Traumatic Brain Injury and Artificial Intelligence: Shaping the Future of Neurorehabilitation—A Review
Abstract
1. Introduction
Search Strategy
2. Considerations of Managing TBI: Current and Future Perspectives
3. Artificial Intelligence in Healthcare: Its History and Applications Within the Field of Neurology
4. The Use of AI as a Diagnostic Tool for TBI
5. The Use of AI as a Predictor for TBI Health Outcomes
5.1. The Current Predictive Models: CRASH and IMPACT
5.2. AI Predicting Functional Outcomes
6. Clinical Trials of AI in Traumatic Brain Injury
7. Long-Term Considerations for TBI Patients and AI-Assisted Treatments
7.1. Management of Patient Lifestyle
7.2. Management of Caregiver Ethics
7.3. Management of Ethics in the Implementation of Artificial Intelligence
7.4. Future Directions in AI and Emerging Technology Research for TBI Rehabilitation
8. Conclusions
Funding
Conflicts of Interest
References
- Maas, A.I.R.; Menon, D.K.; Manley, G.T.; Abrams, M.; Åkerlund, C.; Andelic, N.; Aries, M.; Bashford, T.; Bell, M.J.; Bodien, Y.G.; et al. Traumatic brain injury: Progress and challenges in prevention, clinical care, and research. Lancet Neurol. 2022, 21, 1004–1060. [Google Scholar] [CrossRef] [PubMed]
- Elhaddad, M.; Hamam, S.; Elhaddad, M.; Hamam, S. AI-Driven Clinical Decision Support Systems: An Ongoing Pursuit of Potential. Cureus 2024, 16, e57728. [Google Scholar] [CrossRef] [PubMed]
- Patel, U.K.; Anwar, A.; Saleem, S.; Malik, P.; Rasul, B.; Patel, K.; Yao, R.; Seshadri, A.; Yousufuddin, M.; Arumaithurai, K. Artificial intelligence as an emerging technology in the current care of neurological disorders. J. Neurol. 2021, 268, 1623–1642. [Google Scholar] [CrossRef] [PubMed]
- Bielanin, J.P.; Metwally, S.A.H.; Paruchuri, S.S.; Sun, D. An overview of mild traumatic brain injuries and emerging therapeutic targets. Neurochem. Int. 2024, 172, 105655. [Google Scholar] [CrossRef]
- Iaccarino, M.A.; Bhatnagar, S.; Zafonte, R. Rehabilitation after traumatic brain injury. Handb. Clin. Neurol. 2015, 127, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Adil, S.M.; Elahi, C.; Patel, D.N.; Seas, A.; Warman, P.I.; Fuller, A.T.; Haglund, M.M.; Dunn, T.W. Deep Learning to Predict Traumatic Brain Injury Outcomes in the Low-Resource Setting. World Neurosurg. 2022, 164, e8–e16. [Google Scholar] [CrossRef] [PubMed]
- Peterson, A.B.; Zhou, H.; Thomas, K.E. Disparities in traumatic brain injury-related deaths—United States, 2020. J. Saf. Res. 2022, 83, 419–426. [Google Scholar] [CrossRef]
- Coronado, V.G.; McGuire, L.C.; Sarmiento, K.; Bell, J.; Lionbarger, M.R.; Jones, C.D.; Geller, A.I.; Khoury, N.; Xu, L. Trends in Traumatic Brain Injury in the U.S. and the public health response: 1995–2009. J. Saf. Res. 2012, 43, 299–307. [Google Scholar] [CrossRef]
- Sussman, E.S.; Pendharkar, A.V.; Ho, A.L.; Ghajar, J. Mild traumatic brain injury and concussion: Terminology and classification. Handb. Clin. Neurol. 2018, 158, 21–24. [Google Scholar] [CrossRef]
- Ownbey, M.R.; Pekari, T.B. Acute Mild Traumatic Brain Injury Assessment and Management in the Austere Setting—A Review. Mil. Med. 2022, 187, e47–e51. [Google Scholar] [CrossRef]
- Heslot, C.; Cogné, M.; Guillouët, E.; Perdrieau, V.; Lefevre-Dognin, C.; Glize, B.; Bonan, I.; Azouvi, P. Management of unfavorable outcome after mild traumatic brain injury: Review of physical and cognitive rehabilitation and of psychological care in post-concussive syndrome. Neurochirurgie 2021, 67, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Heslot, C.; Azouvi, P.; Perdrieau, V.; Granger, A.; Lefèvre-Dognin, C.; Cogné, M. A Systematic Review of Treatments of Post-Concussion Symptoms. J. Clin. Med. 2022, 11, 6224. [Google Scholar] [CrossRef] [PubMed]
- Tessler, J.; Horn, L.J. Posttraumatic Headache. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: http://www.ncbi.nlm.nih.gov/books/NBK556134/ (accessed on 24 January 2025).
- Gong, C.; Hu, H.; Peng, X.-M.; Li, H.; Xiao, L.; Liu, Z.; Zhong, Y.-B.; Wang, M.-Y.; Luo, Y. Therapeutic Effects of Repetitive Transcranial Magnetic Stimulation on Cognitive Impairment in Stroke Patients: A Systematic Review and Meta-Analysis. Front. Hum. Neurosci. 2023, 17, 1177594. [Google Scholar] [CrossRef]
- Patel, R.; Silla, F.; Pierce, S.; Theule, J.; Girard, T.A. Cognitive functioning before and after repetitive transcranial magnetic stimulation (rTMS): A quantitative meta-analysis in healthy adults. Neuropsychologia 2020, 141, 107395. [Google Scholar] [CrossRef] [PubMed]
- Lucke-Wold, B.P.; Logsdon, A.F.; Nguyen, L.; Eltanahay, A.; Turner, R.C.; Bonasso, P.; Knotts, C.; Moeck, A.; Maroon, J.C.; Bailes, J.E.; et al. Supplements, nutrition, and alternative therapies for the treatment of traumatic brain injury. Nutr. Neurosci. 2018, 21, 79–91. [Google Scholar] [CrossRef]
- Horn, S.D.; Corrigan, J.D.; Beaulieu, C.L.; Bogner, J.; Barrett, R.S.; Giuffrida, C.G.; Ryser, D.K.; Cooper, K.; Carroll, D.M.; Deutscher, D. Traumatic Brain Injury Patient, Injury, Therapy, and Ancillary Treatments Associated with Outcomes at Discharge and 9 Months Postdischarge. Arch. Phys. Med. Rehabil. 2015, 96, S304–S329. [Google Scholar] [CrossRef]
- Ponsford, J.L.; Downing, M.G.; Olver, J.; Ponsford, M.; Acher, R.; Carty, M.; Spitz, G. Longitudinal Follow-Up of Patients with Traumatic Brain Injury: Outcome at Two, Five, and Ten Years Post-Injury. J. Neurotrauma 2014, 31, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Schwab, K.A.; Gudmudsson, L.S.; Lew, H.L. Chapter 40—Long-Term Functional Outcomes of Traumatic Brain Injury. In Handbook of Clinical Neurology; Grafman, J., Salazar, A.M., Eds.; Traumatic Brain Injury, Part II; Elsevier: Amsterdam, The Netherlands, 2015; Volume 128, pp. 649–659. Available online: https://www.sciencedirect.com/science/article/pii/B9780444635211000406 (accessed on 25 November 2024).
