Efficacy of Neurorehabilitation Approaches in Traumatic Brain Injury Patients: A Comprehensive Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Scope
2.2. Literature Search Strategy
2.3. Data Extraction
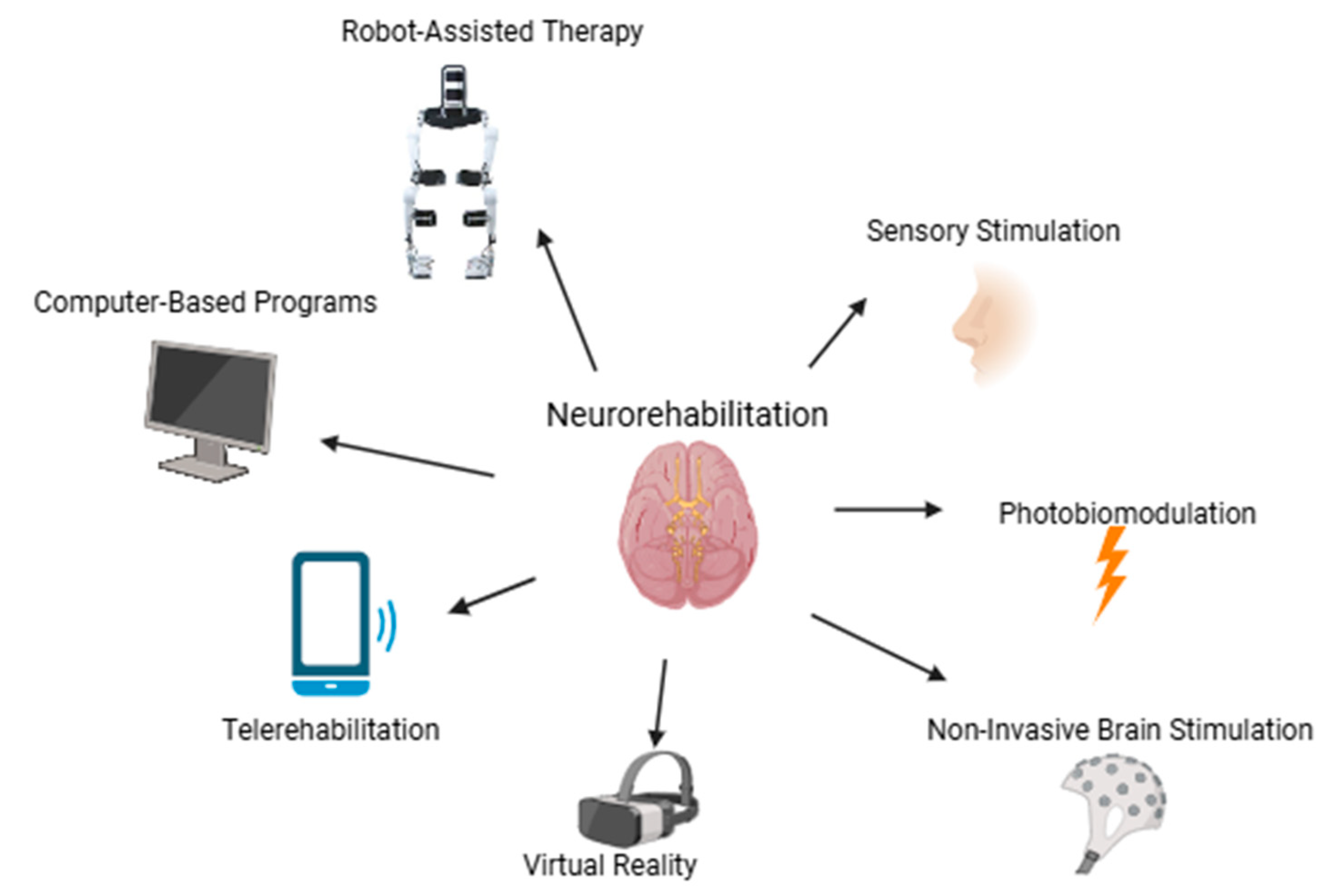
3. Neurorehabilitation Approaches
3.1. Summary of Selected Studies
3.2. Non-Invasive Brain Stimulation
| Study | Sample Size Control/Experimental Group | Method | Cognitive Outcomes | Duration | Motor Outcomes | Other |
|---|---|---|---|---|---|---|
| Flint et al., 2023 [28] | 3/3 | High-γ/μ–β signals | Motor planning, learning, and adaptability improved in the experimental group. | N/A | Motor output (force production), thumb compliance, and physical execution of tasks improved. | Differences in success rates between participants (T1: 28% vs. T2: 99%) |
| Quinn et al., 2020 [30] | 14/10 | Computerized executive function training combined with tDCS | Participants demonstrated significant improvements in depression, anxiety, post-concussive symptoms, complex attention, and executive functions from baseline to post-treatment visits (p < 0.01). | 30-min sessions, 10 weekdays | N/A | Global cerebral blood flow decreased significantly from baseline to post-treatment visits (p = 0.02), with no significant differences between mild and moderate TBI participants. |
| Lee et al., 2018 [36] | 6/7 | Neurodevelopmental therapy and rTMS intervention | The experimental group demonstrated significant improvements in post-intervention scores for the Montgomery-Asberg Depression Rating Scale (MADRS), Trail Making Test (TMT), and Stroop Color Word Test; the control group showed no significant changes in these measures. | 30-min sessions 5 days per week for 2 weeks | N/A | N/A |
| Li et al., 2019 [31] | 24/31 | tDCS sessions | Anodal tDCS did not improve response inhibition in TBI patients; control showed significant improvement in SSRT under anodal tDCS. TBI participants showed no significant improvement in Stop Signal Delay under any stimulation condition. | Single visit, three stimulation conditions | N/A | N/A |
| Motes et al., 2019 [32] | 6/8 | HD-tDCS sessions | Significant differences between the active and sham groups in the changes in the total score of the Rey Auditory Verbal Learning Test (RAVLT), the time taken for the Delis–Kaplan Executive Function System (DKEFS) Inhibition test, and the time taken for the DKEFS Inhibition/Switching Test. | Ten sessions of 20 min of 1 mA anodal HD-tDCS | N/A | N/A |
| Neville et al., 2019 [37] | 13/17 | rTMS was applied using a magnetic stimulator (MagPro X100). | N/A | 5 s of rhythmic high-frequency daily for 10 sessions | No consistent improvement in executive function of controls versus patients. | N/A |
| O’Neil-Pirozz et al., 2017 [34] | 4/4 | tDCS sessions | For the TBI group, word recall increased in the anodal condition for all participants (+3 to +6 words) and in the sham condition for two participants. | Three 90-min sessions, a minimum of 48 h apart | N/A | P300 latency increased across all conditions for the TBI group. |
| Sacco et al., 2016 [29] | 16/16 | tDCS stimulation (HDCstim device) | Within the experimental group, significant improvements were observed between pre-training and post-training, with faster reaction times (p = 0.004) and fewer omission errors; the control group did not exhibit any significant changes; there was borderline improvement in attention performance (p = 0.057) within the experimental group, although no significant changes were found in visual-spatial abilities, semantic fluency, working memory, or long-term memory. | Ten sessions, with each session including 20 min of tDCS stimulation followed by 30 min of cognitive training | N/A | N/A |
3.3. Virtual Reality
| Study | Sample Size Control/Experimental Group | Method | Cognitive Outcomes | Duration | Motor Outcomes | Other |
|---|---|---|---|---|---|---|
| De Luca et al., 2023 [51] | 10/10 | Standard cognitive rehabilitation/Virtual Reality Rehabilitation System | The VR group demonstrated significant improvements in cognitive functions, emotional well-being, and coping strategies compared to the standard rehabilitation group, particularly in attention, executive functioning, and problem-solving. | 3 months standard neurorehabilitation (6 weekly sessions of 60 min), 3 months advanced rehabilitative (3 weekly sessions of 60 min) | N/A | N/A |
| De Luca et al., 2022 [46] | 15/15 | Standard cognitive rehabilitation/Virtual Reality Based-Attention Processes Training (VB_APT) | The experimental group showed significantly greater improvements in global cognition, attention, and depression compared to conventional rehabilitation and significant improvements in executive, visual-spatial, and attention subtests. | First phase: 3 times a week for 8 weeks Second phase: 24 sessions of 60 min each, 3 times a week for 8 weeks | N/A | N/A |
