Investigation and Study on the Biology and Morphology of Apis florea and Apis dorsata in Southern China
Abstract
1. Introduction
2. Materials and Methods
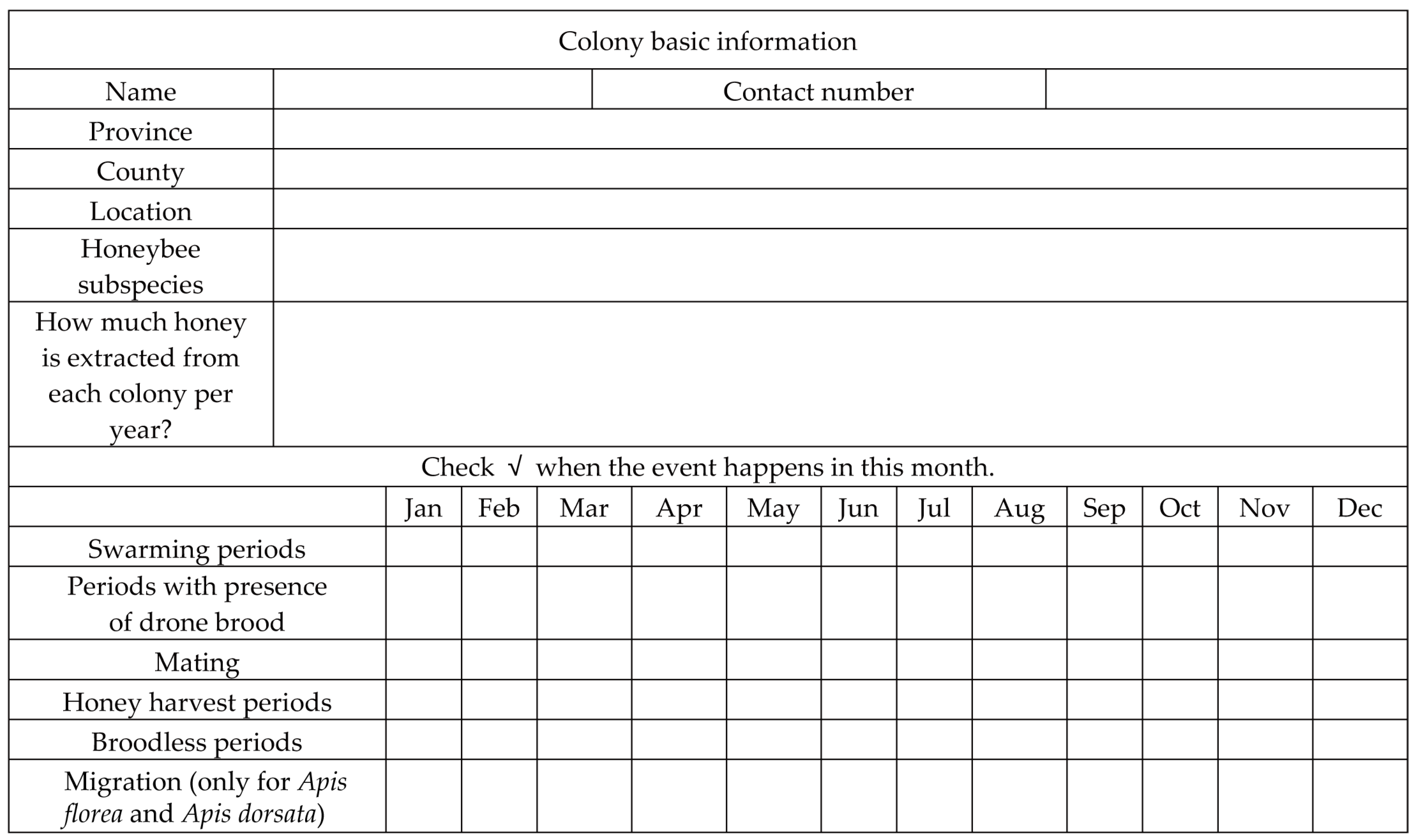
2.1. Survey and Data Acquisition
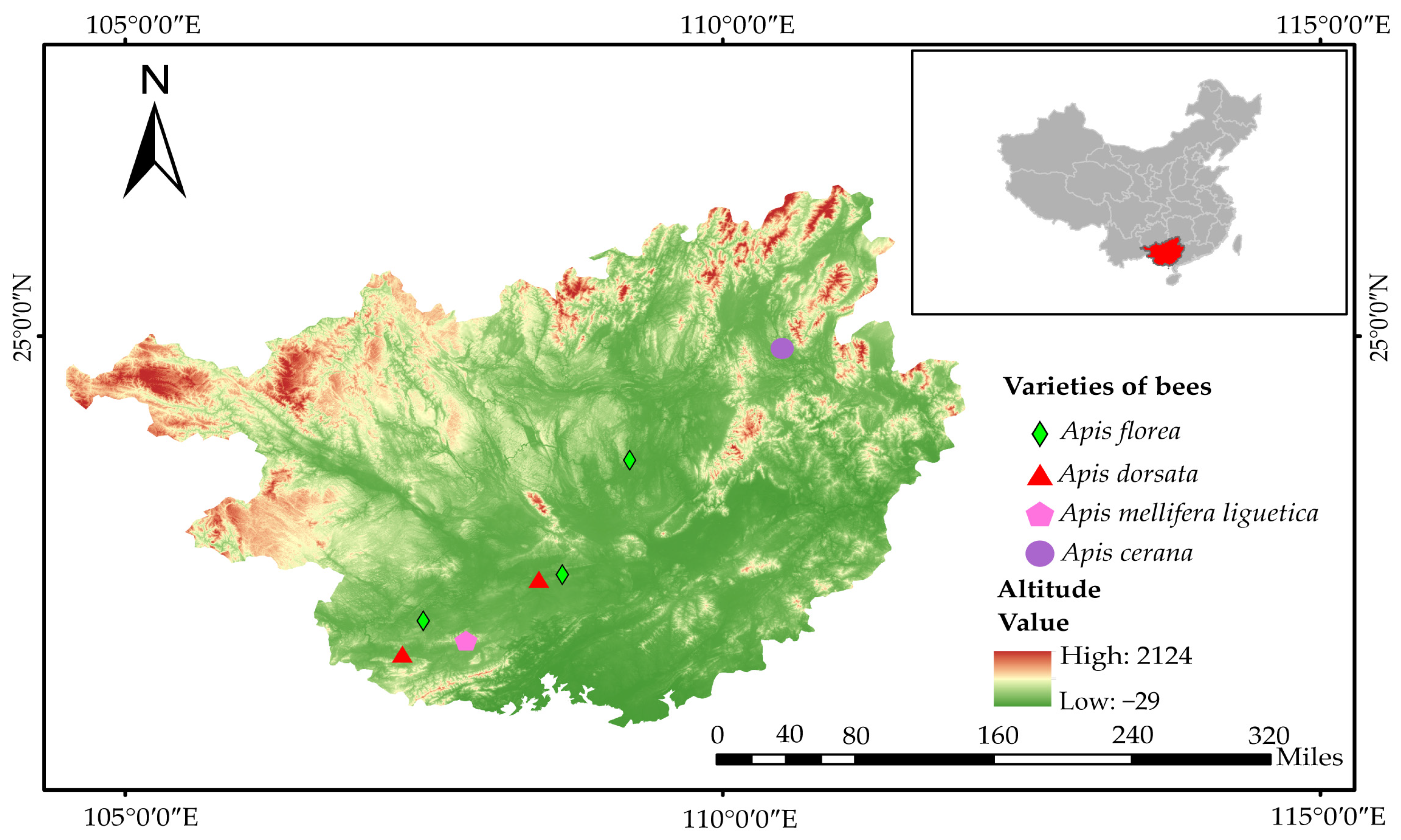
2.2. Description of the Area of Study
2.3. Sampling and Morphometric Measurements
2.4. Statistical Analyses
3. Results
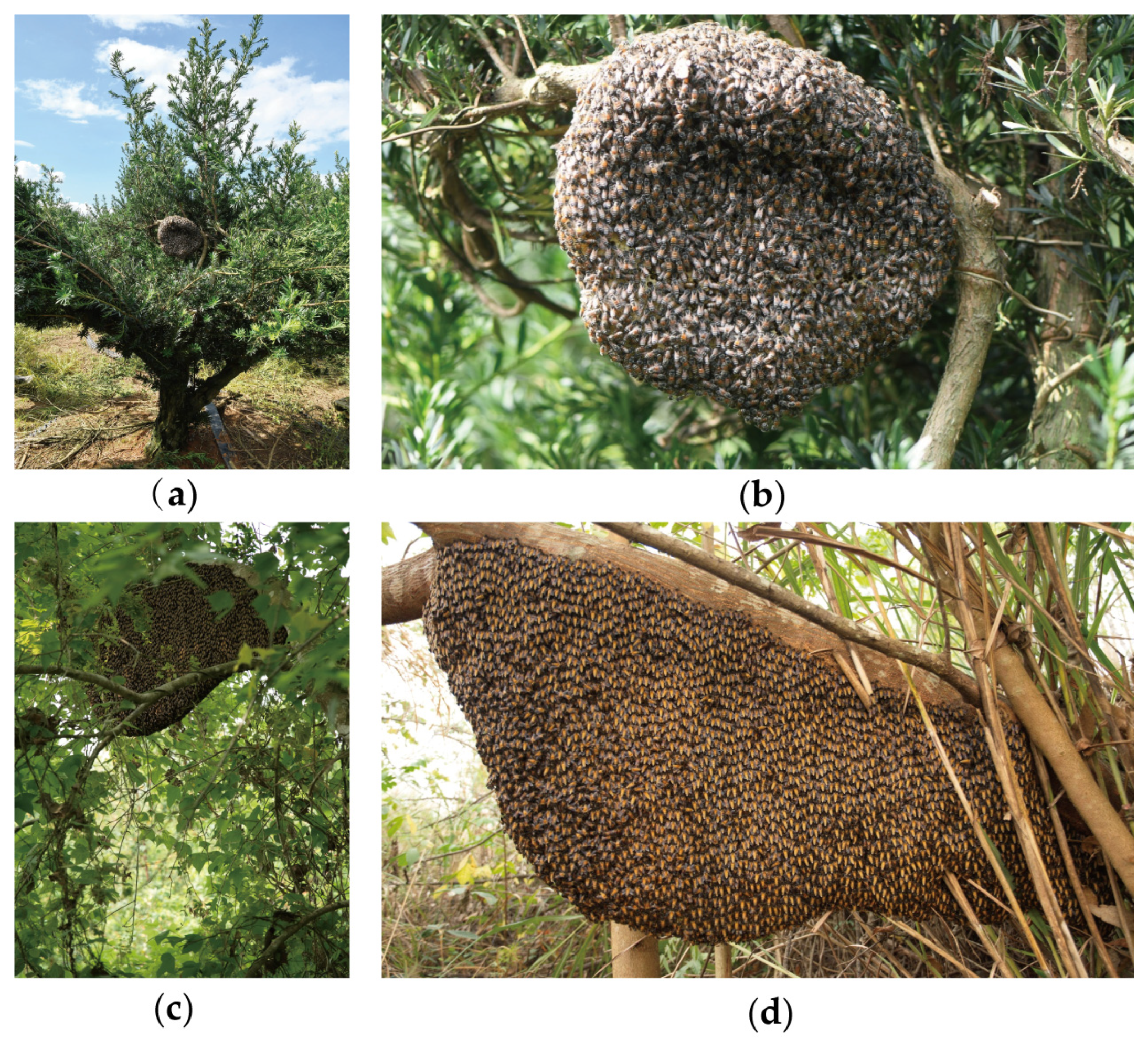
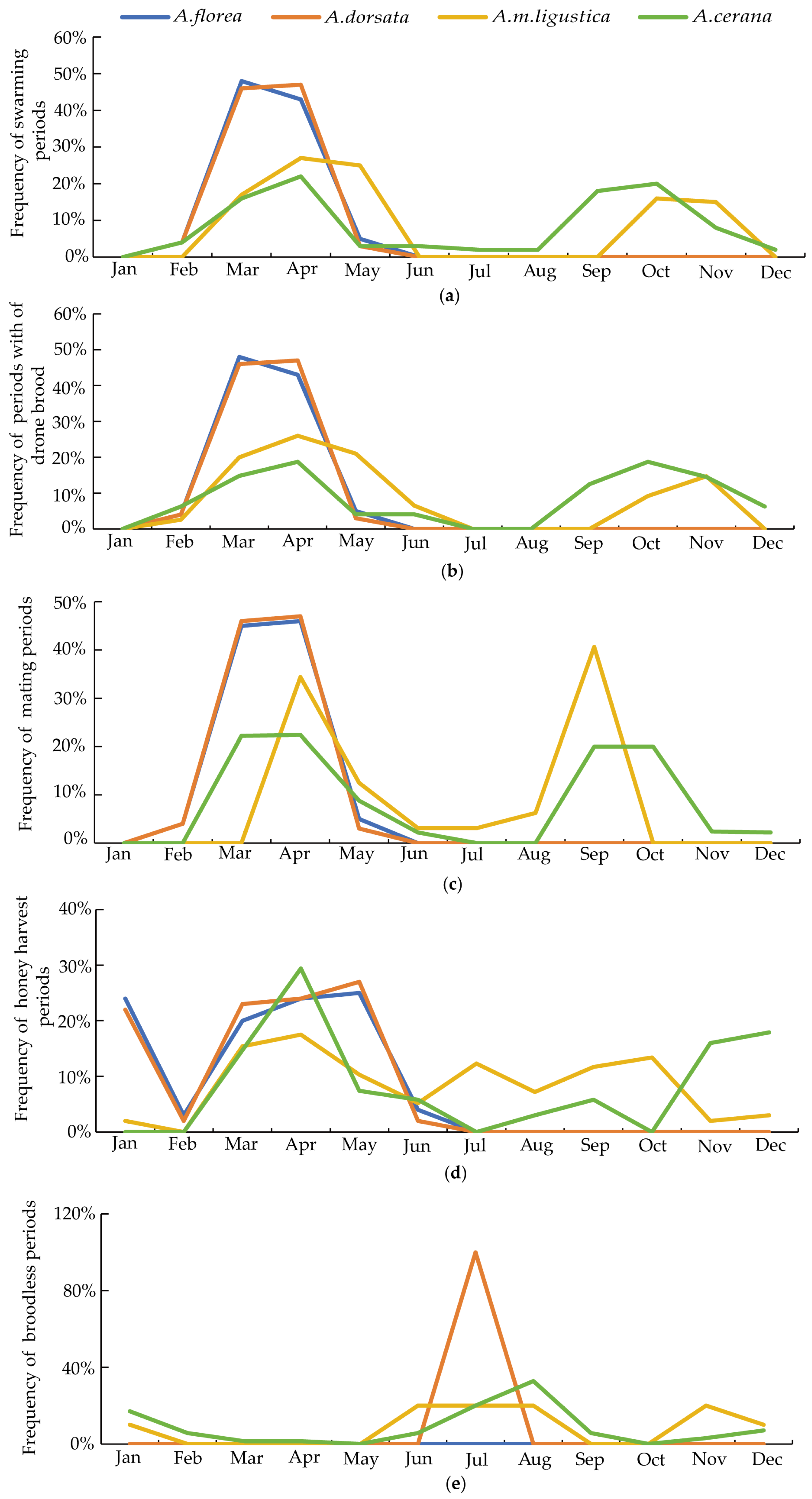
3.1. Biological Characteristics
3.2. Overall Description of the Morphology of A. florea and A. dorsata
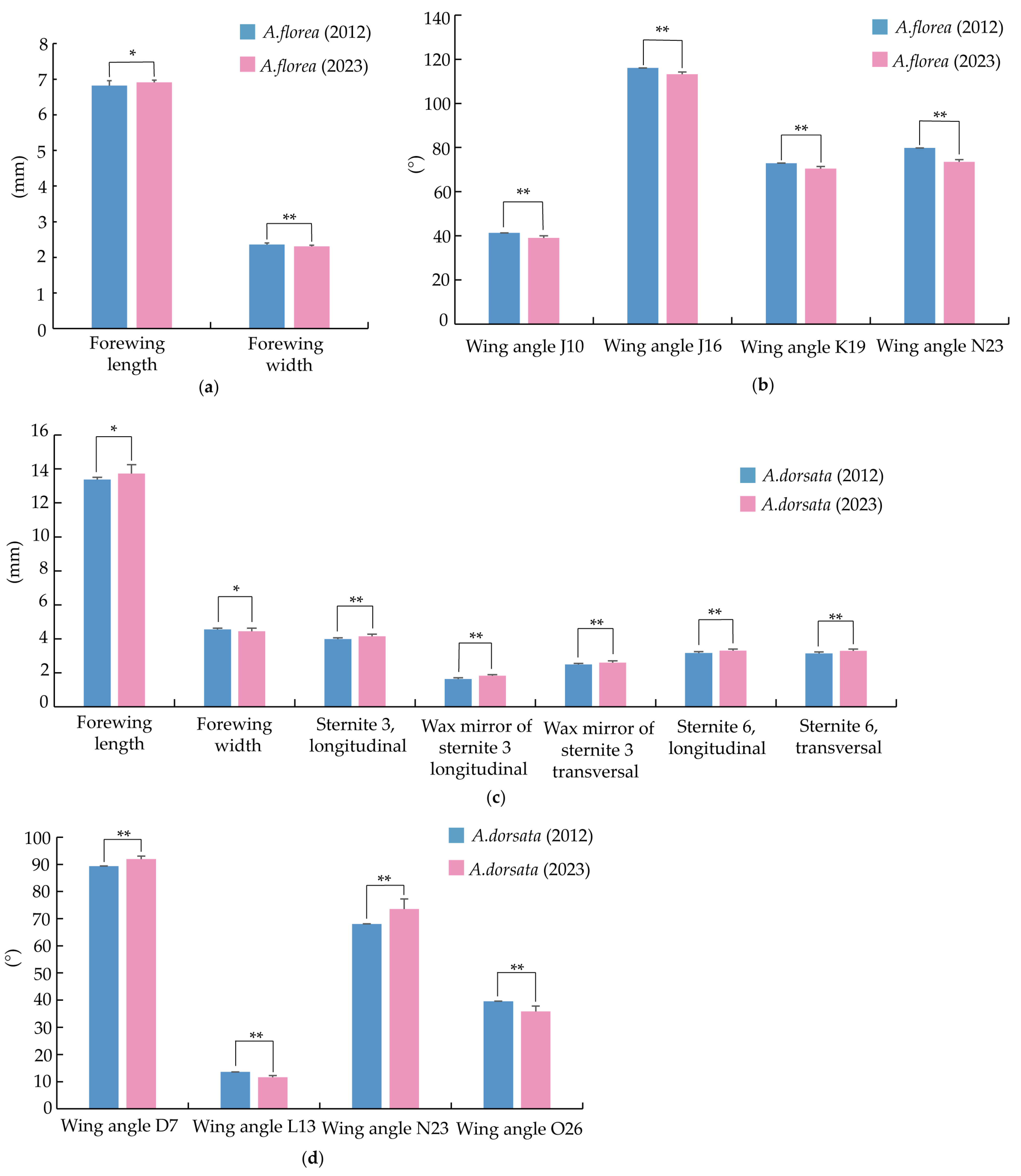
3.3. Morphological Changes in A. florea and A. dorsata over the Past Decade
3.4. Morphological Comparison of Four Species of Honey Bees in Guangxi
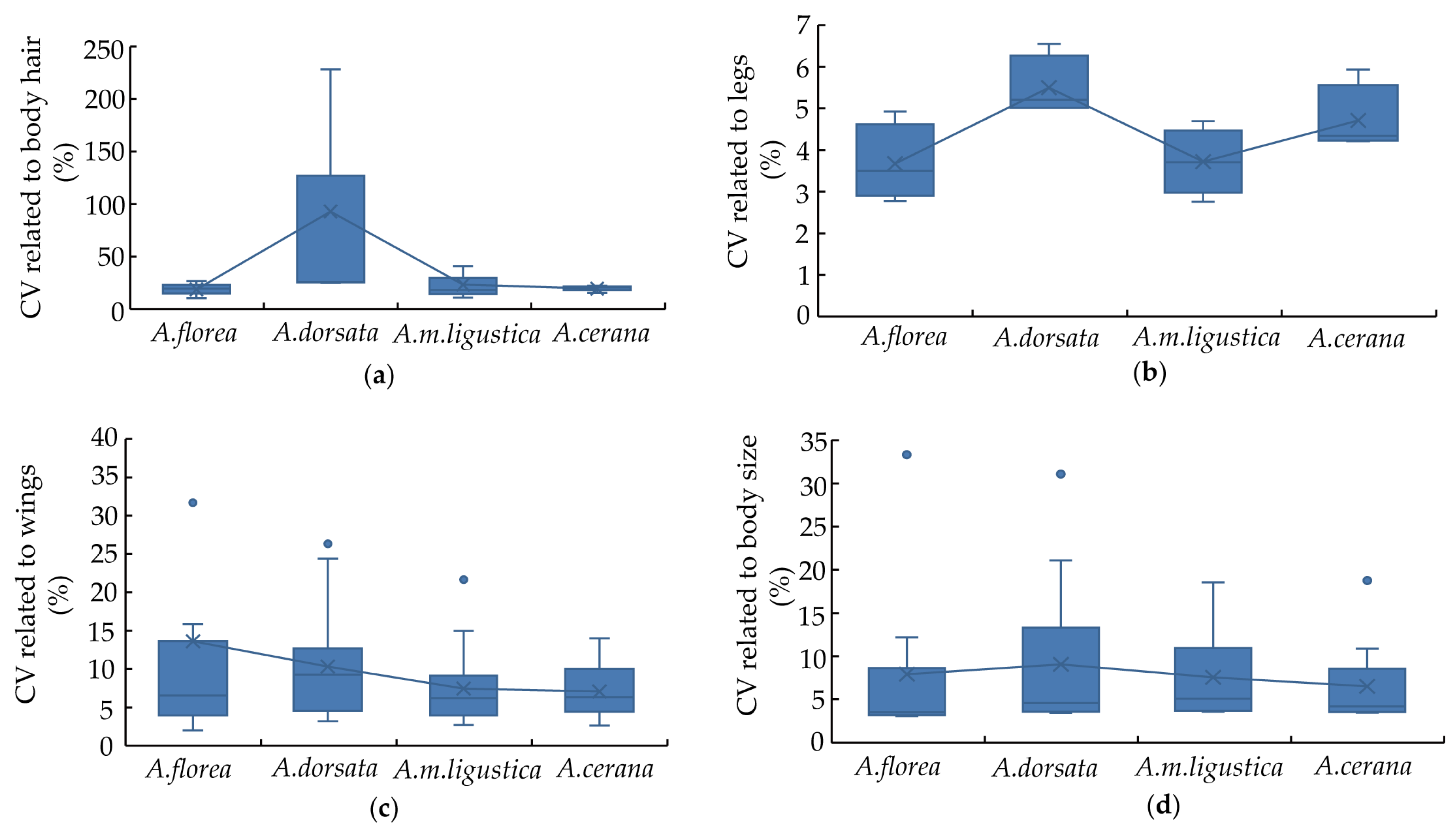
4. Variation Analysis of Morphometric Characteristics
5. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gallai, N.; Salles, J.M.; Settele, J.; Vaissière, B.E. Economic valuation of the vulnerability of world agriculture confronted with pollinator decline. Ecol. Econ. 2009, 68, 810–821. [Google Scholar] [CrossRef]
- Van der Sluijs, J.P.; Simon-Delso, N.; Goulson, D.; Maxim, L.; Bonmatin, J.-M.; Belzunces, L.P. Neonicotinoids, bee disorders and the sustainability of pollinator services. Curr. Opin. Environ. Sustain. 2013, 5, 293–305. [Google Scholar] [CrossRef]
