MASLD: Lipotoxicity and Imaging Parallels from Liver Steatosis to Kidney Injury
Abstract
1. Introduction: Interconnected Epidemics of MASLD and Renal Dysfunction
2. Search Strategy
3. Kidney Involvement in MASLD: Clinical and Epidemiological
4. Lipotoxicity in MASLD and Renal Injury: Shared Metabolic Parallels
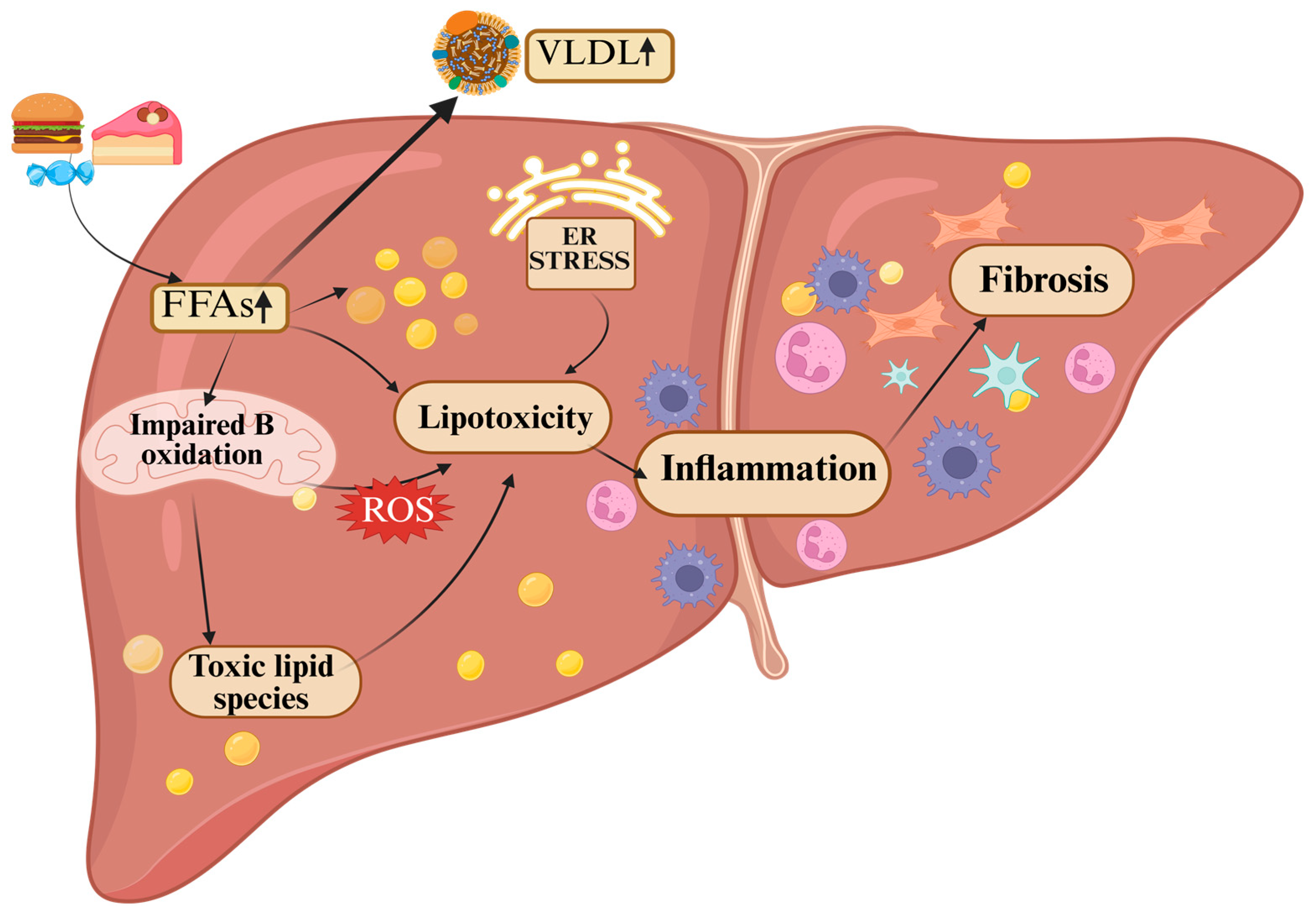
4.1. Hepatic Lipotoxicity in MASLD
4.1.1. CD36: The Inflammatory Entry Gate
4.1.2. Palmitate, Ceramides, and Diacylglycerols: Turning Fuel into Toxins
4.1.3. Organellar Breakdown
4.1.4. Immune Escalation and the Death Spiral
4.2. Renal Lipotoxicity: MASLD Parallels in Metabolic Injury
Renal Lipotoxicity Across Nephron Segments
5. Hepatorenal Biomarkers: From Liver Signals to Kidney Injury
5.1. Biomarkers of Kidney Injury
5.2. MASLD and Kidney Injury: Candidate Signals Behind the Parallels
5.2.1. Fetuin A
5.2.2. FGF21: A Stress-Induced Protector with Renal Relevance
6. Lessons from MASLD: Non-Invasive Imaging of Renal Fat
7. Discussion
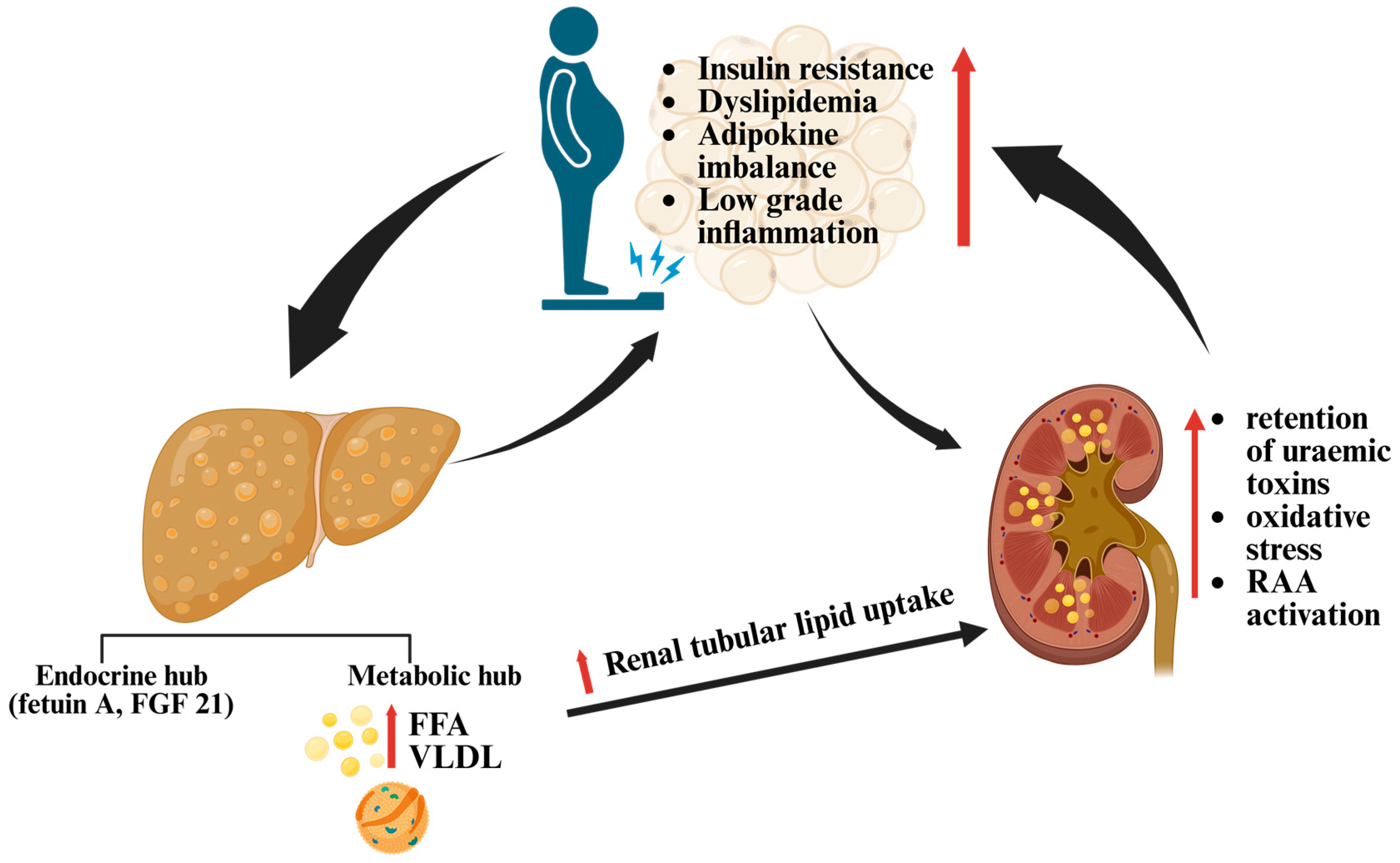
7.1. The Hepatorenal Metabolic Axis
7.2. Why Renal Fat Imaging Lags Behind Hepatic
7.3. Clinical Implications
7.4. Emerging Therapeutic Convergence
7.4.1. Lifestyle Interventions
7.4.2. Pharmacotherapy with Hepatorenal Benefits
7.5. Research Gaps and Limitations
7.6. Future Perspectives
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AKI | Acute Kidney Injury |
| BMI | Body Mass Index |
| CAP | Controlled Attenuation Parameter |
| CD36 | Cluster of Differentiation 36 |
| CKD | Chronic Kidney Disease |
| CT | Computed Tomography |
| DAG | Diacylglycerol |
| DKD | Diabetic Kidney Disease |
| EASL | European Association for the Study of the Liver |
| ECM | Extracellular Matrix |
| ER | Endoplasmic Reticulum |
| ESKD | End-Stage Kidney Disease |
| eGFR | Estimated Glomerular Filtration Rate |
| FFA | Free Fatty Acids |
| FGF21 | Fibroblast Growth Factor 21 |
| GLP-1 RAs | Agonists of the glucagon-like peptide-1 receptor |
| GLUT4 | Glucose Transporter Type 4 |
| IDEAL | Iterative Decomposition of water and fat with Echo Asymmetry and Least-squares estimation (MRI sequence) |
| IL-1β | Interleukin-1 beta |
| IL-6 | Interleukin-6 |
| IL-18 | Interleukin-18 |
| IR | Insulin Resistance |
| KDIGO | Kidney Disease: Improving Global Outcomes |
| KIM-1 | Kidney Injury Molecule-1 |
| L-FABP | Liver-type Fatty Acid-Binding Protein |
| LDL | Low-Density Lipoprotein |
| MASLD | Metabolic Dysfunction-Associated Steatotic Liver Disease |
| MASH | Metabolic Dysfunction-Associated Steatohepatitis |
| MRI | Magnetic Resonance Imaging |
| NAFLD | Non-Alcoholic Fatty Liver Disease |
| NGAL | Neutrophil Gelatinase-Associated Lipocalin |
| NLRP3 | NOD-Like Receptor Protein 3 (inflammasome) |
| Ox-LDL | Oxidized Low-Density Lipoprotein |
| PNPLA3 | Patatin-Like Phospholipase Domain-Containing 3 |
| ROS | Reactive Oxygen Species |
| RSF | Renal Sinus Fat |
| SGLT2 | Sodium-glucose cotransporter-2 |
| T2DM | Type 2 Diabetes Mellitus |
| TAG | Triacylglycerol |
| TLR4 | Toll-Like Receptor 4 |
| TNF-α | Tumor Necrosis Factor Alpha |
| UDFF | Ultrasound-Derived Fat Fraction |
| UPR | Unfolded Protein Response |
| uL-FABP | Urinary Liver-Type Fatty Acid-Binding Protein |
| uPTM-FetA | Urinary Post-Translationally Modified Fetuin-A |
| VLDL | Very-Low-Density Lipoprotein |
References
- Younossi, Z.M.; Kalligeros, M.; Henry, L. Epidemiology of metabolic dysfunction-associated steatotic liver disease. Clin. Mol. Hepatol. 2025, 31, S32–S50. [Google Scholar] [CrossRef]
- Le, M.H.; Yeo, Y.H.; Zou, B.; Barnet, S.; Henry, L.; Cheung, R.; Nguyen, M.H. Forecasted 2040 global prevalence of nonalcoholic fatty liver disease using hierarchical bayesian approach. Clin. Mol. Hepatol. 2022, 28, 841–850. [Google Scholar] [CrossRef]
- Lee, E.J.; Choi, M.; Ahn, S.B.; Yoo, J.J.; Kang, S.H.; Cho, Y.; Song, D.S.; Koh, H.; Jun, D.W.; Lee, H.W. Prevalence of nonalcoholic fatty liver disease in pediatrics and adolescents: A systematic review and meta-analysis. World J. Pediatr. WJP 2024, 20, 569–580. [Google Scholar] [CrossRef] [PubMed]
- EASL; EASD; EASO. Easl-Easd-Easo clinical practice guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD). J. Hepatol. 2024, 81, 492–542. [Google Scholar] [CrossRef] [PubMed]
- GBD 2017 DALYs and HALE Collaborators. Global, regional, and national disability-adjusted life-years (DALYS) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2017: A systematic analysis for the global burden of disease study 2017. Lancet 2018, 392, 1859–1922. [Google Scholar] [CrossRef] [PubMed]
- Kovesdy, C.P. Epidemiology of chronic kidney disease: An update 2022. Kidney Int. Suppl. 2022, 12, 7–11. [Google Scholar] [CrossRef]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology 2023, 78, 1966–1986. [Google Scholar] [CrossRef]
- Mantovani, A.; Zusi, C.; Dalbeni, A.; Grani, G.; Buzzetti, E. Risk of kidney dysfunction in NAFLD. Curr. Pharm. Des. 2020, 26, 1045–1061. [Google Scholar] [CrossRef]
- Stevens, P.E.; Ahmed, S.B.; Carrero, J.J.; Foster, B.; Francis, A.; Hall, R.K.; Levin, A. Kdigo 2024 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. 2024, 105, S117–S314. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Jafar, T.H.; Nitsch, D.; Neuen, B.L.; Perkovic, V. Chronic kidney disease. Lancet 2021, 398, 786–802. [Google Scholar] [CrossRef]
- Agustanti, N.; Soetedjo, N.N.M.; Damara, F.A.; Iryaningrum, M.R.; Permana, H.; Bestari, M.B.; Supriyadi, R. The association between metabolic dysfunction-associated fatty liver disease and chronic kidney disease: A systematic review and meta-analysis. Diabetes Metab. Syndr. 2023, 17, 102780. [Google Scholar] [CrossRef] [PubMed]
