOL [41,42].
3. Results
3.1. Descriptive Data
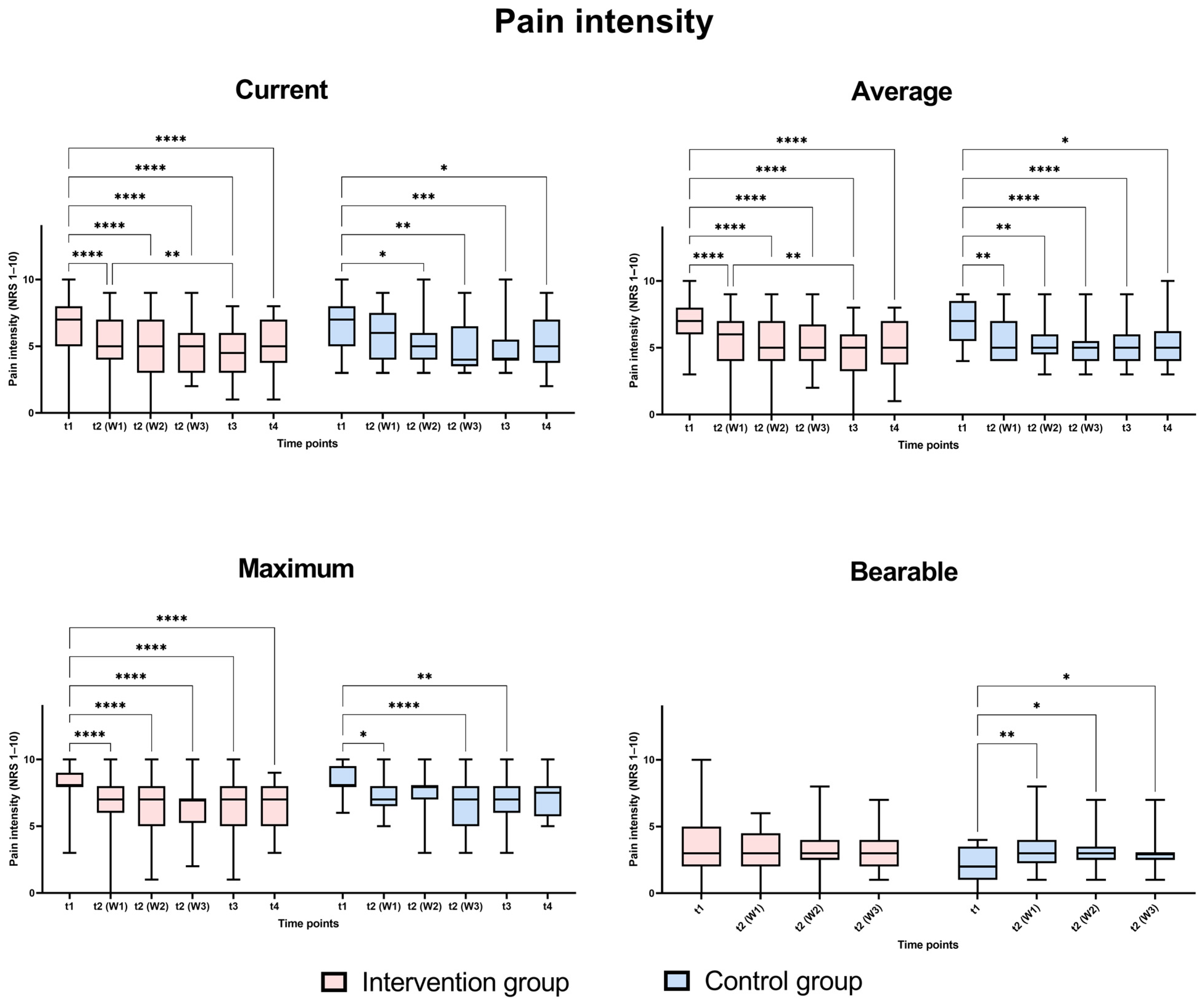
3.2. Pain
3.3. Patient Satisfaction and Quality of Life
EQ-5D-3L
3.4. Psychological Parameters
3.4.1. Short Form-36
3.4.2. Depression Anxiety Stress Score (DASS)
4. Discussion
Limitations and Strengths
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Turk, D.C. Psychological Approaches to Pain Management, Third Edition: A Practitioner’s Handbook, 3rd ed.; Guilford Publications: New York, NY, USA, 2018; ISBN 1462528538. [Google Scholar]
- Butler, D.S.; Moseley, G.L. Explain Pain; Noigroup Publ.: Adelaide, Australia, 2003; ISBN 978-0975091005. [Google Scholar]
- Kröner-Herwig, B. Schmerzpsychotherapie: Grundlagen—Diagnostik—Krankheitsbilder—Behandlung; Springer Medizin: Berlin/Heidelberg, Germany, 2011; ISBN 9783642127823. [Google Scholar]
- Erdmann, C.; Glöß, S.; Grieshammer, U.; Haberl, A.; Kutscher, S.; Linsmeier, B. Schmerzpatienten Behandeln: Nichtmedikamentöses und Komplementäres Schmerzmanagement; Georg Thieme Verlag: Stuttgart, Germany; New York, NY, USA, 2019; ISBN 9783132421820. [Google Scholar]
- International Association for the Study of Pain. International Association for the Study of Pain. Available online: https://www.iasp-pain.org (accessed on 2 February 2025).
- Loeser, J.D.; Butler, S.H.; Chapman, C.R.; Turk, D.C. Bonica’s Management of Pain, 3rd ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2001; ISBN 978-0683304626. [Google Scholar]
- Andersson, H.I.; Ejlertsson, G.; Leden, I.; Rosennberg, C. Chronic Pain in a Geographically Defined General Population. Clin. J. Pain 1993, 9, 174–182. [Google Scholar]
- Bundesinstitut für Arzneimittel und Medizinprodukte (BfArM) im Auftrag des Bundesministeriums für Gesundheit (BMG) unter Beteiligung der Arbeitsgruppe ICD des Kuratoriums für Fragen der Klassifikation im Gesundheitswesen. ICD-10-GM Version 2021, Systematisches Verzeichnis, Internationale Statistische Klassifikation der Krankheiten und Verwandter Gesundheitsprobleme, 10. Revision, Stand: 18 September 2020. Available online: https://www.bfarm.de/SharedDocs/Downloads/DE/Kodiersysteme/klassifikationen/icd-10-gm/vorgaenger/icd10gm2021_zip.html?nn=841246&cms_dlConfirm=true&cms_calledFromDoc=841246 (accessed on 5 January 2023).
