Effect of Butyric Salt Supplementations, as Metabiotics, on Rumen Functions in Pre-Weaned Dairy Calves
Abstract
1. Introduction
2. Materials and Methods
2.1. Herd and Animals
2.2. Experimental Design
2.3. Quantification of Feed Consumption
2.4. Determination of the Calves’ Body Weight
2.5. Blood Sampling and Determination of the Complete Blood Count
2.6. Determination of Total Proteins
2.7. Fecal Sampling and Parasitological Procedures
2.8. Rumen Tissue Sampling and Histomorphological Examination
2.9. Statistical Analysis
3. Results
3.1. Zootechnical Findings
3.2. Hematological Findings
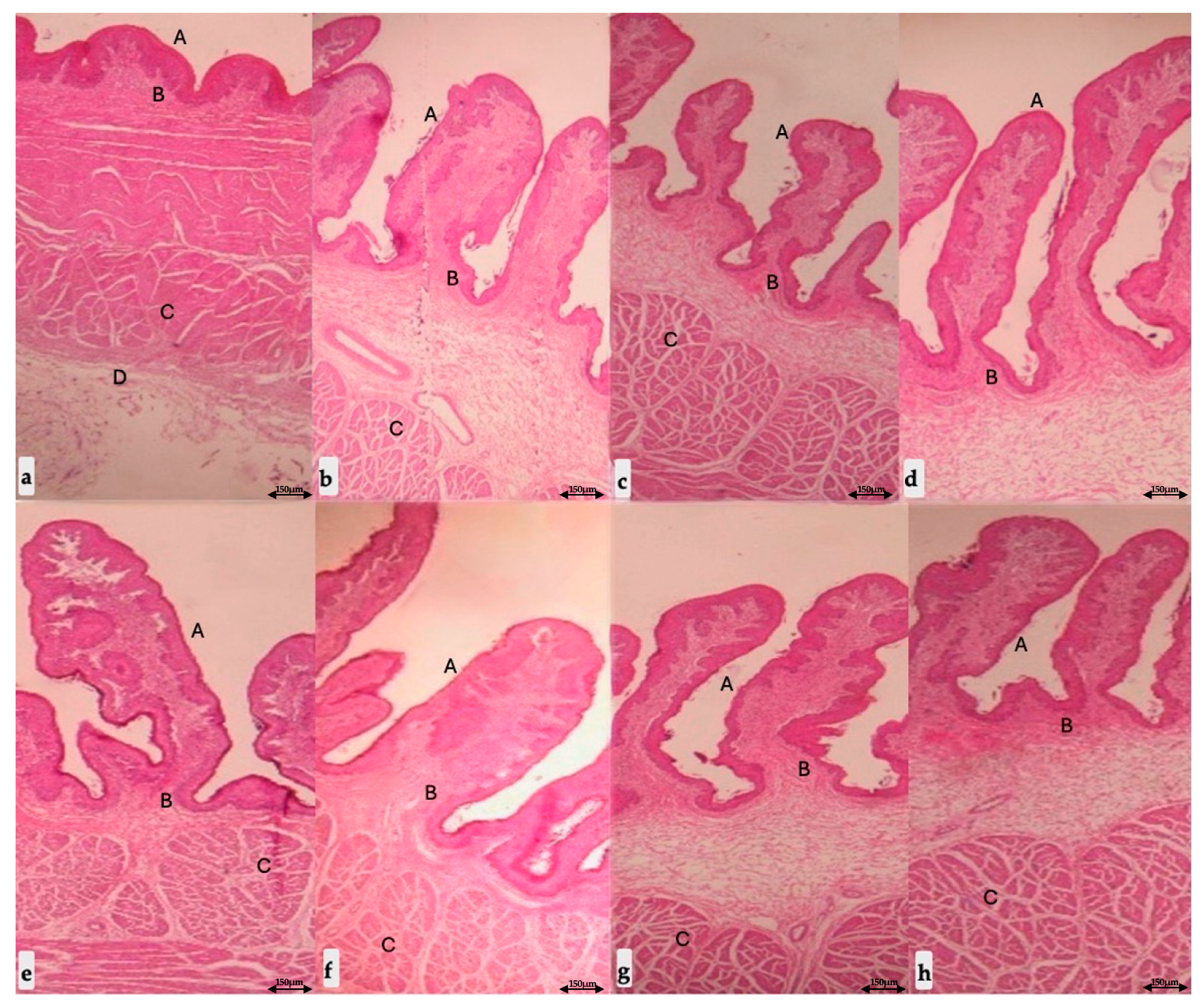
3.3. Gross Measurements of Rumen Tissue and Histomorphological Findings
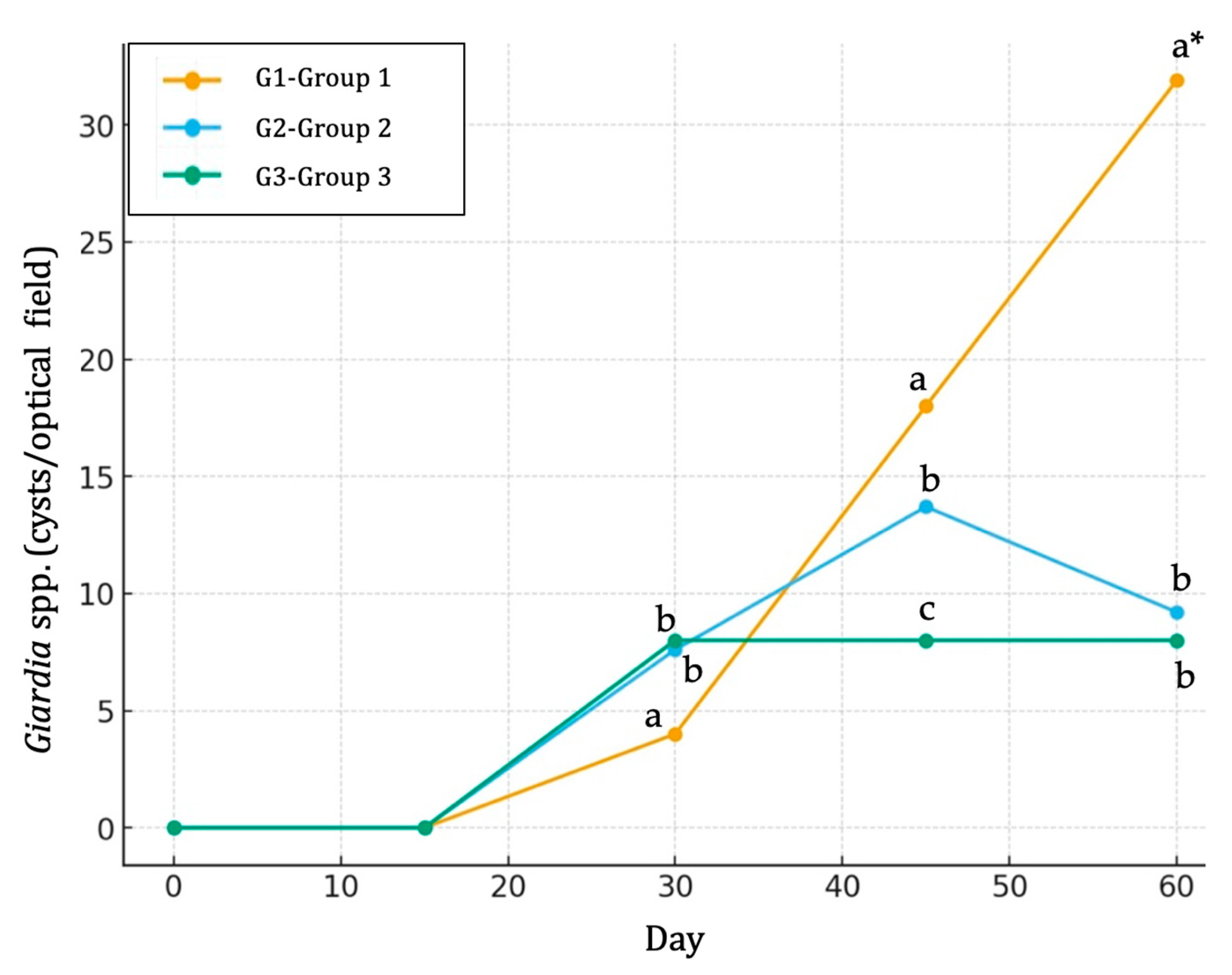
3.4. Parasitological Findings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Parsons, S.D.; Allison, C.D. Grazing management as it affects nutrition, animal production and economics of beef production. Vet. Clin. N. Am. Food Anim. Pract. 1999, 7, 77–94. [Google Scholar] [CrossRef] [PubMed]
- Van Ackeren, C.; Steinga, H.; Hartung, K.; Funk, R.; Drochner, W. Effect of roughage level in a total mixed ration on feed intake, ruminal fermentation patterns and chewing activity of early-weaned calves with ad libitum access to grass hay. Anim. Feed Sci. Technol. 2009, 153, 48–59. [Google Scholar] [CrossRef]
- Wickramasinghe, H.; Kramer, A.; Appuhamy, J. Drinking water intake of newborn dairy calves and its effects on feed intake, growth performance, health status and nutrient digestibility. J. Dairy Sci. 2019, 102, 377–387. [Google Scholar] [CrossRef]
