Interplay Among Synaptic Glutamate Release and Excitotoxicity: Neuronal Damage and Graphene-Based Materials Related Protection
Abstract
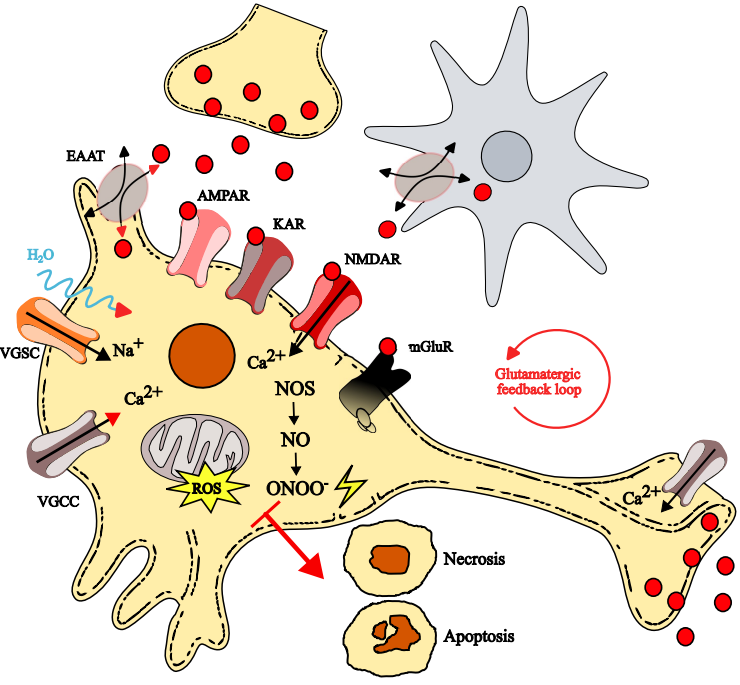
1. Glutamate-Induced Excitotoxicity
2. Glutamate Receptors and Their Role in Excitotoxicity
2.1. Ionotropic Glutamatergic Receptors: An Overview
2.2. Metabotropic Glutamatergic Receptors: An Overview
3. Highlights of the Excitotoxicity Mechanisms
3.1. Sodium-Driven Osmotic Dysregulation and Its Role in Neuronal Injury
3.2. Amplification of Neuronal Damage via the Glutamatergic Feedback Loop
3.3. Calcium-Dependent Mechanisms Driving Excitotoxic Neuronal Death
3.4. Astrocytic Dysfunction and Its Contribution to Glutamate Excitotoxicity
4. Excitotoxicity Role in Secondary Damage Development: The Case of Ischemic Stroke
5. Excitotoxicity in Chronic Neurodegenerative Diseases: Some Examples
6. New Therapeutic Approaches and Graphene-Based Materials in the CNS
6.1. Neural Interfaces
6.2. Neuroregeneration
6.3. Drug Delivery
6.4. Glutamate Release Modulation via GO Efficiently Limits Excitotoxicity
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| AIF | Apoptosis-inducing factor |
| AMPA | α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid |
| AMPARs | AMPA receptors |
| BBB | Blood–brain barrier |
| CBF | Cerebral blood flow |
| CBP | CREB-binding protein |
| CNS | Central nervous system |
| DOX | Doxorubicin |
| EAAT/EAATs | Excitatory amino acid transporter(s) |
| EAE | Experimental autoimmune encephalomyelitis |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| GBMs | Graphene-based materials |
| GO | Graphene oxide |
| GQDs | Graphene quantum dots |
| HCs | Hemichannels |
| HD | Huntington’s disease |
| IP3 | Inositol triphosphate |
| KA | Kainic acid |
| KARs | Kainate receptors |
| MEAs | Microelectrode arrays |
| mHTT | Mutant huntingtin |
| NCE | Na+/Ca2+ exchanger |
| NMDA | N-methyl-d-aspartate |
| NMDAR/NMDARs | NMDA receptor(s) |
| NO | Nitric oxide |
| NOS | Nitric oxide synthase |
| OGD | Oxygen–glucose deprivation |
| ONOO− | Peroxynitrite |
| PAR | Poly(ADP-ribose) |
| PARP | Poly(ADP-ribose) polymerase |
| PD | Parkinson’s disease |
| PID | Peri-infarct depolarization |
| PLC | Phospholipase C |
| PSD/PSD95 | Postsynaptic density/Postsynaptic density protein 95 |
| ROS | Reactive oxygen species |
| SNpc | Substantia nigra pars compacta |
| VGCCs | Voltage-gated calcium channels |
| VGSCs | Voltage-gated sodium channels |
References
- Meldrum, B.S. Glutamate as a neurotransmitter in the brain: Review of physiology and pathology. J. Nutr. 2000, 130, 1007S–1015S. [Google Scholar] [CrossRef]
- Miladinovic, T.; Nashed, M.G.; Singh, G. Overview of glutamatergic dysregulation in central pathologies. Biomolecules 2015, 5, 3112–3141. [Google Scholar] [CrossRef]
- Lau, A.; Tymianski, M. Glutamate receptors, neurotoxicity and neurodegeneration. Pflug. Arch. 2010, 460, 525–542. [Google Scholar] [CrossRef]
- Lewerenz, J.; Maher, P. Chronic glutamate toxicity in neurodegenerative diseases—What is the evidence? Front. Neurosci. 2015, 9, 469. [Google Scholar] [CrossRef] [PubMed]