- Agoston, D.V.; Langford, D. Big Data in Traumatic Brain Injury; Promise and Challenges. Concussion 2017, 2, CNC45. [Google Scholar] [CrossRef]
- Kim, K.A.; Kim, H.; Ha, E.J.; Yoon, B.C.; Kim, D.-J. Artificial Intelligence-Enhanced Neurocritical Care for Traumatic Brain Injury: Past, Present and Future. J. Korean Neurosurg. Soc. 2024, 67, 493–509. [Google Scholar] [CrossRef]
- Hale, A.T.; Stonko, D.P.; Lim, J.; Guillamondegui, O.D.; Shannon, C.N.; Patel, M.B. Using an artificial neural network to predict traumatic brain injury. J. Neurosurg. Pediatr. 2019, 23, 219–226. [Google Scholar] [CrossRef]
- Tu, K.-C.; Eric Nyam, T.-T.; Wang, C.-C.; Chen, N.-C.; Chen, K.-T.; Chen, C.-J.; Liu, C.-F.; Kuo, J.-R. A Computer-Assisted System for Early Mortality Risk Prediction in Patients with Traumatic Brain Injury Using Artificial Intelligence Algorithms in Emergency Room Triage. Brain Sci. 2022, 12, 612. [Google Scholar] [CrossRef] [PubMed]
- Khalili, H.; Rismani, M.; Nematollahi, M.A.; Masoudi, M.S.; Asadollahi, A.; Taheri, R.; Pourmontaseri, H.; Valibeygi, A.; Roshanzamir, M.; Alizadehsani, R.; et al. Prognosis prediction in traumatic brain injury patients using machine learning algorithms. Sci. Rep. 2023, 13, 960. [Google Scholar] [CrossRef]
- Pease, M.; Arefan, D.; Barber, J.; Yuh, E.; Puccio, A.; Hochberger, K.; Nwachuku, E.; Roy, S.; Casillo, S.; Temkin, N.; et al. Outcome Prediction in Patients with Severe Traumatic Brain Injury Using Deep Learning from Head CT Scans. Radiology 2022, 304, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Raj, R.; Luostarinen, T.; Pursiainen, E.; Posti, J.P.; Takala, R.S.K.; Bendel, S.; Konttila, T.; Korja, M. Machine learning-based dynamic mortality prediction after traumatic brain injury. Sci. Rep. 2019, 9, 17672. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, J.; Liu, X.; Oliveira, H.P.; Pereira, T. Learning Models for Traumatic Brain Injury Mortality Prediction on Pediatric Electronic Health Records. Front. Neurol. 2022, 13, 859068. [Google Scholar] [CrossRef]
- Farzaneh, N.; Williamson, C.A.; Gryak, J.; Najarian, K. A hierarchical expert-guided machine learning framework for clinical decision support systems: An application to traumatic brain injury prognostication. npj Digit. Med. 2021, 4, 78. [Google Scholar] [CrossRef] [PubMed]
- Bark, D.; Boman, M.; Depreitere, B.; Wright, D.W.; Lewén, A.; Enblad, P.; Hånell, A.; Rostami, E. Refining outcome prediction after traumatic brain injury with machine learning algorithms. Sci. Rep. 2024, 14, 8036. [Google Scholar] [CrossRef] [PubMed]
- Hamet, P.; Tremblay, J. Artificial intelligence in medicine. Metabolism 2017, 69S, S36–S40. [Google Scholar] [CrossRef]
- Turing, A.M. Computing machinery and intelligence. Mind 1950, LIX, 433–460. [Google Scholar] [CrossRef]
- Cordeschi, R. Ai Turns Fifty: Revisiting Its Origins. Appl. Artif. Intell. 2007, 21, 259–279. [Google Scholar] [CrossRef]
- Miller, R.A.; Pople, H.E.; Myers, J.D. Internist-1, an experimental computer-based diagnostic consultant for general internal medicine. N. Engl. J. Med. 1982, 307, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Shortliffe, E.H. Mycin: A Knowledge-Based Computer Program Applied to Infectious Diseases. In Proceedings of the Annual Symposium on Computer Application in Medical Care, Washington, DC, USA, 3–5 October 1977; pp. 66–69. [Google Scholar]
- Trivedi, M.C. A Classical Approach to Artificial Intelligence; Khanna Publishing House: Delhi, India, 2014; 540p, ISBN 978-81-906988-9-4. [Google Scholar]
- The Laboratory of Computer Science. The Laboratory of Computer Science | DXplain. Available online: http://www.mghlcs.org/projects/dxplain (accessed on 25 November 2024).