- Musso, G.; Cassader, M.; Cohney, S.; De Michieli, F.; Pinach, S.; Saba, F.; Gambino, R. Fatty liver and chronic kidney disease: Novel mechanistic insights and therapeutic opportunities. Diabetes Care 2016, 39, 1830–1845. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.; Wang, Z.; Kennedy, D.J.; Wu, Y.; Buffa, J.A.; Agatisa-Boyle, B.; Li, X.S.; Levison, B.S.; Hazen, S.L. Gut microbiota-dependent trimethylamine n-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ. Res. 2015, 116, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Taliento, A.; Zusi, C.; Baselli, G.; Prati, D.; Granata, S.; Zaza, G.; Colecchia, A.; Maffeis, C.; Byrne, C.D.; et al. PNPLA3 I148M gene variant and chronic kidney disease in type 2 diabetic patients with NAFLD: Clinical and experimental findings. Liver Int. 2020, 40, 1130–1141. [Google Scholar] [CrossRef]
- Yang, T.; Yang, B.; Yin, J.; Hou, C.; Wang, Q. Targeting insulin resistance and liver fibrosis: CKD screening priorities in MASLD. Biomedicines 2025, 13, 842. [Google Scholar] [CrossRef]
- Lonardo, A.; Nascimbeni, F.; Mantovani, A.; Targher, G. Hypertension, diabetes, atherosclerosis and NASH: Cause or consequence? J. Hepatol. 2018, 68, 335–352. [Google Scholar] [CrossRef]
- Ştefan, A.G.; Clenciu, D.; Mitrea, A.; Vladu, I.M.; Protasiewicz-Timofticiuc, D.C.; Roşu, M.M.; Maria, D.T.; Dinu, I.R.; Gheonea, T.C.; Vladu, B.E.; et al. Metabolic syndrome and insulin resistance in Romania. Int. J. Mol. Sci. 2025, 26, 2389. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, C.; Xiong, J. Pathological connections between nonalcoholic fatty liver disease and chronic kidney disease. Kidney Dis. 2022, 8, 458–465. [Google Scholar] [CrossRef]
- Kushwaha, R.; Vardhan, P.S.; Kushwaha, P.P. Chronic kidney disease interplay with comorbidities and carbohydrate metabolism: A review. Life 2023, 14, 13. [Google Scholar] [CrossRef]
- Pei, K.; Gui, T.; Li, C.; Zhang, Q.; Feng, H.; Li, Y.; Wu, J.; Gai, Z. Recent progress on lipid intake and chronic kidney disease. BioMed Res. Int. 2020, 2020, 3680397. [Google Scholar] [CrossRef]
- Sandireddy, R.; Sakthivel, S.; Gupta, P.; Behari, J.; Tripathi, M.; Singh, B.K. Systemic impacts of metabolic dysfunction-associated steatotic liver disease (MASLD) and metabolic dysfunction-associated steatohepatitis (MASH) on heart, muscle, and kidney related diseases. Front. Cell Dev. Biol. 2024, 12, 1433857. [Google Scholar] [CrossRef]
- Targher, G.; Byrne, C.D.; Tilg, H. MASLD: A systemic metabolic disorder with cardiovascular and malignant complications. Gut 2024, 73, 691–702. [Google Scholar] [CrossRef]
- Cheung, A.; Ahmed, A. Nonalcoholic fatty liver disease and chronic kidney disease: A review of links and risks. Clin. Exp. Gastroenterol. 2021, 14, 457–465. [Google Scholar] [CrossRef]
- Dissayabutra, T.; Chuaypen, N.; Somnark, P.; Boonkaew, B.; Udomkarnjananun, S.; Kittiskulnam, P.; Charoenchittang, P.; Prombutara, P.; Tangkijvanich, P. Characterization of gut dysbiosis and intestinal barrier dysfunction in patients with metabolic dysfunction-associated steatotic liver disease and chronic kidney disease: A comparative study. Sci. Rep. 2025, 15, 15481. [Google Scholar] [CrossRef] [PubMed]
- Caussy, C.; Rieusset, J.; Koppe, L. The gut microbiome and the gut-liver-kidney axis in metabolic-associated steatotic liver disease and chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2025, 20, 1626–1629. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Targher, G. PNPLA3 variation and kidney disease. Liver Int. 2025, 45, e16010. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.H.; Lee, M.Y.; Kim, S.H.; Zheng, M.H.; Byrne, C.D.; Targher, G.; Sung, K.C. Comparative associations of non-alcoholic fatty liver disease and metabolic dysfunction-associated steatotic liver disease with risk of incident chronic kidney disease: A cohort study. Hepatobiliary Surg. Nutr. 2024, 13, 801–813. [Google Scholar] [CrossRef]
- Kim, D.W.; Son, M.; Lee, H.J.; Choi, C.H.; Kang, Y.W.; Moon, S.Y.; Koh, M.; Lee, J.Y.; Baek, Y.H.; An, W.S. Chronic kidney disease risk associated with metabolic dysfunction-associated steatotic liver disease: A nationwide cohort study in Korea. Hepatol. Res. 2025, 55, 1239–1250. [Google Scholar] [CrossRef]
- Mantovani, A.; Petracca, G.; Beatrice, G.; Csermely, A.; Lonardo, A.; Schattenberg, J.M.; Tilg, H.; Byrne, C.D.; Targher, G. Non-alcoholic fatty liver disease and risk of incident chronic kidney disease: An updated meta-analysis. Gut 2022, 71, 156–162. [Google Scholar] [CrossRef]
- Liu, W.; Sun, X. Does metabolic dysfunction-associated fatty liver disease increase the risk of chronic kidney disease? A meta-analysis of cohort studies. BMC Nephrol. 2024, 25, 467. [Google Scholar] [CrossRef]
- Gurun, M.; Brennan, P.; Handjiev, S.; Khatib, A.; Leith, D.; Dillon, J.F.; Byrne, C.J. Increased risk of chronic kidney disease and mortality in a cohort of people diagnosed with metabolic dysfunction associated steatotic liver disease with hepatic fibrosis. PLoS ONE 2024, 19, e0299507. [Google Scholar] [CrossRef]
- Gao, J.; Li, Y.; Zhang, Y.; Zhan, X.; Tian, X.; Li, J.; Wang, R.; He, Y.; Wang, A.; Wu, S. Severity and remission of metabolic dysfunction-associated fatty/steatotic liver disease with chronic kidney disease occurrence. J. Am. Heart Assoc. 2024, 13, e032604. [Google Scholar] [CrossRef]
- Calinoiu, A.; Guluta, E.C.; Rusu, A.; Minca, A.; Minca, D.; Tomescu, L.; Gheorghita, V.; Minca, D.G.; Negreanu, L. Accessory renal arteries—A source of hypertension: A case report. World J. Clin. Cases 2023, 11, 1506–1512. [Google Scholar] [CrossRef]
