Proteasomes and Ubiquitin C-Terminal Hydrolase L1 as Biomarkers of Tissue Damage and Inflammatory Response to Different Types of Injury—A Short Review
Abstract
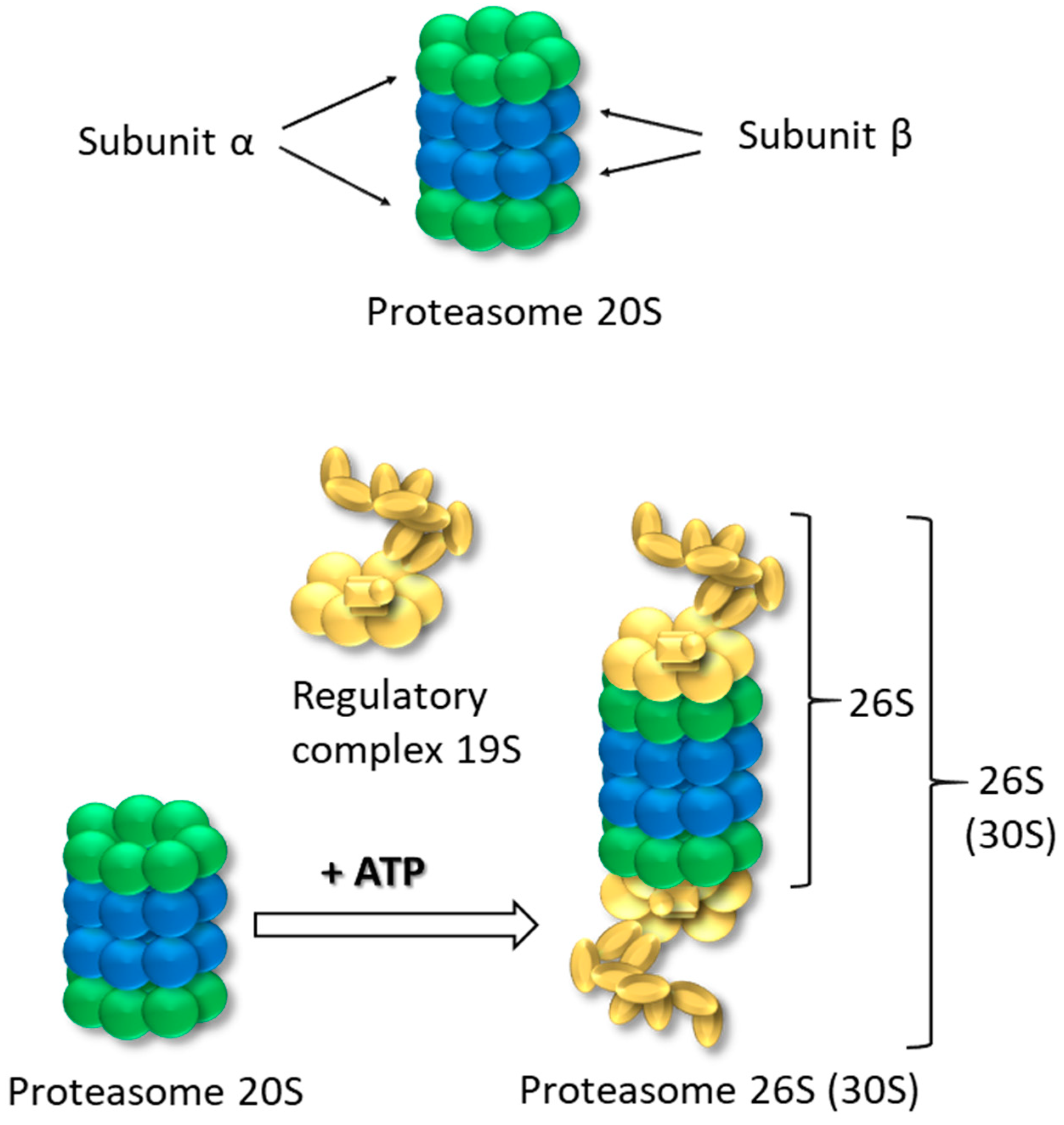
1. Proteasomes 20S and 26S-Structure, Role and Function
2. 20S Proteasome Ubiquitin-Independent Protein Degradation
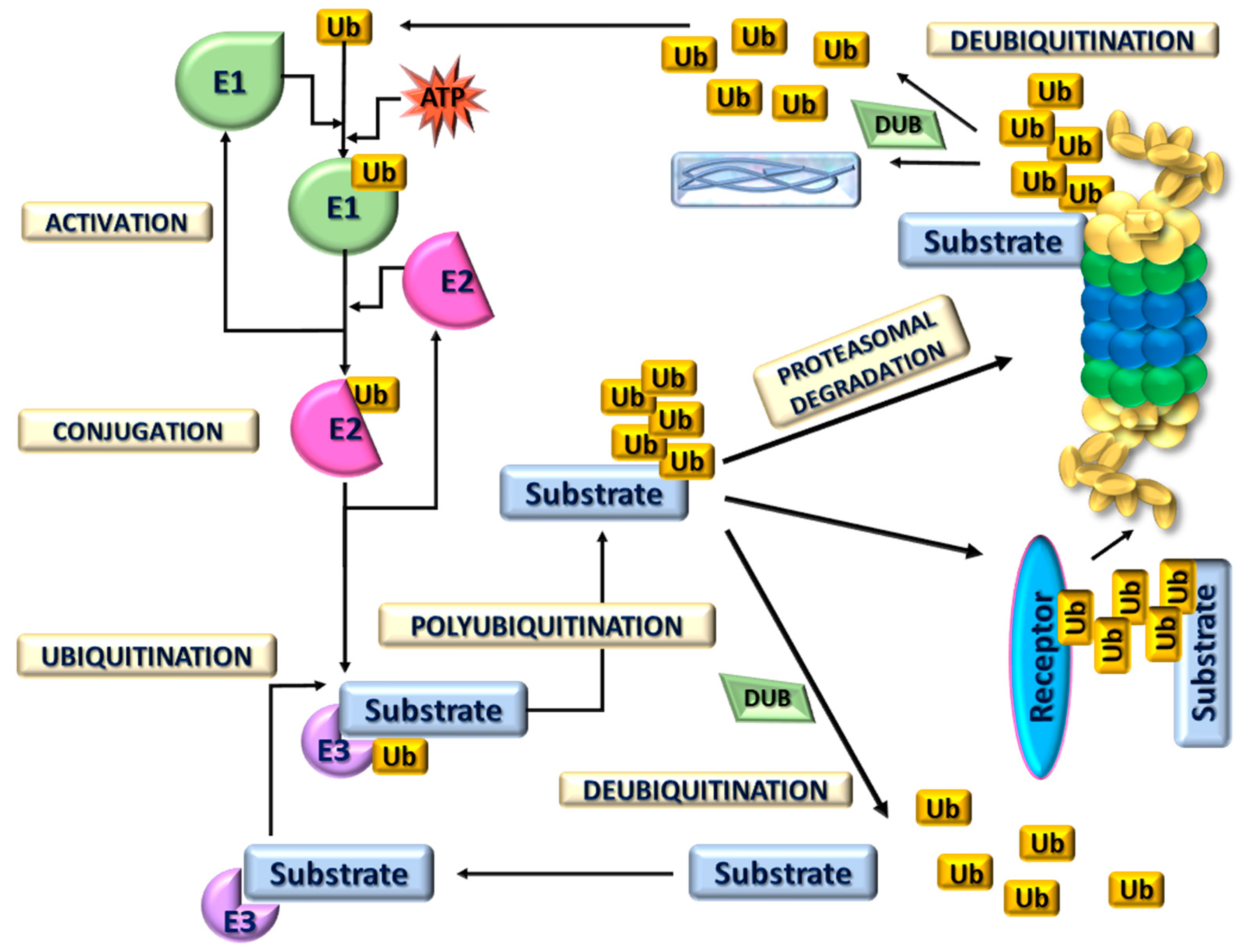
3. 26S Proteasome Ubiquitin-Dependent Protein Degradation
4. Proteasomal System of Protein Degradation in Response to Different Types of Injury
4.1. Central Nervous System Injury
4.2. Thermal Injury
4.3. Abdominal Injury
4.4. Tissue Injury
4.5. Inflammation-Associated Injury
4.6. Ischemia-Reperfusion Injury
| Type of Injury | Key Findings |
|---|---|
| Central nervous system injury |
|
| Thermal injury |
|
| Abdominal injury |
|
| Tissue injury |
|
| Inflammation-associated injury |
|
| Ischemia-reperfusion injury |
|
5. Possible Clinical Implications of Proteasome Inhibition
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| APGN | acute proliferative glomerulonephritis |
| Bcl-2 | B-cell lymphoma 2 regulatory protein |
| Bax | proapoptotic protein |
| CNS | central nervous system |
| COX2 | cyclooxygenase |
| c-proteasome | circulating proteasome |
| CT | computed tomography |
| DUB | deubiquitinating enzymes |
| FSGS | focal segmental glomerulosclerosis |
| IAP | inhibitor of apoptosis |
| IkB | Inhibitor of κB |
| IFNγ | interferon gamma |
| GFAP | glial fibrillary acidic protein |
| HECT | homologous to the E6AP carboxyl terminus |
| HIE | hypoxic-ischemic encephalopathy |
| HIF1A | hypoxia-inducible factor 1-alpha |
| IκB | inhibitor of nuclear factor kappa B |
| JAMM | Jab1/Mov34/Mpr1 metalloenzyme |
| MAP-2 | microtubule-associated protein 2 |
| MBP | myelin basic protein |
| MGN | membranous glomerulonephritis |
| MRI | magnetic resonance imaging |
| LN | lupus nephritis |
| NF-kβ | nuclear factor kβ |
| NOXA | proapoptotic protein |
| NSE | Neuron-Specific Enolase |
| NF-L | neurofilament Light |
| NSI | neurological severity index |
| OUT | ovarian tumor-like proteases |
| p53 | tumor suppressor p53 |
| PROTAC | proteolysis-targeting chimera |
| RING | really interesting new gene |
| SCI | spinal cord injury |
| TBI | traumatic brain injury |
| TNF-α | tumor necrosis factor |
| TBI | traumatic brain injury |
| Ub | ubiquitin |
| UCH | ubiquitin C-terminal hydrolases |
| UCHL1 | ubiquitin carboxy-terminal hydrolase L1 |
| UPP | ubiquitin-proteasome pathway |
| UPS | ubiquitin-proteasome system |
| USP | ubiquitin-specific proteases |
References
- Adams, J. The proteasome: Structure, function, and role in the cell. Cancer Treat. Rev. 2003, 29, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Almond, J.; Cohen, G. The proteasome: A novel target for cancer chemotherapy. Leukemia 2002, 16, 433–443. [Google Scholar] [CrossRef]
- Marshall, R.S.; Vierstra, R.D. Dynamic regulation of the 26S proteasome: From synthesis to degradation. Front. Mol. Biosci. 2019, 6, 40. [Google Scholar] [CrossRef] [PubMed]
- Golab, J.; Bauer, T.M.; Daniel, V.; Naujokat, C. Role of the ubiquitin-proteasome pathway in the diagnosis of human diseases. Clin. Chim. Acta 2004, 340, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Hasselgren, P.O. Role of the ubiquitin-proteasome pathway in sepsis-induced muscle catabolism. Mol. Biol. Rep. 1999, 26, 71–76. [Google Scholar] [CrossRef]
- Gerards, W.L.H.; De Jong, W.W.; Boelens, W.; Bloemendal, H. Structure and assembly of the 20S proteasome. Cell. Mol. Life Sci. 1998, 54, 253–262. [Google Scholar] [CrossRef]