| Ettenhofer et al., 2019 [47] | 6/11 | VR driving simulator NEUROdrive | Intervention group had greater improvement in in working memory (p = 0.004) and visual search/selective attention (p = 0.01). No significant differences between groups in other cognitive measures. | Six 90-min sessions, 4 weeks | No significant change between groups (p > 0.05) in VR tactical and operational scores. Scores remained “average” at both time points in the intervention group. | Greater improvement in intervention group (p < 0.05) for physical functioning. No significant difference (p > 0.05) for mental functioning. |
| Liu et al., 2023 [45] | 13/7 | Participants used instrumented glove on their dominant hand and performed a grasp-and-place maneuver; they also experienced VR environment. | N/A | One single session, three blocks of trials | Multimodal feedback was effective in enhancing neural activity and improving motor performance in TBI participants, as demonstrated by increased EEG power, improved motion pathlength, and greater EMG-EEG coherence. Neurotypical participants responded better to unimodal feedback, with faster task completion and reduced EMG coherence. | N/A |
| Teterfiller et al., 2019 [48] | 32/31 | Xbox Kinect games | N/A | 3–4 times per week for 12 weeks, lasting for 30 min | No statistically significant difference between the two groups; both treatment groups showed improved balance responses to these therapies. | N/A |
3.4. Computer-Based Programs
| Study | Sample Size Control/Experimental Group | Method | Cognitive Outcomes | Duration | Motor Outcomes | Other |
|---|---|---|---|---|---|---|
| Liu et al., 2021 [55] | 30/30 | Computer-assisted cognitive rehabilitation (CACR system) | Significantly better cognitive scores in social cognitive ability, self-care ability, sphincter control, and comprehensive ability compared to the control group (p < 0.05). | N/A | N/A | N/A |
| Rodriguez-Rajo et al., 2024 [54] | 26/28 | Computerized tasks module designed for the rehabilitation of social cognition | Experimental group showed better results for almost all measures. Improved ability to recognize facial emotions in the control group. Experimental group demonstrated better ability to recognize emotions or mental states from eyes post-treatment compared to the control group. | Weekly sessions | N/A | N/A |
3.5. Telerehabilitation
| Study, Year | Sample Size Control/Experimental Group | Method | Cognitive Outcomes | Duration | Motor Outcomes | Other |
|---|---|---|---|---|---|---|
| Bell et al., 2017 [62] | 178/178 | Telephone-Delivered Problem-Solving Treatment | Experimental group had short-term benefits for psychological distress, sleep quality, depression, PTSD symptoms, and physical health compared to control group (p < 0.05). However, these improvements were not sustained at 12 months. | Baseline, 6 months, 12 months | N/A | Participants in the experimental group reported higher satisfaction with the intervention and perceived it as more helpful in addressing their challenges. |
| Raso et al., 2021 [61] | 11/11 | Scheduled videoconferences patient-clinical unit; wearable monitoring devices | Higher mortality in the LSH program (36%) compared to the telemonitoring group (18%). Bedsores (18% vs. 0%) and infections (36% vs. 18%) were more common in the LSH group, but differences were not significant. No significant differences between groups in neuropsychological scores. | N/A | 4-year intervention period, with monthly consultations for the telemonitoring group | Lower daily health care costs |
| Vuletic et al., 2016 [63] | 178/178 | Control group: mailing of educational brochures Experimental group: telephone calls | - | Biweekly over a 6-month period | N/A | Experimental group showed significant improvements in overall sleep quality, sleep duration, latency, and habitual sleep efficiency at 6 months. Improvements in sleep were not sustained at 12 months. |
3.6. Robot-Assisted Therapy
3.6.1. Robot-Assisted Motor Therapy
| Study, Year | Sample Size Control/Experimental Group | Method | Cognitive Outcomes | Duration | Motor Outcomes | Other |
|---|---|---|---|---|---|---|
| Esquenazi et al., 2017 [66] | 7/8/7 (all experimental l: G-EO system/Lokomat/PBWSTT groups) | G-EO; Lokomat; manual assisted BWSTT | N/A | 18 sessions of gait training for 6 to 8 weeks, generally 3 times per week. Each session lasted up to 75 min. | Functional mobility improved significantly in the G-EO and PBWSTT groups. | All three interventions (G-EO, Lokomat, and PBWSTT) significantly improved self-selected velocity, but only Lokomat and PBWSTT improved maximal velocity; improvements in the stroke impact scale mobility domain were seen only in the Lokomat and PBWSTT groups. |
