Bioactive Compounds, Antibacterial, Antioxidant, Anticancer, and Antidiabetic Potential of the Seed and Leaves of Tribulus terrestris
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Ethanol SE and LE of T. terrestris
2.2. Determination of Bioactive Components
2.3. Determination of Total Phenolic Content (TPC) and Total Flavonoid Content (TFC)
2.4. Antibacterial Activity
2.4.1. Disc Diffusion Method
2.4.2. Determination of Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)Values
2.5. Antioxidant Activity
2.5.1. Diphenyl-1-Picrylhydrazyl (DPPH) Radical Scavenging Activity
2.5.2. 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) Assay
2.6. Cell Culture and Cytotoxicity Assays
2.7. Antidiabetic Activity
2.7.1. Determination of α-Amylase Inhibitory Activity
2.7.2. Determination of α-Glucosidase Inhibitory Activity
2.8. Statistical Analysis
3. Results
3.1. Extraction Yields
3.2. Chemical Composition of the T. terrestris LE and SE
3.3. TPC and TFC of T. terrestris LE and SE
3.4. Antibacterial Effects of T. terrestris LE and SE
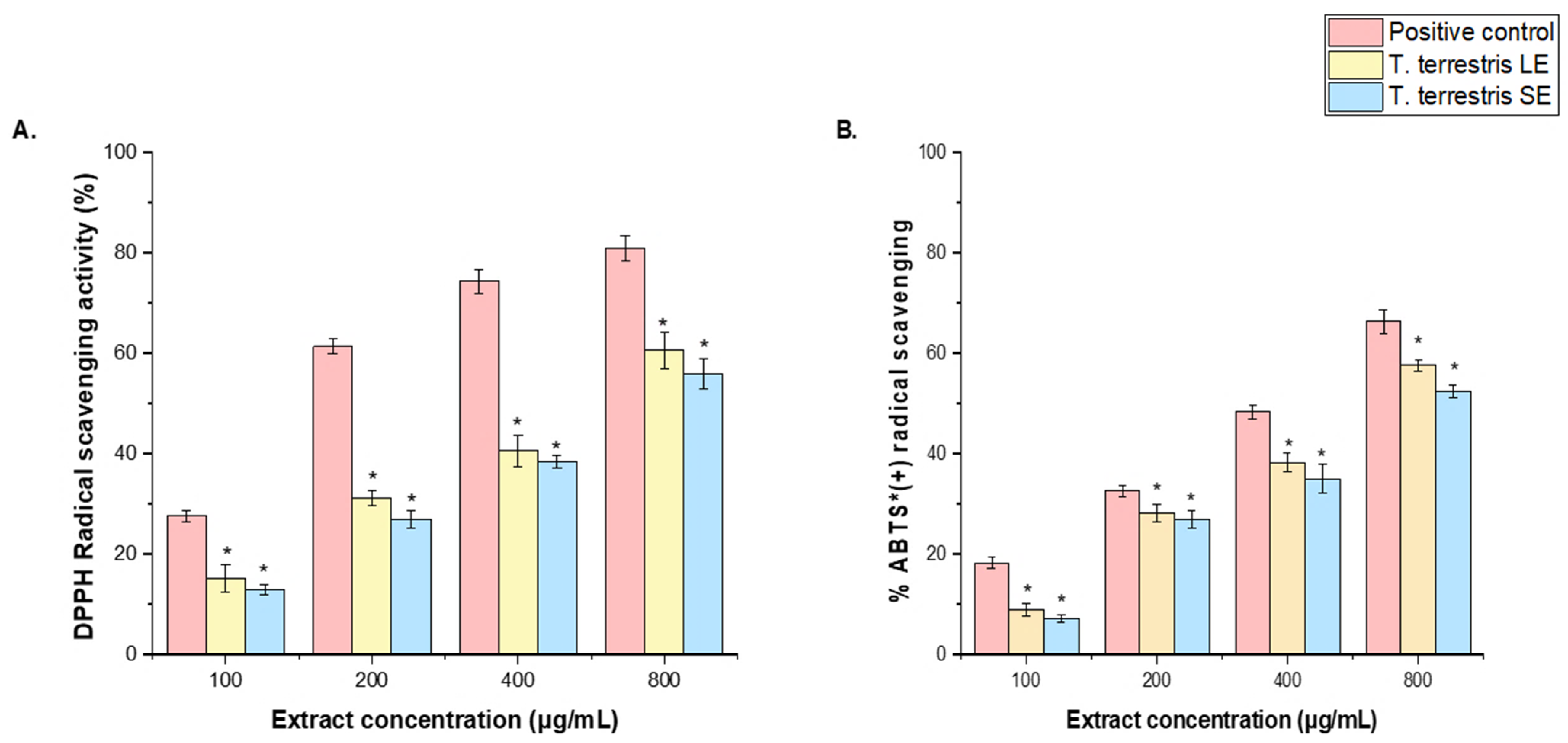
3.5. DPPH and ABTS+ Radical Scavenging Activity
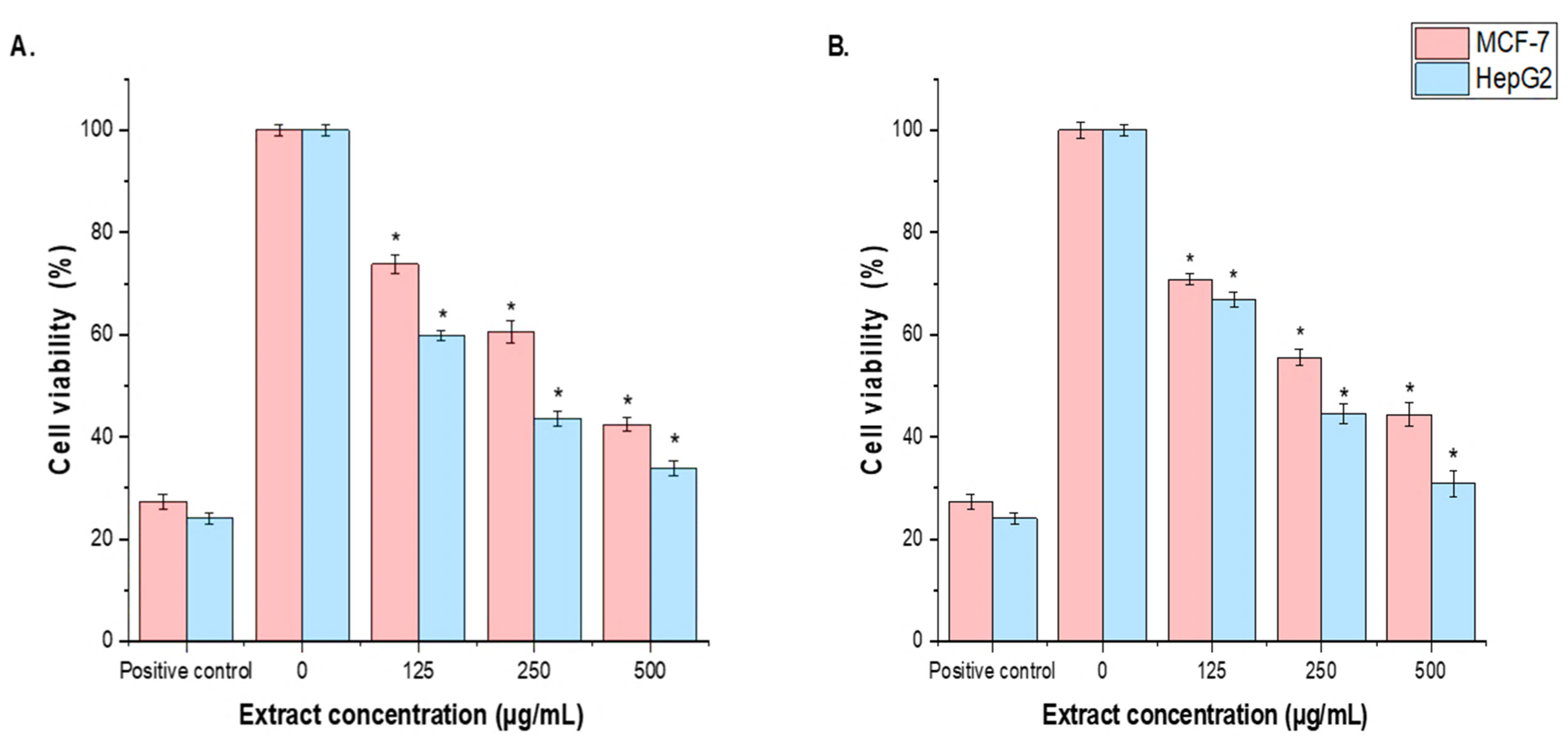
3.6. Cell Cytotoxicity
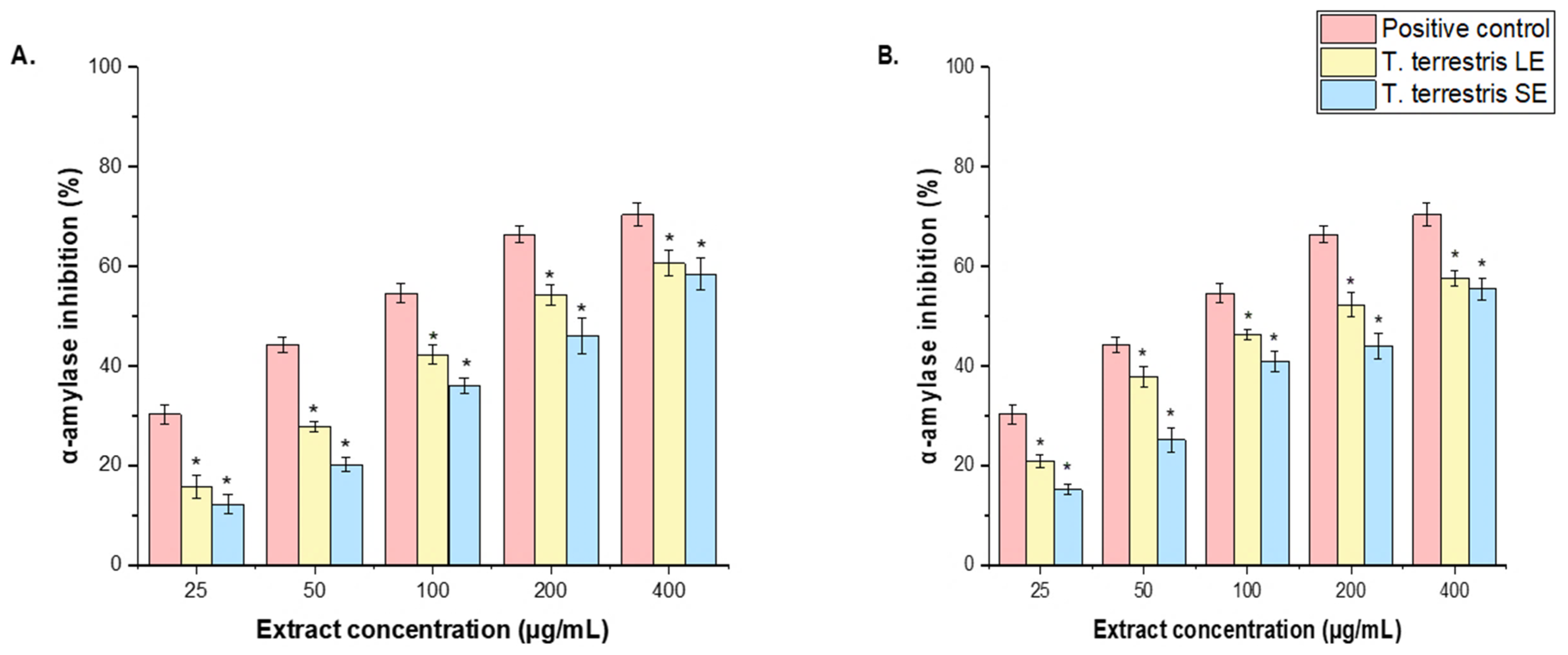
3.7. In Vitro Antidiabetic Activities of T. terrestris LE and SE
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chhatre, S.; Nesari, T.; Somani, G.; Kanchan, D.; Sathaye, S. Phytopharmacological overview of Tribulus terrestris. Pharmacogn. Rev. 2014, 8, 45. [Google Scholar] [CrossRef]
- Gupta, R. Ethnobotanical studies on medicinal plant: Gokhru (Tribulus terrestris). Int. J. Herb. Med. 2017, 5, 73–74. [Google Scholar]
- Nebieridze, V.; Skhirtladze, A.; Kemertelidze, E.; Ganzera, M. Nucleosides from Tribulus terrestris. Chem. Nat. Compd. 2017, 53, 1010–1011. [Google Scholar] [CrossRef]
- Zhu, W.; Du, Y.; Meng, H.; Dong, Y.; Li, L. A review of traditional pharmacological uses, phytochemistry, and pharmacological activities of Tribulus terrestris. Chem. Cent. J. 2017, 11, 60. [Google Scholar] [CrossRef]
- Ștefănescu, R.; Tero-Vescan, A.; Negroiu, A.; Aurică, E.; Vari, C.-E. A comprehensive review of the phytochemical, pharmacological, and toxicological properties of Tribulus terrestris L. Biomolecules 2020, 10, 752. [Google Scholar] [CrossRef]
- Dovchinsuren, B.; Damdinsuren, A.; Bayarkhuu, B.; Purevkhuu, M.; Batjargal, O.; Badarch, B.; Dorjbal, E. Tribulus terrestris L. in traditional Mongolian medicine: Medicinal Applications, Phytochemistry, Pharmacology. Pharmacogn. J. 2025, 17, 171–178. [Google Scholar] [CrossRef]
- Dinchev, D.; Janda, B.; Evstatieva, L.; Oleszek, W.; Aslani, M.R.; Kostova, I. Distribution of steroidal saponins in Tribulus terrestris from different geographical regions. Phytochemistry 2008, 69, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Batovska, D.; Panova, N.; Gerasimova, A.; Tumbarski, Y.; Ivanov, I.; Dincheva, I.; Yotkovska, I.; Gentscheva, G.; Nikolova, K. Chamomile Matters: Species-and Producer-Dependent Variation in Bulgarian Matricaria recutita L. and Chamaemelum nobile L. Essential Oils and Their Cosmetic Potential. Cosmetics 2025, 12, 123. [Google Scholar] [CrossRef]
- Lazarova, I.; Ivanova, A.; Mechkarova, P.; Peev, D.; Valyovska, N. Intraspecific variability of biologically active compounds of different populations of Tribulus terrestris L. (Zygophyllaceae) in South Bulgaria. Biotechnol. Biotechnol. Equip. 2011, 25, 2352–2356. [Google Scholar] [CrossRef]
- Sarvin, B.; Stekolshchikova, E.; Rodin, I.; Stavrianidi, A.; Shpigun, O. Optimization and comparison of different techniques for complete extraction of saponins from T. terrestris. J. Appl. Res. Med. Aromat. Plants 2018, 8, 75–82. [Google Scholar] [CrossRef]