- Coverdale, J.; Tyler, H.; Quigley, J.; Brumm, J. Effect of various levels of forage and form of diet on rumen development and growth in calves. J. Dairy Sci. 2004, 87, 2554–2562. [Google Scholar] [CrossRef]
- Dieho, K.; Bannink, A.; Geurts, I.A.L.; Schonewille, J.T.; Gort, G.; Dijkstra, J. Morphological adaptation of rumen papillae during the dry period and early lactation as affected by rate of increase of concentrate allowance. J. Dairy Sci. 2016, 99, 2339–2352. [Google Scholar] [CrossRef] [PubMed]
- Suarez, B.; Van Reenen, C.; Stockhofe, N.; Dijkstra, J.; Gerrits, W. Effect of roughage source and roughage to concentrate ratio on animal performance and rumen development in veal calves. J. Dairy Sci. 2007, 90, 2390–2403. [Google Scholar] [CrossRef] [PubMed]
- Van Niekerk, J.; Middeldorp, M.; Guan, L.; Steele, M. Preweaning to postweaning rumen papillae structural growth, ruminal fermentation characteristics, and acute-phase proteins in calves. J. Dairy Sci. 2021, 104, 3632–3645. [Google Scholar] [CrossRef]
- Lam, S.; Munro, J.; Zhou, M.; Guan, L.; Schenkel, F.S.; Steele, M.A.; Miller, S.P.; Montanholi, Y.R. Associations of rumen parameters with feed efficiency and sampling routine in beef cattle. Animal 2018, 12, 1442–1450. [Google Scholar] [CrossRef]
- Nemati, M.; Amanlou, H.; Khorvash, M.; Moshiri, B.; Mirzaei, M.; Khan, M.A.; Ghaffari, M.H. Rumen fermentation, blood metabolites, and growth performance of calves during transition from liquid to solid feed: Effects of dietary level and particle size of alfalfa hay. J. Dairy Sci. 2015, 98, 7131–7141. [Google Scholar] [CrossRef]