- von Korff, M.; Ormel, J.; Keefe, F.J.; Dworkin, S.F. Grading the severity of chronic pain. Pain 1992, 50, 133–149. [Google Scholar] [CrossRef] [PubMed]
- van Hecke, O.; Torrance, N.; Smith, B.H. Chronic pain epidemiology and its clinical relevance. Br. J. Anaesth. 2013, 111, 13–18. [Google Scholar] [CrossRef]
- Gatchel, R.J.; Peng, Y.B.; Peters, M.L.; Fuchs, P.N.; Turk, D.C. The biopsychosocial approach to chronic pain: Scientific advances and future directions. Psychol. Bull. 2007, 133, 581–624. [Google Scholar] [CrossRef]
- Kamper, S.J.; Apeldoorn, A.T.; Chiarotto, A.; Smeets, R.J.E.M.; Ostelo, R.W.J.G.; Guzman, J.; van Tulder, M.W. Multidisciplinary biopsychosocial rehabilitation for chronic low back pain: Cochrane systematic review and meta-analysis. BMJ 2015, 350, h444. [Google Scholar] [CrossRef] [PubMed]
- Flor, H.; Fydrich, T.; Turk, D.C. Efficacy of multidisciplinary pain treatment centers: A meta-analytic review. Pain 1992, 49, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Elliott, T.E.; Renier, C.M.; Palcher, J.A. Chronic pain, depression, and quality of life: Correlations and predictive value of the SF-36. Pain Med. 2003, 4, 331–339. [Google Scholar] [CrossRef]
- Fayaz, A.; Croft, P.; Langford, R.M.; Donaldson, L.J.; Jones, G.T. Prevalence of chronic pain in the UK: A systematic review and meta-analysis of population studies. BMJ Open 2016, 6, e010364. [Google Scholar] [CrossRef]
- Todd, A.; McNamara, C.L.; Balaj, M.; Huijts, T.; Akhter, N.; Thomson, K.; Kasim, A.; Eikemo, T.A.; Bambra, C. The European epidemic: Pain prevalence and socioeconomic inequalities in pain across 19 European countries. Eur. J. Pain 2019, 23, 1425–1436. [Google Scholar] [CrossRef]
- Breivik, H.; Collett, B.; Ventafridda, V.; Cohen, R.; Gallacher, D. Survey of chronic pain in Europe: Prevalence, impact on daily life, and treatment. Eur. J. Pain 2006, 10, 287–333. [Google Scholar] [CrossRef]