- Saeed, M.; Munawar, M.; Bi, J.B.; Ahmed, S.; Ahmad, M.Z.; Kamboh, A.A.; Arain, M.A.; Naveed, M.; Chen, H. Promising phytopharmacology, nutritional potential, health benefits, and traditional usage of Tribulus terrestris L. herb. Heliyon 2024, 10, e25549. [Google Scholar] [CrossRef]
- Wang, Z.-F.; Wang, B.-B.; Zhao, Y.; Wang, F.-X.; Sun, Y.; Guo, R.-J.; Song, X.-B.; Xin, H.-L.; Sun, X.-G. Furostanol and spirostanol saponins from Tribulus terrestris. Molecules 2016, 21, 429. [Google Scholar] [CrossRef]
- Shahid, M.; Riaz, M.; Talpur, M.; Pirzada, T. Phytopharmacology of Tribulus terrestris. J. Biol. Regul. Homeost. Agents 2016, 30, 785–788. [Google Scholar]
- Zhang, H.; Kuang, Y.; Shahrajabian, M.H.; Wang, N.; Sun, W. Study of puncture vine (Tribulus terrestris L.), and the important roles of its chemical ingredients. Not. Sci. Biol. 2025, 17, 12194. [Google Scholar] [CrossRef]
- Sirotkin, A.V.; Kolesarova, A. Puncture vine (Tribulus terrestris L.) in control of health and reproduction. Physiol. Res. 2021, 70, S657. [Google Scholar]
- Vilar Neto, J.d.O.; de Moraes, W.M.A.M.; Pinto, D.V.; da Silva, C.A.; Caminha, J.d.S.R.; Nunes Filho, J.C.C.; Reis, C.E.G.; Prestes, J.; Santos, H.O.; De Francesco Daher, E. Effects of Tribulus (Tribulus terrestris L.) Supplementation on Erectile Dysfunction and Testosterone Levels in Men—A Systematic Review of Clinical Trials. Nutrients 2025, 17, 1275. [Google Scholar] [CrossRef]
- Khalid, A.; Algarni, A.S.; Homeida, H.E.; Sultana, S.; Javed, S.A.; Rehman, Z.U.; Abdalla, H.; Alhazmi, H.A.; Albratty, M.; Abdalla, A.N. Phytochemical, cytotoxic, and antimicrobial evaluation of Tribulus terrestris L., Typha domingensis pers., and Ricinus communis L.: Scientific evidences for folkloric uses. Evid. Based Complement. Altern. Med. 2022, 2022, 6519712. [Google Scholar] [CrossRef]
- Abbas, A.; Naqvi, S.A.R.; Rasool, M.H.; Noureen, A.; Mubarik, M.S.; Tareen, R.B. Phytochemical analysis, antioxidant and antimicrobial screening of Seriphidium oliverianum plant extracts. Dose Response 2021, 19, 15593258211004739. [Google Scholar] [CrossRef]
- Wolfe, K.L.; Liu, R.H. Apple peels as a value-added food ingredient. J. Agric. Food Chem. 2003, 51, 1676–1683. [Google Scholar] [CrossRef]
- Ordonez, A.; Gomez, J.; Vattuone, M. Antioxidant activities of Sechium edule (Jacq.) Swartz extracts. Food Chem. 2006, 97, 452–458. [Google Scholar] [CrossRef]
- Salem, N.; Kefi, S.; Tabben, O.; Ayed, A.; Jallouli, S.; Feres, N.; Hammami, M.; Khammassi, S.; Hrigua, I.; Nefisi, S. Variation in chemical composition of Eucalyptus globulus essential oil under phenological stages and evidence synergism with antimicrobial standards. Ind. Crops Prod. 2018, 124, 115–125. [Google Scholar] [CrossRef]
- Basri, D.F.; Sandra, V. Synergistic interaction of methanol extract from Canarium odontophyllum Miq. Leaf in combination with oxacillin against methicillin-resistant Staphylococcus aureus (MRSA) ATCC 33591. Int. J. Microbiol. 2016, 2016, 5249534. [Google Scholar] [CrossRef]
- Aljeldah, M.M.; Yassin, M.T.; Mostafa, A.A.-F.; Aboul-Soud, M.A. Synergistic Antibacterial Potential of Greenly Synthesized Silver Nanoparticles with Fosfomycin Against Some Nosocomial Bacterial Pathogens. Infect. Drug Resist. 2022, 15, 125–142. [Google Scholar] [CrossRef]
- Tian, M.; Wu, X.; Lu, T.; Zhao, X.; Wei, F.; Deng, G.; Zhou, Y. Phytochemical analysis, antioxidant, antibacterial, cytotoxic, and enzyme inhibitory activities of Hedychium flavum rhizome. Front. Pharmacol. 2020, 11, 572659. [Google Scholar] [CrossRef]