- Stanley, J.; Sah, K.; Subbanna, A.R.; Preetha, G.; Gupta, J. How efficient is Apis cerana (Hymenoptera: Apidae) in pollinating cabbage, Brassica oleracea var. capitata? Pollination behavior, pollinator effectiveness, pollinator requirement, and impact of pollination. J. Econ. Entomol. 2017, 110, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Ollerton, J.; Winfree, R.; Tarrant, S. How many flowering plants are pollinated by animals? Oikos 2011, 120, 321–326. [Google Scholar] [CrossRef]
- Khalifa, S.A.; Elshafiey, E.H.; Shetaia, A.A.; El-Wahed, A.A.A.; Algethami, A.F.; Musharraf, S.G.; AlAjmi, M.F.; Zhao, C.; Masry, S.H.; Abdel-Daim, M.M. Overview of bee pollination and its economic value for crop production. Insects 2021, 12, 688. [Google Scholar] [CrossRef] [PubMed]
- Sáez, A.; Negri, P.; Viel, M.; Aizen, M.A. Pollination efficiency of artificial and bee pollination practices in kiwifruit. Sci. Hortic. 2019, 246, 1017–1021. [Google Scholar] [CrossRef]
- Kafantaris, I.; Amoutzias, G.D.; Mossialos, D. Foodomics in bee product research: A systematic literature review. Eur. Food Res. Technol. 2021, 247, 309–331. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Ruiz-Navajas, Y.; Fernández-López, J.; Pérez-Álvarez, J. Functional properties of honey, propolis, and royal jelly. J. Food Sci. 2008, 73, R117–R124. [Google Scholar] [CrossRef]