- Kunjappu, M.J.; Hochstrasser, M. Assembly of the 20S proteasome. Biochim. Biophys. Acta-Mol. Cell Res. 2014, 1843, 2–12. [Google Scholar] [CrossRef]
- Sahu, I.; Mali, S.M.; Sulkshane, P.; Xu, C.; Rozenberg, A.; Morag, R.; Sahoo, M.P.; Singh, S.K.; Ding, Z.; Wang, Y.; et al. The 20S as a stand-alone proteasome in cells can degrade the ubiquitin tag. Nat. Commun. 2021, 12, 6173. [Google Scholar] [CrossRef]
- Ben-Nissan, G.; Sharon, M. Regulating the 20S proteasome ubiquitin-independent degradation pathway. Biomolecules 2014, 4, 862–884. [Google Scholar] [CrossRef]
- Türker, F.; Cook, E.K.; Margolis, S.S. The proteasome and its role in the nervous system. Cell Chem. Biol. 2021, 28, 903–917. [Google Scholar] [CrossRef]
- Ben-Nissan, G.; Katzir, N.; Füzesi-Levi, M.G.; Sharon, M. Biology of the Extracellular Proteasome. Biomolecules 2022, 12, 619. [Google Scholar] [CrossRef] [PubMed]
- Hatakeyama, S. Ubiquitylation as a Quality Control System for Intracellular Proteins. J. Biochem. 2003, 134, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Amerik, A.Y.; Hochstrasser, M. Mechanism and function of deubiquitinating enzymes. Biochim. Biophys. Acta-Mol. Cell Res. 2004, 1695, 189–207. [Google Scholar] [CrossRef]
- Toma-Fukai, S.; Shimizu, T. Structural diversity of ubiquitin e3 ligase. Molecules 2021, 26, 6682. [Google Scholar] [CrossRef]
- Deng, N.H.; Tian, Z.; Zou, Y.J.; Quan, S.B. E3 ubiquitin ligase TRIM31: A potential therapeutic target. Biomed. Pharmacother. 2024, 176, 116846. [Google Scholar] [CrossRef]
- Yang, Q.; Zhao, J.; Chen, D.; Wang, Y. E3 ubiquitin ligases: Styles, structures and functions. Mol. Biomed. 2021, 2, 23. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, N.; Li, M.; Hong, T.; Meng, W.; Ouyang, T. Ubiquitin C-terminal hydrolase-L1: A new cancer marker and therapeutic target with dual effects (Review). Oncol. Lett. 2023, 25, 123. [Google Scholar] [CrossRef]
- Xu, Z. Potential roles of UCH family deubiquitinases in tumorigenesis and chemical inhibitors developed against them. Am. J. Cancer Res. 2024, 14, 2666–2694. [Google Scholar] [CrossRef]
- Bishop, P.; Rocca, D.; Henley, J.M. Ubiquitin C-Terminal hydrolase L1 (UCH-L1): Structure, distribution and roles in brain function and dysfunction. Biochem. J. 2016, 473, 2453–2462. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, M.; Zhou, W.; Ly, P.T.T.; Cai, F.; Song, W. NF-κB signaling inhibits ubiquitin carboxyl-terminal hydrolase L1 gene expression. J. Neurochem. 2011, 116, 1160–1170. [Google Scholar] [CrossRef]
- Graham, S.H.; Liu, H. Life and death in the trash heap: The ubiquitin proteasome pathway and UCHL1 in brain aging, neurodegenerative disease and cerebral Ischemia. Ageing Res. Rev. 2017, 34, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Sulistio, Y.A.; Heese, K. The Ubiquitin-Proteasome System and Molecular Chaperone Deregulation in Alzheimer’s Disease. Mol. Neurobiol. 2016, 53, 905–931. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, T.D. Introduction to NF-κB: Players, pathways, perspectives. Oncogene 2006, 25, 6680–6684. [Google Scholar] [CrossRef] [PubMed]
- Gong, B.; Radulovic, M.; Figueiredo-Pereira, M.E.; Cardozo, C. The ubiquitin-proteasome system: Potential therapeutic targets for alzheimer’s disease and spinal cord injury. Front. Mol. Neurosci. 2016, 9, 4. [Google Scholar] [CrossRef]
- Palomo, V.; Nozal, V.; Rojas-Prats, E.; Gil, C.; Martinez, A. Protein kinase inhibitors for amyotrophic lateral sclerosis therapy. Br. J. Pharmacol. 2021, 178, 1316–1335. [Google Scholar] [CrossRef]