- Yu, X.; Zhao, M.; Liu, F.; Zeng, S.; Hu, J. Antioxidants in volatile Maillard reaction products: Identification and interaction. LWT-Food Sci. Technol. 2013, 53, 22–28. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Alkhudhayri, A.A.; Wahab, R.; Siddiqui, M.A.; Ahmad, J. Selenium nanoparticles induce cytotoxicity and apoptosis in human breast cancer (MCF-7) and liver (HEPG2) cell lines. Nanosci. Nanotechnol. Lett. 2020, 12, 324–330. [Google Scholar] [CrossRef]
- Ghasemi, M.; Turnbull, T.; Sebastian, S.; Kempson, I. The MTT assay: Utility, limitations, pitfalls, and interpretation in bulk and single-cell analysis. Int. J. Mol. Sci. 2021, 22, 12827. [Google Scholar] [CrossRef]
- Al-Dhabi, N.A.; Valan Arasu, M. Quantification of phytochemicals from commercial Spirulina products and their antioxidant activities. Evid. Based Complement. Altern. Med. 2016, 2016, 7631864. [Google Scholar] [CrossRef]
- Wickramaratne, M.N.; Punchihewa, J.; Wickramaratne, D. In-vitro alpha amylase inhibitory activity of the leaf extracts of Adenanthera pavonina. BMC Complement. Altern. Med. 2016, 16, 466. [Google Scholar] [CrossRef]
- Kim, Y.-M.; Wang, M.-H.; Rhee, H.-I. A novel α-glucosidase inhibitor from pine bark. Carbohydr. Res. 2004, 339, 715–717. [Google Scholar] [CrossRef]
- Al-Bayati, F.A.; Al-Mola, H.F. Antibacterial and antifungal activities of different parts of Tribulus terrestris L. growing in Iraq. J. Zhejiang Univ. Sci. B 2008, 9, 154–159. [Google Scholar] [CrossRef]
- Soleimanpour, S.; Sedighinia, F.S.; Afshar, A.S.; Zarif, R.; Ghazvini, K. Antibacterial activity of Tribulus terrestris and its synergistic effect with Capsella bursa-pastoris and Glycyrrhiza glabra against oral pathogens: An in-vitro study. Avicenna J. Phytomed. 2015, 5, 210. [Google Scholar]
- Alzahrani, K.J. Tribulus terrestris Fruit Extract: Bioactive Compounds, ADMET Analysis, and Molecular Docking with Penicillin-Binding Protein 2a Transpeptidase of Methicillin-Resistant Staphylococcus epidermidis. Curr. Issues Mol. Biol. 2025, 47, 52. [Google Scholar] [CrossRef]
- Ammar, N.M.; El-Hawary, S.; Mohamed, D.A.; Afifi, M.S.; Ghanem, D.M.; Awad, G. Phytochemical and biological studies of Tribulus terrestris L. growing in Egypt. Int. J. Pharmacol. 2018, 14, 248–259. [Google Scholar] [CrossRef]
- Abbas, M.W.; Hussain, M.; Akhtar, S.; Ismail, T.; Qamar, M.; Shafiq, Z.; Esatbeyoglu, T. Bioactive compounds, antioxidant, anti-inflammatory, anti-cancer, and toxicity assessment of Tribulus terrestris—In vitro and in vivo studies. Antioxidants 2022, 11, 1160. [Google Scholar] [CrossRef]
- Kaya, E.; Aydın, T.; Sağlamtaş, R. Evaluation of antioxidant activities and inhibition effects of Tribulus terrestris L. extracts on some metabolic enzymes. S. Afr. J. Bot. 2024, 170, 156–162. [Google Scholar] [CrossRef]
- Kaliaperumal, K.; Bhat, B.A.; Subramanian, K.; Ramakrishnan, T.; Chakravarthy, E.; Al-Keridis, L.A.; Ahmad, I.; Alabdallah, N.M.; Saeed, M.; Karunakaran, R. In-vivo anti-hyperglycemic effect of herbal extracts Tribulus terrestris (L.) and Curcuma amada (R) on streptozotocin-induced diabetic rats and its associated histopathological studies. Heliyon 2024, 10, e24009. [Google Scholar] [CrossRef]
- Saxena, S.; Chakraborty, D. Antimicrobial Activity and GC-MS Analysis of Bioactive Compounds of Tribulus terrestris L. Asian J. Res. Bot. 2023, 6, 352–361. [Google Scholar]
- Hal, D.M.; Eltamany, E.; Abdelhameed, R.F.; Ibrahim, A.K.; Badr, J. Chemical review on Zygophyllum genus. Rec. Pharm. Biomed. Sci. 2022, 6, 105–129. [Google Scholar] [CrossRef]
- Williams, C.; Mbuyane, L.L.; Bauer, F.F.; Mokwena, L.; Divol, B.; Buica, A. A gas chromatography-mass spectrometry method for the determination of fatty acids and sterols in yeast and grape juice. Appl. Sci. 2021, 11, 5152. [Google Scholar] [CrossRef]