- Ferrucci, D.; Levas, A.; Bagchi, S.; Gondek, D.; Mueller, E.T. Watson: Beyond Jeopardy! Artif. Intell. 2013, 199–200, 93–105. [Google Scholar] [CrossRef]
- Mintz, Y.; Brodie, R. Introduction to artificial intelligence in medicine. Minim. Invasive Ther. Allied Technol. 2019, 28, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Bakkar, N.; Kovalik, T.; Lorenzini, I.; Spangler, S.; Lacoste, A.; Sponaugle, K.; Ferrante, P.; Argentinis, E.; Sattler, R.; Bowser, R. Artificial intelligence in neurodegenerative disease research: Use of IBM Watson to identify additional RNA-binding proteins altered in amyotrophic lateral sclerosis. Acta Neuropathol. 2018, 135, 227–247. [Google Scholar] [CrossRef] [PubMed]
- Castellino, R.A. Computer aided detection (CAD): An overview. Cancer Imaging 2005, 5, 17–19. [Google Scholar] [CrossRef]
- Ghafoorian, M.; Karssemeijer, N.; Heskes, T.; van Uden, I.W.M.; Sanchez, C.I.; Litjens, G.; de Leeuw, F.-E.; van Ginneken, B.; Marchiori, E.; Platel, B. Location Sensitive Deep Convolutional Neural Networks for Segmentation of White Matter Hyperintensities. Sci. Rep. 2017, 7, 5110. [Google Scholar] [CrossRef]
- Chan, S.; Reddy, V.; Myers, B.; Thibodeaux, Q.; Brownstone, N.; Liao, W. Machine Learning in Dermatology: Current Applications, Opportunities, and Limitations. Dermatol. Ther. 2020, 10, 365–386. [Google Scholar] [CrossRef]
- Chen, S.C.; Bravata, D.M.; Weil, E.; Olkin, I. A comparison of dermatologists’ and primary care physicians’ accuracy in diagnosing melanoma: A systematic review. Arch. Dermatol. 2001, 137, 1627–1634. [Google Scholar] [CrossRef]
- Habehh, H.; Gohel, S. Machine Learning in Healthcare. Curr. Genom. 2021, 22, 291–300. [Google Scholar] [CrossRef]
- Hirani, R.; Noruzi, K.; Khuram, H.; Hussaini, A.S.; Aifuwa, E.I.; Ely, K.E.; Lewis, J.M.; Gabr, A.E.; Smiley, A.; Tiwari, R.K.; et al. Artificial Intelligence and Healthcare: A Journey through History, Present Innovations, and Future Possibilities. Life 2024, 14, 557. [Google Scholar] [CrossRef]
- Vinny, P.W.; Vishnu, V.Y.; Padma Srivastava, M.V. Artificial Intelligence shaping the future of neurology practice. Med. J. Armed Forces India 2021, 77, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Chilamkurthy, S.; Ghosh, R.; Tanamala, S.; Biviji, M.; Campeau, N.G.; Venugopal, V.K.; Mahajan, V.; Rao, P.; Warier, P. Deep learning algorithms for detection of critical findings in head CT scans: A retrospective study. Lancet 2018, 392, 2388–2396. [Google Scholar] [CrossRef]
- Arbabshirani, M.R.; Fornwalt, B.K.; Mongelluzzo, G.J.; Suever, J.D.; Geise, B.D.; Patel, A.A.; Moore, G.J. Advanced machine learning in action: Identification of intracranial hemorrhage on computed tomography scans of the head with clinical workflow integration. NPJ Digit. Med. 2018, 1, 9. [Google Scholar] [CrossRef] [PubMed]
- Dewan, M.C.; Rattani, A.; Gupta, S.; Baticulon, R.E.; Hung, Y.-C.; Punchak, M.; Agrawal, A.; Adeleye, A.O.; Shrime, M.G.; Rubiano, A.M.; et al. Estimating the global incidence of traumatic brain injury. J. Neurosurg. 2019, 130, 1080–1097. [Google Scholar] [CrossRef] [PubMed]
- National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Health Care Services; Committee on the Review of the Department of Veterans Affairs Examinations for Traumatic Brain Injury. Evaluation of the Disability Determination Process for Traumatic Brain Injury in Veterans; National Academies Press: Washington, DC, USA, 2019; ISBN 978-0-309-48686-6. Available online: http://www.ncbi.nlm.nih.gov/books/NBK542602/ (accessed on 26 November 2024).
- Diaz-Arrastia, R.; Wang, K.K.W.; Papa, L.; Sorani, M.D.; Yue, J.K.; Puccio, A.M.; McMahon, P.J.; Inoue, T.; Yuh, E.L.; Lingsma, H.F.; et al. Acute biomarkers of traumatic brain injury: Relationship between plasma levels of ubiquitin C-terminal hydrolase-L1 and glial fibrillary acidic protein. J. Neurotrauma 2014, 31, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Maas, A.I.R.; Stocchetti, N.; Bullock, R. Moderate and severe traumatic brain injury in adults. Lancet Neurol. 2008, 7, 728–741. [Google Scholar] [CrossRef] [PubMed]
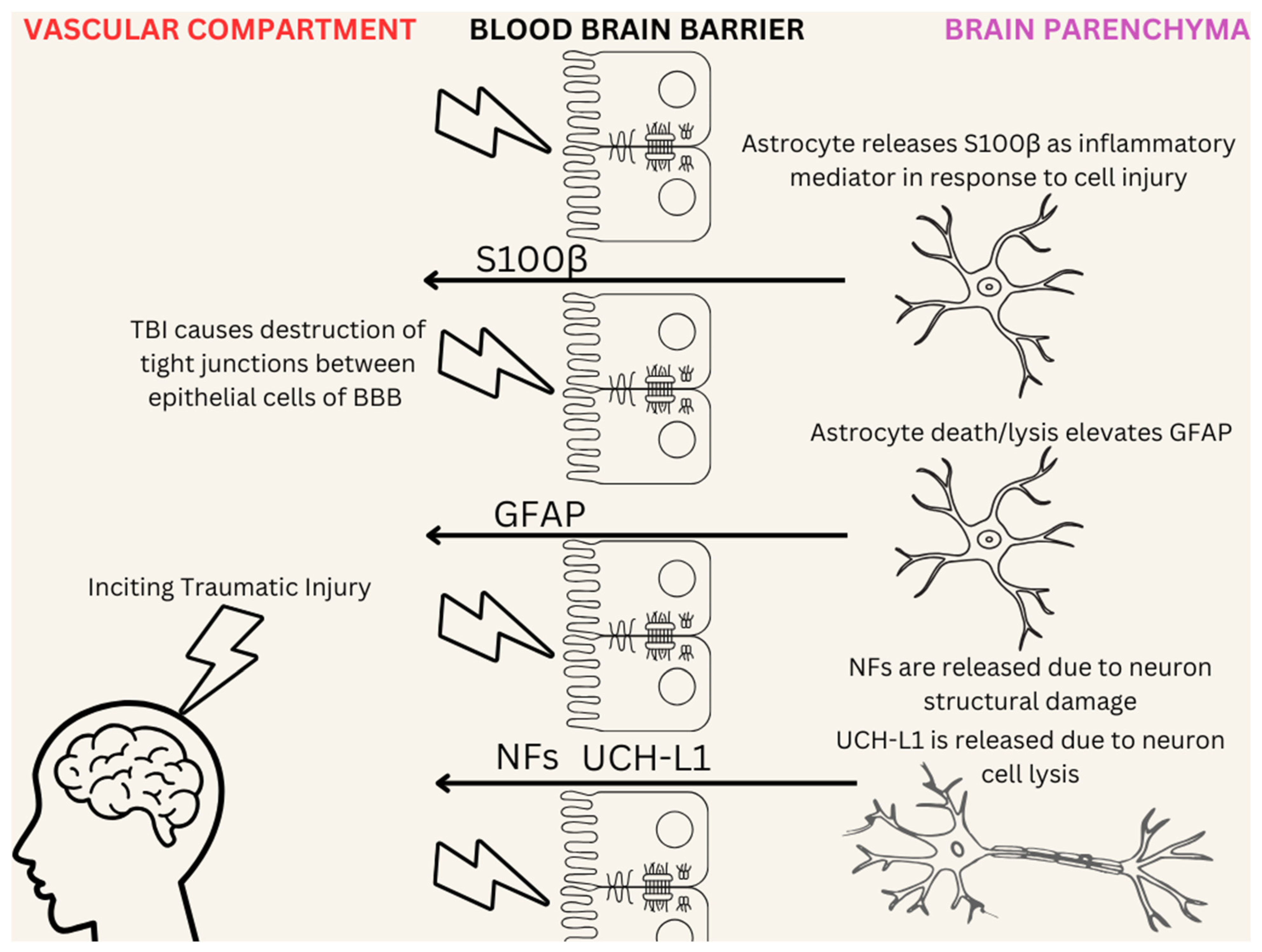
- Najem, D.; Rennie, K.; Ribecco-Lutkiewicz, M.; Ly, D.; Haukenfrers, J.; Liu, Q.; Nzau, M.; Fraser, D.D.; Bani-Yaghoub, M. Traumatic brain injury: Classification, models, and markers. Biochem. Cell Biol. 2018, 96, 391–406. [Google Scholar] [CrossRef]
- Tomaiuolo, R.; Zibetti, M.; Di Resta, C.; Banfi, G. Challenges of the Effectiveness of Traumatic Brain Injuries Biomarkers in the Sports-Related Context. J. Clin. Med. 2023, 12, 2563. [Google Scholar] [CrossRef]
- Ghaith, H.S.; Nawar, A.A.; Gabra, M.D.; Abdelrahman, M.E.; Nafady, M.H.; Bahbah, E.I.; Ebada, M.A.; Ashraf, G.M.; Negida, A.; Barreto, G.E. A Literature Review of Traumatic Brain Injury Biomarkers. Mol. Neurobiol. 2022, 59, 4141–4158. [Google Scholar] [CrossRef]
- Reyes, J.; Spitz, G.; Major, B.P.; O’Brien, W.T.; Giesler, L.P.; Bain, J.W.P.; Xie, B.; Rosenfeld, J.V.; Law, M.; Ponsford, J.L.; et al. Utility of Acute and Subacute Blood Biomarkers to Assist Diagnosis in CT-Negative Isolated Mild Traumatic Brain Injury. Neurology 2023, 101, e1992–e2004. [Google Scholar] [CrossRef]