- Lucas, D.R.; Newhouse, J.P. The toxic effect of sodium L-glutamate on the inner layers of the retina. Arch. Ophthalmol. 1957, 58, 193–201. [Google Scholar] [CrossRef]
- Olney, J.W. Brain lesions, obesity, and other disturbances in mice treated with monosodium glutamate. Science 1969, 164, 719–721. [Google Scholar] [CrossRef]
- Olney, J.W.; Sharpe, L.G. Brain lesions in an infant rhesus monkey treated with monosodium glutamate. Science 1969, 166, 386–388. [Google Scholar] [CrossRef] [PubMed]
- Olney, J.W.; Labruyere, J.; Price, M.T. Pathological changes induced in cerebrocortical neurons by phencyclidine and related drugs. Science 1989, 244, 1360–1362. [Google Scholar] [CrossRef]
- Jørgensen, M.B.; Diemer, N.H. Selective neuron loss after cerebral ischemia in the rat: Possible role of transmitter glutamate. Acta Neurol. Scand. 1982, 66, 536–546. [Google Scholar] [CrossRef] [PubMed]
- Rothman, S. Synaptic release of excitatory amino acid neurotransmitter mediates anoxic neuronal death. J. Neurosci. 1984, 4, 1884–1891. [Google Scholar] [CrossRef]
- Sattler, R.; Tymianski, M. Molecular mechanisms of glutamate receptor-mediated excitotoxic neuronal cell death. Mol. Neurobiol. 2001, 24, 107–129. [Google Scholar] [CrossRef]
- Sheldon, A.L.; Robinson, M.B. The role of glutamate transporters in neurodegenerative diseases and potential opportunities for intervention. Neurochem. Int. 2007, 51, 333–355. [Google Scholar] [CrossRef]
- Lu, W.; Shi, Y.; Jackson, A.C.; Bjorgan, K.; During, M.J.; Sprengel, R.; Seeburg, P.H.; Nicoll, R.A. Subunit composition of synaptic AMPA receptors revealed by a single-cell genetic approach. Neuron 2009, 62, 254–268. [Google Scholar] [CrossRef]
- Baranovic, J.; Plested, A.J.R. How to build the fastest receptor on earth. Biol. Chem. 2016, 397, 195–205. [Google Scholar] [CrossRef]
- Hollmann, M.; Hartley, M.; Heinemann, S. Ca2+ permeability of KA-AMPA–gated glutamate receptor channels depends on subunit composition. Science 1991, 252, 851–853. [Google Scholar] [CrossRef]
- Wright, A.; Vissel, B. The essential role of AMPA receptor GluR2 subunit RNA editing in the normal and diseased brain. Front. Mol. Neurosci. 2012, 5, 34. [Google Scholar] [CrossRef]
- Fortin, D.A.; Davare, M.A.; Srivastava, T.; Brady, J.D.; Nygaard, S.; Derkach, V.A.; Soderling, T.R. Long-term potentiation-dependent spine enlargement requires synaptic Ca2+-permeable AMPA receptors recruited by CaM-kinase I. J. Neurosci. 2010, 30, 11565–11575. [Google Scholar] [CrossRef] [PubMed]
- Cull-Candy, S.; Kelly, L.; Farrant, M. Regulation of Ca2+-permeable AMPA receptors: Synaptic plasticity and beyond. Curr. Opin. Neurobiol. 2006, 16, 288–297. [Google Scholar] [CrossRef]
- Liu, S.J.; Zukin, R.S. Ca2+-permeable AMPA receptors in synaptic plasticity and neuronal death. Trends Neurosci. 2007, 30, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Chałupnik, P.; Szymańska, E. Kainate receptor antagonists: Recent advances and therapeutic perspective. Int. J. Mol. Sci. 2023, 24, 1908. [Google Scholar] [CrossRef] [PubMed]