- Gastaldelli, A.; Cusi, K. From NASH to diabetes and from diabetes to NASH: Mechanisms and treatment options. JHEP Rep. 2019, 1, 312–328. [Google Scholar] [CrossRef]
- Ren, L.; Cui, H.; Wang, Y.; Ju, F.; Cai, Y.; Gang, X.; Wang, G. The role of lipotoxicity in kidney disease: From molecular mechanisms to therapeutic prospects. Biomed. Pharmacother. 2023, 161, 114465. [Google Scholar] [CrossRef]
- Schaffer, J.E. Lipotoxicity: When tissues overeat. Curr. Opin. Lipidol. 2003, 14, 281–287. [Google Scholar] [CrossRef]
- Engin, A.B. What is lipotoxicity? Adv. Exp. Med. Biol. 2017, 960, 197–220. [Google Scholar] [PubMed]
- Bobulescu, I.A. Renal lipid metabolism and lipotoxicity. Curr. Opin. Nephrol. Hypertens. 2010, 19, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Marra, F.; Svegliati-Baroni, G. Lipotoxicity and the gut-liver axis in NASH pathogenesis. J. Hepatol. 2018, 68, 280–295. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Korf, H.; Vidal-Puig, A. An adipocentric perspective on the development and progression of non-alcoholic fatty liver disease. J. Hepatol. 2023, 78, 1048–1062. [Google Scholar] [CrossRef]
- Iturbe-Rey, S.; Maccali, C.; Arrese, M.; Aspichueta, P.; Oliveira, C.P.; Castro, R.E.; Lapitz, A.; Izquierdo-Sanchez, L.; Bujanda, L.; Perugorria, M.J.; et al. Lipotoxicity-driven metabolic dysfunction-associated steatotic liver disease (MASLD). Atherosclerosis 2025, 400, 119053. [Google Scholar] [CrossRef] [PubMed]
- Rada, P.; González-Rodríguez, Á.; García-Monzón, C.; Valverde, Á.M. Understanding lipotoxicity in NAFLD pathogenesis: Is cd36 a key driver? Cell Death Dis. 2020, 11, 802. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, C.; Luo, X.; Wang, P.; Zhou, W.; Zhong, S.; Xie, Y.; Jiang, Y.; Yang, P.; Tang, R.; et al. CD36 palmitoylation disrupts free fatty acid metabolism and promotes tissue inflammation in non-alcoholic steatohepatitis. J. Hepatol. 2018, 69, 705–717. [Google Scholar] [CrossRef] [PubMed]
- Nassir, F.; Adewole, O.L.; Brunt, E.M.; Abumrad, N.A. CD36 deletion reduces VLDL secretion, modulates liver prostaglandins, and exacerbates hepatic steatosis in ob/ob mice. J. Lipid Res. 2013, 54, 2988–2997. [Google Scholar] [CrossRef]
- Bieghs, V.; Verheyen, F.; van Gorp, P.J.; Hendrikx, T.; Wouters, K.; Lütjohann, D.; Gijbels, M.J.; Febbraio, M.; Binder, C.J.; Hofker, M.H.; et al. Internalization of modified lipids by CD36 and SR-A leads to hepatic inflammation and lysosomal cholesterol storage in Kupffer cells. PLoS ONE 2012, 7, e34378. [Google Scholar] [CrossRef]
- Schneiderhan, W.; Schmid-Kotsas, A.; Zhao, J.; Grünert, A.; Nüssler, A.; Weidenbach, H.; Menke, A.; Schmid, R.M.; Adler, G.; Bachem, M.G. Oxidized low-density lipoproteins bind to the scavenger receptor, CD36, of hepatic stellate cells and stimulate extracellular matrix synthesis. Hepatology 2001, 34, 729–737. [Google Scholar] [CrossRef]
- García-Monzón, C.; Lo Iacono, O.; Crespo, J.; Romero-Gómez, M.; García-Samaniego, J.; Fernández-Bermejo, M.; Domínguez-Díez, A.; Rodríguez de Cía, J.; Sáez, A.; Porrero, J.L.; et al. Increased soluble CD36 is linked to advanced steatosis in nonalcoholic fatty liver disease. Eur. J. Clin. Investig. 2014, 44, 65–73. [Google Scholar] [CrossRef]
- Ramos-Molina, B.; Rossell, J.; Pérez-Montes de Oca, A.; Pardina, E.; Genua, I.; Rojo-López, M.I.; Julián, M.T.; Alonso, N.; Julve, J.; Mauricio, D. Therapeutic implications for sphingolipid metabolism in metabolic dysfunction-associated steatohepatitis. Front. Endocrinol. 2024, 15, 1400961. [Google Scholar] [CrossRef]
- Schweiger, M.; Romauch, M.; Schreiber, R.; Grabner, G.F.; Hütter, S.; Kotzbeck, P.; Benedikt, P.; Eichmann, T.O.; Yamada, S.; Knittelfelder, O.; et al. Pharmacological inhibition of adipose triglyceride lipase corrects high-fat diet-induced insulin resistance and hepatosteatosis in mice. Nat. Commun. 2017, 8, 14859. [Google Scholar] [CrossRef]
- Hirsova, P.; Ibrabim, S.H.; Gores, G.J.; Malhi, H. Lipotoxic lethal and sublethal stress signaling in hepatocytes: Relevance to nash pathogenesis. J. Lipid Res. 2016, 57, 1758–1770. [Google Scholar] [CrossRef]
- Hajduch, E.; Lachkar, F.; Ferré, P.; Foufelle, F. Roles of ceramides in non-alcoholic fatty liver disease. J. Clin. Med. 2021, 10, 792. [Google Scholar] [CrossRef]
- Sunny, N.E.; Parks, E.J.; Browning, J.D.; Burgess, S.C. Excessive hepatic mitochondrial TCA cycle and gluconeogenesis in humans with nonalcoholic fatty liver disease. Cell Metab. 2011, 14, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Koliaki, C.; Szendroedi, J.; Kaul, K.; Jelenik, T.; Nowotny, P.; Jankowiak, F.; Herder, C.; Carstensen, M.; Krausch, M.; Knoefel, W.T.; et al. Adaptation of hepatic mitochondrial function in humans with non-alcoholic fatty liver is lost in steatohepatitis. Cell Metab. 2015, 21, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Fromenty, B.; Roden, M. Mitochondrial alterations in fatty liver diseases. J. Hepatol. 2023, 78, 415–429. [Google Scholar] [CrossRef] [PubMed]
- Radosavljevic, T.; Brankovic, M.; Samardzic, J.; Djuretić, J.; Vukicevic, D.; Vucevic, D.; Jakovljevic, V. Altered mitochondrial function in MASLD: Key features and promising therapeutic approaches. Antioxidants 2024, 13, 906. [Google Scholar] [CrossRef]
- Li, X.; Chen, W.; Jia, Z.; Xiao, Y.; Shi, A.; Ma, X. Mitochondrial dysfunction as a pathogenesis and therapeutic strategy for metabolic-dysfunction-associated steatotic liver disease. Int. J. Mol. Sci. 2025, 26, 4256. [Google Scholar] [CrossRef]