- Tiwari, S.; Singh, S. Reciprocal Upshot of Nitric Oxide, Endoplasmic Reticulum Stress, and Ubiquitin Proteasome System in Parkinson’s Disease Pathology. Neuroscientist 2021, 27, 340–354. [Google Scholar] [CrossRef]
- Ghaith, H.S.; Nawar, A.A.; Gabra, M.D.; Abdelrahman, M.E.; Nafady, M.H.; Bahbah, E.I.; Ebada, M.A.; Ashraf, G.M.; Negida, A.; Barreto, G.E. A Literature Review of Traumatic Brain Injury Biomarkers. Mol. Neurobiol. 2022, 59, 4141–4158. [Google Scholar] [CrossRef]
- McDonald, S.J.; Shultz, S.R.; Agoston, D.V. The Known Unknowns: An Overview of the State of Blood-Based Protein Biomarkers of Mild Traumatic Brain Injury. J. Neurotrauma 2021, 38, 2652–2666. [Google Scholar] [CrossRef]
- Papa, L.; McKinley, W.I.; Valadka, A.B.; Newman, Z.C.; Nordgren, R.K.; Pramuka, P.E.; Barbosa, C.E.; Brito, A.M.P.; Loss, L.J.; Tinoco-Garcia, L.; et al. Diagnostic Performance of GFAP, UCH-L1, and MAP-2 Within 30 and 60 Minutes of Traumatic Brain Injury. JAMA Netw. Open 2024, 7, e2431115. [Google Scholar] [CrossRef]
- Hier, D.B.; Obafemi-Ajayi, T.; Thimgan, M.S.; Olbricht, G.R.; Azizi, S.; Allen, B.; Hadi, B.A.; Wunsch, D.C. Blood biomarkers for mild traumatic brain injury: A selective review of unresolved issues. Biomark. Res. 2021, 9, 70. [Google Scholar] [CrossRef]
- Chayoua, W.; Visser, K.; de Koning, M.E.; Beishuizen, A.; IJmker, R.; van der Naalt, J.; Krabbe, J.G.; van der Horn, H.J. Evaluation of Glial Fibrillary Acidic Protein and Ubiquitin C-Terminal Hydrolase-L1 Using a Rapid Point of Care Test for Predicting Head Computed Tomography Lesions After Mild Traumatic Brain Injury in a Dutch Multi-Center Cohort. J. Neurotrauma 2024, 41, e1630–e1640. [Google Scholar] [CrossRef] [PubMed]
- Kobeissy, F.; Arja, R.D.; Munoz, J.C.; Shear, D.A.; Gilsdorf, J.; Zhu, J.; Yadikar, H.; Haskins, W.; Tyndall, J.A.; Wang, K.K. The game changer: UCH-L1 and GFAP-based blood test as the first marketed in vitro diagnostic test for mild traumatic brain injury. Expert Rev. Mol. Diagn. 2024, 24, 67–77. [Google Scholar] [CrossRef]
- Lagares, A.; de la Cruz, J.; Terrisse, H.; Mejan, O.; Pavlov, V.; Vermorel, C.; Payen, J.-F.; Maignan, M.; Viglino, D.; Jacquin, L.; et al. An automated blood test for glial fibrillary acidic protein (GFAP) and ubiquitin carboxy-terminal hydrolase L1 (UCH-L1) to predict the absence of intracranial lesions on head CT in adult patients with mild traumatic brain injury: BRAINI, a multicentre obs. eBioMedicine 2024, 110, 105477. [Google Scholar] [CrossRef]
- Yadav, D.; Lee, J.Y.; Puranik, N.; Chauhan, P.S.; Chavda, V.; Jin, J.O.; Lee, P.C.W. Modulating the Ubiquitin–Proteasome System: A Therapeutic Strategy for Autoimmune Diseases. Cells 2022, 11, 1093. [Google Scholar] [CrossRef] [PubMed]
- Torghabe, S.Y.; Alavi, P.; Rostami, S.; Davies, N.M.; Kesharwani, P.; Karav, S.; Sahebkar, A. Modulation of the ubiquitin-proteasome system by curcumin: Therapeutic implications in cancer. Pathol. Res. Pract. 2025, 265, 155741. [Google Scholar] [CrossRef]
- Dagar, G.; Kumar, R.; Yadav, K.K.; Singh, M.; Pandita, T.K. Ubiquitination and deubiquitination: Implications on cancer therapy. Biochim. Biophys. Acta-Gene Regul. Mech. 2023, 1866, 194979. [Google Scholar] [CrossRef]
- Seyoum Tola, F. The Role of Ubiquitin-Proteasome System in the Pathogenesis of Severe Acute Respiratory Syndrome Coronavirus-2 Disease. Int. J. Inflam. 2023, 2023, 6698069. [Google Scholar] [CrossRef]
- Ma, Z.; Hao, J.; Yang, Z.; Zhang, M.; Xin, J.; Bi, H.; Guo, D. Research Progress on the Role of Ubiquitination in Eye Diseases. Cell Biochem. Biophys. 2024, 82, 1825–1836. [Google Scholar] [CrossRef] [PubMed]