- Amoo, M.; Henry, J.; O’Halloran, P.J.; Brennan, P.; Husien, M.B.; Campbell, M.; Caird, J.; Javadpour, M.; Curley, G.F. S100B, GFAP, UCH-L1 and NSE as predictors of abnormalities on CT imaging following mild traumatic brain injury: A systematic review and meta-analysis of diagnostic test accuracy. Neurosurg. Rev. 2022, 45, 1171–1193. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Puvenna, V.; Janigro, D. Biomarkers of Traumatic Brain Injury and Their Relationship to Pathology. In Translational Research in Traumatic Brain Injury; Laskowitz, D., Grant, G., Eds.; Frontiers in Neuroscience; CRC Press: Boca Raton, FL, USA; Taylor and Francis Group: Boca Raton, FL, USA, 2016; ISBN 978-1-4665-8491-4. Available online: http://www.ncbi.nlm.nih.gov/books/NBK326724/ (accessed on 26 November 2024).
- Visser, K.; Koggel, M.; Blaauw, J.; van der Horn, H.J.; Jacobs, B.; van der Naalt, J. Blood-based biomarkers of inflammation in mild traumatic brain injury: A systematic review. Neurosci. Biobehav. Rev. 2022, 132, 154–168. [Google Scholar] [CrossRef] [PubMed]
- Marchi, N.; Cavaglia, M.; Fazio, V.; Bhudia, S.; Hallene, K.; Janigro, D. Peripheral markers of blood-brain barrier damage. Clin. Chim. Acta 2004, 342, 1–12. [Google Scholar] [CrossRef]
- Lin, E.; Yuh, E.L. Computational Approaches for Acute Traumatic Brain Injury Image Recognition. Front. Neurol. 2022, 13, 791816. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.G.F.; Milliron, E.; Ho, M.-L.; Hu, H.H.; Rusin, J.; Leonard, J.; Sribnick, E.A. Advanced neuroimaging in traumatic brain injury: An overview. Neurosurg. Focus 2019, 47, E17. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Utriainen, D.; Chai, C.; Chen, Y.; Wang, L.; Sethi, S.K.; Xia, S.; Haacke, E.M. Cerebral microbleed detection using Susceptibility Weighted Imaging and deep learning. Neuroimage 2019, 198, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Bullock, M.R.; Chesnut, R.; Ghajar, J.; Gordon, D.; Hartl, R.; Newell, D.W.; Servadei, F.; Walters, B.C.; Wilberger, J.E.; Surgical Management of Traumatic Brain Injury Author Group. Surgical management of acute subdural hematomas. Neurosurgery 2006, 58, S16–S24; discussion Si-iv. [Google Scholar] [CrossRef]
- Yao, H.; Williamson, C.; Soroushmehr, R.; Gryak, J.; Najarian, K. Hematoma Segmentation Using Dilated Convolutional Neural Network. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; Volume 2018, pp. 5902–5905. [Google Scholar] [CrossRef]
- Kerley, C.I.; Huo, Y.; Chaganti, S.; Bao, S.; Patel, M.B.; Landman, B.A. Montage based 3D Medical Image Retrieval from Traumatic Brain Injury Cohort using Deep Convolutional Neural Network. Proc. SPIE Int. Soc. Opt. Eng. 2019, 10949, 109492U. [Google Scholar] [CrossRef]
- Agrawal, D.; Joshi, S.; Bahel, V.; Poonamallee, L.; Agrawal, A. Three dimensional convolutional neural network-based automated detection of midline shift in traumatic brain injury cases from head computed tomography scans. J. Neurosci. Rural. Pract. 2024, 15, 293–299. [Google Scholar] [CrossRef]
- Chen, W.; Belle, A.; Cockrell, C.; Ward, K.R.; Najarian, K. Automated midline shift and intracranial pressure estimation based on brain CT images. J. Vis. Exp. 2013, 3871. [Google Scholar] [CrossRef]
- Courville, E.; Kazim, S.F.; Vellek, J.; Tarawneh, O.; Stack, J.; Roster, K.; Roy, J.; Schmidt, M.; Bowers, C. Machine learning algorithms for predicting outcomes of traumatic brain injury: A systematic review and meta-analysis. Surg. Neurol. Int. 2023, 14, 262. [Google Scholar] [CrossRef] [PubMed]
- Iderdar, Y.; Arraji, M.; Wachami, N.A.; Guennouni, M.; Boumendil, K.; Mourajid, Y.; Elkhoudri, N.; Saad, E.; Chahboune, M. Predictors of outcomes 3 to 12 months after traumatic brain injury: A systematic review and meta-analysis. Osong Public Health Res. Perspect. 2024, 15, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Zarei, H.; Vazirizadeh-Mahabadi, M.; Adel Ramawad, H.; Sarveazad, A.; Yousefifard, M. Prognostic Value of CRASH and IMPACT Models for Predicting Mortality and Unfavorable Outcome in Traumatic Brain Injury; a Systematic Review and Meta-Analysis. Arch. Acad. Emerg. Med. 2023, 11, e27. [Google Scholar] [CrossRef] [PubMed]
- MRC CRASH Trial Collaborators; Perel, P.; Arango, M.; Clayton, T.; Edwards, P.; Komolafe, E.; Poccock, S.; Roberts, I.; Shakur, H.; Steyerberg, E.; et al. Predicting outcome after traumatic brain injury: Practical prognostic models based on large cohort of international patients. BMJ 2008, 336, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Roozenbeek, B.; Lingsma, H.F.; Lecky, F.E.; Lu, J.; Weir, J.; Butcher, I.; McHugh, G.S.; Murray, G.D.; Perel, P.; Maas, A.I.; et al. Prediction of outcome after moderate and severe traumatic brain injury: External validation of the International Mission on Prognosis and Analysis of Clinical Trials (IMPACT) and Corticoid Randomisation After Significant Head injury (CRASH) prognostic models. Crit. Care Med. 2012, 40, 1609–1617. [Google Scholar] [CrossRef]
- IMPACT Database Table | TBI-IMPACT.org. Available online: http://www.tbi-impact.org/?p=impact/dbtable (accessed on 26 November 2024).