- Shin, S.; Kim, J.; Lee, J.Y.; Kim, J.; Oh, C.M. Mitochondrial quality control: Its role in metabolic dysfunction-associated steatotic liver disease (MASLD). J. Obes. Metab. Syndr. 2023, 32, 289–302. [Google Scholar] [CrossRef]
- Chu, Q.; Gu, X.; Zheng, Q.; Wang, J.; Zhu, H. Mitochondrial mechanisms of apoptosis and necroptosis in liver diseases. Anal. Cell. Pathol. 2021, 2021, 8900122. [Google Scholar] [CrossRef]
- Venkatesan, N.; Doskey, L.C.; Malhi, H. The role of endoplasmic reticulum in lipotoxicity during metabolic dysfunction-associated steatotic liver disease (MASLD) pathogenesis. Am. J. Pathol. 2023, 193, 1887–1899. [Google Scholar] [CrossRef]
- Tsuchida, T.; Friedman, S.L. Mechanisms of hepatic stellate cell activation. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 397–411. [Google Scholar] [CrossRef]
- Mridha, A.R.; Wree, A.; Robertson, A.A.B.; Yeh, M.M.; Johnson, C.D.; Van Rooyen, D.M.; Haczeyni, F.; Teoh, N.C.; Savard, C.; Ioannou, G.N.; et al. NLRP3 inflammasome blockade reduces liver inflammation and fibrosis in experimental NASH in mice. J. Hepatol. 2017, 66, 1037–1046. [Google Scholar] [CrossRef]
- Roy, S.; Saha, P.; Bose, D.; Trivedi, A.; More, M.; Xiao, S.; Diehl, A.M.; Chatterjee, S. Hepatic NLRP3-derived HSP70 binding to tlr4 mediates MASLD to mash progression upon inhibition of PP2A by harmful algal bloom toxin microcystin, a second hit. Int. J. Mol. Sci. 2023, 24, 16354. [Google Scholar] [CrossRef]
- Meyer, M.; Schwärzler, J.; Jukic, A.; Tilg, H. Innate immunity and MASLD. Biomolecules 2024, 14, 476. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.; Jeon, J.H.; Hong, C.W. Neutrophils in MASLD and MASH. BMB Rep. 2025, 58, 116–123. [Google Scholar] [CrossRef]
- Sawada, K.; Chung, H.; Softic, S.; Moreno-Fernandez, M.E.; Divanovic, S. The bidirectional immune crosstalk in metabolic dysfunction-associated steatotic liver disease. Cell Metab. 2023, 35, 1852–1871. [Google Scholar] [CrossRef] [PubMed]
- Mladenić, K.; Lenartić, M.; Marinović, S.; Polić, B.; Wensveen, F.M. The “domino effect” in MASLD: The inflammatory cascade of steatohepatitis. Eur. J. Immunol. 2024, 54, e2149641. [Google Scholar] [CrossRef] [PubMed]
- Moorhead, J.F.; Chan, M.K.; El-Nahas, M.; Varghese, Z. Lipid nephrotoxicity in chronic progressive glomerular and tubulo-interstitial disease. Lancet 1982, 2, 1309–1311. [Google Scholar] [CrossRef]
- Gewin, L.S. Sugar or fat? Renal tubular metabolism reviewed in health and disease. Nutrients 2021, 13, 1580. [Google Scholar] [CrossRef]
- Jiang, T.; Wang, Z.; Proctor, G.; Moskowitz, S.; Liebman, S.E.; Rogers, T.; Lucia, M.S.; Li, J.; Levi, M. Diet-induced obesity in C57BL/6J mice causes increased renal lipid accumulation and glomerulosclerosis via a sterol regulatory element-binding protein-1c-dependent pathway. J. Biol. Chem. 2005, 280, 32317–32325. [Google Scholar] [CrossRef]
- Sucedaram, Y.; Johns, E.J.; Husain, R.; Abdul Sattar, M.; Abdulla, M.H.; Nelli, G.; Rahim, N.S.; Khalilpourfarshbafi, M.; Abdullah, N.A. Exposure to high-fat style diet induced renal and liver structural changes, lipid accumulation and inflammation in intact and ovariectomized female rats. J. Inflamm. Res. 2021, 14, 689–710. [Google Scholar] [CrossRef]
- Asghari, G.; Momenan, M.; Yuzbashian, E.; Mirmiran, P.; Azizi, F. Dietary pattern and incidence of chronic kidney disease among adults: A population-based study. Nutr. Metab. 2018, 15, 88. [Google Scholar] [CrossRef] [PubMed]
- Schelling, J.R. The contribution of lipotoxicity to diabetic kidney disease. Cells 2022, 11, 3236. [Google Scholar] [CrossRef] [PubMed]
- Hao, M.; Lv, Y.; Liu, S.; Guo, W. The new challenge of obesity—Obesity-associated nephropathy. Diabetes Metab. Syndr. Obes. 2024, 17, 1957–1971. [Google Scholar] [CrossRef]
- Yang, X.; Okamura, D.M.; Lu, X.; Chen, Y.; Moorhead, J.; Varghese, Z.; Ruan, X.Z. CD36 in chronic kidney disease: Novel insights and therapeutic opportunities. Nat. Rev. Nephrol. 2017, 13, 769–781. [Google Scholar] [CrossRef]
- Xu, W.; Zhu, Y.; Wang, S.; Liu, J.; Li, H. From adipose to ailing kidneys: The role of lipid metabolism in obesity-related chronic kidney disease. Antioxidants 2024, 13, 1540. [Google Scholar] [CrossRef]
- Niu, H.; Ren, X.; Tan, E.; Wan, X.; Wang, Y.; Shi, H.; Hou, Y.; Wang, L. CD36 deletion ameliorates diabetic kidney disease by restoring fatty acid oxidation and improving mitochondrial function. Ren. Fail. 2023, 45, 2292753. [Google Scholar] [CrossRef]
- Panov, A.; Mayorov, V.I.; Dikalov, S. Metabolic syndrome and β-oxidation of long-chain fatty acids in the brain, heart, and kidney mitochondria. Int. J. Mol. Sci. 2022, 23, 4047. [Google Scholar] [CrossRef]
- Kang, H.M.; Ahn, S.H.; Choi, P.; Ko, Y.A.; Han, S.H.; Chinga, F.; Park, A.S.; Tao, J.; Sharma, K.; Pullman, J.; et al. Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nat. Med. 2015, 21, 37–46. [Google Scholar] [CrossRef]
- Opazo-Ríos, L.; Mas, S.; Marín-Royo, G.; Mezzano, S.; Gómez-Guerrero, C.; Moreno, J.A.; Egido, J. Lipotoxicity and diabetic nephropathy: Novel mechanistic insights and therapeutic opportunities. Int. J. Mol. Sci. 2020, 21, 2632. [Google Scholar] [CrossRef]
- Oh, S.H.; Kim, Y.J.; Bae, S.; Jung, H.Y.; Park, S.Y.; Lim, J.H.; Cho, J.H.; Kim, C.D.; Park, S.H.; Kwon, T.H.; et al. High-fat diet promotes lipotoxicity in the podocytes of uninephrectomized mice: A targeted lipidomics and kidney podocyte-specific analysis. Cell Death Discov. 2025, 11, 193. [Google Scholar] [CrossRef]