- Qiu, M.; Chen, J.; Li, X.; Zhuang, J. Intersection of the Ubiquitin–Proteasome System with Oxidative Stress in Cardiovascular Disease. Int. J. Mol. Sci. 2022, 23, 12197. [Google Scholar] [CrossRef]
- Matuszczak, E.; Tylicka, M.; Dębek, W.; Tokarzewicz, A.; Gorodkiewicz, E.; Hermanowicz, A. Concentration of UHCL1 in the Serum of Children with Acute Appendicitis, Before and After Surgery, and Its Correlation with CRP and Prealbumin. J. Investig. Surg. 2018, 31, 136–141. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, J.; Qin, X. Advances in early biomarkers of diabetic nephropathy. Rev. Assoc. Med. Bras. 2018, 64, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Song, I.-K.; Kim, H.J.; Magesh, V.; Lee, K.-J. Ubiquitin C-terminal hydrolase-L1 plays a key role in angiogenesis by regulating hydrogen peroxide generated by NADPH oxidase 4. Biochem. Biophys. Res. Commun. 2018, 495, 1567–1572. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Appukuttan, B.; Moh, K.; Ashander, L.M.; Smith, J.R. Ubiquitin carboxyl-terminal esterase l1 promotes proliferation of human choroidal and retinal endothelial cells. Asia-Pac. J. Ophthalmol. 2015, 4, 51–55. [Google Scholar] [CrossRef]
- Tezel, E.; Hibi, K.; Nagasaka, T.; Nakao, A. PGP9.5 as a prognostic factor in pancreatic cancer. Clin. Cancer Res. 2000, 6, 4764–4767. [Google Scholar]
- Yamazaki, T.; Hibi, K.; Takase, T.; Tezel, E.; Nakayama, H.; Kasai, Y.; Ito, K.; Akiyama, S.; Nagasaka, T.; Nakao, A. PGP9.5 as a marker for invasive colorectal cancer. Clin. Cancer Res. 2002, 8, 192–195. [Google Scholar] [PubMed]
- Miyoshi, Y.; Nakayama, S.; Torikoshi, Y.; Tanaka, S.; Ishihara, H.; Taguchi, T.; Tamaki, Y.; Noguchi, S. High expression of ubiquitin carboxy-terminal hydrolase-L1 and -L3 mRNA predicts early recurrence in patients with invasive breast cancer. Cancer Sci. 2006, 97, 523–529. [Google Scholar] [CrossRef]
- Yu, J.; Tao, Q.; Cheung, K.F.; Jin, H.; Poon, F.F.; Wang, X.; Li, H.; Cheng, Y.Y.; Röcken, C.; Ebert, M.P.A.; et al. Epigenetic identification of ubiquitin carboxyl-terminal hydrolase L1 as a functional tumor suppressor and biomarker for hepatocellular carcinoma and other digestive tumors. Hepatology 2008, 48, 508–518. [Google Scholar] [CrossRef]
- Yamashita, K.; Park, H.L.; Kim, M.S.; Osada, M.; Tokumaru, Y.; Inoue, H.; Mori, M.; Sidransky, D. PGP9.5 Methylation in Diffuse-Type Gastric Cancer. Cancer Res. 2006, 66, 3921–3927. [Google Scholar] [CrossRef]
- Jin, C.; Yu, W.; Lou, X.; Zhou, F.; Han, X.; Zhao, N.; Lin, B. UCHL1 is a putative tumor suppressor in ovarian cancer cells and contributes to cisplatin resistance. J. Cancer 2013, 4, 662–670. [Google Scholar] [CrossRef]
- Jara, J.H.; Frank, D.D.; Özdinler, P.H. Could Dysregulation of UPS be a Common Underlying Mechanism for Cancer and Neurodegeneration? Lessons from UCHL1. Cell Biochem. Biophys. 2013, 67, 45–53. [Google Scholar] [CrossRef]
- Sun, J.; Shi, X.; Mamun, M.; Gao, Y. The role of deubiquitinating enzymes in gastric cancer (Review). Oncol. Lett. 2019, 19, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Luo, Q.; Liu, Z. Ubiquitination and deubiquitination of MCL1 in cancer: Deciphering chemoresistance mechanisms and providing potential therapeutic options. Cell Death Dis. 2020, 11, 556. [Google Scholar] [CrossRef]
- Li, L.; Tao, Q.; Jin, H.; Van Hasselt, A.; Poon, F.F.; Wang, X.; Zeng, M.S.; Jia, W.H.; Zeng, Y.X.; Chan, A.T.C.; et al. The tumor suppressor UCHL1 forms a complex with p53/MDM2/ARF to promote p53 signaling and is frequently silenced in nasopharyngeal carcinoma. Clin. Cancer Res. 2010, 16, 2949–2958. [Google Scholar] [CrossRef]