- Hu, R.; Zou, H.; Wang, Z.; Cao, B.; Peng, Q.; Jing, X.; Wang, Y.; Shao, Y.; Pei, Z.; Zhang, X.; et al. Nutritional interventions improved rumen functions and promoted compensatory growth of growth-retarded yaks as revealed by integrated transcripts and microbiome analyses. Front. Microbiol. 2019, 10, 318. [Google Scholar] [CrossRef]
- Oleskin, A.V.; Shenderov, B.A. Neuromodulatory effects and targets of the SCFAs and gasotransmitters produced by the human symbiotic microbiota. Microb. Ecol. Health Dis. 2016, 27, 30971. [Google Scholar] [CrossRef]
- Guilloteau, P.; Martin, L.; Eeckhaut, V.; Ducatelle, R.; Zabielski, R.; Van Immerseel, F. From the gut to the peripheral tissues: The multiple effects of butyrate. Nutr. Res. Rev. 2010, 23, 366–384. [Google Scholar] [CrossRef] [PubMed]
- Górka, P.; Kowalski, Z.M.; Pietrzak, P.; Kotunia, A.; Jagusiak, W.; Holst, J.J.; Guilloteau, P.; Zabielski, R. Effect of method of delivery of sodium butyrate on rumen development in newborn calves. J. Dairy Sci. 2011, 94, 5578–5588. [Google Scholar] [CrossRef] [PubMed]
- Malhi, M.; Gui, H.; Yao, L.; Aschenbach, J.R.; Gabel, G.; Shen, Z. Increased papillae growth and enhanced short-chain fatty acid absorption in the rumen of goats are associated with transient increases in cyclin D1 expression after ruminal butyrate infusion. J. Dairy Sci. 2013, 96, 7603–7616. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Agriculture Fisheries and Food (MAFF). Manual of Veterinary Parasitological Laboratory Techniques. Reference Book 418; HMSO: London, UK, 1986.