- Schlag, S.; Huang, Y.; Vetter, W. GC/EI-MS method for the determination of phytosterols in vegetable oils. Anal. Bioanal. Chem. 2022, 414, 1061–1071. [Google Scholar] [CrossRef]
- Siddique, M.H.; Andleeb, R.; Ashraf, A.; Zubair, M.; Fakhar-e-Alam, M.; Hayat, S.; Muzammil, S.; Atif, M.; Shafeeq, S.; Afzal, M. Integration of in silico and in vitro approaches to evaluate antioxidant and anticancer properties of Tribulus terrestris extracts. Arab. J. Chem. 2022, 15, 103984. [Google Scholar] [CrossRef]
- Uysal, S.; Senkardes, I.; Jekő, J.; Cziáky, Z.; Zengin, G. Chemical characterization and pharmacological profile of Tribulus terrestris extracts: A novel source of cosmeceuticals and pharmaceuticals. Biochem. Syst. Ecol. 2023, 107, 104600. [Google Scholar] [CrossRef]
- Affuf, A.; Karpenko, J.N.; Gulyaev, D.K.; Molokhova, E.I.; Blinova, O.L.; Gileva, A.A.; Belonogova, V.D. Phytochemical study of Tribulus terrestris L. Pharm. Pharmacol. 2019, 7, 346–355. [Google Scholar] [CrossRef]
- Kostova, I.; Dinchev, D. Saponins in Tribulus terrestris–chemistry and bioactivity. Phytochem. Rev. 2005, 4, 111–137. [Google Scholar] [CrossRef]
- Xu, Y.-J.; Xu, T.-H.; Zhou, H.-O.; Li, B.; Xie, S.-X.; Si, Y.-S.; Liu, Y.; Liu, T.-H.; Xu, D.-M. Two new furostanol saponins from Tribulus terrestris. J. Asian Nat. Prod. Res. 2010, 12, 349–354. [Google Scholar] [CrossRef]
- Wu, T.-S.; Shi, L.-S.; Kuo, S.-C. Alkaloids and other constituents from Tribulus terrestris. Phytochemistry 1999, 50, 1411–1415. [Google Scholar] [CrossRef]
- Louveaux, A.; Jay, M.; El Hadi, O.T.M.; Roux, G. Variability in flavonoid compounds of four Tribulus species: Does it play a role in their identification by desert locust Schistocerca gregaria? J. Chem. Ecol. 1998, 24, 1465–1481. [Google Scholar] [CrossRef]
- Platzer, M.; Kiese, S.; Herfellner, T.; Schweiggert-Weisz, U.; Miesbauer, O.; Eisner, P. Common trends and differences in antioxidant activity analysis of phenolic substances using single electron transfer based assays. Molecules 2021, 26, 1244. [Google Scholar] [CrossRef]
- Figueiredo, C.C.M.; Gomes, A.C.; Granero, F.O.; Junior, J.L.B.; Silva, L.P.; Ruiz, A.L.T.G.; da Silva, R.M.G. Antiglycation and antitumoral activity of Tribulus terrestris dry extract. Avicenna J. Phytomed. 2021, 11, 224. [Google Scholar] [PubMed]
- Figueiredo, C.C.M.; da Costa Gomes, A.; Granero, F.O.; Junior, J.L.B.; Silva, L.P.; Ximenes, V.F.; da Silva, R.M.G. Antiglycation and antioxidant activities of the crude extract and saponin fraction of Tribulus terrestris before and after microcapsule release. J. Integr. Med. 2022, 20, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Touaibia, M.; Dhoha, A. Biochemical characterization, antioxidant and antidiabetic properties of Zygophyllum geslini Coss extracts. Int. J. Biol. Chem. Sci. 2024, 18, 1105–1114. [Google Scholar] [CrossRef]
- Abdallah, H.M.; Esmat, A. Antioxidant and anti-inflammatory activities of the major phenolics from Zygophyllum simplex L. J. Ethnopharmacol. 2017, 205, 51–56. [Google Scholar] [CrossRef] [PubMed]
- El-Afify, S.M.; El-Metwaly, M.A.; Abbas, M.A.; El-Amier, Y.A. In vitro assessment of antioxidant and cytotoxic activities of Zygophyllum coccineum L. methanolic extract. Egypt. J. Chem. 2024, 67, 393–401. [Google Scholar]
- Shetty, N.; Holla, S.; Nayak, V.; Shenoy, V.B.; Mohandas, R.K. Antioxidant and anti-inflammatory activity by modulating IL-6 as a potential mechanism in the nephroprotective and hepatoprotective properties of Tribulus terrestris. Res. Pharm. Sci. 2024, 19, 376–386. [Google Scholar] [CrossRef]