| Maggio et al., 2019 [67] | 28/28 | Lokomat Pro, equipped with a VR screen/Lokomat Nanos, without VR | Significant improvement in global cognitive function, cognitive flexibility and shifting skills, selective attention, and visual search for experimental group. Both groups showed significant improvements in mood and well-being. | 40 one-hour sessions (8 weeks, 5 times/week) | Experimental group showed significant improvement in executive functions. Both groups showed significant improvement in physical well-being. | Experimental group showed significant improvement in overall quality of life. |
3.6.2. Robot-Assisted Cognitive Therapy
3.7. Photobiomodulation
| Study, Year | Sample Size Control/Experimental Group | Method | Cognitive Outcomes | Duration | Motor Outcomes | Other |
|---|---|---|---|---|---|---|
| Carneiro et al., 2019 [74] | 10 (only experimental group) | Transcranial PBM | Sustained gains in visuospatial ability and planning, improved processing speed and divided attention, modest improvement in inhibition and selective attention | 18 sessions, delivered three times per week for six weeks. | N/A | Improved cerebral bloodflow |
| Henderson et al., 2017 [75] | 39 (only experimental group) | Multi-Watt Near-Infrared Phototherapy | N/A | Each session lasted 30 min, with 9–12 min of application per target area (8 to 34 sessions/participant). | N/A | Significant improvement in mood and reduced depression symptoms, reduction in fatigue, and enhanced overall well-being |
| Hipskind et al., 2018 [76] | 12 (only experimental group) | Pulsed Transcranial Red/Near-Infrared Light Therapy Using LED | Significant improvement in symbol search, coding, and processing speed; six of the 15 neuropsychological scales showed significant improvement (p < 0.05), particularly in verbal memory and processing speed. | 20 min per session, 3 times per week for 6 weeks (total of 18 sessions) | N/A | N/A |
| Lin et al., 2023 [73] | 15/15 | Intravenous PBM | Improved short-term memory and attention, reduced confusion and disorganized behavior | 60 min session performed on weekdays over two consecutive weeks for each of the three courses | No significant change in motor outcomes | N/A |
3.8. Sensory Stimulation
| Study, Year | Sample Size Control/Experimental Group | Method | Cognitive Outcomes | Duration | Motor Outcomes | Other |
|---|---|---|---|---|---|---|
| Moattari et al., 2016 [84] | 20/20/20(control/experimental/placebo groups) | Sensory stimulation (auditory, visual, tactile, olfactory) | Gradual increase in cognitive function and basic cognitive sensory recovery especially in family-conducted sensory stimulation group (RLA scale, WNSSP) | 7 days, 2 times per day (30 min) | N/A | Improved level of consciousness (GCS) |
| Salmai et al., 2017 [83] | 30/30/30 (family centered/nurse/control groups) | Sensory stimulation (auditory, sensory, kinetic, affective-only in the family centered stimulation) | Enhanced patients’ responsiveness and cognitive functions (CRS-R scores), statistically significant after 4 days | 7 days, 2 times per day (30–45 min) | N/A | Statistically significant GCS improvement after 4 days |
3.9. Combined Approaches
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dewan, M.C.; Rattani, A.; Gupta, S.; Baticulon, R.E.; Hung, Y.-C.; Punchak, M.; Agrawal, A.; Adeleye, A.O.; Shrime, M.G.; Rubiano, A.M.; et al. Estimating the Global Incidence of Traumatic Brain Injury. J. Neurosurg. 2019, 130, 1080–1097. [Google Scholar] [CrossRef] [PubMed]
- Min, J.H.; Shin, Y.-I. Treatment and Rehabilitation for Traumatic Brain Injury: Current Update. Brain Neurorehabilit. 2022, 15, e14. [Google Scholar] [CrossRef]
- Oberholzer, M.; Müri, R.M. Neurorehabilitation of Traumatic Brain Injury (TBI): A Clinical Review. Med. Sci. 2019, 7, 47. [Google Scholar] [CrossRef]
- Naik, A.; Bederson, M.M.; Detchou, D.; Dharnipragada, R.; Hassaneen, W.; Arnold, P.M.; Germano, I.M. Traumatic Brain Injury Mortality and Correlates in Low- and Middle-Income Countries: A Meta-Epidemiological Study. Neurosurgery 2023, 93, 736–744. [Google Scholar] [CrossRef]
- James, S.L.; Theadom, A.; Ellenbogen, R.G.; Bannick, M.S.; Montjoy-Venning, W.; Lucchesi, L.R. GBD 2016 Traumatic Brain Injury and Spinal Cord Injury Collaborators. Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 56–87, Erratum in Lancet Neurol. 2021, 20, e7. https://doi.org/10.1016/S1474-4422(21)00383-5. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gardner, R.C.; Dams-O’Connor, K.; Morrissey, M.R.; Manley, G.T. Geriatric Traumatic Brain Injury: Epidemiology, Outcomes, Knowledge Gaps, and Future Directions. J. Neurotrauma 2018, 35, 889–906. [Google Scholar] [CrossRef]
- Dams-O’Connor, K.; Ketchum, J.M.; Cuthbert, J.P.; Corrigan, J.D.; Hammond, F.M.; Haarbauer-Krupa, J.; Kowalski, R.G.; Miller, A.C. Functional Outcome Trajectories Following Inpatient Rehabilitation for TBI in the United States: A NIDILRR TBIMS and CDC Interagency Collaboration. J. Head Trauma Rehabil. 2020, 35, 127–139. [Google Scholar] [CrossRef]