- Utaaker, K.S. Intestinal Protozoan Parasites in Northern India–Investigations on Transmission Routes. Ph.D. Thesis, Norwegian University of Life Sciences, Oslo, Norway, 2018. [Google Scholar]
- Steel, R.G.D.; Torrie, J.H. Principles and Procedures of Statistics. A Biometrical Approach, 2nd ed.; McGraw-Hill Book Co.: New York, NY, USA, 1980. [Google Scholar]
- Aiello, S.E.; Moses, M.A. Merck Veterinary Manual. Reference Guides, 11th ed.; Merck & Co. Inc.: Rahway, NJ, USA, 2016. [Google Scholar]
- Constable, P.D.; Hinchcliff, K.W.; Done, S.H.; Grünberg, W. Veterinary Medicine. A Textbook of the Diseases of Cattle, Horses, Sheep, Pigs, and Goats, 11th ed.; Elsevier Ltd.: Maryland Heights, MO, USA, 2017. [Google Scholar]
- Lesmeister, K.; Tozer, P.; Heinrichs, A. Development and analysis of a rumen tissue sampling procedure. J. Dairy Sci. 2004, 87, 1336–1344. [Google Scholar] [CrossRef]
- Sato, T.; Hidaka, K.; Mishima, T.; Nibe, K.; Kitahara, G.; Hidaka, Y.; Katamoto, H.; Kamimura, S. Effect of sugar supplementation on rumen protozoa profile and papillae development in retarded growth calves. J. Vet. Med. Sci. 2010, 72, 1471–1474. [Google Scholar] [CrossRef]
- Reddy, K.; Jeong, J.; Baek, Y.C.; Oh, Y.; Kim, M.; So, K.; Kim, M.J.; Kim, D.W.; Park, S.; Lee, H.J. Early weaning of calves after different dietary regimens affects later rumen development, growth, and carcass traits in Hanwoo cattle. Asian-Australas. J. Anim. Sci. 2017, 30, 1425–1434. [Google Scholar] [CrossRef]
- Castells, L.; Bach, A.; Aris, A.; Terre, M. Effects of forage provision to young calves on rumen fermentation and development of the gastrointestinal tract. J. Dairy Sci. 2013, 96, 5226–5236. [Google Scholar] [CrossRef]
- Lin, X.; Wang, Y.; Wang, J.; Hou, Q.; Hu, Z.; Shi, K.; Yan, Z.; Wang, Z. Effect of initial time of forage supply on growth and rumen development in preweaning calves. Anim. Prod. Sci. 2018, 58, 2224–22232. [Google Scholar] [CrossRef]
- Beiranvand, H.; Ghorbani, G.; Khorvash, M.; Nabipour, A.; Dehghan-Banadaky, M.; Homayouni, A.; Kargar, S. Interactions of alfalfa hay and sodium propionate on dairy calf performance and rumen development. J. Dairy Sci. 2014, 97, 2270–2280. [Google Scholar] [CrossRef]
- Novak, T.; Rodriguez-Zas, S.; Southey, B.; Starkey, J.; Stockler, R.; Alfaro, G.; Moisa, S. Jersey steer ruminal papillae histology and nutrigenomics with diet changes. J. Anim. Physiol. Anim. Nutr. 2019, 103, 1694–1707. [Google Scholar] [CrossRef]
- Beharka, A.; Nagaraja, T.; Morrill, J.; Kennedy, G.; Klemm, R. Effects of form of the diet on anatomical, microbial, and fermentative development of the rumen of neonatal calves. J. Dairy Sci. 1998, 81, 1946–1955. [Google Scholar] [CrossRef]
- Castro-Flores, P.; Elizondo-Salazar, J.A. Growth and rumen development in calves fed starter submitted to different processing. Agron. Mesoam. 2012, 23, 343–352. [Google Scholar] [CrossRef]