- Perdomo, C.M.; Martin-Calvo, N.; Ezponda, A.; Mendoza, F.J.; Bastarrika, G.; Garcia-Fernandez, N.; Herrero, J.I.; Colina, I.; Escalada, J.; Frühbeck, G. Epicardial and liver fat implications in albuminuria: A retrospective study. Cardiovasc. Diabetol. 2024, 23, 308. [Google Scholar] [CrossRef]
- Kang, S.H.; Cho, K.H.; Do, J.Y. Non-alcoholic fatty liver disease is associated with low-grade albuminuria in men without diabetes mellitus. Int. J. Med. Sci. 2019, 16, 285–291. [Google Scholar] [CrossRef]
- Zhang, B.; Song, Y.; Ma, Q.; Yang, J.; Bai, L. Expression and significance of KIM-1, NGAL, and HO-1 in patients with acute kidney injury after cardiac valve replacement. J. Inflamm. Res. 2023, 16, 2755–2761. [Google Scholar] [CrossRef] [PubMed]
- Goknar, N.; Oktem, F.; Ozgen, I.T.; Torun, E.; Kuçukkoc, M.; Demir, A.D.; Cesur, Y. Determination of early urinary renal injury markers in obese children. Pediatr. Nephrol. 2015, 30, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Rico-Fontalvo, J.; Aroca-Martínez, G.; Daza-Arnedo, R.; Cabrales, J.; Rodríguez-Yanez, T.; Cardona-Blanco, M.; Montejo-Hernández, J.; Rodelo Barrios, D.; Patiño-Patiño, J.; Osorio Rodríguez, E. Novel biomarkers of diabetic kidney disease. Biomolecules 2023, 13, 633. [Google Scholar] [CrossRef]
- Gunasekara, T.; De Silva, P.; Chandana, E.P.S.; Jayasinghe, S.; Herath, C.; Siribaddana, S.; Jayasundara, N. Body mass index and implications for pediatric kidney health: A cross-sectional study with urinary biomarkers. Pediatr. Nephrol. 2024, 39, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Panduru, N.M.; Forsblom, C.; Saraheimo, M.; Thorn, L.; Bierhaus, A.; Humpert, P.M.; Groop, P.H. Urinary liver-type fatty acid-binding protein and progression of diabetic nephropathy in type 1 diabetes. Diabetes Care 2013, 36, 2077–2083. [Google Scholar] [CrossRef]
- Kamijo-Ikemori, A.; Sugaya, T.; Yasuda, T.; Kawata, T.; Ota, A.; Tatsunami, S.; Kaise, R.; Ishimitsu, T.; Tanaka, Y.; Kimura, K. Clinical significance of urinary liver-type fatty acid-binding protein in diabetic nephropathy of type 2 diabetic patients. Diabetes Care 2011, 34, 691–696. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, Z.; Li, C.; Li, X.; Cao, Y.; Wang, Y.; Li, J.; Wang, Y.; Wang, K.; Liu, Y.; et al. Lipidomics reveals potential biomarkers and pathophysiological insights in the progression of diabetic kidney disease. Metab. Open 2025, 25, 100354. [Google Scholar] [CrossRef]
- Afshinnia, F.; Rajendiran, T.M.; Karnovsky, A.; Soni, T.; Wang, X.; Xie, D.; Yang, W.; Shafi, T.; Weir, M.R.; He, J.; et al. Lipidomic signature of progression of chronic kidney disease in the chronic renal insufficiency cohort. Kidney Int. Rep. 2016, 1, 256–268. [Google Scholar] [CrossRef]
- Watt, M.J.; Miotto, P.M.; De Nardo, W.; Montgomery, M.K. The liver as an endocrine organ-linking NAFLD and insulin resistance. Endocr. Rev. 2019, 40, 1367–1393. [Google Scholar] [CrossRef] [PubMed]
- Milani, I.; Codini, M.; Guarisco, G.; Chinucci, M.; Gaita, C.; Leonetti, F.; Capoccia, D. Hepatokines and MASLD: The GLP1-RAS-FGF21-fetuin-A crosstalk as a therapeutic target. Int. J. Mol. Sci. 2024, 25, 10795. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Chattopadhyay, D.; Chatterjee, S.K.; Mondal, S.A.; Majumdar, S.S.; Mukhopadhyay, S.; Saha, N.; Velayutham, R.; Bhattacharya, S.; Mukherjee, S. Increase in PPARγ inhibitory phosphorylation by fetuin-a through the activation of RAS-MEK-ERK pathway causes insulin resistance. Biochim. Biophys. Acta Mol. Basis Dis. 2021, 1867, 166050. [Google Scholar] [CrossRef] [PubMed]
- Elhoseeny, M.M.; Abdulaziz, B.A.; Mohamed, M.A.; Elsharaby, R.M.; Rashad, G.M.; Othman, A.A.A. Fetuin-a: A relevant novel serum biomarker for non-invasive diagnosis of metabolic dysfunction-associated steatotic liver disease (MASLD): A retrospective case-control study. BMC Gastroenterol. 2024, 24, 226. [Google Scholar] [CrossRef]
- Liu, S.; Xiao, J.; Zhao, Z.; Wang, M.; Wang, Y.; Xin, Y. Systematic review and meta-analysis of circulating fetuin-a levels in nonalcoholic fatty liver disease. J. Clin. Transl. Hepatol. 2021, 9, 3–14. [Google Scholar] [CrossRef]
- Vallés, P.G.; Gil Lorenzo, A.F.; Garcia, R.D.; Cacciamani, V.; Benardon, M.E.; Costantino, V.V. Toll-like receptor 4 in acute kidney injury. Int. J. Mol. Sci. 2023, 24, 1415. [Google Scholar] [CrossRef]
- Dagher, P.C.; Hato, T.; Mang, H.E.; Plotkin, Z.; Richardson, Q.V.; Massad, M.; Mai, E.; Kuehl, S.E.; Graham, P.; Kumar, R.; et al. Inhibition of toll-like receptor 4 signaling mitigates microvascular loss but not fibrosis in a model of ischemic acute kidney injury. Int. J. Mol. Sci. 2016, 17, 647. [Google Scholar] [CrossRef]
- Ix, J.H.; Chertow, G.M.; Shlipak, M.G.; Brandenburg, V.M.; Ketteler, M.; Whooley, M.A. Fetuin-A and kidney function in persons with coronary artery disease—Data from the heart and soul study. Nephrol. Dial. Transplant. 2006, 21, 2144–2151. [Google Scholar] [CrossRef]
- Bassey, P.E.; Numthavaj, P.; Rattanasiri, S.; Sritara, P.; McEvoy, M.; Ongphiphadhanakul, B.; Thakkinstian, A. Causal association pathways between fetuin-a and kidney function: A mediation analysis. J. Int. Med. Res. 2022, 50, 3000605221082874. [Google Scholar] [CrossRef]
- Caglar, K.; Yilmaz, M.I.; Saglam, M.; Cakir, E.; Kilic, S.; Sonmez, A.; Eyileten, T.; Yenicesu, M.; Oguz, Y.; Tasar, M.; et al. Serum fetuin-a concentration and endothelial dysfunction in chronic kidney disease. Nephron Clin. Pract. 2008, 108, c233–c240. [Google Scholar] [CrossRef]