- Steyerberg, E.W.; Mushkudiani, N.; Perel, P.; Butcher, I.; Lu, J.; McHugh, G.S.; Murray, G.D.; Marmarou, A.; Roberts, I.; Habbema, J.D.F.; et al. Predicting outcome after traumatic brain injury: Development and international validation of prognostic scores based on admission characteristics. PLoS Med. 2008, 5, e165; discussion e165. [Google Scholar] [CrossRef] [PubMed]
- Roozenbeek, B.; Chiu, Y.-L.; Lingsma, H.F.; Gerber, L.M.; Steyerberg, E.W.; Ghajar, J.; Maas, A.I.R. Predicting 14-day mortality after severe traumatic brain injury: Application of the IMPACT models in the brain trauma foundation TBI-trac® New York State database. J. Neurotrauma 2012, 29, 1306–1312. [Google Scholar] [CrossRef]
- Eagle, S.R.; Nwachuku, E.; Elmer, J.; Deng, H.; Okonkwo, D.O.; Pease, M. Performance of CRASH and IMPACT Prognostic Models for Traumatic Brain Injury at 12 and 24 Months Post-Injury. Neurotrauma Rep. 2023, 4, 118–123. [Google Scholar] [CrossRef]
- Say, I.; Chen, Y.E.; Sun, M.Z.; Li, J.J.; Lu, D.C. Machine learning predicts improvement of functional outcomes in traumatic brain injury patients after inpatient rehabilitation. Front. Rehabil. Sci. 2022, 3, 1005168. [Google Scholar] [CrossRef]
- Linacre, J.M.; Heinemann, A.W.; Wright, B.D.; Granger, C.V.; Hamilton, B.B. The structure and stability of the Functional Independence Measure. Arch. Phys. Med. Rehabil. 1994, 75, 127–132. [Google Scholar] [CrossRef]
- Ottenbacher, K.J.; Hsu, Y.; Granger, C.V.; Fiedler, R.C. The reliability of the functional independence measure: A quantitative review. Arch. Phys. Med. Rehabil. 1996, 77, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- Satyadev, N.; Warman, P.I.; Seas, A.; Kolls, B.J.; Haglund, M.M.; Fuller, A.T.; Dunn, T.W. Machine Learning for Predicting Discharge Disposition After Traumatic Brain Injury. Neurosurgery 2022, 90, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Abujaber, A.; Fadlalla, A.; Gammoh, D.; Abdelrahman, H.; Mollazehi, M.; El-Menyar, A. Prediction of in-hospital mortality in patients on mechanical ventilation post traumatic brain injury: Machine learning approach. BMC Med. Inform. Decis. Mak. 2020, 20, 336. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.-D.; Chao, E.; Chen, S.-J.; Hueng, D.-Y.; Lan, H.-Y.; Chiang, H.-H. Machine Learning Algorithms to Predict In-Hospital Mortality in Patients with Traumatic Brain Injury. J. Pers. Med. 2021, 11, 1144. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Guo, M.; Wang, Z.; Liu, H.; Bai, X.; Cui, S.; Guo, X.; Gao, L.; Gao, L.; Liao, A.; et al. Predictive model for early functional outcomes following acute care after traumatic brain injuries: A machine learning-based development and validation study. Injury 2023, 54, 896–903. [Google Scholar] [CrossRef]
- Christodoulou, E.; Ma, J.; Collins, G.S.; Steyerberg, E.W.; Verbakel, J.Y.; Van Calster, B. A systematic review shows no performance benefit of machine learning over logistic regression for clinical prediction models. J. Clin. Epidemiol. 2019, 110, 12–22. [Google Scholar] [CrossRef] [PubMed]
- University Hospital, Grenoble. Artificial Intelligence Analysis of Initial Scan Evolution of Traumatic Brain Injured Patient to Predict Neurological Outcome: Pilot Translational an Exploratory Study; Report No.: NCT04058379; ClinicalTrials.gov: Bethesda, MD, USA, 2021. Available online: https://clinicaltrials.gov/study/NCT04058379 (accessed on 26 November 2024).
- Rajaei, F.; Cheng, S.; Williamson, C.A.; Wittrup, E.; Najarian, K. AI-Based Decision Support System for Traumatic Brain Injury: A Survey. Diagnostics 2023, 13, 1640. [Google Scholar] [CrossRef]
- Guy’s and St Thomas’ NHS Foundation Trust. A Mixed Methods Study to Assess the Clinical Effectiveness and Acceptability of qER Artificial Intelligence Software to Prioritise CT Head Interpretation; Report No.: NCT06027411; ClinicalTrials.gov: Bethesda, MD, USA, 2024. Available online: https://clinicaltrials.gov/study/NCT06027411 (accessed on 26 November 2024).
- Agrawal, D.; Joshi, S.; Poonamallee, L. Automated Midline Shift Detection and Quantification in Traumatic Brain Injury: A Comprehensive Review. Indian J. Neurotrauma 2024, 21, 6–12. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, W.; Qu, Y.; Feng, C.; Wang, D.; Yin, H.; Li, C.; Sun, Z.; Sun, D. Development and Application of Medicine-Engineering Integration in the Rehabilitation of Traumatic Brain Injury. Biomed. Res. Int. 2021, 2021, 9962905. [Google Scholar] [CrossRef]
- Understanding Microelectromechanical System (MEMS) Sensors—Technical Articles. Available online: https://control.com/technical-articles/understanding-microelectromechanical-system-mems-sensors/ (accessed on 23 January 2025).