- Hensler, S.; Heinemann, D.; Becker, M.T.; Ackermann, H.; Wiesemann, A.; Abholz, H.H.; Engeser, P. Chronic pain in German general practice. Pain Med. 2009, 10, 1408–1415. [Google Scholar] [CrossRef] [PubMed]
- Deutsche Gesellschaft für Schmerzmedizin e.V. Schmerzmedizin 2020_Versorgung Verbessern. 2020. Available online: https://www.eickhoff-kommunikation.de/pdf/PressemappeDGS.pdf (accessed on 5 January 2023).
- Hayden, J.A.; van Tulder, M.W.; Malmivaara, A.; Koes, B.W. Exercise therapy for treatment of non-specific low back pain. Cochrane Database Syst. Rev. 2005, 2005, CD000335. [Google Scholar] [CrossRef]
- Hall, J.E. Guyton and Hall Textbook of Medical Physiology E-Book: Guyton and Hall Textbook of Medical Physiology E-Book, 13th ed.; Elsevier—Health Sciences Division: Chantilly, VA, USA, 2015; ISBN 978-1455770052. [Google Scholar]
- Sabatowski, R.; Kaiser, U.; Scharnagel, R. Interdisziplinäre multimodale Schmerztherapie—Grundlagen und Fallstricke. Anästh. Intensivmed. 2021, 62, 334–344. [Google Scholar]
- Gatchel, R.J.; McGeary, D.D.; McGeary, C.A.; Lippe, B. Interdisciplinary Chronic pain management. Am. Psychol. 2014, 69, 119–130. [Google Scholar] [CrossRef]
- Bojinca, M.; Bida, D.; Mihai, C.; Milicescu, M.; Cornea, R. Efficacy of Exercise Program with the Huber System compared with classic excercise Program in Rehbailitation for patients with chronic low back pain. In Proceedings of the Annual European Congres of Rheumatology of the European League Against Rheumatism (EULAR), Amsterdam, The Netherlands, 21–24 June 2006. [Google Scholar]
- DJO, LLC. BEDIENUNGSANLEITUNG HUBER® 360 MD. Available online: https://enovis-medtech.eu/media/storage.djoglobal.eu/de_DE/Documents/Administration_documents/1786-Guide_HUBER_360-MD-DE-DJO-HD.PDF (accessed on 9 April 2023).
- DJO Global. Medizintechnik Produkt-Information HUBER® 360 Neuro Physical TrainingTM: Von der Rehabilitation bis zum Training: Analysiert, Therapiert und. Available online: https://cdn.competec.ch/documents2/0/4/0/203100040/203100040.pdf (accessed on 6 April 2023).