- Devi, Y.; Khan, S.; Rana, P.; Deepak, D.; Dhandapani, M.; Ghai, S.; Gopichandran, L.; Dhandapani, S. Cognitive, Behavioral, and Functional Impairments among Traumatic Brain Injury Survivors: Impact on Caregiver Burden. J. Neurosci. Rural Pract. 2020, 11, 629–635. [Google Scholar] [CrossRef]
- Kumar, K.S.; Samuelkamaleshkumar, S.; Viswanathan, A.; Macaden, A.S. Cognitive rehabilitation for adults with traumatic brain injury to improve occupational outcomes. Cochrane Database Syst. Rev. 2017, 6, CD007935. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cicerone, K.D.; Goldin, Y.; Ganci, K.; Rosenbaum, A.; Wethe, J.V.; Langenbahn, D.M.; Malec, J.F.; Bergquist, T.F.; Kingsley, K.; Nagele, D.; et al. Evidence-Based Cognitive Rehabilitation: Systematic Review of the Literature From 2009 Through 2014. Arch. Phys. Med. Rehabil. 2019, 100, 1515–1533. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.; Kim, D.Y.; Park, S.W.; Lee, B.S.; Han, H.W.; Jeon, N.; Kim, M.; Kang, M.; Kim, S. A systematic review of cognitive telerehabilitation in patients with cognitive dysfunction. Front. Neurol. 2025, 15, 1450977. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cicerone, K.D.; Dahlberg, C.; Kalmar, K.; Langenbahn, D.M.; Malec, J.F.; Bergquist, T.F.; Felicetti, T.; Giacino, J.T.; Harley, J.P.; Harrington, D.E.; et al. Evidence-based cognitive rehabilitation: Recommendations for clinical practice. Arch. Phys. Med. Rehabil. 2000, 81, 1596–1615. [Google Scholar] [CrossRef] [PubMed]
- Bland, D.C.; Zampieri, C.; Damiano, D.L. Effectiveness of Physical Therapy for Improving Gait and Balance in Individuals with Traumatic Brain Injury: A Systematic Review. Brain Inj. 2011, 25, 664–679. [Google Scholar] [CrossRef]
- Cumplido-Trasmonte, C.; Barquín-Santos, E.; Gor-García-Fogeda, M.D.; Plaza-Flores, A.; García-Varela, D.; Ibáñez-Herrán, L.; González-Alted, C.; Díaz-Valles, P.; López-Pascua, C.; Castrillo-Calvillo, A.; et al. STELO: A New Modular Robotic Gait Device for Acquired Brain Injury—Exploring Its Usability. Sensors 2023, 24, 198. [Google Scholar] [CrossRef] [PubMed]
- Lippert, J.; Guggisberg, A.G. Diagnostic and Therapeutic Approaches in Neurorehabilitation after Traumatic Brain Injury and Disorders of Consciousness. Clin. Transl. Neurosci. 2023, 7, 21. [Google Scholar] [CrossRef]
- Maas, A.I.R.; Menon, D.K.; Manley, G.T.; InTBIR Participants and Investigators. Traumatic Brain Injury: Progress and Challenges in Prevention, Clinical Care, and Research. Lancet Neurol. 2022, 21, 1004–1060. [Google Scholar] [CrossRef]
- Andelic, N.; Ye, J.; Tornas, S.; Roe, C.; Lu, J.; Bautz-Holter, E.; Moger, T.; Sigurdardottir, S.; Schanke, A.-K.; Aas, E. Cost-Effectiveness Analysis of an Early-Initiated, Continuous Chain of Rehabilitation after Severe Traumatic Brain Injury. J. Neurotrauma 2014, 31, 1313–1320. [Google Scholar] [CrossRef]
- Jacob, L.; Cogné, M.; Tenovuo, O.; Røe, C.; Andelic, N.; Majdan, M.; Ranta, J.; Ylen, P.; Dawes, H.; Azouvi, P.; et al. Predictors of Access to Rehabilitation in the Year Following Traumatic Brain Injury: A European Prospective and Multi-center Study. Neurorehabil. Neural Repair 2020, 34, 814–830. [Google Scholar] [CrossRef]
- Ponsford, J.; Harrison-Felix, C.; Ketchum, J.M.; Spitz, G.; Miller, A.C.; Corrigan, J.D. Outcomes 1 and 2 Years After Moderate to Severe Traumatic Brain Injury: An International Comparative Study. Arch. Phys. Med. Rehabil. 2021, 102, 371–377. [Google Scholar] [CrossRef]
- Turner-Stokes, L.; Pick, A.; Nair, A.; Disler, P.B.; Wade, D.T. Multi-Disciplinary Rehabilitation for Acquired Brain Injury in Adults of Working Age. Cochrane Database Syst. Rev. 2015, 12, CD004170. [Google Scholar] [CrossRef]
- Truijen, S.; Abdullahi, A.; Bijsterbosch, D.; Van Zoest, E.; Conijn, M.; Wang, Y.; Struyf, N.; Saeys, W. Effect of Home-Based Virtual Reality Training and Telerehabilitation on Balance in Individuals with Parkinson Disease, Multiple Sclerosis, and Stroke: A Systematic Review and Meta-Analysis. Neurol. Sci. 2022, 43, 2995–3006. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, A.S.; Fagundes, C.V.; Mendes, F.A.D.S.; Leal, J.C. Effectiveness of Virtual Reality Rehabilitation in Persons with Multiple Sclerosis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Mult. Scler. Relat. Disord. 2021, 54, 103128. [Google Scholar] [CrossRef] [PubMed]
- Kesikburun, S. Non-Invasive Brain Stimulation in Rehabilitation. Turk. J. Phys. Med. Rehabil. 2022, 68, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hara, T.; Shanmugalingam, A.; McIntyre, A.; Burhan, A.M. The Effect of Non-Invasive Brain Stimulation (NIBS) on Executive Functioning, Attention and Memory in Rehabilitation Patients with Traumatic Brain Injury: A Systematic Review. Diagnostics 2021, 11, 627. [Google Scholar] [CrossRef]
- Korupolu, R.; Miller, A.; Park, A.; Yozbatiran, N. Neurorehabilitation with Vagus Nerve Stimulation: A Systematic Review. Front. Neurol. 2024, 15, 1390217. [Google Scholar] [CrossRef]