- Weathington, N.M.; Mallampalli, R.K. Emerging therapies targeting the ubiquitin proteasome system in cancer. J. Clin. Investig. 2014, 124, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, O.; Beaufrere, B.; Boirie, Y.; Ralliere, C.; Taillandier, D.; Aurousseau, E.; Schoeffler, P.; Arnal, M.; Attaix, D. Increased mRNA levels for components of the lysosomal, Ca2+-activated, and ATP-ubiquitin-dependent proteolytic pathways in skeletal muscle from head trauma patients. Proc. Natl. Acad. Sci. USA 1996, 93, 2714–2718. [Google Scholar] [CrossRef]
- Seiffert, M.; Gosenca, D.; Ponelies, N.; Ising, N.; Patel, M.B.; Obertacke, U.; Majetschak, M. Regulation of the ubiquitin proteasome system in mechanically injured human skeletal muscle. Physiol. Res. 2007, 56, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Farges, M.C.; Balcerzak, D.; Fisher, B.D.; Attaix, D.; Béchet, D.; Ferrara, M.; Baracos, V.E. Increased muscle proteolysis after local trauma mainly reflects macrophage-associated lysosomal proteolysis. Am. J. Physiol.-Endocrinol. Metab. 2002, 282, 326–335. [Google Scholar] [CrossRef]
- Çetin, G.; Klafack, S.; Studencka-Turski, M.; Krüger, E.; Ebstein, F. The ubiquitin–proteasome system in immune cells. Biomolecules 2021, 11, 60. [Google Scholar] [CrossRef]
- Szabo, Z.; Ying, Z.; Radak, Z.; Gomez-Pinilla, F. Voluntary exercise may engage proteasome function to benefit the brain after trauma. Brain Res. 2010, 1341, 25–31. [Google Scholar] [CrossRef]
- Urso, M.L.; Chen, Y.W.; Scrimgeour, A.G.; Lee, P.C.; Lee, K.F.; Clarkson, P.M. Alterations in mRNA expression and protein products following spinal cord injury in humans. J. Physiol. 2007, 579, 877–892. [Google Scholar] [CrossRef]
- Tylicka, M.; Matuszczak, E.; Dębek, W.; Hermanowicz, A.; Ostrowska, H. Circulating proteasome activity following mild head injury in children. Child’s Nerv. Syst. 2014, 30, 1191–1196. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.P.; Nigam, R.; Tomar, G.S.; Monisha, M.; Bhoi, S.K.; Subramanian, A.; Sengar, K.; Akula, D.; Panta, P.; Anindya, R. Early and rapid detection of UCHL1 in the serum of brain-trauma patients: A novel gold nanoparticle-based method for diagnosing the severity of brain injury. Analyst 2018, 143, 3366–3373. [Google Scholar] [CrossRef] [PubMed]
- Thelin, E.; Al Nimer, F.; Frostell, A.; Zetterberg, H.; Blennow, K.; Nyström, H.; Svensson, M.; Bellander, B.M.; Piehl, F.; Nelson, D.W. A Serum protein biomarker panel improves outcome prediction in human traumatic brain injury. J. Neurotrauma 2019, 36, 2850–2862. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.; Jarvis, P. Review of the potential use of blood neuro-biomarkers in the diagnosis of mild traumatic brain injury. Clin. Exp. Emerg. Med. 2017, 4, 121–127. [Google Scholar] [CrossRef]
- Glushakova, O.; Glushakov, A.; Hayes, R. Finding effective biomarkers for pediatric traumatic brain injury. Brain Circ. 2016, 2, 129. [Google Scholar] [CrossRef]
- Mondello, S.; Kobeissy, F.; Vestri, A.; Hayes, R.L.; Kochanek, P.M.; Berger, R.P. Serum Concentrations of Ubiquitin C-Terminal Hydrolase-L1 and Glial Fibrillary Acidic Protein after Pediatric Traumatic Brain Injury. Sci. Rep. 2016, 6, 28203. [Google Scholar] [CrossRef]
- Douglas-Escobar, M.V.; Heaton, S.C.; Bennett, J.; Young, L.J.; Glushakova, O.; Xu, X.; Barbeau, D.Y.; Rossignol, C.; Miller, C.; Crow, A.M.O.; et al. UCH-L1 and GFAP serum levels in neonates with hypoxic-ischemic encephalopathy: A single center pilot study. Front. Neurol. 2014, 5, 273. [Google Scholar] [CrossRef]