- Khan, M.; Lee, H.J.; Lee, W.S.; Kim, H.S.; Kim, S.B.; Park, S.; Baek, K.; Ha, J.; Choi, Y. Starch Source Evaluation in Calf Starter: Ii. Ruminal Parameters, Rumen Development, Nutrient Digestibilities, and Nitrogen Utilization in Holstein Calves. J. Dairy Sci. 2008, 91, 1140–1149. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.; Kincaid, R.; Hodgson, A.; Harrison, J.; Hillers, J.; Cronrath, J. Dietary fiber and early weaning on growth and rumen development of calves. J. Dairy Sci. 1987, 70, 2095–2104. [Google Scholar] [CrossRef]
- Zitnan, R.; Kuhla, S.; Sanftleben, P.; Bilska, A.; Scheneider, F.; Zupcanova, M.; Voigt, J. Diet induced ruminal papillae development in neonatal calves not correlating with rumen butyrate. Vet. Med. 2005, 20, 472–479. [Google Scholar] [CrossRef]
- Bach, A.; Gimenez, A.; Juaristi, J.; Ahedo, J. Effects of physical from of a starter for dairy replacement calves on feed intake and performance. J. Dairy Sci. 2007, 90, 3028–3033. [Google Scholar] [CrossRef]
- Elizondo-Salazar, J.A. Alimentación y manejo del calostro en el ganado de leche. Agron. Mesoam. 2007, 18, 271–281. [Google Scholar] [CrossRef]
- Laarman, A.; Oba, M. Effect of calf starter on rumen pH of Holstein dairy calves at weaning. J. Dairy Sci. 2011, 94, 5661–5664. [Google Scholar] [CrossRef]
- Elizondo-Salazar, J.A.; Sanchez-Alvarez, M. Efecto del consumo de dieta líquida y alimento balanceado sobre el crecimiento y desarrollo ruminal en terneras de lechería. Agron. Costarric. 2012, 36, 81–90. [Google Scholar] [CrossRef]
- Schaff, C.; Gruse, J.; Maciej, J.; Pfuhl, R.; Zitnan, R.; Rajsky, M.; Hammon, H. Effects of feeding unlimited amounts of milk replacer for the first 5 weeks of age on rumen and small intestinal growth and development in dairy calves. J. Dairy Sci. 2018, 101, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Aschenbach, J.; Zebeli, Q.; Patra, A.; Greco, G.; Amasheh, S.; Penner, G. Symposium review: The importance of the ruminal epithelial barrier for a healthy and productive cow. J. Dairy Sci. 2019, 102, 1866–1882. [Google Scholar] [CrossRef]
- Vital, M.; Howe, A.C.; Tiedje, J.M. Revealing the bacterial butyrate synthesis pathways by analyzing (meta)genomic data. mBio 2018, 5, e00889. [Google Scholar] [CrossRef] [PubMed]
- Robi, D.T.; Mossie, T.; Temteme, S. Eukaryotic infections in dairy calves: Impacts, diagnosis and strategies for prevention and control. Vet. Med. 2023, 14, 195–208. [Google Scholar] [CrossRef]
- Urie, N.J.; Lombard, J.E.; Shivley, C.B.; Adams, A.E.; Kopral, C.A.; Santin, M. Preweaned heifer management on US dairy operations: Part III. Factors associated with Cryptosporidium and Giardia in preweaned dairy heifer calves. J. Dairy Sci. 2018, 101, 9199–9213. [Google Scholar] [CrossRef]