- Azarm, A.; Ayoobi, F.; Zare-Bidaki, M.; Taheri, M.; Zarandi, E.R. Antibacterial and antibiofilm activities of tribulus terrestris methanolic extract against Streptococcus mutans, Streptococcus sobrinus, and Lactobacillus acidophilus: An in vitro study. Dent. Res. J. 2024, 21, 57. [Google Scholar] [CrossRef]
- Alshabi, A.M.; Alkahtani, S.A.; Shaikh, I.A.; Orabi, M.A.; Abdel-Wahab, B.A.; Walbi, I.A.; Habeeb, M.S.; Khateeb, M.M.; Shettar, A.K.; Hoskeri, J.H. Tribulus terrestris cytotoxicity against breast cancer MCF-7 and lung cancer A549 cell lines is mediated via activation of apoptosis, caspase-3, DNA degradation, and suppressing Bcl-2 activity. Separations 2022, 9, 383. [Google Scholar] [CrossRef]
- Pourali, M.; Yaghoobi, M.M.; Sormaghi, M.H.S. Cytotoxic, anti-proliferative and apoptotic effects of Tribulus terrestris L. fruit extract on human prostate cancer Lncap and colon cancer HT-29 Cell Lines. Jundishapur J. Nat. Pharm. Prod. 2017, 12, e33561. [Google Scholar]
- Patel, A.; Soni, A.; Siddiqi, N.J.; Sharma, P. An insight into the anticancer mechanism of Tribulus terrestris extracts on human breast cancer cells. 3 Biotech 2019, 9, 58. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, J.C.; Min, J.S.; Kim, M.-j.; Kim, J.A.; Kor, M.H.; Yoo, H.S.; Ahn, J.K. Aqueous extract of Tribulus terrestris Linn induces cell growth arrest and apoptosis by down-regulating NF-κB signaling in liver cancer cells. J. Ethnopharmacol. 2011, 136, 197–203. [Google Scholar] [CrossRef]
- Qari, S.H.; El-Assouli, S. Evaluation of cytological and genetic effects of Tribulus terrestris fruit aqueous extract on cultured human lymphocytes. Saudi J. Biol. Sci. 2019, 26, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Parekh, S.; Arkatkar, A.; Soni, A.; Patel, P.; Mishra, K. Nyctanthes arbor-tristis alkaloids activates p53 independent cell death receptor and necroptosis pathways in HepG2 cells. 3 Biotech 2023, 13, 167. [Google Scholar] [CrossRef] [PubMed]
- Al-Eisa, R.A.; Tag, H.M.; ElNaggar, M.S.; Abdelrazek, H.M.; El-Shenawy, N.S. Evaluation of Tribulus terrestris extracts relative to metformin on oxidative stress and Histopathology of the liver for diabetic male rats. Diabetology 2022, 3, 46–55. [Google Scholar] [CrossRef]
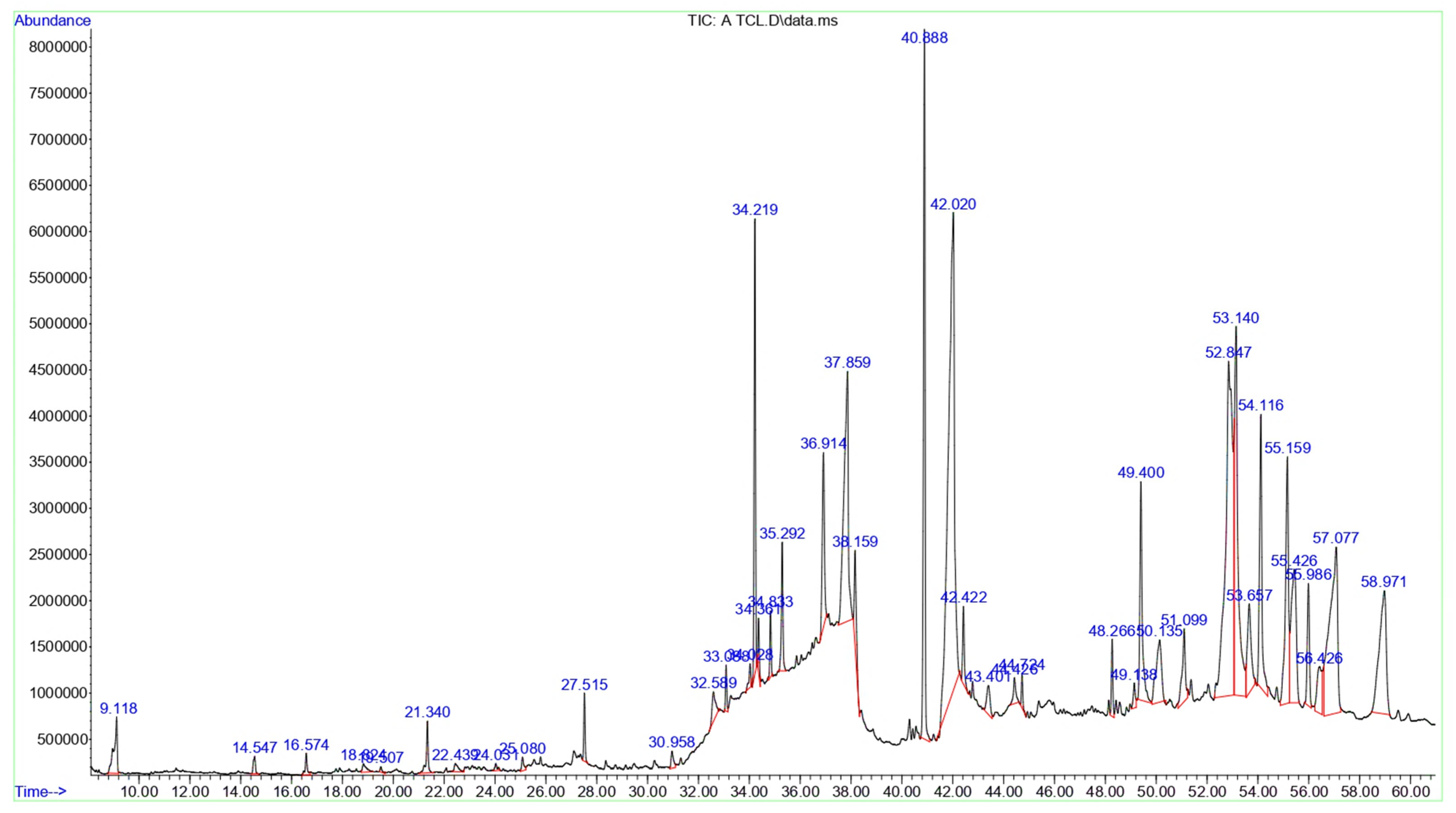
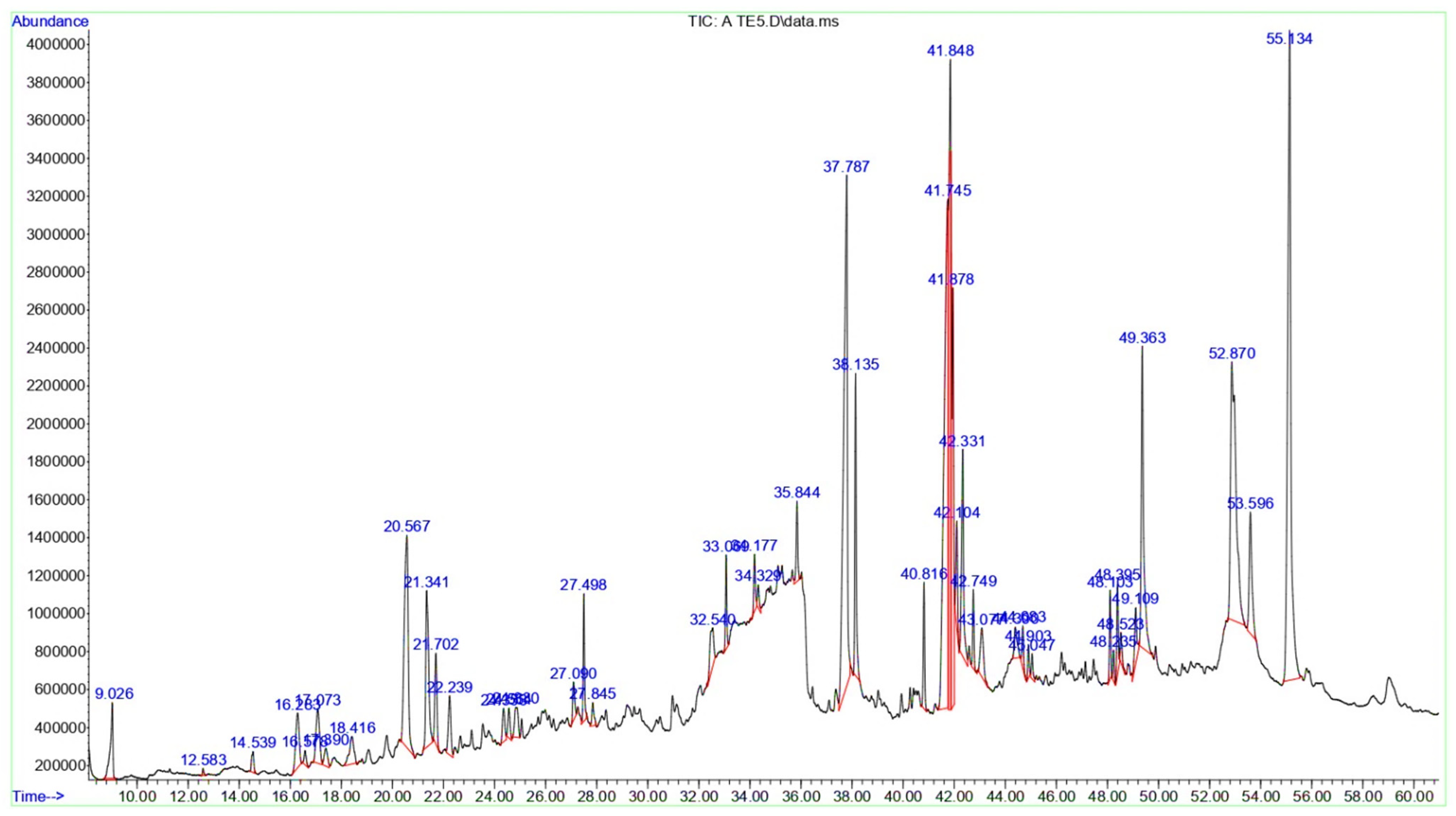

| Peak | RT | Area | Area % | Name | MF | MW | Classification |
|---|---|---|---|---|---|---|---|
| 1 | 9.118 | 51,513,682 | 0.97 | D-Limonene | C10H16 | 136 | Monoterpenoids |
| 2 | 14.547 | 10,595,389 | 0.19 | 2-Decenal, (E)- | C10H18O | 154 | Aldehyde |
| 3 | 16.574 | 12,551,830 | 0.23 | Linalyl acetate | C12H20O2 | 196 | Monoterpenoids |
| 4 | 18.824 | 9,470,910 | 0.17 | 3,5-Heptadienal, 2-ethylidene-6-methyl- | C10H14O | 150 | Monoterpenoids |
| 5 | 19.507 | 2,957,458 | 0.05 | 2,6-Dimethyl-8-oxoocta-2,6-dienoic acid, methyl ester | C11H16O3 | 196 | Monoterpenoids |
| 6 | 21.34 | 28,681,887 | 0.54 | (E)-Tetradec-2-enal | C14H26O | 210 | Fatty aldehydes |
| 7 | 22.439 | 9,097,545 | 0.17 | trans-2-undecenoic acid | C11H20O2 | 184 | Fatty acids and conjugates |
| 8 | 24.031 | 4,632,250 | 0.08 | [5,5-Dimethyl-6-(3-methyl-buta-1,3-dienyl)-7-oxa-bicyclo [4.1.0]hept-1-yl]-methanol | C14H22O2 | 222 | Oxepanes |
| 9 | 25.08 | 7,892,209 | 0.14 | 2,4-Di-tert-butylphenol | C14H22O | 206 | Phenylpropanes |
| 10 | 27.515 | 26,603,932 | 0.50 | 1-Hexadecanol, 2-methyl- | C17H36O | 256 | Fatty alcohols |
| 11 | 30.958 | 12,217,607 | 0.23 | 5-O-Methyl-d-gluconic acid dimethylamide | C9H19NO6 | 237 | Fatty amides |
| 12 | 32.589 | 28,502,221 | 0.53 | 6-O-Methyl-2,4-methylene-β-sedoheptitol | C9H18O7 | 238 | 1,3-dioxanes |