- Weremijewicz, A.; Matuszczak, E.; Sankiewicz, A.; Tylicka, M.; Komarowska, M.; Tokarzewicz, A.; Debek, W.; Gorodkiewicz, E.; Hermanowicz, A. Matrix metalloproteinase-2 and its correlation with basal membrane components laminin-5 and collagen type IV in paediatric burn patients measured with Surface Plasmon Resonance Imaging (SPRI) biosensors. Burns 2018, 44, 931–940. [Google Scholar] [CrossRef]
- Matuszczak, E.; Tylicka, M.; Hermanowicz, A.; Debek, W.; Sankiewicz, A.; Gorodkiewicz, E. Application of SPR imaging biosensor for the measurement of 20S proteasomes in blood plasma of children with thermal injury. Ann. Clin. Lab. Sci. 2016, 46, 407–411. [Google Scholar]
- Matuszczak, E.; Tylicka, M.; Dȩbek, W.; Hermanowicz, A.; Ostrowska, H. Correlation between circulating proteasome activity, total protein and c-reactive protein levels following burn in children. Burns 2014, 40, 842–847. [Google Scholar] [CrossRef]
- Matuszczak, E.; Tylicka, M.; Dębek, W.; Sankiewicz, A.; Gorodkiewicz, E.; Hermanowicz, A. Overexpression of ubiquitin carboxyl-terminal hydrolase L1 (UCHL1) in serum of children after thermal injury. Adv. Med. Sci. 2017, 62, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Matuszczak, E.; Tylicka, M.; Dębek, W.; Hermanowicz, A.; Ostrowska, H. The comparison of C-proteasome activity in the plasma of children after burn injury, mild head injury and blunt abdominal trauma. Adv. Med. Sci. 2015, 60, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.B.; Earle, S.A.; Majetschak, M. Dynamics of tissue ubiquitin pools and ubiquitin-proteasome pathway component activities during the systemic response to traumatic shock. Physiol. Res. 2007, 56, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, J.; Wu, H.; Wang, T.; Gan, H.; Zhang, X.; Liu, Y.; Li, R.; Zhao, Z.; Chen, Q.; et al. UCH-LI expression of podocytes in diseased glomeruli and in vitro. J. Pathol. 2009, 217, 642–653. [Google Scholar] [CrossRef]
- Zhang, H.; Mao, X.; Sun, Y.; Hu, R.; Luo, W.; Zhao, Z.; Chen, Q.; Zhang, Z. NF-κB upregulates ubiquitin C-terminal hydrolase 1 in diseased podocytes in glomerulonephritis. Mol. Med. Rep. 2015, 12, 2893–2901. [Google Scholar] [CrossRef]
- Radón, V.; Czesla, M.; Reichelt, J.; Fehlert, J.; Hammel, A.; Rosendahl, A.; Knop, J.-H.; Wiech, T.; Wenzel, U.O.; Sachs, M.; et al. Ubiquitin C-Terminal Hydrolase L1 is required for regulated protein degradation through the ubiquitin proteasome system in kidney. Kidney Int. 2018, 93, 110–127. [Google Scholar] [CrossRef]
- Brackeva, B.; De Punt, V.; Kramer, G.; Costa, O.; Verhaeghen, K.; Stangé, G.; Sadones, J.; Xavier, C.; Aerts, J.M.F.G.; Gorus, F.K.; et al. Potential of UCHL1 as biomarker for destruction of pancreatic beta cells. J. Proteom. 2015, 117, 156–167. [Google Scholar] [CrossRef]
- Higashi, T.; Friedman, S.L.; Hoshida, Y. Hepatic stellate cells as key target in liver fibrosis. Adv. Drug Deliv. Rev. 2017, 121, 27–42. [Google Scholar] [CrossRef]
- Brown, H.; Patel, J.; Barsamian, E.M.; Collins, S.C.; Mcdermott, W. V Cold preservation of liver for homotransplantation. Surg. Forum 1964, 15, 215–217. [Google Scholar]
- Zaouali, M.A.; Panisello-Roselló, A.; Lopez, A.; Benítez, C.C.; Folch-Puy, E.; García-Gil, A.; Carbonell, T.; Adam, R.; Roselló-Catafau, J. Relevance of proteolysis and proteasome activation in fatty liver graft preservation: An Institut Georges Lopez-1 vs University of Wisconsin appraisal. World J. Gastroenterol. 2017, 23, 4211–4221. [Google Scholar] [CrossRef]
- Kandilis, A.N.; Karidis, N.P.; Kouraklis, G.; Patsouris, E.; Vasileiou, I.; Theocharis, S. Proteasome inhibitors: Possible novel therapeutic strategy for ischemia–reperfusion injury? Expert Opin. Investig. Drugs 2014, 23, 67–80. [Google Scholar] [CrossRef]