- Zhang, L.; Jin, Y.; Wang, H.; Guo, X.; Yang, Z.; Zhao, L. Dietary sodium butyrate attenuates intestinal inflammation in weaned pigs by modulating intestinal microbiota and barrier function. Sci. Rep. 2019, 9, 1–11. [Google Scholar]
- Khan, M.A.; Bach, A.; Weary, D.M.; von Keyserlingk, M.A.G. Invited review: Transitioning from milk to solid feed in dairy heifers. J. Dairy Sci. 2016, 99, 885–902. [Google Scholar] [CrossRef]
- Louis, P.; Flint, H.J. Formation of propionate and butyrate by the human colonic microbiota. Environ. Microbiol. 2017, 19, 29–41. [Google Scholar] [CrossRef]
- Peng, L.; Li, Z.R.; Green, R.S.; Holzman, I.R.; Lin, J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J. Nutr. 2009, 139, 1619–1625. [Google Scholar] [CrossRef]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [PubMed]
- Corrêa-Oliveira, R.; Fachi, J.L.; Vieira, A.; Sato, F.T.; Vinolo, M.A.R. Regulation of immune cell function by short-chain fatty acids. Clin. Transl. Immunol. 2016, 5, e73. [Google Scholar] [CrossRef] [PubMed]


| Week | WM/MR (L) |
|---|---|
| 1st | 4.5–6.0 |
| 2nd | 7.0 |
| 3rd | 7.0 |
| 4th | 7.0 |
| 5th | 7.0 |
| 6th | 7.0 |
| 7th | 5.0 |
| 8th | 2.0 |
| 9th | 0.0 |
| Treatment 1 | SEM | |||
|---|---|---|---|---|
| G1 | G2 | G3 | ||
| Initial BW (day of birth, kg) | 47.1 a | 47.4 a | 46.0 a | 1.10 |
| Final BW (60 day of age, kg) | 80.1 a | 87.3 b | 93.9 c | 1.73 |
| BW gain (g/day) | 550 a | 665 b | 798 c,* | 71.66 |
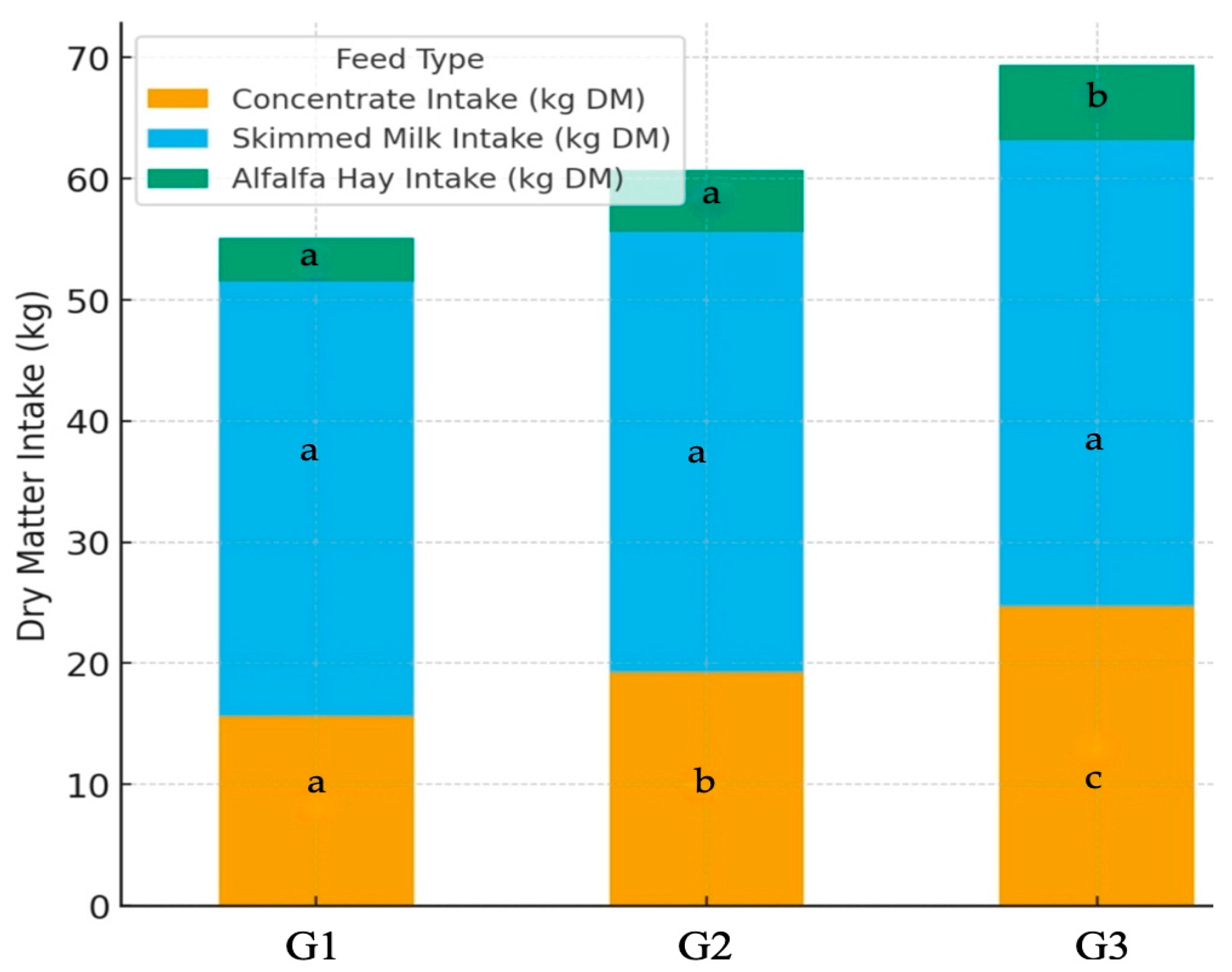
| Concentrate mixture (expressed as DM intake, kg) | 15.6 a | 19.2 b | 24.7 c | 2.65 |
| Milk replacer (expressed as DM intake, kg) | 35.9 a | 36.4 a | 38.5 a | 0.79 |
| Alfalfa hay (expressed as DM intake, kg) | 3.6 a | 5.1 a | 6.2 b | 0.75 |
| Sum of total DM intake 2 (kg) | 55.1 a | 60.7 a | 69.4 b | 4.16 |
| FCR (kg DM intake/kg BW gain) | 1.67 a | 1.52b | 1.45 c,* | 0.07 |
| Hematological Parameters | Reference Ranges 1 | Treatment 2 | SEM | ||
|---|---|---|---|---|---|
| G1 | G2 | G3 | |||
| Hematocrit (PCV, %) | 24–46 | 26.0 a | 27.1 a | 30.7 b | 1.27 |
| Hemoglobin (HGB, n × 10 g/L) | 8–15 | 10.1 a | 9.4 a | 10.0 a | 0.46 |
| Red blood cells (RBC, n × 1012/L) | 5–10 | 7.4 a | 6.8 a | 7.8 a | 0.30 |
| Mean corpuscular volume (MCV, fL = 10−15 L) | 40–60 | 37.9 a | 37.8 a | 39.6 a | 0.56 |
| Mean corpuscular hemoglobin (MCH, pg) | 11–17 | 12.0 a | 13.0 a | 13.4 a | 0.39 |
| Mean corpuscular hemoglobin concentration (MCHC, n × 10 g/L) | 30–36 | 32.1 a | 33.4 a | 33.7 a | 0.56 |
| Red cell distribution width (RDW, %) | 15.5–19.7 | 21.5 a | 22.0 a | 20.2 a | 0.57 |
| Platelets (n × 109/L) | 100–800 | 597 a | 628 a | 432 a | 47.9 |
| Mean platelet volume (MPV, fL = 10−15 L) | 3.5–6.5 | 6.2 a | 6.4 a | 7.3 a | 0.20 |
| White blood cells (WBC, n × 109/L) | 4–12 | 9.1 a | 10.2 a | 10.2 a | 0.58 |
| Neutrophils (%) | 15–33 | 47.3 a | 48.9 a | 55.9 b | 1.96 |
| Neutrophils (n × 109/L) | 0.6–4.0 | 4.3 a | 4.1 a | 5.8 a | 0.43 |