| 13 | 33.088 | 17,233,900 | 0.32 | Octose | C8H16O8 | 240 | Monosaccharides |
| 14 | 34.028 | 11,721,796 | 0.22 | d-Glycero-d-ido-heptose | C7H14O7 | 210 | Monosaccharides |
| 15 | 34.219 | 187,227,793 | 3.52 | β-Lactose | C12H22O11 | 342 | Glycosyl compounds |
| 16 | 34.361 | 20,427,543 | 0.38 | d-Gala-l-ido-octonic amide | C8H17NO8 | 255 | Monosaccharides |
| 17 | 34.833 | 25,284,815 | 0.47 | β-D-Mannofuranoside, 1-O-(10-undecenyl)- | C17H32O6 | 332 | Fatty acyl glycosides of mono- and disaccharides |
| 18 | 35.292 | 59,864,528 | 1.12 | Desulphosinigrin | C10H17NO6S | 279 | Thioglycosides |
| 19 | 36.914 | 109,968,411 | 2.07 | Methyl 6-O-[1-methylpropyl]-β-d-galactopyranoside | C11H22O6 | 250 | O-glycosyl compounds |
| 20 | 37.859 | 323,542,928 | 6.09 | n-Hexadecanoic acid | C16H32O2 | 256 | Long-chain fatty acids |
| 21 | 38.159 | 56,195,332 | 1.05 | Hexadecanoic acid, ethyl ester | C18H36O2 | 284 | Fatty acid esters |
| 22 | 40.888 | 343,368,273 | 6.47 | Phytol | C20H40O | 296 | Diterpenoid |
| 23 | 42.02 | 865,971,414 | 16.32 | Linolenic acid | C18H30O2 | 278 | Linoleic acids and derivatives |
| 24 | 42.422 | 38,940,585 | 0.73 | Stearic acid | C18H36O2 | 284 | Long-chain fatty acids |
| 25 | 43.401 | 29,301,050 | 0.55 | 8,11,14-Eicosatrienoic acid, (Z,Z,Z)- | C20H34O2 | 306 | Long-chain fatty acids |
| 26 | 44.426 | 19,150,433 | 0.36 | Arachidonic acid methyl ester | C21H34O2 | 318 | Fatty acid methyl esters |
| 27 | 44.724 | 14,142,888 | 0.26 | Methyl 13,16-docosadienoate | C23H42O2 | 350 | Fatty acid methyl esters |
| 28 | 48.266 | 32,799,596 | 0.61 | Corynan-17-ol, 18,19-didehydro-10-methoxy-, acetate (ester) | C22H28N2O3 | 368 | Indoles and derivatives |
| 29 | 49.138 | 14,894,988 | 0.28 | 18,19-Secoyohimban-19-oic acid, 16,17,20,21-tetradehydro-16-(hydroxymethyl)-, methyl ester, (15β,16E)- | C21H24N2O3 | 352 | Indoles and derivatives |
| 30 | 49.4 | 156,996,266 | 2.95 | Palmitic acid β-monoglyceride | C19H38O4 | 330 | Glycerolipids |
| 31 | 50.135 | 100,188,763 | 1.88 | 26-Nor-5-cholesten-3β-ol-25-one | C26H42O2 | 386 | Oxosteroids |
| 32 | 51.099 | 67,851,955 | 1.27 | 2-[4-methyl-6-(2,6,6-trimethylcyclohex-1-enyl)hexa-1,3,5-trienyl]cyclohex-1-en-1-carboxaldehyde | C23H32O | 324 | Retinoids |
| 33 | 52.847 | 715,821,052 | 13.49 | Vitamin E | C29H50O2 | 430 | Vitamin E compounds |
| 34 | 53.14 | 371,308,434 | 6.99 | Linolenic acid, methyl ester | C21H36O4 | 352 | Linoleic acids and derivatives |
| 35 | 53.657 | 100,137,742 | 1.88 | α-Monostearin | C21H42O4 | 358 | Monoacylglycerols |
| 36 | 54.116 | 195,379,212 | 3.68 | Lupeol | C30H50O | 426 | Triterpenoid |
| 37 | 55.159 | 223,293,715 | 4.21 | Oleamide | C18H35NO | 281 | Fatty amides |
| 38 | 55.426 | 204,209,299 | 3.84 | Estrone methyl ether | C23H28O6 | 400 | Estrane steroids |
| 39 | 55.986 | 77,621,180 | 1.46 | 2,2,4-Trimethyl-3-(3,8,12,16-tetramethyl-heptadeca-3,7,11,15-tetraenyl)-cyclohexanol | C30H52O | 428 | Triterpenoids |
| 40 | 56.426 | 67,809,797 | 1.27 | Cholest-22-ene-21-ol, 3,5-dehydro-6-methoxy-, pivalate | C33H54O3 | 498 | Cholestane steroids |
| 41 | 57.077 | 396,141,007 | 7.46 | Campesterol | C28H48O | 400 | Ergosterols and derivatives |
| 42 | 58.971 | 254,003,805 | 4.78 | Stigmasterol | C29H48O | 412 | Stigmastanes and derivatives |
| Peak | RT | Area | Area% | Name | MF | MW | Classification |
|---|---|---|---|---|---|---|---|
| 1 | 9.026 | 27,674,175 | 1.18 | D-Limonene | C10H16 | 136 | Monoterpenoids |
| 2 | 12.583 | 1,292,485 | 0.05 | 1-Azabicyclo [3.2.1]octan-6-ol, | C7H13NO | 127 | Azepanes |
| 3 | 14.539 | 6,362,755 | 0.27 | 2-Decenal, (E)- | C10H18O | 154 | Aldehyde |