- Tantot, M.; Le Moal, V.; Mévellec, É.; Nouy-Trollé, I.; Lemoine-Josse, E.; Besnier, F.; Guiraud, T. Effects of an Intensive 6-Week Rehabilitation Program with the HUBER Platform in the Treatment of Non-Specific Chronic Low Back Pain: A Pilot Study. Clin. Pract. 2022, 12, 609–618. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Casser, H.R.; Hüppe, M.; Kohlmann, T.; Korb, J.; Lindena, G.; Maier, C.; Nagel, B.; Pfingsten, M.; Thoma, R. Deutscher Schmerzfragebogen (DSF) und standardisierte Dokumentation mit KEDOQ-Schmerz. Auf dem Weg zur gemeinsamen Qualitätsentwicklung der Schmerztherapie. Schmerz 2012, 26, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Deutsche Schmerzliga e.V. Pressemappe Chronischer Schmerz: Daten, Fakten, Hintergründe. 2013. Available online: https://schmerzliga.de/ewhihaje/2019/07/Dossier_Schmerzliga.pdf (accessed on 5 January 2023).
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Petzke, F.; Hüppe, M.; Kohlmann, T.; Kükens-Höner, S.; Lindena, G.; Pfingsten, M.; Nagel, N. Handbuch Deutscher Schmerz-Fragebogen. 2020. Available online: https://www.schmerzgesellschaft.de/fileadmin/pdf/DSF_Handbuch_2020.pdf (accessed on 5 January 2023).
- Beck, H.; Martin, E.; Motsch, J.; Am Schulte Esch, J. Schmerztherapie: 171 Tabellen; Thieme: Stuttgart, Germany, 2002; ISBN 3131148810. [Google Scholar]
- Sendera, M.; Sendera, A. Chronischer Schmerz: Schulmedizinische, Komplementärmedizinische und Psychotherapeutische Aspekte; Springer: Vienna, Austria, 2015. [Google Scholar]
- Gerbershagen, U. Organisierte Schmerzbehandlung. Eine Standortbestimmung. Internist 1986, 27, 459–469. [Google Scholar]
- Pfingsten, M.; Schöps, P.; Wille, T.; Terp, L.; Hildebrandt, J. Chronifizierungsausmaß von Schmerzerkrankungen. Schmerz 2000, 14, 10–17. [Google Scholar] [CrossRef]
- Baron, R.; Koppert, W.; Strumpf, M.; Willweber-Strumpf, A. Praktische Schmerzmedizin; Springer: Berlin/Heidelberg, Germany, 2013; ISBN 978-3-642-37604-7. [Google Scholar]
- Rabin, R.; de Charro, F. EQ-5D: A measure of health status from the EuroQol Group. Ann. Med. 2001, 33, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Ellert, U.; Bellach, B.M. Der SF-36 im Bundes-Gesundheitssurvey--Beschreibung einer aktuellen Normstichprobe. Gesundheitswesen 1999, 61, S184–S190. [Google Scholar] [PubMed]
- Bullinger, M. Erfassung der gesundheitsbezogenen Lebensqualität mit dem SF-36. Health Surv. Rehabil. 1996, 35, XVII–XXVII; quiz XXVII–XXIX. [Google Scholar]
- Kurth, B.-M.; Ellert, U. The SF-36 questionnaire and its usefulness in population studies: Results of the German Health Interview and Examination Survey 1998. Soz. Praventivmed. 2002, 47, 266–277. [Google Scholar] [CrossRef]
- Ellert, U.; Kurth, B.-M. Methodische Betrachtungen zu den Summenscores des SF-36 anhand der erwachsenen bundesdeutschen Bevölkerung. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2004, 47, 1027–1032. [Google Scholar] [CrossRef]