- Bernard, A.; Ferhat, L.; Dessi, F.; Charton, G.; Represa, A.; Ben-Ari, Y.; Khrestchatisky, M. Q/R editing of the rat GluR5 and GluR6 kainate receptors in vivo and in vitro: Evidence for independent developmental, pathological and cellular regulation. Eur. J. Neurosci. 1999, 11, 604–616. [Google Scholar] [CrossRef]
- Chittajallu, R.; Vignes, M.; Dev, K.K.; Barnes, J.M.; Collingridge, G.L.; Henley, J.M. Regulation of glutamate release by presynaptic kainate receptors in the hippocampus. Nature 1996, 379, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Castillo, P.E.; Malenka, R.C.; Nicoll, R.A. Kainate receptors mediate a slow postsynaptic current in hippocampal CA3 neurons. Nature 1997, 388, 182–186. [Google Scholar] [CrossRef]
- Brorson, J.R.; Manzolillo, P.A.; Miller, R.J. Ca2+ entry via AMPA/KA receptors and excitotoxicity in cultured cerebellar Purkinje cells. J. Neurosci. 1994, 14, 187–197. [Google Scholar] [CrossRef]
- Lakhan, S.E.; Caro, M.; Hadzimichalis, N. NMDA receptor activity in neuropsychiatric disorders. Front. Psychiatry 2013, 4, 52. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.W.; Ascher, P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature 1987, 325, 529–531. [Google Scholar] [CrossRef]
- Mayer, M.L.; Westbrook, G.L.; Guthrie, P.B. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature 1984, 309, 261–263. [Google Scholar] [CrossRef]
- Kuner, T.; Schoepfer, R. Multiple structural elements determine subunit specificity of Mg2+ block in NMDA receptor channels. J. Neurosci. 1996, 16, 3549–3558. [Google Scholar] [CrossRef]
- Wyllie, D.J.; Livesey, M.R.; Hardingham, G.E. Influence of GluN2 subunit identity on NMDA receptor function. Neuropharmacology 2013, 74, 4–17. [Google Scholar] [CrossRef] [PubMed]
- Voglis, G.; Tavernarakis, N. The role of synaptic ion channels in synaptic plasticity. EMBO Rep. 2006, 7, 1104–1110. [Google Scholar] [CrossRef]
- Hansen, K.B.; Wollmuth, L.P.; Bowie, D.; Furukawa, H.; Menniti, F.S.; Sobolevsky, A.I.; Swanson, G.T.; Swanger, S.A.; Greger, I.H.; Nakagawa, T.; et al. Structure, function, and pharmacology of glutamate receptor ion channels. Pharmacol. Rev. 2021, 73, 298–487. [Google Scholar] [CrossRef]
- Yin, S.; Niswender, C.M. Progress toward advanced understanding of metabotropic glutamate receptors: Structure, signaling and therapeutic indications. Cell. Signal. 2014, 26, 2284–2297. [Google Scholar] [CrossRef]
- Conn, P.J.; Pin, J.P. The metabotropic glutamate receptors: Structure and functions. Annu. Rev. Pharmacol. Toxicol. 1997, 37, 205–237. [Google Scholar] [CrossRef] [PubMed]
- Heuss, C.; Scanziani, M.; Gähwiler, B.H.; Gerber, U. G-protein-independent signaling mediated by metabotropic glutamate receptors. Nat. Neurosci. 1999, 2, 1070–1077. [Google Scholar] [CrossRef] [PubMed]
- Krieger, P.; Hellgren-Kotaleski, J.; Kettunen, P.; El Manira, A.J. Interaction between metabotropic and ionotropic glutamate receptors regulates neuronal network activity. J. Neurosci. 2000, 20, 5382–5391. [Google Scholar] [CrossRef]
- Niswender, C.M.; Conn, P.J. Metabotropic glutamate receptors: Physiology, pharmacology, and disease. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 295–322. [Google Scholar] [CrossRef]