- Chen, J.; Dong, Y.; Guo, H.; Zhao, T.; Zhang, D.; Jin, S. Efficacy of rTMS Combined with Cognitive Training in TBI with Cognition Disorder: A Systematic Review and Meta-Analysis. Neurol. Sci. 2024, 45, 3683–3697. [Google Scholar] [CrossRef]
- Schiff, N.D.; Giacino, J.T.; Butson, C.R.; Choi, E.Y.; Baker, J.L.; O’Sullivan, K.P.; Janson, A.P.; Bergin, M.; Bronte-Stewart, H.M.; Chua, J.; et al. Thalamic Deep Brain Stimulation in Traumatic Brain Injury: A Phase 1, Randomized Feasibility Study. Nat. Med. 2023, 29, 3162–3174. [Google Scholar] [CrossRef]
- Flint, R.D.; Li, Y.; Wang, P.T.; Vaidya, M.; Barry, A.; Ghassemi, M.; Tomic, G.; Brkic, N.; Ripley, D.; Liu, C.; et al. Noninvasively Recorded High-Gamma Signals Improve Synchrony of Force Feedback in a Novel Neurorehabilitation Brain–Machine Interface for Brain Injury. J. Neural Eng. 2022, 19, 036024. [Google Scholar] [CrossRef]
- Sacco, K.; Galetto, V.; Dimitri, D.; Geda, E.; Perotti, F.; Zettin, M.; Geminiani, G.C. Concomitant Use of Transcranial Direct Current Stimulation and Computer-Assisted Training for the Rehabilitation of Attention in Traumatic Brain Injured Patients: Behavioral and Neuroimaging Results. Front. Behav. Neurosci. 2016, 10, 57. [Google Scholar] [CrossRef]
- Quinn, D.K.; Upston, J.; Jones, T.; Brandt, E.; Story-Remer, J.; Fratzke, V.; Wilson, J.K.; Rieger, R.; Hunter, M.A.; Gill, D.; et al. Cerebral Perfusion Effects of Cognitive Training and Transcranial Direct Current Stimulation in Mild-Moderate TBI. Front. Neurol. 2020, 11, 545174. [Google Scholar] [CrossRef]
- Li, L.M.; Violante, I.R.; Zimmerman, K.; Leech, R.; Hampshire, A.; Patel, M.; Opitz, A.; McArthur, D.; Jolly, A.; Carmichael, D.W.; et al. Traumatic Axonal Injury Influences the Cognitive Effect of Non-Invasive Brain Stimulation. Brain 2019, 142, 3280–3293. [Google Scholar] [CrossRef] [PubMed]
- Motes, M.A.; Spence, J.S.; Yeatman, K.; Jones, P.M.; Lutrell, M.; O’Hair, R.; Shakal, S.; DeLaRosa, B.L.; To, W.; Vanneste, S.; et al. High-Definition Transcranial Direct Current Stimulation to Improve Verbal Retrieval Deficits in Chronic Traumatic Brain Injury. J. Neurotrauma 2020, 37, 170–177. [Google Scholar] [CrossRef] [PubMed]
- De Freitas, D.J.; De Carvalho, D.; Paglioni, V.M.; Brunoni, A.R.; Valiengo, L.; Thome-Souza, M.S.; Guirado, V.M.P.; Zaninotto, A.L.; Paiva, W.S. Effects of Transcranial Direct Current Stimulation (tDCS) and Concurrent Cognitive Training on Episodic Memory in Patients with Traumatic Brain Injury: A Double-Blind, Randomised, Placebo-Controlled Study. BMJ Open 2021, 11, e045285. [Google Scholar] [CrossRef] [PubMed]
- O’Neil-Pirozzi, T.M.; Doruk, D.; Thomson, J.M.; Fregni, F. Immediate Memory and Electrophysiologic Effects of Prefrontal Cortex Transcranial Direct Current Stimulation on Neurotypical Individuals and Individuals with Chronic Trau-matic Brain Injury: A Pilot Study. Int. J. Neurosci. 2017, 127, 592–600. [Google Scholar] [CrossRef]
- Vallejo, P.; Cueva, E.; Martínez-Lozada, P.; García-Ríos, C.A.; Miranda-Barros, D.H.; Leon-Rojas, J.E. Repetitive Transcranial Magnetic Stimulation in Stroke: A Literature Review of the Current Role and Controversies of Neurorehabilitation Through Electromagnetic Pulses. Cureus 2023, 15, e41714. [Google Scholar] [CrossRef]
- Lee, S.A.; Kim, M.-K. Effect of Low Frequency Repetitive Transcranial Magnetic Stimulation on Depression and Cognition of Patients with Traumatic Brain Injury: A Randomized Controlled Trial. Med. Sci. Monit. 2018, 24, 8789–8794. [Google Scholar] [CrossRef]
- Neville, I.S.; Zaninotto, A.L.; Hayashi, C.Y.; Rodrigues, P.A.; Galhardoni, R.; Ciampi De Andrade, D.; Brunoni, A.R.; Amorim, R.L.O.; Teixeira, M.J.; Paiva, W.S. Repetitive TMS Does Not Improve Cognition in Patients with TBI: A Randomized Double-Blind Trial. Neurology 2019, 93, e190–e199. [Google Scholar] [CrossRef]
- Chiang, H.-S.; Shakal, S.; Vanneste, S.; Kraut, M.; Hart, J. Case Report: Improving Verbal Retrieval Deficits With High Definition Transcranial Direct Current Stimulation Targeting the Pre-Supplementary Motor Area in a Patient With Chronic Traumatic Brain Injury. Front. Neurol. 2021, 12, 678518. [Google Scholar] [CrossRef]
- Eilam-Stock, T.; George, A.; Charvet, L.E. Cognitive Telerehabilitation with Transcranial Direct Current Stimulation Improves Cognitive and Emotional Functioning Following a Traumatic Brain Injury: A Case Study. Arch. Clin. Neuro Psychol. 2021, 36, 442–453. [Google Scholar] [CrossRef]
- Bonanno, M.; De Luca, R.; De Nunzio, A.M.; Quartarone, A.; Calabrò, R.S. Innovative Technologies in the Neurorehabilitation of Traumatic Brain Injury: A Systematic Review. Brain Sci. 2022, 12, 1678. [Google Scholar] [CrossRef]
- Nieto-Escamez, F.; Cortés-Pérez, I.; Obrero-Gaitán, E.; Fusco, A. Virtual Reality Applications in Neurorehabilitation: Current Panorama and Challenges. Brain Sci. 2023, 13, 819. [Google Scholar] [CrossRef] [PubMed]