- Falkhamn, L.M.; Stenberg, G.; Enthoven, P.; Stålnacke, B.-M. Interdisciplinary Multimodal Pain Rehabilitation in Patients with Chronic Musculoskeletal Pain in Primary Care-A Cohort Study from the Swedish Quality Registry for Pain Rehabilitation (SQRP). Int. J. Environ. Res. Public Health 2023, 20, 5051. [Google Scholar] [CrossRef]
- Pöhlmann, K.; Tonhauser, T.; Joraschky, P.; Arnold, B. Die Multimodale Schmerztherapie Dachau (MSD). Daten zur Wirksamkeit eines diagnose-unabhängigen multimodalen Therapieprogramms bei Rückenschmerzen und anderen Schmerzen. Schmerz 2009, 23, 40–46. [Google Scholar] [CrossRef]
- Arnold, B.; Casser, H.-R.; Klimczyk, K.; Lutz, J.; Brinkschmidt, T.; Gralow, I.; Irnich, D.; Kaiser, U.; Nagel, B.; Schiltenwolf, M.; et al. Akutstationäre multimodale Schmerztherapie und Rehabilitation: Rahmenbedingungen, Aufgaben und differenzierte Patientenzuweisung. Schmerz 2015, 29, 641–648. [Google Scholar] [CrossRef]
- Fillingim, R.B.; King, C.D.; Ribeiro-Dasilva, M.C.; Rahim-Williams, B.; Riley, J.L. Sex, gender, and pain: A review of recent clinical and experimental findings. J. Pain 2009, 10, 447–485. [Google Scholar] [CrossRef]
- Mills, S.E.E.; Nicolson, K.P.; Smith, B.H. Chronic pain: A review of its epidemiology and associated factors in population-based studies. Br. J. Anaesth. 2019, 123, e273–e283. [Google Scholar] [CrossRef]
- Courtenay, W.H. Constructions of masculinity and their influence on men’s well-being: A theory of gender and health. Soc. Sci. Med. 2000, 50, 1385–1401. [Google Scholar] [CrossRef] [PubMed]
- Samulowitz, A.; Gremyr, I.; Eriksson, E.; Hensing, G. “Brave Men” and “Emotional Women”: A Theory-Guided Literature Review on Gender Bias in Health Care and Gendered Norms towards Patients with Chronic Pain. Pain Res. Manag. 2018, 2018, 6358624. [Google Scholar] [CrossRef] [PubMed]
- Das Mainzer Stadienmodell der Schmerzchronifizierung (Mainz Pain Staging System (MPSS)) [Nach Prof. H. U. Gerbershagen]. Available online: https://www.klinikum-ab-alz.de/fileadmin/user_upload/Stadien_Gerbershagen.pdf (accessed on 7 July 2024).
- Kaiser, U.; Petzke, F.; Nagel, B.; Marschall, U.; Casser, H.-R.; Isenberg, T.; Kohlmann, T.; Lindena, G. Evaluation eines frühen interdisziplinären multimodalen Assessments für Patienten mit Schmerzen: Protokoll einer randomisierten kontrollierten Studie (PAIN2020). Schmerz 2021, 35, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Shaddoud, A.A. Validity and Responsiveness of EuroQol-5 Dimension (EQ-5D-3L) Versus SF-36 Questionnaire in Chronic Pelvic Pain. SSRN J. 2021. [Google Scholar] [CrossRef]