- Cohen, E.; Fraser, A.; Goldberg, E.; Milo, G.; Garty, M.; Krause, I. Association between the body mass index and chronic kidney disease in men and women. A population-based study from Israel. Nephrol. Dial. Transplant. 2013, 28 (Suppl. S4), iv130–iv135. [Google Scholar] [CrossRef]
- Kambham, N.; Markowitz, G.S.; Valeri, A.M.; Lin, J.; D’Agati, V.D. Obesity-related glomerulopathy: An emerging epidemic. Kidney Int. 2001, 59, 1498–1509. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.T.; Tseng, W.C.; Lee, K.H.; Lin, C.C.; Ou, S.M.; Li, S.Y. Associations of urinary fetuin-a with histopathology and kidney events in biopsy-proven kidney disease. Clin. Kidney J. 2024, 17, sfae065. [Google Scholar] [CrossRef] [PubMed]
- Musolino, M.; Greco, M.; D’Agostino, M.; Tripodi, L.; Misiti, R.; Dragone, F.; Cianfrone, P.; Zicarelli, M.; Foti, D.P.; Andreucci, M.; et al. Urinary post-translationally modified fetuin-a (uPTM-FetA) in chronic kidney disease patients with and without diabetic kidney disease. Medicina 2024, 60, 363. [Google Scholar] [CrossRef] [PubMed]
- Welliam, Y.; Witarto, B.S.; Visuddho, V.; Wungu, C.D.K.; Budiarto, R.M.; Susilo, H.; Multazam, C. Osteopontin, kidney injury molecule-1, and fetuin-a as prognostic markers of end-stage renal disease: A systematic review and meta-analysis. PLoS ONE 2025, 20, e0320804. [Google Scholar] [CrossRef]
- Chuang, G.T.; Kremer, D.; Huang, C.H.; Alkaff, F.F.; Lin, C.H.; Tseng, T.L.; Laverman, G.D.; Bakker, S.J.L.; Chuang, L.M. Urinary fetuin-a fragments predict progressive estimated glomerular filtration rate decline in two independent type 2 diabetes cohorts of different ethnicities. Am. J. Nephrol. 2024, 55, 106–114. [Google Scholar] [CrossRef]
- Magalhães, P.; Zürbig, P.; Mischak, H.; Schleicher, E. Urinary fetuin-a peptides as a new marker for impaired kidney function in patients with type 2 diabetes. Clin. Kidney J. 2021, 14, 269–276. [Google Scholar] [CrossRef]
- Negroiu, C.E.; Tudoraşcu, R.I.; Beznă, M.C.; Ungureanu, A.I.; Honţaru, S.O.; Dănoiu, S. The role of FGF21 in the interplay between obesity and non-alcoholic fatty liver disease: A narrative review. Rom. J. Morphol. Embryol. 2024, 65, 159–172. [Google Scholar] [CrossRef]
- Flippo, K.H.; Potthoff, M.J. Metabolic messengers: FGF21. Nat. Metab. 2021, 3, 309–317. [Google Scholar] [CrossRef]
- Tucker, B.; McClelland, R.L.; Allison, M.A.; Budoff, M.J.; Wu, B.J.; Barter, P.J.; Rye, K.A.; Ong, K.L. Relationship of fibroblast growth factor 21 levels with inflammation, lipoproteins and non-alcoholic fatty liver disease. Atherosclerosis 2020, 299, 38–44. [Google Scholar] [CrossRef]
- Barb, D.; Bril, F.; Kalavalapalli, S.; Cusi, K. Plasma fibroblast growth factor 21 is associated with severity of nonalcoholic steatohepatitis in patients with obesity and type 2 diabetes. J. Clin. Endocrinol. Metab. 2019, 104, 3327–3336. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Yue, T.; Chen, Z.; Wu, W.; Xu, S.; Weng, J. Targeting FGF21 in cardiovascular and metabolic diseases: From mechanism to medicine. Int. J. Biol. Sci. 2023, 19, 66–88. [Google Scholar] [CrossRef] [PubMed]
- Fisher, F.M.; Chui, P.C.; Antonellis, P.J.; Bina, H.A.; Kharitonenkov, A.; Flier, J.S.; Maratos-Flier, E. Obesity is a fibroblast growth factor 21 (FGF21)-resistant state. Diabetes 2010, 59, 2781–2789. [Google Scholar] [CrossRef] [PubMed]
- Kohara, M.; Masuda, T.; Shiizaki, K.; Akimoto, T.; Watanabe, Y.; Honma, S.; Sekiguchi, C.; Miyazawa, Y.; Kusano, E.; Kanda, Y.; et al. Association between circulating fibroblast growth factor 21 and mortality in end-stage renal disease. PLoS ONE 2017, 12, e0178971. [Google Scholar] [CrossRef]
- Lin, Z.; Zhou, Z.; Liu, Y.; Gong, Q.; Yan, X.; Xiao, J.; Wang, X.; Lin, S.; Feng, W.; Li, X. Circulating FGF21 levels are progressively increased from the early to end stages of chronic kidney diseases and are associated with renal function in Chinese. PLoS ONE 2011, 6, e18398. [Google Scholar] [CrossRef]
- Suassuna, P.G.A.; de Paula, R.B.; Sanders-Pinheiro, H.; Moe, O.W.; Hu, M.C. Fibroblast growth factor 21 in chronic kidney disease. J. Nephrol. 2019, 32, 365–377. [Google Scholar] [CrossRef]
- Kim, H.W.; Lee, J.E.; Cha, J.J.; Hyun, Y.Y.; Kim, J.E.; Lee, M.H.; Song, H.K.; Nam, D.H.; Han, J.Y.; Han, S.Y.; et al. Fibroblast growth factor 21 improves insulin resistance and ameliorates renal injury in db/db mice. Endocrinology 2013, 154, 3366–3376. [Google Scholar] [CrossRef]
- Shi, Y.C.; Lu, W.W.; Hou, Y.L.; Fu, K.; Gan, F.; Cheng, S.J.; Wang, S.P.; Qi, Y.F.; Liu, J.H. Protection effect of exogenous fibroblast growth factor 21 on the kidney injury in vascular calcification rats. Chin. Med. J. 2018, 131, 532–538. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, F.; Zhang, C.; Yao, F.; Zhang, B.; Zhang, Y.; Sun, X. FGF21 ameliorates diabetic nephropathy through CDK1-dependently regulating the cell cycle. Front. Pharmacol. 2024, 15, 1500458. [Google Scholar] [CrossRef]
- Giontella, A.; Zagkos, L.; Geybels, M.; Larsson, S.C.; Tzoulaki, I.; Mantzoros, C.S.; Andersen, B.; Gill, D.; Cronjé, H.T. Renoprotective effects of genetically proxied fibroblast growth factor 21: Mendelian randomization, proteome-wide and metabolome-wide association study. Metabolism 2023, 145, 155616. [Google Scholar] [CrossRef]
- Minami, S.; Sakai, S.; Yamamoto, T.; Takabatake, Y.; Namba-Hamano, T.; Takahashi, A.; Matsuda, J.; Yonishi, H.; Nakamura, J.; Maeda, S.; et al. FGF21 and autophagy coordinately counteract kidney disease progression during aging and obesity. Autophagy 2024, 20, 489–504. [Google Scholar] [CrossRef] [PubMed]