| Lymphocytes (%) | 45–75 | 39.5 a | 34.9 a | 34.4 a | 1.77 |
| Lymphocytes (n × 109/L) | 2.5–7.5 | 3.7 a | 3.4 a | 3.4 a | 0.26 |
| Monocytes (%) | 0–8 | 3.2 a | 3.5 a | 6.7 b | 0.56 |
| Monocytes (n × 109/L) | 0–0.9 | 0.3 a | 0.3 a | 0.7 b | 0.06 |
| Eosinophils (%) | 0–20 | 5.6 b | 1.9 a | 2.0 a | 0.61 |
| Eosinophils (n × 109/L) | 0–2.4 | 0.4 b | 0.2 a | 0.2 a | 0.04 |
| Basophils (%) | 0–2 | 0.3 a | 0.5 a | 1.0 b | 0.06 |
| Basophils (n × 109/L) | 0–0.2 | 0.1 a | 0.1 a | 0.1 a | 0.01 |
| Total proteins (g/dL) | 5.7–8.1 | 6.6 a | 6.7 a | 7.8 b | 0.19 |
| Hematological Parameters | Reference Ranges 1 | Treatment 2 | SEM | ||
|---|---|---|---|---|---|
| G1 | G2 | G3 | |||
| Hematocrit (PCV, %) | 24–46 | 28.8 a | 35.6 b | 32.6 b | 0.96 |
| Hemoglobin (HGB, n × 10 g/L) | 8–15 | 10.6 a | 11.7 b | 11.9 b | 0.20 |
| Red blood cells (RBC, n × 1012/L) | 5–10 | 11.6 a | 11.9 a | 11.1 a | 0.18 |
| Mean corpuscular volume (MCV, fL = 10−15 L) | 40–60 | 29.3 a | 30.8 a | 30.7 a | 0.47 |
| Mean corpuscular hemoglobin (MCH, pg) | 11–17 | 11.1 a | 10.6 a | 11.2 a | 0.30 |
| Mean corpuscular hemoglobin concentration (MCHC, n × 10 g/L) | 30–36 | 31.7 a | 33.9 a,b | 35.7 b | 0.50 |
| Red cell distribution width (RDW, %) | 15.5–19.7 | 20.5 a | 22.0 a | 21.8 a | 0.38 |
| Platelets (n × 109/L) | 100–800 | 508 a | 582 a | 565 a | 22.5 |
| Mean platelet volume (MPV, fL = 10−15 L) | 3.5–6.5 | 6.3 a | 6.3 a | 6.6 a | 0.21 |
| White blood cells (WBC, n × 109/L) | 4–12 | 11.1 a | 11.4 a | 10.5 a | 0.49 |
| Neutrophils (%) | 15–33 | 39.0 a | 34.3 a | 27.7 b | 2.32 |
| Neutrophils (n × 109/L) | 0.6–4.0 | 4.3 a | 4.3 a | 3.0 a | 0.42 |
| Lymphocytes (%) | 45–75 | 52.5 a | 56.5 a | 60.3 a | 2.02 |
| Lymphocytes (n × 109/L) | 2.5–7.5 | 5.9 a | 6.1 a | 6.2 a | 0.14 |
| Monocytes (%) | 0–8 | 5.4 a | 5.9 a | 8.3 b | 0.60 |
| Monocytes (n × 109/L) | 0–0.9 | 0.6 a | 0.7 a | 0.8 a | 0.05 |
| Eosinophils (%) | 0–20 | 1.6 a | 1.7 a | 1.9 a | 0.16 |
| Eosinophils (n × 109/L) | 0–2.4 | 0.2 a | 0.2a | 0.2 a | 0.02 |
| Basophils (%) | 0–2 | 1.4 a | 1.6 a | 1.8 a | 0.15 |
| Basophils (n × 109/L) | 0–0.2 | 0.2 a | 0.2 a | 0.2 a | 0.02 |
| Total proteins (g/dL) | 5.7–8.1 | 6.7 a | 6.8 a | 6.7 a | 0.18 |
| Rumen | Group | Anatomical Region | Mean (mm) | SD | ||||
|---|---|---|---|---|---|---|---|---|
| Parameter | Structure | Dorsal Sac (mm) | Ventral Sac (mm) | Caudodorsal Sac (mm) | Caudoventral Sac (mm) | |||
| Length | Rumen papillae | G1 (n = 10) | 1.00 | 1.55 | 1.44 | 1.44 | 1.36 a | 0.244 |
| G2 (n = 10) | 1.55 | 1.95 | 1.94 | 2.21 | 1.91 b | 0.272 | ||
| G3 (n = 10) | 2.60 | 3.57 | 3.12 | 3.50 | 3.20 c,* | 0.444 | ||
| Thickness | Wall | G1 (n = 10) | 3.54 | 3.46 | 3.51 | 4.00 | 3.62 a | 0.251 |
| G2 (n = 10) | 4.32 | 5.34 | 5.34 | 5.23 | 5.06 b | 0.494 | ||
| G3 (n = 10) | 5.87 | 8.06 | 6.87 | 7.51 | 7.08 c,* | 0.941 | ||
| Muscular | G1 (n = 10) | 2.00 | 1.43 | 1.53 | 2.00 | 1.74 a | 0.303 | |