- Khalifa, S.A.; Elashal, M.H.; Yosri, N.; Du, M.; Musharraf, S.G.; Nahar, L.; Sarker, S.D.; Guo, Z.; Cao, W.; Zou, X. Bee pollen: Current status and therapeutic potential. Nutrients 2021, 13, 1876. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.-Y.; Zhu, Q.-Q.; Guan, C.-X.; Xiong, G.-L.; Chen, X.-X.; Gong, H.-B.; Li, J.-W.; Ouyang, S.-H.; Kurihara, H.; Li, Y.-F. Antioxidant and Anti-Inflammatory Properties of Hydrolyzed Royal Jelly Peptide in Human Dermal Fibroblasts: Implications for Skin Health and Care Applications. Bioengineering 2024, 11, 496. [Google Scholar] [CrossRef] [PubMed]
- Diao, Q.; Yang, D.; Zhao, H.; Deng, S.; Wang, X.; Hou, C.; Wilfert, L. Prevalence and population genetics of the emerging honey bee pathogen DWV in Chinese apiculture. Sci. Rep. 2019, 9, 12042. [Google Scholar] [CrossRef]
- Goulson, D.; Nicholls, E.; Botías, C.; Rotheray, E.L. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 2015, 347, 1255957. [Google Scholar] [CrossRef]
- Kennedy, C.M.; Lonsdorf, E.; Neel, M.C.; Williams, N.M.; Ricketts, T.H.; Winfree, R.; Bommarco, R.; Brittain, C.; Burley, A.L.; Cariveau, D. A global quantitative synthesis of local and landscape effects on wild bee pollinators in agroecosystems. Ecol. Lett. 2013, 16, 584–599. [Google Scholar] [CrossRef] [PubMed]