| 4 | 16.283 | 27,132,745 | 1.16 | 2-Ethyl-2-hexen-1-ol | C8H16O | 128 | Fatty alcohols |
| 5 | 16.578 | 4,377,573 | 0.18 | 2,2-Dimethyl-5-(3-methyl-2-oxiranyl)cyclohexanone | C11H18O2 | 182 | Cyclic ketones |
| 6 | 17.073 | 27,542,799 | 1.17 | Diplodialide B | C10H16O3 | 184 | Oxocins |
| 7 | 17.39 | 8,535,962 | 0.36 | R-Limonene | C10H16O3 | 184 | Monoterpenoids |
| 8 | 18.416 | 18,070,103 | 0.77 | 9-Tetradecenal, (Z)- | C14H26O | 210 | Fatty aldehydes |
| 9 | 20.567 | 119,036,249 | 5.09 | 13-Tetradece-11-yn-1-ol | C14H24O | 208 | Fatty alcohols |
| 10 | 21.341 | 64,046,996 | 2.74 | (E)-Tetradec-2-enal | C14H26O | 210 | Fatty aldehydes |
| 11 | 21.702 | 27,011,428 | 1.15 | 3-Nonynoic acid | C9H14O2 | 154 | Fatty acids and conjugates |
| 12 | 22.239 | 21,603,631 | 0.92 | cis,cis-7,10,-Hexadecadienal | C16H28O | 236 | Fatty aldehydes |
| 13 | 24.353 | 11,191,061 | 0.47 | 11-Hexadecyn-1-ol | C16H30O | 238 | Fatty alcohol |
| 14 | 24.564 | 9,064,484 | 0.38 | 13-Heptadecyn-1-ol | C17H32O | 252 | Fatty alcohol |
| 15 | 24.83 | 14,909,604 | 0.63 | E-11-Hexadecenal | C16H30O | 238 | Fatty aldehydes |
| 16 | 27.09 | 10,712,686 | 0.45 | 7-Methyl-Z-tetradecen-1-ol acetate | C17H32O2 | 268 | Fatty alcohol esters |
| 17 | 27.498 | 25,117,256 | 1.07 | 1-Hexadecanol, 2-methyl- | C17H36O | 256 | Fatty alcohol |
| 18 | 27.845 | 6,916,745 | 0.29 | 9-Hexadecenoic acid | C16H30O2 | 254 | Fatty acids and conjugates |
| 19 | 32.54 | 22,489,431 | 0.96 | 6-O-Methyl-2,4-methylene-β-sedoheptitol | C9H18O7 | 238 | Dioxanes |
| 20 | 33.069 | 17,832,901 | 0.76 | Octose | C8H16O8 | 240 | Monosaccharides |
| 21 | 34.177 | 12,983,214 | 0.55 | β-Lactose | C12H22O11 | 342 | Glycosyl compounds |
| 22 | 34.329 | 5,968,022 | 0.25 | d-Gala-l-ido-octonic amide | C8H17NO8 | 255 | Monosaccharides |
| 23 | 35.844 | 18,000,733 | 0.77 | Desulphosinigrin | C10H17NO6S | 279 | Thioglycosides |
| 24 | 37.787 | 266,140,032 | 11.38 | n-Hexadecanoic acid | C16H32O2 | 256 | Long-chain fatty acids |
| 25 | 38.135 | 61,588,053 | 2.63 | Hexadecanoic acid, ethyl ester | C18H36O2 | 284 | Fatty acid esters |
| 26 | 40.816 | 25,044,348 | 1.07 | 2-(Octadec-9-enyloxy)ethanol | C20H40O2 | 312 | Ethers |
| 27 | 41.745 | 281,852,469 | 12.05 | cis,cis-Linoleic acid | C18H32O2 | 280 | Linoleic acids and derivatives |
| 28 | 41.848 | 179,843,343 | 7.69 | Linolenic acid | C18H30O2 | 278 | Linoleic acids and derivatives |
| 29 | 41.878 | 124,694,063 | 5.33 | Linoleic acid ethyl ester | C20H36O2 | 308 | Linoleic acids and derivatives |
| 30 | 42.104 | 24,879,985 | 1.06 | Ethyl Oleate | C20H38O2 | 310 | Fatty acid esters |
| 31 | 42.331 | 62,232,484 | 2.66 | Oleic Acid | C18H34O2 | 282 | Fatty acids and conjugates |
| 32 | 42.749 | 15,226,202 | 0.65 | (E)-9-Octadecenoic acid ethyl ester | C20H38O2 | 310 | Fatty acid esters |
| 33 | 43.077 | 22,693,098 | 0.97 | Linolenic acid, methyl ester | C21H36O4 | 352 | Linoleic acids and derivatives |
| 34 | 44.39 | 13,034,909 | 0.55 | Arachidonic acid methyl ester | C21H34O2 | 318 | Fatty acid methyl esters |
| 35 | 44.683 | 9,712,486 | 0.41 | Methyl 13,16-docosadienoate | C23H42O2 | 350 | Fatty acid methyl esters |
| 36 | 44.903 | 6,506,007 | 0.27 | Glyceryl diacetate 2-linolenate | C25H40O6 | 436 | Triacylglycerols |
| 37 | 45.047 | 5,778,806 | 0.24 | Ethyl iso-allocholate | C26H44O5 | 436 | Hydroxy bile acids, alcohols and derivatives |
| 38 | 48.103 | 16,807,940 | 0.71 | Cholestan-3-ol, 2-methylene-, (3β,5α)- | C28H48O | 400 | Cholesterols and derivatives |
| 39 | 48.235 | 5,805,416 | 0.24 | Corynan-17-ol, 18,19-didehydro-10-methoxy-, acetate (ester) | C22H28N2O3 | 368 | Indoles and derivatives |