- Mende, C.W.; Einhorn, D. Fatty kidney disease: A new renal and endocrine clinical entity? Describing the role of the kidney in obesity, metabolic syndrome, and type 2 diabetes. Endocr. Pract. 2019, 25, 854–858. [Google Scholar] [CrossRef] [PubMed]
- Foster, M.C.; Hwang, S.J.; Porter, S.A.; Massaro, J.M.; Hoffmann, U.; Fox, C.S. Fatty kidney, hypertension, and chronic kidney disease: The Framingham heart study. Hypertension 2011, 58, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Petzold, G. Role of ultrasound methods for the assessment of NAFLD. J. Clin. Med. 2022, 11, 4581. [Google Scholar] [CrossRef]
- Araújo, N.C.; Rioja Lda, S.; Rebelo, M.A. Renal parenchymal disease: Histopathologic-sonographic correlation. Rev. Assoc. Medica Bras. 2008, 54, 48–54. [Google Scholar] [CrossRef]
- Lamacchia, O.; Nicastro, V.; Camarchio, D.; Valente, U.; Grisorio, R.; Gesualdo, L.; Cignarelli, M. Para- and perirenal fat thickness is an independent predictor of chronic kidney disease, increased renal resistance index and hyperuricaemia in type-2 diabetic patients. Nephrol. Dial. Transplant. 2011, 26, 892–898. [Google Scholar] [CrossRef]
- Karlas, T.; Petroff, D.; Sasso, M.; Fan, J.G.; Mi, Y.Q.; de Lédinghen, V.; Kumar, M.; Lupsor-Platon, M.; Han, K.H.; Cardoso, A.C.; et al. Individual patient data meta-analysis of controlled attenuation parameter (cap) technology for assessing steatosis. J. Hepatol. 2017, 66, 1022–1030. [Google Scholar] [CrossRef]
- Huang, Y.; Li, J.; Liu, C.; Song, D.; Zhu, C.; Ren, Y.; Lv, J.; Jiang, L.; Shan, R.; Wang, H.; et al. Noninvasive quantification of hepatic steatosis using ultrasound-derived fat fraction (CHESS2303): A prospective multicenter study. MedComm 2025, 6, e70123. [Google Scholar] [CrossRef]
- Berzigotti, A.; Tsochatzis, E.; Boursier, J.; Castera, L.; Cazzagon, N.; Friedrich-Rust, M.; European Association for the Study of the Liver. EASL clinical practice guidelines on non-invasive tests for evaluation of liver disease severity and prognosis—2021 update. J. Hepatol. 2021, 75, 659–689. [Google Scholar] [CrossRef]
- Raphael, H.; Klang, E.; Konen, E.; Leibowitz, A.; Frenkel-Nir, Y.; Apter, S.; Grossman, E. Renal fat accumulation assessed by MRI or CT and its association with clinical and metabolic disorders: A systematic imaging review. J. Clin. Med. 2025, 14, 4305. [Google Scholar] [CrossRef]
- Lăpădat, A.M.; Florescu, L.M.; Manea, N.C.; Gheonea, D.I.; Pirici, D.; Tudoraşcu, D.R.; Ene, R.; Gheonea, I.A. MR spectroscopy of the liver—A reliable non-invasive alternative for evaluating non-alcoholic fatty liver disease. Rom. J. Morphol. Embryol. 2020, 61, 73–80. [Google Scholar] [CrossRef]
- Su, H.; Wan, C.; Lei, C.T.; Zhang, C.Y.; Ye, C.; Tang, H.; Qiu, Y.; Zhang, C. Lipid deposition in kidney diseases: Interplay among redox, lipid mediators, and renal impairment. Antioxid. Redox Signal. 2018, 28, 1027–1043. [Google Scholar] [CrossRef]
- Constantinescu, C.; SĂndulescu, L.; SĂftoiu, A. The role of elastography in non-alcoholic fatty liver disease. Curr. Health Sci. J. 2020, 46, 255–269. [Google Scholar]
- Liu, J.; Wu, Y.; Tian, C.; Zhang, X.; Su, Z.; Nie, L.; Wang, R.; Zeng, X. Quantitative assessment of renal steatosis in patients with type 2 diabetes mellitus using the iterative decomposition of water and fat with echo asymmetry and least squares estimation quantification sequence imaging: Repeatability and clinical implications. Quant. Imaging Med. Surg. 2024, 14, 7341–7352. [Google Scholar] [CrossRef]
- Dong, Y.; Zhang, Q.; Wang, X.; Liu, Y.; An, Q.; Zhang, Z.; Hu, L.; Lin, L.; Liu, A. Preliminary quantitative analysis of renal sinus fat dysfunction in t2dm patients using MRI fat fraction and r2* mapping. Front. Endocrinol. 2025, 16, 1486839. [Google Scholar] [CrossRef] [PubMed]
- Lew, Q.J.; Jafar, T.H.; Talaei, M.; Jin, A.; Chow, K.Y.; Yuan, J.M.; Koh, W.P. Increased body mass index is a risk factor for end-stage renal disease in the Chinese Singapore population. Kidney Int. 2017, 92, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.T.; Yang, P.Y.; Yang, Y.W.; Sun, H.Y.; Lin, I.C. The association of nephrolithiasis with metabolic syndrome and its components: A cross-sectional analysis. Ther. Clin. Risk Manag. 2017, 13, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Gati, A.; Kouidhi, S.; Marrakchi, R.; El Gaaied, A.; Kourda, N.; Derouiche, A.; Chebil, M.; Caignard, A.; Perier, A. Obesity and renal cancer: Role of adipokines in the tumor-immune system conflict. Oncoimmunology 2014, 3, e27810. [Google Scholar] [CrossRef]
- Liu, X.; Sun, Q.; Hou, H.; Zhu, K.; Wang, Q.; Liu, H.; Zhang, Q.; Ji, L.; Li, D. The association between bmi and kidney cancer risk: An updated dose-response meta-analysis in accordance with PRISMA guideline. Medicine 2018, 97, e12860. [Google Scholar] [CrossRef]
- Urhut, C.M.; Sandulescu, L.D.; Streba, L.; Iovanescu, V.F.; Sandulescu, S.M.; Danoiu, S. Hepatocellular carcinoma with gastrointestinal involvement: A systematic review. Diagnostics 2022, 12, 1270. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Streja, E.; Kovesdy, C.P.; Oreopoulos, A.; Noori, N.; Jing, J.; Nissenson, A.R.; Krishnan, M.; Kopple, J.D.; Mehrotra, R.; et al. The obesity paradox and mortality associated with surrogates of body size and muscle mass in patients receiving hemodialysis. Mayo Clin. Proc. 2010, 85, 991–1001. [Google Scholar] [CrossRef]
- Yurista, S.R.; Eder, R.A.; Feeley, M.; Kodur, N.; Tang, W.H.W.; Nguyen, C.T. A closer look at ACC/AHA and ESC guidelines for managing obesity and overweight in adults. JACC Adv. 2023, 2, 100570. [Google Scholar] [CrossRef] [PubMed]