- Abe, T.; Sugihara, H.; Nawa, H.; Shigemoto, R.; Mizuno, N.; Nakanishi, S. Molecular characterization of a novel metabotropic glutamate receptor mGluR5 coupled to inositol phosphate/Ca2+ signal transduction. J. Biol. Chem. 1992, 267, 13361–13368. [Google Scholar] [CrossRef]
- Aramori, I.; Nakanishi, S. Signal transduction and pharmacological characteristics of a metabotropic glutamate receptor, mGluR1, in transfected CHO cells. Neuron 1992, 8, 757–765. [Google Scholar] [CrossRef]
- Snyder, E.M.; Philpot, B.D.; Huber, K.M.; Dong, X.; Fallon, J.R.; Bear, M.F. Internalization of ionotropic glutamate receptors in response to mGluR activation. Nat. Neurosci. 2001, 4, 1079–1085. [Google Scholar] [CrossRef]
- Bruno, V.; Copani, A.; Knöpfel, T.; Kuhn, R.; Casabona, G.; Dell’Albani, P.; Condorelli, D.F.; Nicoletti, F. Activation of metabotropic glutamate receptors coupled to inositol phospholipid hydrolysis amplifies NMDA-induced neuronal degeneration in cultured cortical cells. Neuropharmacology 1995, 34, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Ohishi, H.; Ogawa-Meguro, R.; Shigemoto, R.; Kaneko, T.; Nakanishi, S.; Mizuno, N. Immunohistochemical localization of metabotropic glutamate receptors, mGluR2 and mGluR3, in rat cerebellar cortex. Neuron 1994, 13, 55–66. [Google Scholar] [CrossRef]
- Chavis, P.; Shinozaki, H.; Bockaert, J.; Fagni, L. The metabotropic glutamate receptor types 2/3 inhibit L-type calcium channels via a pertussis toxin-sensitive G-protein in cultured cerebellar granule cells. J. Neurosci. 1994, 14, 7067–7076. [Google Scholar] [CrossRef]
- Tanabe, Y.; Nomura, A.; Masu, M.; Shigemoto, R.; Mizuno, N.; Nakanishi, S. Signal transduction, pharmacological properties, and expression patterns of two rat metabotropic glutamate receptors, mGluR3 and mGluR4. J. Neurosci. 1993, 13, 1372–1378. [Google Scholar] [CrossRef]
- Bradley, S.R.; Levey, A.I.; Hersch, S.M.; Conn, P.J. Immunocytochemical localization of group III metabotropic glutamate receptors in the hippocampus with subtype-specific antibodies. J. Neurosci. 1996, 16, 2044–2056. [Google Scholar] [CrossRef]
- Blackshaw, L.A.; Page, A.J.; Young, R.L. Metabotropic glutamate receptors as novel therapeutic targets on visceral sensory pathways. Front. Neurosci. 2011, 5, 40. [Google Scholar] [CrossRef] [PubMed]
- Kiedrowski, L.; Wroblewski, J.T.; Costa, E. Intracellular sodium concentration in cultured cerebellar granule cells challenged with glutamate. Mol. Pharmacol. 1994, 45, 1050–1054. [Google Scholar] [CrossRef] [PubMed]
- Rothman, S.M. The neurotoxicity of excitatory amino acids is produced by passive chloride influx. J. Neurosci. 1985, 5, 1483–1489. [Google Scholar] [CrossRef] [PubMed]
- Rungta, R.L.; Choi, H.B.; Tyson, J.R.; Malik, A.; Dissing-Olesen, L.; Lin, P.J.C.; Cain, S.M.; Cullis, P.R.; Snutch, T.P.; MacVicar, B.A. The cellular mechanisms of neuronal swelling underlying cytotoxic edema. J. Physiol. 2015, 161, 610–621. [Google Scholar] [CrossRef]
- Choi, D.W. Ionic dependence of glutamate neurotoxicity. J. Neurosci. 1987, 7, 369–379. [Google Scholar] [CrossRef]