- Koh, I.; Lonsdorf, E.V.; Williams, N.M.; Brittain, C.; Isaacs, R.; Gibbs, J.; Ricketts, T.H. Modeling the status, trends, and impacts of wild bee abundance in the United States. Proc. Natl. Acad. Sci. USA 2016, 113, 140–145. [Google Scholar] [CrossRef]
- Settele, J.; Bishop, J.; Potts, S.G. Climate change impacts on pollination. Nat. Plants 2016, 2, 16092. [Google Scholar] [CrossRef]
- Quinlan, G.M.; Doser, J.W.; Kammerer, M.A.; Grozinger, C.M. Estimating genus-specific effects of non-native honey bees and urbanization on wild bee communities: A case study in Maryland, United States. Sci. Total Environ. 2024, 953, 175783. [Google Scholar] [CrossRef]
- Iwasaki, J.M.; Hogendoorn, K. Mounting evidence that managed and introduced bees have negative impacts on wild bees: An updated review. Curr. Res. Insect Sci. 2022, 2, 100043. [Google Scholar] [CrossRef]
- MacInnis, G.; Normandin, E.; Ziter, C.D. Decline in wild bee species richness associated with honey bee (Apis mellifera L.) abundance in an urban ecosystem. PeerJ 2023, 11, e14699. [Google Scholar] [CrossRef] [PubMed]
- Matsuzawa, T.; Kohsaka, R. Status and trends of urban beekeeping regulations: A global review. Earth 2021, 2, 933–942. [Google Scholar] [CrossRef]
- Sørensen, P.B.; Strandberg, B.; Bruus, M.; Kjær, C.; Larsen, S.; Hansen, R.R.; Damgaard, C.F.; Strandberg, M. Modelling risk of competitive effects from honeybees on wild bees. Ecol. Indic. 2020, 118, 106749. [Google Scholar] [CrossRef]