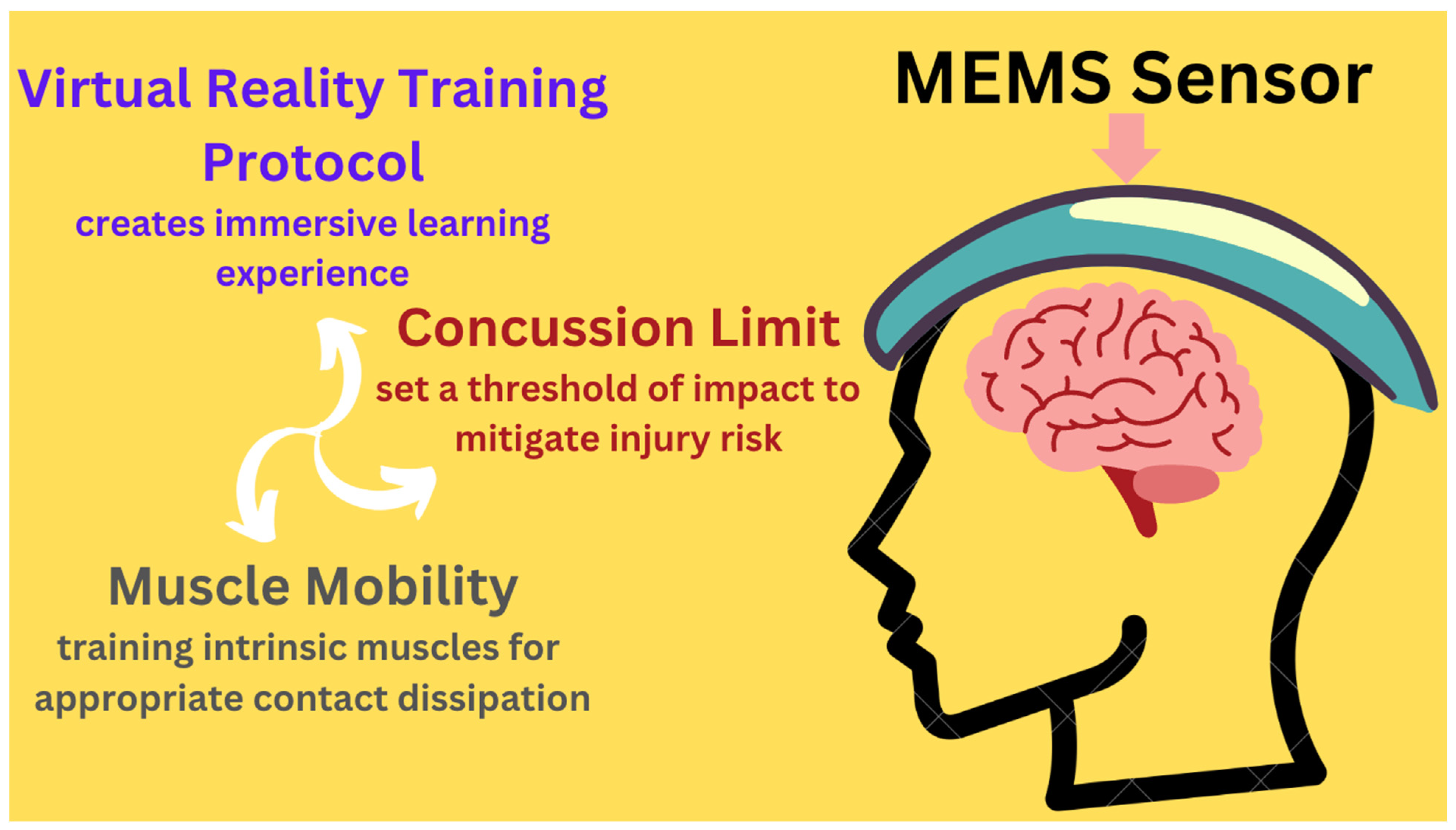
- Huang, C.-M. Detection and Prevention of Concussive Injuries with Smart Technology; Report No.: NCT04946747; ClinicalTrials.gov: Bethesda, MD, USA, 2021. Available online: https://clinicaltrials.gov/study/NCT04946747 (accessed on 26 November 2024).
- Sheridan, D.C.; Pettersson, D.; Newgard, C.D.; Selden, N.R.; Jafri, M.A.; Lin, A.; Rowell, S.; Hansen, M.L. Can QuickBrain MRI replace CT as first-line imaging for select pediatric head trauma? J. Am. Coll. Emerg. Physicians Open 2020, 1, 965–973. [Google Scholar] [CrossRef]
- Eagle, S.R.; Pease, M.; Nwachuku, E.; Deng, H.; Okonkwo, D.O. Prognostic Models for Traumatic Brain Injury Have Good Discrimination but Poor Overall Model Performance for Predicting Mortality and Unfavorable Outcomes. Neurosurgery 2023, 92, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Ran, K.R.; Azad, T.D. Letter: Prognostic Models for Traumatic Brain Injury Have Good Discrimination But Poor Overall Model Performance for Predicting Mortality and Unfavorable Outcomes. Neurosurgery 2023, 92, e69. [Google Scholar] [CrossRef] [PubMed]
- Bryant, A.M.; Rose, N.B.; Temkin, N.R.; Barber, J.K.; Manley, G.T.; McCrea, M.A.; Nelson, L.D.; TRACK-TBI Investigators; Badjatia, N.; Gopinath, S.; et al. Profiles of Cognitive Functioning at 6 Months After Traumatic Brain Injury Among Patients in Level I Trauma Centers: A TRACK-TBI Study. JAMA Netw. Open 2023, 6, e2349118. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.-C.; Liu, C.-J.; Huang, H.-C.; Lin, J.-H.; Chen, P.-Y.; Su, Y.-K.; Chen, C.-T.; Chiu, H.-Y. A Meta-analysis of Dynamic Prevalence of Cognitive Deficits in the Acute, Subacute, and Chronic Phases After Traumatic Brain Injury. J. Neurosci. Nurs. 2021, 53, 63. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.; Cornwell, P.; Hewetson, R.; Copley, A. The pervasive and unyielding impacts of cognitive-communication changes following traumatic brain injury. Int. J. Lang. Commun. Disord. 2023, 58, 2131–2143. [Google Scholar] [CrossRef]
- Togher, L.; Douglas, J.; Turkstra, L.S.; Welch-West, P.; Janzen, S.; Harnett, A.; Kennedy, M.; Kua, A.; Patsakos, E.; Ponsford, J.; et al. INCOG 2.0 Guidelines for Cognitive Rehabilitation Following Traumatic Brain Injury, Part IV: Cognitive-Communication and Social Cognition Disorders. J. Head. Trauma. Rehabil. 2023, 38, 65–82. [Google Scholar] [CrossRef]
- Physiopedia [Internet]. Traumatic Brain Injury Clinical Guidelines. Available online: https://www.physio-pedia.com/Traumatic_Brain_Injury_Clinical_Guidelines (accessed on 1 March 2025).