- Doble, A. The role of excitotoxicity in neurodegenerative disease: Implications for therapy. Pharmacol. Ther. 1999, 81, 163–221. [Google Scholar] [CrossRef]
- Malik, A.R.; Willnow, T.E. Excitatory amino acid transporters in physiology and disorders of the central nervous system. Int. J. Mol. Sci. 2019, 20, 5671. [Google Scholar] [CrossRef]
- Choi, D.W. Calcium-mediated neurotoxicity: Relationship to specific channel types and role in ischemic damage. Trends Neurosci. 1988, 11, 465–469. [Google Scholar] [CrossRef]
- Sattler, R.; Charlton, M.P.; Hafner, M.; Tymianski, M. Distinct influx pathways, not calcium load, determine neuronal vulnerability to calcium neurotoxicity. J. Neurochem. 1998, 71, 2349–2364. [Google Scholar] [CrossRef]
- Zündorf, G.; Reiser, G. Calcium dysregulation and homeostasis of neural calcium in the molecular mechanisms of neurodegenerative diseases provide multiple targets for neuroprotection. Antioxid. Redox Signal. 2011, 14, 1275–1288. [Google Scholar] [CrossRef]
- Kumagai, A.; Sasaki, T.; Matsuoka, K.; Abe, M.; Tabata, T.; Itoh, Y.; Takemori, H. Monitoring of glutamate-induced excitotoxicity by mitochondrial oxygen consumption. Synapse 2019, 73, e22067. [Google Scholar] [CrossRef] [PubMed]
- Sattler, R.; Xiong, Z.; Lu, W.Y.; Hafner, M.; MacDonald, J.F.; Tymianski, M. Specific coupling of NMDA receptor activation to nitric oxide neurotoxicity by PSD-95 protein. Science 1999, 284, 1845–1848. [Google Scholar] [CrossRef] [PubMed]
- Stamler, J.S.; Lamas, S.; Fang, F.C. Nitrosylation: The prototypic redox-based signaling mechanism. Cell 2001, 106, 675–683. [Google Scholar] [CrossRef]
- Radi, R.; Beckman, J.S.; Bush, K.M.; Freeman, B.A. Peroxynitrite oxidation of sulfhydryls: The cytotoxic potential of superoxide and nitric oxide. J. Biol. Chem. 1991, 266, 4244–4250. [Google Scholar] [CrossRef] [PubMed]
- Radi, R.; Beckman, J.S.; Bush, K.M.; Freeman, B.A. Peroxynitrite-induced membrane lipid peroxidation: The cytotoxic potential of superoxide and nitric oxide. Arch. Biochem. Biophys. 1991, 288, 481–487. [Google Scholar] [CrossRef]
- Hara, M.R.; Agrawal, N.; Kim, S.F.; Cascio, M.B.; Fujimuro, M.; Ozeki, Y.; Takahashi, M.; Cheah, J.H.; Tankou, S.K.; Hester, L.D.; et al. S-nitrosylated GAPDH initiates apoptotic cell death by nuclear translocation following Siah1 binding. Nat. Cell Biol. 2005, 7, 665–674. [Google Scholar] [CrossRef]
- Tristan, C.; Shahani, N.; Sedlak, T.W.; Sawa, A. The diverse functions of GAPDH: Views from different subcellular compartments. Cell Signal. 2010, 23, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Vedia, L.M.; McDonald, B.; Reep, B.; Brüne, B.; Di Silvio, M.; Billiar, T.R.; Lapetina, E.G. Nitric oxide-induced S-nitrosylation of glyceraldehyde-3-phosphate dehydrogenase inhibits enzymatic activity and increases endogenous ADP-ribosylation. J. Biol. Chem. 1992, 267, 24929–24932. [Google Scholar] [CrossRef]
- Thayer, S.A.; Wang, G.J. Glutamate-induced calcium loads: Effects on energy metabolism and neuronal viability. Clin. Exp. Pharmacol. Physiol. 1995, 22, 303–304. [Google Scholar] [CrossRef] [PubMed]