- Casanelles-Abella, J.; Moretti, M. Challenging the sustainability of urban beekeeping using evidence from Swiss cities. NPJ Urban Sustain. 2022, 2, 3. [Google Scholar] [CrossRef]
- Casanelles-Abella, J.; Fontana, S.; Fournier, B.; Frey, D.; Moretti, M. Low resource availability drives feeding niche partitioning between wild bees and honeybees in a European city. Ecol. Appl. 2023, 33, e2727. [Google Scholar] [CrossRef]
- Qin, H.R.; Bi, Z.H. SWOT Analysis and Development Countermeasures of Chinese Honey Bee Industry in Guangxi. J. Anhui Agric. Sci. 2022, 50, 227–231. (In Chinese) [Google Scholar]
- Qu, X.; Zhang, X.; Zhang, G.; Qin, H.; Zhang, H.; Tian, H.; Chen, X. Investigations on Beekeeping and Breeding of Apis cerana in China. Life 2024, 15, 9. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.F. Phylogenetic Analysis of Honeybees (Hymenoptera: Apidae: Apis) and Genetic Differentiation of Chinese Apis dorsata Fabricius. Ph.D. Thesis, Zhejiang University, Hangzhou, China, 2012. [Google Scholar]
- Friedrich, R. Morphometric Analysis and Classification. In Biogeography and Taxonomy of Honeybees; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1988; pp. 66–78. [Google Scholar]
- Meixner, M.D.; Pinto, M.A.; Bouga, M.; Kryger, P.; Ivanova, E.; Fuchs, S. Standard methods for characterising subspecies and ecotypes of Apis mellifera. J. Apic. Res. 2013, 52, 1–28. [Google Scholar] [CrossRef]