- Voinescu, A.; Sui, J.; Stanton Fraser, D. Virtual Reality in Neurorehabilitation: An Umbrella Review of Me-ta-Analyses. J. Clin. Med. 2021, 10, 1478. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.-S.; Lee, K.; Kim, S.; Cho, S.; Paik, N.-J. Transcranial Direct Current Stimulation for the Treatment of Motor Impairment Following Traumatic Brain Injury. J. Neuroeng. Rehabil. 2019, 16, 14. [Google Scholar] [CrossRef] [PubMed]
- Llorens, R.; Fuentes, M.A.; Borrego, A.; Latorre, J.; Alcañiz, M.; Colomer, C.; Noé, E. Effectiveness of a Combined Transcranial Direct Current Stimulation and Virtual Reality-Based Intervention on Upper Limb Function in Chronic Individuals Post-Stroke with Persistent Severe Hemiparesis: A Randomized Controlled Trial. J. Neuroeng. Rehabil. 2021, 18, 108. [Google Scholar] [CrossRef]
- Liu, M.; Wilder, S.; Sanford, S.; Glassen, M.; Dewil, S.; Saleh, S.; Nataraj, R. Augmented Feedback Modes during Functional Grasp Training with an Intelligent Glove and Virtual Reality for Persons with Traumatic Brain Injury. Front. Robot. AI 2023, 10, 1230086. [Google Scholar] [CrossRef]
- De Luca, R.; Bonanno, M.; Rifici, C.; Pollicino, P.; Caminiti, A.; Morone, G.; Calabrò, R.S. Does Non-Immersive Virtual Reality Improve Attention Processes in Severe Traumatic Brain Injury? Encouraging Data from a Pilot Study. Brain Sci. 2022, 12, 1211. [Google Scholar] [CrossRef]
- Ettenhofer, M.L.; Guise, B.; Brandler, B.; Bittner, K.; Gimbel, S.I.; Cordero, E.; Nelson Schmitt, S.; Williams, K.; Cox, D.; Roy, M.J.; et al. Neurocognitive Driving Rehabilitation in Virtual Environments (NeuroDRIVE): A Pilot Clinical Trial for Chronic Traumatic Brain Injury. NeuroRehabilitation 2019, 44, 531–544. [Google Scholar] [CrossRef]
- Tefertiller, C.; Hays, K.; Natale, A.; O’Dell, D.; Ketchum, J.; Sevigny, M.; Eagye, C.B.; Philippus, A.; Harrison-Felix, C. Results From a Randomized Controlled Trial to Address Balance Deficits After Traumatic Brain Injury. Arch. Phys. Med. Rehabil. 2019, 100, 1409–1416. [Google Scholar] [CrossRef]
- Chanpimol, S.; Seamon, B.; Hernandez, H.; Harris-Love, M.; Blackman, M.R. Using Xbox Kinect Motion Capture Tech-nology to Improve Clinical Rehabilitation Outcomes for Balance and Cardiovascular Health in an Individual with Chronic TBI. Arch. Physiother. 2017, 7, 6. [Google Scholar] [CrossRef]
- Gottshall, K.R.; Sessoms, P.H. Improvements in Dizziness and Imbalance Results from Using a Multi Disciplinary and Multi Sensory Approach to Vestibular Physical Therapy—A Case Study. Front. Syst. Neurosci. 2015, 9, 106. [Google Scholar] [CrossRef]
- De Luca, R.; Bonanno, M.; Marra, A.; Rifici, C.; Pollicino, P.; Caminiti, A.; Castorina, M.V.; Santamato, A.; Quartarone, A.; Calabrò, R.S. Can Virtual Reality Cognitive Rehabilitation Improve Executive Functioning and Coping Strategies in Traumatic Brain Injury? A Pilot Study. Brain Sci. 2023, 13, 578. [Google Scholar] [CrossRef] [PubMed]
- Alt Murphy, M.; Pradhan, S.; Levin, M.F.; Hancock, N.J. Uptake of Technology for Neurorehabilitation in Clinical Practice: A Scoping Review. Phys. Ther. 2024, 104, 140. [Google Scholar] [CrossRef] [PubMed]
- Van Den Berg, M.; Sherrington, C.; Killington, M.; Smith, S.; Bongers, B.; Hassett, L.; Crotty, M. Video and Comput-er-Based Interactive Exercises Are Safe and Improve Task-Specific Balance in Geriatric and Neurological Rehabilitation: A Randomised Trial. J. Physiother. 2016, 62, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Rajo, P.; García-Rudolph, A.; Sánchez-Carrión, R.; Aparicio-López, C.; Enseñat-Cantallops, A.; García-Molina, A. Computerized Social Cognitive Training in the Subacute Phase after Traumatic Brain Injury: A Quasi-Randomized Controlled Trial. Appl. Neuropsychol. Adult 2024, 31, 540–553. [Google Scholar] [CrossRef]
- Liu, B.; Lu, H. Research on Computer Assisted Cognitive Rehabilitation for Cognitive Dysfunction after Traumatic Brain Injury. J. Phys. Conf. Ser. 2021, 1915, 032025. [Google Scholar] [CrossRef]
- Martin, S.; Armstrong, E.; Thomson, E.; Vargiu, E.; Solà, M.; Dauwalder, S.; Miralles, F.; Daly Lynn, J. A Qualitative Study Adopting a User-Centered Approach to Design and Validate a Brain Computer Interface for Cognitive Rehabilitation for People with Brain Injury. Assist. Technol. 2018, 30, 233–241. [Google Scholar] [CrossRef]
- Del Pino, R.; Díez-Cirarda, M.; Ustarroz-Aguirre, I.; Gonzalez-Larragan, S.; Caprino, M.; Busnatu, S.; Gand, K.; Schlieter, H.; Gabilondo, I.; Gómez-Esteban, J.C. Costs and Effects of Telerehabilitation in Neurological and Cardiological Diseases: A Systematic Review. Front. Med. 2022, 9, 832229. [Google Scholar] [CrossRef]
- Federico, S.; Cacciante, L.; Cieślik, B.; Turolla, A.; Agostini, M.; Kiper, P.; Picelli, A. Telerehabilitation for Neurolog-ical Motor Impairment: A Systematic Review and Meta-Analysis on Quality of Life, Satisfaction, and Acceptance in Stroke, Multiple Sclerosis, and Parkinson’s Disease. J. Clin. Med. 2024, 13, 299. [Google Scholar] [CrossRef]