- Drews, O.; Taegtmeyer, H. Targeting the ubiquitin-proteasome system in heart disease: The basis for new therapeutic strategies. Antioxid. Redox Signal. 2014, 21, 2322–2343. [Google Scholar] [CrossRef] [PubMed]
- Oliva, J. Proteasome and organs ischemia-reperfusion injury. Int. J. Mol. Sci. 2018, 19, 106. [Google Scholar] [CrossRef] [PubMed]
- Jialin, B.A.O.; Sato, K.; Min, L.I.; Youhe, G.A.O.; Abid, R.; Aird, W.; Simons, M.; Post, M.J. PR-39 and PR-11 peptides inhibit ischemia-reperfusion injury by blocking proteasome-mediated IκBα degradation. Am. J. Physiol.-Hear. Circ. Physiol. 2001, 281, 2612–2618. [Google Scholar] [CrossRef]
- Pye, J.; Ardeshirpour, F.; McCain, A.; Bellinger, D.A.; Merricks, E.; Adams, J.; Elliott, P.J.; Pien, C.; Fischer, T.H.; Baldwin, A.S.; et al. Proteasome inhibition ablates activation of NF-κB in myocardial reperfusion and reduces reperfusion injury. Am. J. Physiol.-Hear. Circ. Physiol. 2003, 284, 919–926. [Google Scholar] [CrossRef]
- McConkey, D.J.; Zhu, K. Mechanisms of proteasome inhibitor action and resistance in cancer. Drug Resist. Updat. 2008, 11, 164–179. [Google Scholar] [CrossRef] [PubMed]
- Drexler, H.C.A. Activation of the cell death program by inhibition of proteasome function. Proc. Natl. Acad. Sci. USA 1997, 94, 855–860. [Google Scholar] [CrossRef]
- Shinohara, K.; Tomioka, M.; Nakano, H.; Toné, S.; Ito, H.; Kawashima, S. Apoptosis induction resulting from proteasome inhibition. Biochem. J. 1996, 317, 385–388. [Google Scholar] [CrossRef]
- Crawford, L.J.; Walker, B.; Irvine, A.E. Proteasome inhibitors in cancer therapy. J. Cell Commun. Signal. 2011, 5, 101–110. [Google Scholar] [CrossRef]
- Traenckner, E.B.M.; Wilk, S.; Baeuerle, P.A. A proteasome inhibitor prevents activation of NF-κB and stabilizes a newly phosphorylated form of IκB-α that is still bound to NF-κB. EMBO J. 1994, 13, 5433–5441. [Google Scholar] [CrossRef]
- Kamińska, J.; Tylicka, M.; Sutkowska, K.; Gacuta, K.M.; Sawicka, M.M.; Kowalewska, E.; Ćwiklińska-Dworakowska, M.; Maciejczyk, M.; Łysoń, T.; Kornhuber, J.; et al. The preliminary study suggests an association between NF-ĸB pathway activation and increased plasma 20S proteasome activity in intracranial aneurysm patients. Sci. Rep. 2024, 14, 3941. [Google Scholar] [CrossRef] [PubMed]
- Żebrowska-Nawrocka, M.; Szmajda-Krygier, D.; Krygier, A.; Jeleń, A.; Balcerczak, E. Bioinformatic Analysis of IKK Complex Genes Expression in Selected Gastrointestinal Cancers. Int. J. Mol. Sci. 2024, 25, 9868. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, S.; Vargas, J.; Hoffmann, A. Signaling via the NFκB system. WIREs Syst. Biol. Med. 2016, 8, 227–241. [Google Scholar] [CrossRef]
- Ruschak, A.M.; Slassi, M.; Kay, L.E.; Schimmer, A.D. Novel proteasome inhibitors to overcome bortezomib resistance. J. Natl. Cancer Inst. 2011, 103, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Henninger, N.; Sicard, K.M.; Bouley, J.; Fisher, M.; Stagliano, N.E. The proteasome inhibitor VELCADE® reduces infarction in rat models of focal cerebral ischemia. Neurosci. Lett. 2006, 398, 300–305. [Google Scholar] [CrossRef]
- Sterz, J.; Jakob, C.; Kuckelkorn, U.; Heider, U.; Mieth, M.; Kleeberg, L.; Kaiser, M.; Kloetzel, P.M.; Sezer, O.; Von Metzler, I. BSc2118 is a novel proteasome inhibitor with activity against multiple myeloma. Eur. J. Haematol. 2010, 85, 99–107. [Google Scholar] [CrossRef]