- Requier, F.; Pérez-Méndez, N.; Andersson, G.K.S.; Blareau, E.; Merle, I.; Garibaldi, L.A. Bee and non-bee pollinator importance for local food security. Trends Ecol. Evol. 2023, 38, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Willmer, P.G.; Cunnold, H.; Ballantyne, G. Insights from measuring pollen deposition: Quantifying the pre-eminence of bees as flower visitors and effective pollinators. Arthropod-Plant Interact. 2017, 11, 411–425. [Google Scholar] [CrossRef]
- Allsopp, M.H.; De Lange, W.J.; Veldtman, R. Valuing insect pollination services with cost of replacement. PLoS ONE 2008, 3, e3128. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Li, J.; Zhao, Y.; Chen, Y.; Zeng, Z. A descriptive study of the prevalence of parasites and pathogens in Chinese black honeybees. Parasitology 2015, 142, 1364–1374. [Google Scholar] [CrossRef]
- Zhou, S.; Zhu, X.; Xu, X.; Gao, J.; Zhou, B. Multivariate morphometric analysis of local and introduced populations of Apis cerana (Hymenoptera: Apidae) on Hainan Island, China. J. Apic. Res. 2018, 57, 374–381. [Google Scholar] [CrossRef]
- Wang, Y.; Zeng, B.; Deng, M.; Zhao, T.; Liao, Y.; Ren, R.; Wang, H.; Yuan, Y. Whole-genome resequencing reveals genetic diversity and adaptive evolution in Chinese honeybee (Apis cerana cerana) in Guizhou, China. Front. Genet. 2024, 15, 1352455. [Google Scholar] [CrossRef]