| 40 | 48.395 | 18,970,108 | 0.81 | 2-Monolinolenin | C21H36O4 | 352 | Linoleic acids and derivatives |
| 41 | 48.523 | 8,012,896 | 0.34 | 2-Monoolein | C21H40O4 | 356 | Glycerolipids |
| 42 | 49.109 | 18,191,454 | 0.77 | 18,19-Secoyohimban-19-oic acid, 16,17,20,21-tetradehydro-16-(hydroxymethyl)-, methyl ester, (15β,16E)- | C21H24N2O3 | 352 | Indoles and derivatives |
| 43 | 49.363 | 106,419,555 | 4.55 | Palmitic acid β-monoglyceride | C19H38O4 | 330 | Glycerolipids |
| 44 | 52.87 | 202,940,007 | 8.68 | 1-Monolinolein | C21H38O4 | 354 | monoacylglycerol |
| 45 | 53.596 | 48,811,429 | 2.08 | α-Monostearin | C21H42O4 | 358 | Monoacylglycerols |
| 46 | 55.134 | 304,089,901 | 13.01 | Oleamide | C18H35NO | 281 | Fatty amides |
| Bacterium/ Dilution | Positive Control | 1000 μg/mL | 500 μg/mL | 250 μg/mL | 125 μg/mL | MIC (μg/mL) | MBC (μg/mL) |
|---|---|---|---|---|---|---|---|
| S. aureus | 29 ± 0.00 | 24 ± 0.00 * | 20 ± 0.00 * | 16 ± 0.00 * | 11 ± 0.00 * | 25 ± 0.00 | 50 ± 0.00 |
| S. epidermidis | 31 ± 0.00 | 25 ± 0.00 * | 21 ± 0.00 * | 17 ± 0.00 * | 12 ± 0.00 * | 25 ± 0.00 | 50 ± 0.00 |
| B. subtilis | 27 ± 0.00 | 23 ± 0.00 * | 19 ± 0.00 * | 14 ± 0.00 * | 11 ± 0.00 * | 25 ± 0.00 | 50 ± 0.00 |
| E. coli | 30 ± 0.00 | 25 ± 0.00 * | 22 ± 0.00 * | 17 ± 0.00 * | 14 ± 0.00 * | 12.50 ± 0.00 | 25 ± 0.00 |
| K. pneumoniae | 22 ± 0.00 | 17 ± 0.00 * | 14 ± 0.00 * | 10 ± 0.00 * | 8 ± 10.00 | 6.25 ± 0.00 | 12.50 ± 0.00 |
| P. aeruginosa | 26 ± 0.00 | 24 ± 0.00 * | 21 ± 0.00 * | 14 ± 0.00 * | 11 ± 0.00 * | 12.50 ± 0.00 | 25 ± 0.00 |
| Bacterium/ Dilution | Positive Control | 1000 μg/mL | 500 μg/mL | 250 μg/mL | 125 μg/mL | MIC (μg/mL) | MBC (μg/mL) |
|---|---|---|---|---|---|---|---|
| S. aureus | 29 ± 0.00 | 22 ± 0.00 * | 17 ± 0.00 * | 15 ± 0.00 * | 14 ± 0.00 * | 25 ± 0.00 | 50.00 ± 0.00 |
| S. epidermidis | 31 ± 0.00 | 23 ± 0.00 * | 19 ± 0.00 * | 16 ± 0.00 * | 13 ± 0.00 * | 25 ± 0.00 | 50 ± 0.00 |
| B. subtilis | 27 ± 0.00 | 20 ± 0.00 * | 18 ± 0.00 * | 15 ± 0.00 * | 10 ± 0.00 * | 50 ± 0.00 | 100 ± 0.00 |
| E. coli | 30 ± 0.00 | 24 ± 0.00 * | 18 ± 0.00 * | 12 ± 0.00 * | 10 ± 0.00 * | 12.50 ± 0.00 | 50.00 ± 0.00 |
| K. pneumoniae | 22 ± 0.00 | 20 ± 0.00 * | 16 ± 0.00 * | 12 ± 0.00 * | 9 ± 10.00 * | 12.50 ± 0.00 | 50 ± 0.00 |
| P. aeruginosa | 26 ± 0.00 | 26 ± 0.00 | 20 ± 0.00 * | 17 ± 0.00 * | 13 ± 0.00 * | 25 ± 0.00 | 50 ± 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
AlSedairy, S.A.; Aziz, I.M.; Alshalan, R.M.; Farrag, M.A.; Almuqrin, A.M.; Alghamdi, A.K.; Aljowaie, R.M. Bioactive Compounds, Antibacterial, Antioxidant, Anticancer, and Antidiabetic Potential of the Seed and Leaves of Tribulus terrestris. Life 2025, 15, 1799. https://doi.org/10.3390/life15121799
AlSedairy SA, Aziz IM, Alshalan RM, Farrag MA, Almuqrin AM, Alghamdi AK, Aljowaie RM. Bioactive Compounds, Antibacterial, Antioxidant, Anticancer, and Antidiabetic Potential of the Seed and Leaves of Tribulus terrestris. Life. 2025; 15(12):1799. https://doi.org/10.3390/life15121799
Chicago/Turabian StyleAlSedairy, Sahar Abdulaziz, Ibrahim M. Aziz, Rawan M. Alshalan, Mohamed A. Farrag, Abdulaziz M. Almuqrin, Amal Khalaf Alghamdi, and Reem M. Aljowaie. 2025. "Bioactive Compounds, Antibacterial, Antioxidant, Anticancer, and Antidiabetic Potential of the Seed and Leaves of Tribulus terrestris" Life 15, no. 12: 1799. https://doi.org/10.3390/life15121799
APA StyleAlSedairy, S. A., Aziz, I. M., Alshalan, R. M., Farrag, M. A., Almuqrin, A. M., Alghamdi, A. K., & Aljowaie, R. M. (2025). Bioactive Compounds, Antibacterial, Antioxidant, Anticancer, and Antidiabetic Potential of the Seed and Leaves of Tribulus terrestris. Life, 15(12), 1799. https://doi.org/10.3390/life15121799