- Walker, W.C.; Pickett, T.C. Motor impairment after severe traumatic brain injury: A longitudinal multicenter study. J. Rehabil. Res. Dev. 2007, 44, 975–982. [Google Scholar] [CrossRef]
- Snowden, T.; Morrison, J.; Boerstra, M.; Eyolfson, E.; Acosta, C.; Grafe, E.; Reid, H.; Brand, J.; Galati, M.; Gargaro, J.; et al. Brain Changes: Aerobic Exercise for Traumatic Brain Injury Rehabilitation. Front. Hum. Neurosci. 2023, 17, 1307507. [Google Scholar] [CrossRef]
- Johnson, L.; Williams, G.; Sherrington, C.; Pilli, K.; Chagpar, S.; Auchettl, A.; Beard, J.; Gill, R.; Vassallo, G.; Rushworth, N.; et al. The effect of physical activity on health outcomes in people with moderate-to-severe traumatic brain injury: A rapid systematic review with meta-analysis. BMC Public Health 2023, 23, 63. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.-C.; Li, S.-F.; Sun, P.; Bai, G.-T.; Wang, N.; Han, C.; Sun, J.; Li, Y.; Li, H.-T. Early Intensive Rehabilitation for Patients with Traumatic Brain Injury: A Prospective Pilot Trial. World Neurosurg. 2020, 137, e183–e188. [Google Scholar] [CrossRef] [PubMed]
- Tiersky, L.A.; Anselmi, V.; Johnston, M.V.; Kurtyka, J.; Roosen, E.; Schwartz, T.; Deluca, J. A trial of neuropsychologic rehabilitation in mild-spectrum traumatic brain injury. Arch. Phys. Med. Rehabil. 2005, 86, 1565–1574. [Google Scholar] [CrossRef] [PubMed]
- Tornås, S.; Løvstad, M.; Solbakk, A.-K.; Schanke, A.-K.; Stubberud, J. Goal Management Training Combined with External Cuing as a Means to Improve Emotional Regulation, Psychological Functioning, and Quality of Life in Patients with Acquired Brain Injury: A Randomized Controlled Trial. Arch. Phys. Med. Rehabil. 2016, 97, 1841–1852.e3. [Google Scholar] [CrossRef] [PubMed]
- Kreitzer, N.; Kurowski, B.G.; Bakas, T. Systematic Review of Caregiver and Dyad Interventions After Adult Traumatic Brain Injury. Arch. Phys. Med. Rehabil. 2018, 99, 2342–2354. [Google Scholar] [CrossRef]
- Powell, J.M.; Wise, E.K.; Brockway, J.A.; Fraser, R.; Temkin, N.; Bell, K.R. Characteristics and Concerns of Caregivers of Adults with Traumatic Brain Injury. J. Head. Trauma. Rehabil. 2017, 32, E33. [Google Scholar] [CrossRef] [PubMed]
- Triebel, K.L.; Martin, R.C.; Novack, T.A.; Dreer, L.; Turner, C.; Pritchard, P.R.; Raman, R.; Marson, D.C. Treatment consent capacity in patients with traumatic brain injury across a range of injury severity. Neurology 2012, 78, 1472–1478. [Google Scholar] [CrossRef] [PubMed]
- Honeybul, S.; Gillett, G.; Ho, K.; Lind, C. Ethical considerations for performing decompressive craniectomy as a life-saving intervention for severe traumatic brain injury. J. Med. Ethics 2012, 38, 657–661. [Google Scholar] [CrossRef]
- Kjeldgaard, A.; Soendergaard, P.L.; Wolffbrandt, M.M.; Norup, A. Predictors of caregiver burden in caregivers of individuals with traumatic or non-traumatic brain injury: A scoping review. NeuroRehabilitation 2023, 52, 9–28. [Google Scholar] [CrossRef]
- Niemeier, J.P.; Kreutzer, J.S.; Marwitz, J.H.; Sima, A.P. A Randomized Controlled Pilot Study of a Manualized Intervention for Caregivers of Patients with Traumatic Brain Injury in Inpatient Rehabilitation. Arch. Phys. Med. Rehabil. 2019, 100, S65–S75. [Google Scholar] [CrossRef]
- Youssef, A.; Ng, M.Y.; Long, J.; Hernandez-Boussard, T.; Shah, N.; Miner, A.; Larson, D.; Langlotz, C.P. Organizational Factors in Clinical Data Sharing for Artificial Intelligence in Health Care. JAMA Netw. Open 2023, 6, e2348422. [Google Scholar] [CrossRef]
- Daneshjou, R.; Smith, M.P.; Sun, M.D.; Rotemberg, V.; Zou, J. Lack of Transparency and Potential Bias in Artificial Intelligence Data Sets and Algorithms: A Scoping Review. JAMA Dermatol. 2021, 157, 1362–1369. [Google Scholar] [CrossRef]
- Flores, L.; Kim, S.; Young, S.D. Addressing bias in artificial intelligence for public health surveillance. J. Med. Ethics 2024, 50, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Aquino, Y.S.J.; Carter, S.M.; Houssami, N.; Braunack-Mayer, A.; Win, K.T.; Degeling, C.; Wang, L.; Rogers, W.A. Practical, epistemic and normative implications of algorithmic bias in healthcare artificial intelligence: A qualitative study of multidisciplinary expert perspectives. J. Med. Ethics 2023, jme-2022-108850. [Google Scholar] [CrossRef]
- Chen, R.J.; Wang, J.J.; Williamson, D.F.K.; Chen, T.Y.; Lipkova, J.; Lu, M.Y.; Sahai, S.; Mahmood, F. Algorithmic fairness in artificial intelligence for medicine and healthcare. Nat. Biomed. Eng. 2023, 7, 719–742. [Google Scholar] [CrossRef] [PubMed]
- Mollura, D.J.; Culp, M.P.; Pollack, E.; Battino, G.; Scheel, J.R.; Mango, V.L.; Elahi, A.; Schweitzer, A.; Dako, F. Artificial Intelligence in Low- and Middle-Income Countries: Innovating Global Health Radiology. Radiology 2020, 297, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Whyte, J.; Vasterling, J.; Manley, G.T. Common data elements for research on traumatic brain injury and psychological health: Current status and future development. Arch. Phys. Med. Rehabil. 2010, 91, 1692–1696. [Google Scholar] [CrossRef] [PubMed]
- Thurmond, V.A.; Hicks, R.; Gleason, T.; Miller, A.C.; Szuflita, N.; Orman, J.; Schwab, K. Advancing integrated research in psychological health and traumatic brain injury: Common data elements. Arch. Phys. Med. Rehabil. 2010, 91, 1633–1636. [Google Scholar] [CrossRef]
- Yaseen, A.; Robertson, C.; Cruz Navarro, J.; Chen, J.; Heckler, B.; DeSantis, S.M.; Temkin, N.; Barber, J.; Foreman, B.; Diaz-Arrastia, R.; et al. Integrating, Harmonizing, and Curating Studies with High-Frequency and Hourly Physiological Data: Proof of Concept from Seven Traumatic Brain Injury Data Sets. J. Neurotrauma 2023, 40, 2362–2375. [Google Scholar] [CrossRef]
- Beard, K.; Pennington, A.M.; Gauff, A.K.; Mitchell, K.; Smith, J.; Marion, D.W. Potential Applications and Ethical Considerations for Artificial Intelligence in Traumatic Brain Injury Management. Biomedicines 2024, 12, 2459. [Google Scholar] [CrossRef]