- Simmich, J.; Ross, M.H.; Russell, T. Real-Time Video Telerehabilitation Shows Comparable Satisfaction and Simi-lar or Better Attendance and Adherence Compared with in-Person Physiotherapy: A Systematic Review. J. Physiother. 2024, 70, 181–192. [Google Scholar] [CrossRef]
- De Luca, R.; Maggio, M.G.; Naro, A.; Portaro, S.; Cannavò, A.; Calabrò, R.S. Can Patients with Severe Traumatic Brain Injury Be Trained with Cognitive Telerehabilitation? An Inpatient Feasibility and Usability Study. J. Clin. Neurosci. 2020, 79, 246–250. [Google Scholar] [CrossRef]
- Raso, M.G.; Arcuri, F.; Liperoti, S.; Mercurio, L.; Mauro, A.; Cusato, F.; Romania, L.; Serra, S.; Pignolo, L.; Tonin, P.; et al. Telemonitoring of Patients With Chronic Traumatic Brain Injury: A Pilot Study. Front. Neurol. 2021, 12, 598777. [Google Scholar] [CrossRef] [PubMed]
- Bell, K.R.; Fann, J.R.; Brockway, J.A.; Cole, W.R.; Bush, N.E.; Dikmen, S.; Hart, T.; Lang, A.J.; Grant, G.; Gahm, G.; et al. Telephone Problem Solving for Service Members with Mild Traumatic Brain Injury: A Randomized, Clinical Trial. J. Neurotrauma 2017, 34, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Vuletic, S.; Bell, K.R.; Jain, S.; Bush, N.; Temkin, N.; Fann, J.R.; Stanfill, K.E.; Dikmen, S.; Brockway, J.A.; He, F.; et al. Telephone Problem-Solving Treatment Improves Sleep Quality in Service Members With Combat-Related Mild Traumatic Brain Injury: Results From a Randomized Clinical Trial. J. Head Trauma Rehabil. 2016, 31, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Oña, E.D.; Cano-de La Cuerda, R.; Sánchez-Herrera, P.; Balaguer, C.; Jardón, A. A Review of Robotics in Neurorehabilitation: Towards an Automated Process for Upper Limb. J. Healthc. Eng. 2018, 2018, 9758939. [Google Scholar] [CrossRef]
- Chang, W.H.; Kim, Y.-H. Robot-Assisted Therapy in Stroke Rehabilitation. J. Stroke 2013, 15, 174. [Google Scholar] [CrossRef]
- Esquenazi, A.; Lee, S.; Wikoff, A.; Packel, A.; Toczylowski, T.; Feeley, J. A Comparison of Locomotor Therapy Interventions: Partial-Body Weight−Supported Treadmill, Lokomat, and G-EO Training in People With Traumatic Brain Injury. PMR 2017, 9, 839–846. [Google Scholar] [CrossRef]
- Maggio, M.G.; Torrisi, M.; Buda, A.; De Luca, R.; Piazzitta, D.; Cannavò, A.; Leo, A.; Milardi, D.; Manuli, A.; Calabro, R.S. Effects of Robotic Neurorehabilitation through Lokomat plus Virtual Reality on Cognitive Function in Patients with Traumatic Brain Injury: A Retrospective Case-Control Study. Int. J. Neurosci. 2020, 130, 117–123. [Google Scholar] [CrossRef]
- Williams, K.; Christenbury, J.; Niemeier, J.P.; Newman, M.; Pinto, S. Is Robotic Gait Training Feasible in Adults With Disorders of Consciousness? J. Head Trauma Rehabil. 2020, 35, E266–E270. [Google Scholar] [CrossRef]
- Nolan, K.J.; Karunakaran, K.K.; Ehrenberg, N.; Kesten, A.G. Robotic Exoskeleton Gait Training for Inpatient Rehabilitation in a Young Adult with Traumatic Brain Injury. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 17–21 July 2018; IEEE: Piscataway, NJ, USA, 2018; pp. 2809–2812. [Google Scholar]
- Anderl, E.R.; Trammell, H.J. Facilitating Walking Recovery with Use of a Wearable Robotic Exoskeleton in an Individual with Traumatic Brain Injury and Ataxia: A Case Study. In Proceedings of the 2017 International Symposium on Wearable Robotics and Rehabilitation (WeRob), Houston, TX, USA, 5–8 November 2017; IEEE: Piscataway, NJ, USA, 2017; pp. 1–2. [Google Scholar]
- Ilyas, C.M.A.; Schmuck, V.; Haque, M.A.; Nasrollahi, K.; Rehm, M.; Moeslund, T.B. Teaching Pepper Robot to Recognize Emotions of Traumatic Brain Injured Patients Using Deep Neural Networks. In Proceedings of the 2019 28th IEEE International Conference on Robot and Human Interactive Communication (RO-MAN), New Delhi, India, 14–18 October 2019; IEEE: Piscataway, NJ, USA, 2019; pp. 1–7. [Google Scholar]
- Hamblin, M.R.; Huang, Y.Y.; Heiskanen, V. Non-Mammalian Hosts and Photobiomodulation: Do All Life-Forms Respond to Light? Photochem. Photobiol. 2019, 95, 126–139. [Google Scholar] [CrossRef]
- Lin, Y.P.; Ku, C.H.; Chang, C.C.; Chang, S.T. Effects of Intravascular Photobiomodulation on Cognitive Impairment and Crossed Cerebellar Diaschisis in Patients with Traumatic Brain Injury: A Longitudinal Study. Lasers Med. Sci. 2023, 38, 108. [Google Scholar] [CrossRef]
- Carneiro, A.M.C.; Poiani, G.C.; Zaninnoto, A.L.; Lazo Osorio, R.; Oliveira, M.L.; Paiva, W.S.; Zângaro, R.A. Transcranial Photobiomodulation Therapy in the Cognitive Rehabilitation of Patients with Cranioencephalic Trauma. Photobiomodul. Photomed. Laser Surg. 2019, 37, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Henderson, T.A.; Morries, L.D. Multi-Watt Near-Infrared Phototherapy for the Treatment of Comorbid Depression: An Open-Label Single-Arm Study. Front. Psychiatry 2017, 8, 187. [Google Scholar] [CrossRef] [PubMed]