- Walkon, L.L.; Strubbe-Rivera, J.O.; Bazil, J.N. Calcium overload and mitochondrial metabolism. Biomolecules 2022, 12, 1891. [Google Scholar] [CrossRef] [PubMed]
- Hall, E.D. Acute therapeutic interventions: Free radical scavengers and antioxidants. Neurosurg. Clin. N. Am. 1997, 8, 195–206. [Google Scholar] [CrossRef]
- Radi, R.; Rodriguez, M.; Castro, L.; Telleri, R. Inhibition of mitochondrial electron transport by peroxynitrite. Arch. Biochem. Biophys. 1994, 308, 89–95. [Google Scholar] [CrossRef]
- Nakagawa, D.; Ohshima, Y.; Takusagawa, M.; Ikota, N.; Takahashi, Y.; Shimizu, S.; Ozawa, T. Functional modification of cytochrome c by peroxynitrite in an electron transfer reaction. Chem. Pharm. Bull. 2001, 49, 1547–1554. [Google Scholar] [CrossRef]
- Polster, B.M.; Basañez, G.; Etxebarria, A.; Hardwick, J.M.; Nicholls, D.G. Calpain I induces cleavage and release of apoptosis-inducing factor from isolated mitochondria. J. Biol. Chem. 2005, 280, 6447–6454. [Google Scholar] [CrossRef]
- Susin, S.A.; Lorenzo, H.K.; Zamzami, N.; Marzo, I.; Snow, B.E.; Brothers, G.M.; Mangion, J.; Jacotot, E.; Costantini, P.; Loeffler, M.; et al. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature 1999, 397, 441–446. [Google Scholar] [CrossRef]
- Yu, S.W.; Andrabi, S.A.; Wang, H.; Kim, N.S.; Poirier, G.G.; Dawson, T.M.; Dawson, V.L. Apoptosis-inducing factor mediates poly(ADP-ribose) (PAR) polymer-induced cell death. Proc. Natl. Acad. Sci. USA 2006, 103, 18314–18319. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Dawson, V.L.; Dawson, T.M.; Snyder, S.H. Nitric oxide activation of poly(ADP-ribose) synthetase in neurotoxicity. Science 1994, 263, 687–689. [Google Scholar] [CrossRef] [PubMed]
- Moubarak, R.S.; Yuste, V.J.; Artus, C.; Bouharrour, A.; Greer, P.A.; Menissier-de Murcia, J.; Susin, S.A. Sequential activation of poly(ADP-ribose) polymerase 1, calpains, and Bax is essential in apoptosis-inducing factor-mediated programmed necrosis. Mol. Cell. Biol. 2007, 27, 4844–4862. [Google Scholar] [CrossRef]
- Wang, Y.; Qin, Z.-H. Molecular and cellular mechanisms of excitotoxic neuronal death. Apoptosis 2010, 15, 1382–1402. [Google Scholar] [CrossRef]
- Ceprian, M.; Fulton, D. Glial cell AMPA receptors in nervous system health, injury and disease. Int. J. Mol. Sci. 2019, 20, 2450. [Google Scholar] [CrossRef] [PubMed]
- Patani, R.; Hardingham, G.E.; Liddelow, S.A. Functional roles of reactive astrocytes in neuroinflammation and neurodegeneration. Nat. Rev. Neurol. 2023, 19, 395–409. [Google Scholar] [CrossRef] [PubMed]
- Pajarillo, E.; Rizor, A.; Lee, J.; Aschner, M.; Lee, E. The role of astrocytic glutamate transporters GLT-1 and GLAST in neurological disorders: Potential targets for neurotherapeutics. Neuropharmacology 2019, 161, 107559. [Google Scholar] [CrossRef]
- Krebs, C.; Fernandes, H.B.; Sheldon, C.; Raymond, L.A.; Baimbridge, K.G. Functional NMDA receptor subtype 2B is expressed in astrocytes after ischemia in vivo and anoxia in vitro. J. Neurosci. 2003, 23, 3364–3372. [Google Scholar] [CrossRef]
- Mahmoud, S.; Gharagozloo, M.; Simard, C.; Gris, D. Astrocytes maintain glutamate homeostasis in the CNS by controlling the balance between glutamate uptake and release. Cells 2019, 8, 184. [Google Scholar] [CrossRef]