| G2 (n = 10) | 2.14 | 1.98 | 1.66 | 2.57 | 2.09 a | 0.378 | ||
| G3 (n = 10) | 2.50 | 3.02 | 2.89 | 3.49 | 2.97 b,* | 0.408 | ||
| Serous | G1 (n = 10) | 0.50 | 0.50 | 0.53 | 0.50 | 0.51 a | 0.015 | |
| G2 (n = 10) | 0.66 | 0.60 | 0.59 | 0.60 | 0.61 a | 0.032 | ||
| G3 (n = 10) | 0.99 | 1.00 | 1.02 | 1.00 | 1.00 b,* | 0.013 | ||
| Group | Day | Giardia spp. | Eimeria spp. | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prevalence (%) | Score | Mean | SD | Prevalence (%) | Mean | SD | |||||||
| 1 | 2 | 3 | 4 | (cysts/Optical Field) | (OPG) | ||||||||
| G1 | 0 | 0.0 | 0/10 | 0 | 0 | 0 | 0 | 0.0 a | 0.00 | 0.0 | 0/10 | 0.0 a | 0.00 |
| 15 | 0.0 | 0/10 | 0 | 0 | 0 | 0 | 0.0 a | 0.00 | 0.0 | 0/10 | 0.0 a | 0.00 | |
| 30 | 40.0 | 4/10 | 4 | 0 | 0 | 0 | 4.0 a | 0.50 | 80.0 | 8/10 | 1068.8 a | 168.89 | |
| 45 | 60.0 | 6/10 | 4 | 2 | 0 | 0 | 18.0 a | 15.37 | 100.0 | 10/10 | 2435.0 a | 515.35 | |
| 60 | 80.0 | 8/10 | 3 | 4 | 1 | 0 | 31.9 a,* | 23.79 | 100.0 | 10/10 | 4870.0 a,* | 1072.43 | |
| G2 | 0 | 0.0 | 0/10 | 0 | 0 | 0 | 0 | 0.0 a | 0.00 | 0.0 | 0/10 | 0.0 a | 0.00 |
| 15 | 0.0 | 0/10 | 0 | 0 | 0 | 0 | 0.0 a | 0.00 | 0.0 | 0/10 | 0.0 a | 0.00 | |
| 30 | 30.0 | 3/10 | 3 | 0 | 0 | 0 | 7.6 b | 0.57 | 70.0 | 7/10 | 874.4 a | 145.34 | |
| 45 | 60.0 | 6/10 | 5 | 1 | 0 | 0 | 13.7 b | 11.23 | 70.0 | 7/10 | 1453.3 b | 187.34 | |
| 60 | 60.0 | 6/10 | 5 | 1 | 0 | 0 | 9.2 b | 2.93 | 80.0 | 8/10 | 1856.2 b | 123.35 | |
| G3 | 0 | 0.0 | 0/10 | 0 | 0 | 0 | 0 | 0.0 a | 0.00 | 0.0 | 0/10 | 0.0 a | 0.00 |
| 15 | 0.0 | 0/10 | 0 | 0 | 0 | 0 | 0.0 a | 0.00 | 0.0 | 0/10 | 0.0 a | 0.00 | |
| 30 | 40.0 | 4/10 | 4 | 0 | 0 | 0 | 8.0 b | 0.82 | 70.0 | 7/10 | 567.3 b | 123.23 | |
| 45 | 50.0 | 5/10 | 5 | 0 | 0 | 0 | 8.0 c | 0.71 | 90.0 | 9/10 | 1238.6 b | 110.45 | |
| 60 | 10.0 | 1/10 | 1 | 0 | 0 | 0 | 8.0 b | 0.00 | 100.0 | 10/10 | 1756.4 b | 86.67 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arsenopoulos, K.V.; Nikolaou, G.; Papadopoulos, E. Effect of Butyric Salt Supplementations, as Metabiotics, on Rumen Functions in Pre-Weaned Dairy Calves. Life 2025, 15, 1820. https://doi.org/10.3390/life15121820
Arsenopoulos KV, Nikolaou G, Papadopoulos E. Effect of Butyric Salt Supplementations, as Metabiotics, on Rumen Functions in Pre-Weaned Dairy Calves. Life. 2025; 15(12):1820. https://doi.org/10.3390/life15121820
Chicago/Turabian StyleArsenopoulos, Konstantinos V., George Nikolaou, and Elias Papadopoulos. 2025. "Effect of Butyric Salt Supplementations, as Metabiotics, on Rumen Functions in Pre-Weaned Dairy Calves" Life 15, no. 12: 1820. https://doi.org/10.3390/life15121820
APA StyleArsenopoulos, K. V., Nikolaou, G., & Papadopoulos, E. (2025). Effect of Butyric Salt Supplementations, as Metabiotics, on Rumen Functions in Pre-Weaned Dairy Calves. Life, 15(12), 1820. https://doi.org/10.3390/life15121820