- Young, A.T.; Amara, D.; Bhattacharya, A.; Wei, M.L. Patient and general public attitudes towards clinical artificial intelligence: A mixed methods systematic review. Lancet Digit. Health 2021, 3, e599–e611. [Google Scholar] [CrossRef]
- Rojahn, J.; Palu, A.; Skiena, S.; Jones, J.J. American public opinion on artificial intelligence in healthcare. PLoS ONE 2023, 18, e0294028. [Google Scholar] [CrossRef]
- Uparela-Reyes, M.J.; Villegas-Trujillo, L.M.; Cespedes, J.; Velásquez-Vera, M.; Rubiano, A.M. Usefulness of Artificial Intelligence in Traumatic Brain Injury: A Bibliometric Analysis and Mini-review. World Neurosurg. 2024, 188, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Rughani, A.I.; Dumont, T.M.; Lu, Z.; Bongard, J.; Horgan, M.A.; Penar, P.L.; Tranmer, B.I. Use of an artificial neural network to predict head injury outcome. J. Neurosurg. 2010, 113, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.S.; Samuelkamaleshkumar, S.; Viswanathan, A.; Macaden, A.S. Cognitive rehabilitation for adults with traumatic brain injury to improve occupational outcomes. Cochrane Database Syst. Rev. 2017, 6, CD007935. [Google Scholar] [CrossRef]
- Newcombe, V.; Richter, S.; Whitehouse, D.P.; Bloom, B.M.; Lecky, F. Fluid biomarkers and neuroimaging in mild traumatic brain injury: Current uses and potential future directions for clinical use in emergency medicine. Emerg. Med. J. 2023, 40, 671–677. [Google Scholar] [CrossRef]
- Juhn, Y.; Liu, H. Artificial intelligence approaches using natural language processing to advance EHR-based clinical research. J. Allergy Clin. Immunol. 2020, 145, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Vélez-Guerrero, M.A.; Callejas-Cuervo, M.; Mazzoleni, S. Artificial Intelligence-Based Wearable Robotic Exoskeletons for Upper Limb Rehabilitation: A Review. Sensors 2021, 21, 2146. [Google Scholar] [CrossRef]
- Wen, D.; Fan, Y.; Hsu, S.-H.; Xu, J.; Zhou, Y.; Tao, J.; Lan, X.; Li, F. Combining brain-computer interface and virtual reality for rehabilitation in neurological diseases: A narrative review. Ann. Phys. Rehabil. Med. 2021, 64, 101404. [Google Scholar] [CrossRef]
- Onakomaiya, M.M.; Kruger, S.E.; Highland, K.B.; Kodosky, P.N.; Pape, M.M.; Roy, M.J. Expanding Clinical Assessment for Traumatic Brain Injury and Comorbid Post-Traumatic Stress Disorder: A Retrospective Analysis of Virtual Environment Tasks in the Computer-Assisted Rehabilitation Environment. Mil. Med. 2017, 182, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Schröder, J.; van Criekinge, T.; Embrechts, E.; Celis, X.; Van Schuppen, J.; Truijen, S.; Saeys, W. Combining the benefits of tele-rehabilitation and virtual reality-based balance training: A systematic review on feasibility and effectiveness. Disabil. Rehabil. Assist. Technol. 2019, 14, 2–11. [Google Scholar] [CrossRef]
- Winters, J.M.; Wang, Y.; Winters, J.M. Wearable sensors and telerehabilitation. IEEE Eng. Med. Biol. Mag. 2003, 22, 56–65. [Google Scholar] [CrossRef]

| Study | Year | Focus | Key Findings/Objectives | Status |
|---|---|---|---|---|
| Clinical Trial (University Hospital, Grenoble) | 2021-present | Use of AI to analyze CT scans for TBI progression | Aims to differentiate brain tissue evolution post-TBI and correlate with therapeutic intensity; addresses limitations of manual CT analysis | Pending results |
| Review Study [87] | 2023 | AI-based decision support systems for TBI | Highlights comprehensive assessment tools for diagnosis, severity assessment, and long-term prognosis; integrates data from various diagnostic tools | Completed |
| Clinical Trial (UK NHS Hospital Registry) | 2024-present | Effectiveness of qER in reducing CT head scan turnaround times | Multi-center trial across 4 NHS hospitals in UK; assesses impact on reporting time, emergency pathway utility, safety, and cost-effectiveness | Pending results |
| Review Study [90] | 2021 | AI in TBI rehabilitation | Showcases AI’s role alongside brain-computer interfaces and wearable devices; facilitates personalized rehabilitation programs | Completed |
| Clinical Trial (AI-enhanced MEMS Sensors) | 2021-present | Use of AI-enhanced sensors to screen and mitigate concussive risks in soccer players | Establishes personalized concussive thresholds; uses VR training to assess neck stiffness | Pending results |
| Clinical Trial (QuickBrain MRI) | 2017–2019 | Comparison of QuickBrain MRI to CT for pediatric head trauma patients | QuickBrain MRI showed >95% sensitivity for detecting clinically important TBI in pediatric head trauma | Completed |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orenuga, S.; Jordache, P.; Mirzai, D.; Monteros, T.; Gonzalez, E.; Madkoor, A.; Hirani, R.; Tiwari, R.K.; Etienne, M. Traumatic Brain Injury and Artificial Intelligence: Shaping the Future of Neurorehabilitation—A Review. Life 2025, 15, 424. https://doi.org/10.3390/life15030424
Orenuga S, Jordache P, Mirzai D, Monteros T, Gonzalez E, Madkoor A, Hirani R, Tiwari RK, Etienne M. Traumatic Brain Injury and Artificial Intelligence: Shaping the Future of Neurorehabilitation—A Review. Life. 2025; 15(3):424. https://doi.org/10.3390/life15030424
Chicago/Turabian StyleOrenuga, Seun, Philip Jordache, Daniel Mirzai, Tyler Monteros, Ernesto Gonzalez, Ahmed Madkoor, Rahim Hirani, Raj K. Tiwari, and Mill Etienne. 2025. "Traumatic Brain Injury and Artificial Intelligence: Shaping the Future of Neurorehabilitation—A Review" Life 15, no. 3: 424. https://doi.org/10.3390/life15030424
APA StyleOrenuga, S., Jordache, P., Mirzai, D., Monteros, T., Gonzalez, E., Madkoor, A., Hirani, R., Tiwari, R. K., & Etienne, M. (2025). Traumatic Brain Injury and Artificial Intelligence: Shaping the Future of Neurorehabilitation—A Review. Life, 15(3), 424. https://doi.org/10.3390/life15030424