- Tse, V. Astrocytic control of glutamate spillover and extrasynaptic NMDA receptor activation: Implications for neurodegenerative disorders. J. Neurosci. 2024, 44, e0083242024. [Google Scholar] [CrossRef]
- Provenzano, F.; Torazza, C.; Bonifacino, T.; Bonanno, G.; Milanese, M. The key role of astrocytes in amyotrophic lateral sclerosis and their commitment to glutamate excitotoxicity. Int. J. Mol. Sci. 2023, 24, 15430. [Google Scholar] [CrossRef]
- Ben Haim, L.; Carrillo-de Sauvage, M.A.; Ceyzériat, K.; Escartin, C. Elusive roles for reactive astrocytes in neurodegenerative diseases. Front. Cell. Neurosci. 2015, 9, 278. [Google Scholar] [CrossRef]
- Larsen, B.R.; MacAulay, N. Activity-dependent astrocyte swelling is mediated by pH-regulating mechanisms. Glia 2017, 65, 1668–1681. [Google Scholar] [CrossRef]
- Doyle, K.P.; Simon, R.P.; Stenzel-Poore, M.P. Mechanisms of ischemic brain damage. Neuropharmacology 2008, 55, 310–318. [Google Scholar] [CrossRef]
- Puig, B.; Brenna, S.; Magnus, T. Molecular communication of a dying neuron in stroke. Int. J. Mol. Sci. 2018, 19, 2834. [Google Scholar] [CrossRef]
- Yao, X.; Wang, Y.; Zhang, D. Regulation of cerebral blood flow in humans: Physiology and clinical implications of autoregulation. Physiol. Rev. 2021, 101, 1487–1559. [Google Scholar] [CrossRef]
- Traystman, R.J. Animal models of focal and global cerebral ischemia. ILAR J. 2003, 44, 85–95. [Google Scholar] [CrossRef]
- Ginsberg, M.D. Adventures in the pathophysiology of brain ischemia: Penumbra, gene expression, neuroprotection: The 2002 Thomas Willis Lecture. Stroke 2003, 34, 214–223. [Google Scholar] [CrossRef]
- Du, Y.; Wang, W.; Lutton, A.D.; Kiyoshi, C.M.; Ma, B.; Taylor, A.T.; Olesik, J.W.; McTigue, D.M.; Askwith, C.C.; Zhou, M. Dissipation of transmembrane potassium gradient is the main cause of cerebral ischemia induced depolarization in astrocytes and neurons. Exp. Neurol. 2018, 303, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Thrane, A.S.; Thrane, V.R.; Nedergaard, M. Drowning stars: Reassessing the role of astrocytes in brain edema. Trends Neurosci. 2014, 37, 620–628. [Google Scholar] [CrossRef] [PubMed]
- Živančević, K.; Lović, D.; Andjus, P.R.; Radenovic, L. Neuroinflammation in post-ischemic brain. In Cerebral Ischemia; Pluta, R., Ed.; Chapter 7; Exon Publications: Brisbane, Australia, 2021. [Google Scholar]
- Hansen, A.J. Effect of anoxia on ion distribution in the brain. Physiol. Rev. 1985, 65, 101–148. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Agulian, S.; Haddad, G.G. Cl− and Na+ homeostasis during anoxia in rat hypoglossal neurons: Intracellular and extracellular in vitro studies. J. Physiol. 1992, 448, 697–708. [Google Scholar] [CrossRef]
- Szatkowski, M.; Attwell, D. Triggering and execution of neuronal death in brain ischemia: Two phases of glutamate release by different mechanisms. Trends Neurosci. 1994, 17, 359–365. [Google Scholar] [CrossRef]