- Dhaliwal, N.K.; Singh, J.; Chhuneja, P.K. Comparative evaluation of Doolittle, Cupkit and Karl Jenter techniques for rearing Apis mellifera Linnaeus queen bees during breeding season. J. Appl. Nat. Sci. 2017, 9, 1658–1661. [Google Scholar] [CrossRef]
- Koeniger, N.; Koeniger, G. Reproductive isolation among species of the genus Apis. Apidologie 2000, 31, 313–339. [Google Scholar] [CrossRef]
- Ellington, C.P. The aerodynamics of hovering insect flight. I. The quasi-steady analysis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1984, 305, 1–15. [Google Scholar]
- Ferrari, A.; Tommasi, N.; Polidori, C. Urbanisation reduced body size but potentially improved flight performance in bees and wasps. Basic Appl. Ecol. 2024, 74, 57–65. [Google Scholar] [CrossRef]
- Evans, S.W. The wing morphology traits of resident birds that spend a large amount of time per day flying are similar to those of migrant birds. J. Ornithol. 2021, 162, 765–778. [Google Scholar] [CrossRef]
- Noll, F.; Justino, C.; Almeida, E.; Mateus, S.; Billen, J. Would wax glands help us to understand the relationships among corbiculate bees? Insectes Sociaux 2021, 68, 191–197. [Google Scholar] [CrossRef]
- Ken, T.; Fuchs, S.; Koeniger, N.; Ruiguang, Z. Morphological characterization of Apis cerana in the Yunnan Province of China. Apidologie 2003, 34, 553–561. [Google Scholar] [CrossRef]