- Osmanski-Zenk, K.; Ningel, A.; Tischer, T.; Mittelmeier, W. Vergleichende Untersuchung der posturalen Kontrolle bei 20–40-Jährigen und Karate-Kaderathleten mittels eines neuromuskulären Trainingsgerätes. Sportverletz. Sportschaden 2022, 36, 200–207. [Google Scholar] [CrossRef]

| Intervention Group (n = 49) | Control Group (n = 17) | Group Comparison t1 | |||
|---|---|---|---|---|---|
| Mean (±SD) | Median (Range) | Mean (±SD) | Median (Range) | p-Value | |
| Age | 54.7 (±12.4) | 56 (27; 79) | 61.8 (±14.5) | 60 (38; 82) | 0.058 1 |
| BMI | 26.7 (±5.95) | 24.49 (17.9; 40) | 29.07 (±5.6) | 29.2 (18.3; 37.2) | 0.107 2 |
| CCI | 1.57 (±1.58) | 1 (0; 6) | 2.47 (±2.15) | 2 (0; 6) | 0.178 2 |
| NRS day 1 | 5.71 (±1.94) | 6 (1; 9) | 5.76 (±1.79) | 6 (3; 9) | 0.964 2 |
| Intervention Group (n = 49) | Control Group (n = 17) | ||
|---|---|---|---|
| Absolute (Percentage %) | Absolute (Percentage %) | ||
| Sex | female | 40 (81.6%) | 12 (70.6) |
| male | 9 (18.4) | 5 (29.4) | |
| Charlson Comorbidity Index | 0 | 14 (28.6%) | 4 (23.5%) |
| 1 | 15 (30.6%) | 4 (23.5%) | |
| 2 | 9 (18.4%) | 1 (5.9%) | |
| 3 | 6 (12.2%) | 2 (11.8%) | |
| 4 | 0 (0%) | 1 (5.9%) | |
| 5 | 4 (8.2%) | 4 (23.5%) | |
| 6 | 1 (2%) | 1 (5.9%) | |
| Disability | Yes | 27 (55.1%) | 10 (58.8%) |
| No | 22 (44.9%) | 7 (41.2%) | |
| Cause of pain (multiple answer options) | no recognizable cause | 6 (12.2%) | 1 (5.9%) |
| specific disease | 23 (46.9%) | 6 (35.3%) | |
| operation | 16 (32.7%) | 9 (52.9%) | |
| accident | 5 (10.2%) | 3 (17.6%) | |
| physical strain * | 22 (44.9%) | 2 (11.8%) | |
| mental stress | 17 (34.7%) | 3 (17.6%) | |
| other cause | 5 (10.2%) | 0 (0%) | |
| Duration of pain | 1 month to ½ year | 2 (4.1%) | 1 (5.9%) |
| ½ year to 1 year | 4 (8.2%) | 2 (11.8%) | |
| 1 to 2 years | 5 (10.2%) | 1 (5.9%) | |
| 2 to 5 years | 11 (22.4%) | 5 (29.4%) | |
| more than 5 years | 27 (55.1%) | 8 (47.1%) | |
| Mainz Pain Staging System (MPSS) | stage 1 | 0 (0%) | 0 (0%) |
| stage 2 | 3 (6.1%) | 1 (5.9%) | |
| stage 3 | 46 (93.9%) | 16 (94.1%) | |
| Graded Chronic Pain Status (GCPS) by von Korff | grade 1 | 1 (2%) | 1 (5,9%) |
| grade 2 | 8 (16.3%) | 1 (5.9%) | |
| grade 3 | 9 (18.4%) | 2 (11.8%) | |
| grade 4 | 31 (63.3%) | 13 (76.5%) |
| Intervention Group | Control Group | 2-Way ANOVA p-Value (Time X HUBER® 360) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| t1 | t3 | t4 | t1 | t3 | t4 | p-Value | |||
| EQ-5D-3L Index Value | mean (±SD) | 0.55 (±0.30) | 0.78 (±0.18) | 0.68 (±0.28) | 0.64 (±0.26) | 0.73 (±0.22) | 0.65 (±0.29) | <0.001 1 | 0.263 |
| median (range) | 0.564 (0.11; 1) | 0.877 (0.175; 1) | 0.788 (0.11; 1) | 0.701 (0.175; 0.887) | 0.788 (0.175; 0.887) | 0.788 (0.175; 0.887) | 0.036 2 | ||
| EQ-5D-3L Visual Analog Scale | mean (±SD) | 49.82 (±17.38) | 62.14 (±15.1) | 55,5 (±19.13) | 47.53 (±12.5) | 55.12 (±17.76) | 54.57 (±18.22) | 0.003 1 | 0.696 |
| median (range) | 50 (10; 100) | 60 (30; 100) | 55 (4; 95) | 48 (25; 70) | 60 (15; 85) | 55 (9; 75) | 0.014 2 | ||