- Suki, M.; Imam, A.; Amer, J.; Milgrom, Y.; Massarwa, M.; Hazou, W.; Tiram, Y.; Perzon, O.; Sharif, Y.; Sackran, J.; et al. SGLT2 inhibitors in MASLD (metabolic dysfunction-associated steatotic liver disease) associated with sustained hepatic benefits, besides the cardiometabolic. Pharmaceuticals 2025, 18, 1118. [Google Scholar] [CrossRef] [PubMed]
- Khadija, T.; Rizwan, Z.; Joel, P.; Mughal, H.M.F.; Abdul Razzak, A.; Tayyab, M.; Arif, M. The effect of statin therapy on liver enzymes and fibrosis progression in patients with coexisting cardiovascular disease and non-alcoholic fatty liver disease (NAFLD). Cureus 2025, 17, e91301. [Google Scholar] [CrossRef] [PubMed]
- Boutari, C.; Pappas, P.D.; Anastasilakis, D.; Mantzoros, C.S. Statins’ efficacy in non-alcoholic fatty liver disease: A systematic review and meta-analysis. Clin. Nutr. 2022, 41, 2195–2206. [Google Scholar] [CrossRef]
- Sanyal, A.J.; Newsome, P.N.; Kliers, I.; Østergaard, L.H.; Long, M.T.; Kjær, M.S.; Cali, A.M.G.; Bugianesi, E.; Rinella, M.E.; Roden, M.; et al. Phase 3 trial of semaglutide in metabolic dysfunction-associated steatohepatitis. N. Engl. J. Med. 2025, 392, 2089–2099. [Google Scholar] [CrossRef]
- Perkovic, V.; Tuttle, K.R.; Rossing, P.; Mahaffey, K.W.; Mann, J.F.E.; Bakris, G.; Baeres, F.M.M.; Idorn, T.; Bosch-Traberg, H.; Lausvig, N.L.; et al. Effects of semaglutide on chronic kidney disease in patients with type 2 diabetes. N. Engl. J. Med. 2024, 391, 109–121. [Google Scholar] [CrossRef]
- Vuppalanchi, R.; Loomba, R.; Sanyal, A.J.; Nikooie, A.; Tang, Y.; Robins, D.A.; Brouwers, B.; Hartman, M.L. Randomised clinical trial: Design of the SYNERGY-NASH phase 2b trial to evaluate tirzepatide as a treatment for metabolic dysfunction-associated steatohepatitis and modification of screening strategy to reduce screen failures. Aliment. Pharmacol. Ther. 2024, 60, 17–32. [Google Scholar] [CrossRef]
- Del Prato, S.; Kahn, S.E.; Pavo, I.; Weerakkody, G.J.; Yang, Z.; Doupis, J.; Aizenberg, D.; Wynne, A.G.; Riesmeyer, J.S.; Heine, R.J.; et al. Tirzepatide versus insulin glargine in type 2 diabetes and increased cardiovascular risk (surpass-4): A randomised, open-label, parallel-group, multicentre, phase 3 trial. Lancet 2021, 398, 1811–1824. [Google Scholar] [CrossRef]
| Mechanistic Module | Liver (Marker → Direction) | Kidney (Marker → Direction) |
|---|---|---|
| FA uptake | CD36 ↑ in hepatocytes/Kupffer/HSC; steatosis + inflammatory signaling [42,43,44,45,46]. | CD36 ↑ in podocyte/tubule leads to FA influx, ROS, profibrotic signaling [74,75,76] |
| Stress/adaptation hepatokine | FGF21 ↑ with metabolic stress/MASLD severity [110,111] | FGF21 ↑ in CKD [114,115] experimental renoprotection [117,118,119,120,121] |
| Inflammatory hepatokine | Fetuin-A ↑ in MASLD [94,95] | uPTM-FetA ↑ in tubular injury [105,106,107] |
| Tubular stress/injury | KIM-1 ↑ (early proximal tubular injury) [83,84,85] | |
| NAG ↑ in obese patients [84] | ||
| NGAL ↑ (AKI–CKD stress; limited lipotoxic specificity) [83,84,85] | ||
| FA-handling tubular stress | uL-FABP ↑ (early injury; precedes creatinine) [87,88] | |
| Renal lipidomics | DAGs, LPCs, PCs, PEs, LPEs ↑ in DKD [89] |
| Modality | Principle | Assessable Compartment (s) | Quantitative Capability | Clinical Feasibility | Cost |
|---|---|---|---|---|---|
| B-mode US (liver) | Qualitative hepatic echogenity; posterior beam attenuation, blurred vessels/diaphragm | Liver parenchyma | No (qualitative) | Very high | Low |
| CAP | Ultrasound attenuation (dB/m) | Liver parenchyma | Surrogate (continuous) | High | Low–Med |
| UDFF (US) | Quantitative backscatter/speed-of-sound | Liver parenchyma | Yes (fat fraction, %) | Emerging | Low–Med |
| MRI-PDFF/MRS (liver) | Chemical-shift water–fat separation/proton spectroscopy | Liver parenchyma | Yes (reference standard) | Medium | High |
| B-mode US (kidney) | Cortical echogenicity vs. liver/spleen | Renal parenchyma (echogenity; not fat) | No | Not applicable | Low |
| US renal sinus area | 2D planimetry in sinus | RSF | Semi-quantitative (area) | High | Low |
| CT (non-contrast, kidney) | Attenuation (HU) and area/volume segmentation | Perirenal & RSF | Semi-quantitative | High | Medium |
| MRI Dixon/IDEAL-IQ (kidney) | Water–fat separation (chemical-shift) | Perirenal/sinus; parenchymal (research) | Yes (research PDFF) | Low–Med | High |
| MRS (kidney) | Proton spectroscopy | Parenchymal (research) | Yes (research) | Low | High |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Săndulescu, S.M.; Ghiga, D.Ș.; Tudorașcu, D.R.; Săndulescu, D.L.; Mită, A.; Urhuț, M.C.; Taisescu, C.-I. MASLD: Lipotoxicity and Imaging Parallels from Liver Steatosis to Kidney Injury. Life 2025, 15, 1805. https://doi.org/10.3390/life15121805
Săndulescu SM, Ghiga DȘ, Tudorașcu DR, Săndulescu DL, Mită A, Urhuț MC, Taisescu C-I. MASLD: Lipotoxicity and Imaging Parallels from Liver Steatosis to Kidney Injury. Life. 2025; 15(12):1805. https://doi.org/10.3390/life15121805
Chicago/Turabian StyleSăndulescu, Sarmis Marian, Denisa Ștefania Ghiga, Diana Rodica Tudorașcu, Daniela Larisa Săndulescu, Adrian Mită, Marinela Cristiana Urhuț, and Citto-Iulian Taisescu. 2025. "MASLD: Lipotoxicity and Imaging Parallels from Liver Steatosis to Kidney Injury" Life 15, no. 12: 1805. https://doi.org/10.3390/life15121805
APA StyleSăndulescu, S. M., Ghiga, D. Ș., Tudorașcu, D. R., Săndulescu, D. L., Mită, A., Urhuț, M. C., & Taisescu, C.-I. (2025). MASLD: Lipotoxicity and Imaging Parallels from Liver Steatosis to Kidney Injury. Life, 15(12), 1805. https://doi.org/10.3390/life15121805