- Montero-Mendieta, S.; Tan, K.; Christmas, M.J.; Olsson, A.; Vilà, C.; Wallberg, A.; Webster, M.T. The genomic basis of adaptation to high-altitude habitats in the eastern honey bee (Apis cerana). Mol. Ecol. 2019, 28, 746–760. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, X.; Zhang, X.; Sun, T.; Qiu, Z.; Lu, Q.; Bi, Z.; Qin, H.; Hu, J.; Tang, P.; Cao, L.; et al. Investigation and Study on the Biology and Morphology of Apis florea and Apis dorsata in Southern China. Life 2025, 15, 341. https://doi.org/10.3390/life15030341
Qu X, Zhang X, Sun T, Qiu Z, Lu Q, Bi Z, Qin H, Hu J, Tang P, Cao L, et al. Investigation and Study on the Biology and Morphology of Apis florea and Apis dorsata in Southern China. Life. 2025; 15(3):341. https://doi.org/10.3390/life15030341
Chicago/Turabian StyleQu, Xinying, Xinru Zhang, Tian Sun, Zequn Qiu, Qihuang Lu, Zhenghui Bi, Hanrong Qin, Junjun Hu, Peng Tang, Lianfei Cao, and et al. 2025. "Investigation and Study on the Biology and Morphology of Apis florea and Apis dorsata in Southern China" Life 15, no. 3: 341. https://doi.org/10.3390/life15030341
APA StyleQu, X., Zhang, X., Sun, T., Qiu, Z., Lu, Q., Bi, Z., Qin, H., Hu, J., Tang, P., Cao, L., & Chen, X. (2025). Investigation and Study on the Biology and Morphology of Apis florea and Apis dorsata in Southern China. Life, 15(3), 341. https://doi.org/10.3390/life15030341