- Doeppner, T.R.; Mlynarczuk-Bialy, I.; Kuckelkorn, U.; Kaltwasser, B.; Herz, J.; Hasan, M.R.; Hermann, D.M.; Bähr, M. The novel proteasome inhibitor BSc2118 protects against cerebral ischaemia through HIF1A accumulation and enhanced angioneurogenesis. Brain 2012, 135, 3282–3297. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, X.; Wang, Y.; Lei, H.; Su, H.; Zeng, J.; Pei, Z.; Huang, R. Inhibition of immunoproteasome reduces infarction volume and attenuates inflammatory reaction in a rat model of ischemic stroke. Cell Death Dis. 2015, 6, e1626. [Google Scholar] [CrossRef]
- Ramachandran, S.; Liaw, J.M.; Jia, J.; Glasgow, S.C.; Liu, W.; Csontos, K.; Upadhya, G.A.; Mohanakumar, T.; Chapman, W.C. Ischemia–reperfusion injury in rat steatotic liver is dependent on NFκB P65 activation. Transpl. Immunol. 2012, 26, 201–206. [Google Scholar] [CrossRef]
- Fan, T.; Huang, Z.; Wang, W.; Zhang, B.; Xu, Y.; Mao, Z.; Chen, L.; Hu, H.; Geng, Q. Proteasome inhibition promotes autophagy and protects from endoplasmic reticulum stress in rat alveolar macrophages exposed to hypoxia-reoxygenation injury. J. Cell. Physiol. 2018, 233, 6748–6758. [Google Scholar] [CrossRef]
- Fang, C.H.; Wang, J.J.; Hobler, S.; Li, B.G.; Fischer, J.E.; Hasselgren, P.O. Proteasome blockers inhibit protein breakdown in skeletal muscle after burn injury in rats. Clin. Sci. 1998, 95, 225–233. [Google Scholar] [CrossRef]
- Vana, P.G.; LaPorte, H.M.; Wong, Y.M.; Kennedy, R.H.; Gamelli, R.L.; Majetschak, M. Proteasome Inhibition After Burn Injury. J. Burn Care Res. 2016, 37, 207–215. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Saez, I.; Vilchez, D. The Mechanistic Links Between Proteasome Activity, Aging and Agerelated Diseases. Curr. Genom. 2014, 15, 38–51. [Google Scholar] [CrossRef] [PubMed]
- Read, N.C.; Gutsol, A.; Holterman, C.E.; Carter, A.; Coulombe, J.; Gray, D.A.; Kennedy, C.R.J. Ubiquitin C-terminal hydrolase L1 deletion ameliorates glomerular injury in mice with ACTN4-associated focal segmental glomerulosclerosis. Biochim. Biophys. Acta-Mol. Basis Dis. 2014, 1842, 1028–1040. [Google Scholar] [CrossRef][Green Version]
- Manasanch, E.E.; Orlowski, R.Z. Proteasome inhibitors in cancer therapy. Nat. Rev. Clin. Oncol. 2017, 14, 417–433. [Google Scholar] [CrossRef]
- Mancuso, F.; Di Chio, C.; Di Matteo, F.; Smaldone, G.; Iraci, N.; Giofrè, S.V. Recent Advances in the Development of Immunoproteasome Inhibitors as Anti-Cancer Agents: The Past 5 Years. Molecules 2025, 30, 755. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tylicka, M.; Matuszczak, E.; Kamińska, J.; Modzelewska, B.; Koper-Lenkiewicz, O.M. Proteasomes and Ubiquitin C-Terminal Hydrolase L1 as Biomarkers of Tissue Damage and Inflammatory Response to Different Types of Injury—A Short Review. Life 2025, 15, 413. https://doi.org/10.3390/life15030413
Tylicka M, Matuszczak E, Kamińska J, Modzelewska B, Koper-Lenkiewicz OM. Proteasomes and Ubiquitin C-Terminal Hydrolase L1 as Biomarkers of Tissue Damage and Inflammatory Response to Different Types of Injury—A Short Review. Life. 2025; 15(3):413. https://doi.org/10.3390/life15030413
Chicago/Turabian StyleTylicka, Marzena, Ewa Matuszczak, Joanna Kamińska, Beata Modzelewska, and Olga Martyna Koper-Lenkiewicz. 2025. "Proteasomes and Ubiquitin C-Terminal Hydrolase L1 as Biomarkers of Tissue Damage and Inflammatory Response to Different Types of Injury—A Short Review" Life 15, no. 3: 413. https://doi.org/10.3390/life15030413
APA StyleTylicka, M., Matuszczak, E., Kamińska, J., Modzelewska, B., & Koper-Lenkiewicz, O. M. (2025). Proteasomes and Ubiquitin C-Terminal Hydrolase L1 as Biomarkers of Tissue Damage and Inflammatory Response to Different Types of Injury—A Short Review. Life, 15(3), 413. https://doi.org/10.3390/life15030413