| Intervention Group | Control Group | Two-Way ANOVA p-Value (Time X HUBER® 360) | |||||
|---|---|---|---|---|---|---|---|
| t1 | t3 | t1 | t3 | p-Value | |||
| Physical component summary | mean (±SD) | 34.54 (±19.1) | 48.65 (±21.28) | 28.62 (±11.66) | 40.75 (±17.51) | 0.235 1 0.174 2 <0.001 3 0.003 4 | 0.668 |
| median (range) | 31.25 (6.25; 85.5) | 44.75 (7.5; 89) | 28 (15.5; 63) | 37 (9.25; 84.75) | |||
| Mental component summary | mean (±SD) | 48.32 (±22.72) | 58.09 (±23.57) | 53.37 (±19.86) | 69.96 (±16.49) | 0.419 1 0.060 2 <0.001 3 <0.001 4 | 0.191 |
| median (range) | 3.25 (3.75; 91.5) | 61.55 (3.75; 94.25) | 45.17 (21.75; 89.75) | 71.38 (40; 97.75) | |||
| SF-36 overall | mean (±SD) | 41.43 (±18.58) | 53.37 (±20.62) | 40.99 (±13.54) | 55.36 (±15.43) | 0.929 1 0.718 2 <0.001 3 <0.001 4 | 0.567 |
| median (range) | 38.23 (7.75; 88.5) | 53.88 (5.63; 88.25) | 37.88 (19.25; 76.38) | 51.38 (27.13; 91.25) | |||
| Intervention Group | Control Group | Two-Way ANOVA p-Value (Time X HUBER® 360) | |||||
|---|---|---|---|---|---|---|---|
| t1 | t3 | t1 | t3 | p-Value | |||
| Depression | mean (±SD) | 9.65 (±5.11) | 3.63 (±4.02) | 7 (±3.59) | 2.12 (±2.52) | 0.053 1 0.151 2 <0.001 3 <0.001 4 | 0.402 |
| median (range) | 9 (0; 21) | 2 (0; 20) | 8 (2; 12) | 2 (0; 10) | |||
| Anxiety | mean ± (SD) | 5.69 (±3.96) | 3.14 (±3.27) | 3 (±2.94) | 2.71 (±3.31) | 0.013 1 0.637 2 <0.001 3 0.703 4 | 0.014 |
| median (range) | 4 (0; 16) | 2 (0; 15) | 3 (0; 11) | 2 (0; 12) | |||
| Stress | mean (±SD) | 10.8 (±4.86) | 5.88 (±4.15) | 8.35 (±3.71) | 3.71 (±2.31) | 0.064 1 0.045 2 <0.001 3 <0.001 4 | 0.836 |
| median (range) | 10 (2; 21) | 5 (0; 16) | 8 (2; 16) | 3 (1; 10) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paulokat, H.M.; Klinder, A.; Mittelmeier, W.; Bajorat, J.; Osmanski-Zenk, K. Evaluation of a Four Week Interdisciplinary Multimodal Pain Therapy on Chronic Pain Patients—A Comprehensive Approach. Life 2025, 15, 576. https://doi.org/10.3390/life15040576
Paulokat HM, Klinder A, Mittelmeier W, Bajorat J, Osmanski-Zenk K. Evaluation of a Four Week Interdisciplinary Multimodal Pain Therapy on Chronic Pain Patients—A Comprehensive Approach. Life. 2025; 15(4):576. https://doi.org/10.3390/life15040576
Chicago/Turabian StylePaulokat, Henrike Maria, Annett Klinder, Wolfram Mittelmeier, Jörn Bajorat, and Katrin Osmanski-Zenk. 2025. "Evaluation of a Four Week Interdisciplinary Multimodal Pain Therapy on Chronic Pain Patients—A Comprehensive Approach" Life 15, no. 4: 576. https://doi.org/10.3390/life15040576
APA StylePaulokat, H. M., Klinder, A., Mittelmeier, W., Bajorat, J., & Osmanski-Zenk, K. (2025). Evaluation of a Four Week Interdisciplinary Multimodal Pain Therapy on Chronic Pain Patients—A Comprehensive Approach. Life, 15(4), 576. https://doi.org/10.3390/life15040576