- Hipskind, S.G.; Grover, F.L., Jr.; Fort, T.R.; Helffenstein, D.; Burke, T.J.; Quint, S.A.; Bussiere, G.; Stone, M.; Hurtado, T. Pulsed Transcranial Red/Near-Infrared Light Therapy Using Light-Emitting Diodes Improves Cerebral Blood Flow and Cognitive Function in Veterans with Chronic Traumatic Brain Injury: A Case Series. Photomed. Laser Surg. 2018, 37, 77–84. [Google Scholar] [CrossRef]
- Chao, L.L.; Barlow, C.; Karimpoor, M.; Lim, L. Changes in Brain Function and Structure After Self-Administered Home Photobiomodulation Treatment in a Concussion Case. Front. Neurol. 2020, 11, 952. [Google Scholar] [CrossRef]
- Chao, L.L. Improvements in Gulf War Illness Symptoms After Near-Infrared Transcranial and Intranasal Photobiomodulation: Two Case Reports. Mil. Med. 2019, 184, e568–e574. [Google Scholar] [CrossRef]
- Zuo, J.; Tao, Y.; Liu, M.; Feng, L.; Yang, Y.; Liao, L. The effect of family-centered sensory and affective stimulation on comatose patients with traumatic brain injury: A systematic review and meta-analysis. Int. J. Nurs. Stud. 2021, 115, 103846. [Google Scholar] [CrossRef]
- Park, S. Effectiveness of direct and non-direct auditory stimulation on coma arousal after traumatic brain injury. Int. J. Nurs. Pract. 2016, 22, 391–396. [Google Scholar] [CrossRef]
- Norwood, M.F.; Lakhani, A.; Watling, D.P.; Marsh, C.H.; Zeeman, H. Efficacy of Multimodal Sensory Therapy in Adult Acquired Brain Injury: A Systematic Review. Neuropsychol. Rev. 2023, 33, 693–713. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- De Luca, R.; Lauria, P.; Bonanno, M.; Corallo, F.; Rifici, C.; Castorina, M.V.; Trifirò, S.; Gangemi, A.; Lombardo, C.; Quartarone, A.; et al. Neurophysiological and Psychometric Outcomes in Minimal Consciousness State after Advanced Audio–Video Emotional Stimulation: A Retrospective Study. Brain Sci. 2023, 13, 1619. [Google Scholar] [CrossRef]
- Salmani, F.; Mohammadi, E.; Rezvani, M.; Kazemnezhad, A. The effects of family-centered affective stimulation on brain-injured comatose patients’ level of consciousness: A randomized controlled trial. Int. J. Nurs. Stud. 2017, 74, 44–52. [Google Scholar] [CrossRef]
- Moattari, M.; Alizadeh Shirazi, F.; Sharifi, N.; Zareh, N. Effects of a Sensory Stimulation by Nurses and Families on Level of Cognitive Function, and Basic Cognitive Sensory Recovery of Comatose Patients With Severe Traumatic Brain Injury: A Randomized Control Trial. Trauma Mon. 2016, 21, e23531. [Google Scholar] [CrossRef] [PubMed]
- Abbate, C.; Trimarchi, P.D.; Basile, I.; Mazzucchi, A.; Devalle, G. Sensory stimulation for patients with disorders of consciousness: From stimulation to rehabilitation. Front. Hum. Neurosci. 2014, 8, 616. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- De Luca, R.; Bonanno, M.; Vermiglio, G.; Trombetta, G.; Andidero, E.; Caminiti, A.; Pollicino, P.; Rifici, C.; Calabrò, R.S. Robotic Verticalization plus Music Therapy in Chronic Disorders of Consciousness: Promising Results from a Pilot Study. Brain Sci. 2022, 12, 1045. [Google Scholar] [CrossRef] [PubMed]
- Alashram, A.R.; Annino, G.; Padua, E.; Romagnoli, C.; Mercuri, N.B. Cognitive Rehabilitation Post Traumatic Brain Injury: A Systematic Review for Emerging Use of Virtual Reality Technology. J. Clin. Neurosci. 2019, 66, 209–219. [Google Scholar] [CrossRef]
- Suarilah, I.; Zulkarnain, H.; Saragih, I.D.; Lee, B.O. Effectiveness of Telehealth Interventions Among Traumatic Brain Injury Survivors: A Systematic Review and Meta-Analysis. J. Telemed. Telecare 2024, 30, 781–794. [Google Scholar] [CrossRef]
- Aulisio, M.C.; Han, D.Y.; Glueck, A.C. Virtual Reality Gaming as a Neurorehabilitation Tool for Brain Injuries in Adults: A Systematic Review. Brain Inj. 2020, 34, 1322–1330. [Google Scholar] [CrossRef]
- Ownsworth, T.; Arnautovska, U.; Beadle, E.; Shum, D.H.K.; Moyle, W. Efficacy of Telerehabilitation for Adults With Traumatic Brain Injury: A Systematic Review. J. Head Trauma Rehabil. 2018, 33, E33–E46. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrei, D.; Mederle, A.L.; Ghenciu, L.A.; Borza, C.; Faur, A.C. Efficacy of Neurorehabilitation Approaches in Traumatic Brain Injury Patients: A Comprehensive Review. Life 2025, 15, 503. https://doi.org/10.3390/life15030503
Andrei D, Mederle AL, Ghenciu LA, Borza C, Faur AC. Efficacy of Neurorehabilitation Approaches in Traumatic Brain Injury Patients: A Comprehensive Review. Life. 2025; 15(3):503. https://doi.org/10.3390/life15030503
Chicago/Turabian StyleAndrei, Diana, Alexandra Laura Mederle, Laura Andreea Ghenciu, Claudia Borza, and Alexandra Corina Faur. 2025. "Efficacy of Neurorehabilitation Approaches in Traumatic Brain Injury Patients: A Comprehensive Review" Life 15, no. 3: 503. https://doi.org/10.3390/life15030503
APA StyleAndrei, D., Mederle, A. L., Ghenciu, L. A., Borza, C., & Faur, A. C. (2025). Efficacy of Neurorehabilitation Approaches in Traumatic Brain Injury Patients: A Comprehensive Review. Life, 15(3), 503. https://doi.org/10.3390/life15030503