- Luoma, J.I.; Kelley, B.G.; Mermelstein, P.G. Progesterone inhibition of voltage-gated calcium channels is a potential neuroprotective mechanism against excitotoxicity. Steroids 2011, 76, 845–855. [Google Scholar] [CrossRef] [PubMed]
- Verma, M.; Wills, Z.; Chu, C.T. Excitatory dendritic mitochondrial calcium toxicity: Implications for Parkinson’s and other neurodegenerative diseases. Front. Neurosci. 2018, 12, 523. [Google Scholar] [CrossRef]
- stemBlaustein, M.P.; Lederer, W.J. Sodium/calcium exchange: Its physiological implications. Physiol. Rev. 1999, 79, 763–854. [Google Scholar] [CrossRef] [PubMed]
- Swan, J.H.; Meldrum, B.S. Protection by NMDA antagonists against selective cell loss following transient ischemia. J. Cereb. Blood Flow Metab. 1990, 10, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Zipfel, G.J.; Lee, J.M.; Choi, D.W. Reducing calcium overload in the ischemic brain. N. Engl. J. Med. 1999, 341, 1543–1544. [Google Scholar] [CrossRef]
- Witcher, M.R.; Park, Y.D.; Lee, M.R.; Sharma, S.; Harris, K.M.; Kirov, S.A. Three-dimensional relationships between perisynaptic astroglia and human hippocampal synapses. Glia 2010, 58, 572–587. [Google Scholar] [CrossRef]
- Eulenburg, V.; Gomeza, J. Neurotransmitter transporters expressed in glial cells as regulators of synapse function. Brain Res. Rev. 2010, 63, 103–112. [Google Scholar] [CrossRef]
- Seki, Y.; Feustel, P.J.; Keller, R.W., Jr.; Tranmer, B.I.; Kimelberg, H.K. Inhibition of ischemia-induced glutamate release in rat striatum by dihydrokinate and an anion channel blocker. Stroke 1999, 30, 433–440. [Google Scholar] [CrossRef]
- Yu, S.P.; Jiang, M.Q.; Shim, S.S.; Wei, Z.Z.; Chen, D.; Wei, L. Extrasynaptic NMDA receptors in acute and chronic excitotoxicity: Implications for preventive treatments of ischemic stroke and late-onset Alzheimer’s disease. Mol. Neurodegener. 2023, 18, 43. [Google Scholar] [CrossRef]
- Dreier, J.P. The role of spreading depression, spreading depolarization and spreading ischemia in neurological disease. Nat. Med. 2011, 17, 439–447. [Google Scholar] [CrossRef]
- Gadhave, D.G.; Sugandhi, V.V.; Jha, S.K.; Nangare, S.N.; Gupta, G.; Singh, S.K.; Dua, K.; Cho, H.; Hansbro, P.M.; Paudel, K.R. Neurodegenerative disorders: Mechanisms of degeneration and therapeutic approaches with their clinical relevance. Ageing Res. Rev. 2024, 99, 102357. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; Hardy, J.; Blennow, K.; Chen, C.; Perry, G.; Kim, S.H.; Villemagne, V.L.; Aisen, P.; Vendruscolo, M.; Iwatsubo, T.; et al. The Amyloid-β Pathway in Alzheimer’s Disease. Mol. Psychiatry 2021, 26, 5481–5503. [Google Scholar] [CrossRef]
- Texidó, L.; Martín-Satué, M.; Alberdi, E.; Solsona, C.; Matute, C. Amyloid β peptide oligomers directly activate NMDA receptors. Cell Calcium 2011, 49, 184–190. [Google Scholar] [CrossRef] [PubMed]
- De Felice, F.G.; Velasco, P.T.; Lambert, M.P.; Viola, K.; Fernandez, S.J.; Ferreira, S.T.; Klein, W.L. A-beta oligomers induce neuronal oxidative stress through an N-methyl-D-aspartate receptor-dependent mechanism that is blocked by the Alzheimer drug memantine. J. Biol. Chem. 2007, 282, 11590–115601. [Google Scholar] [CrossRef]
- Liu, J.; Chang, L.; Song, Y.; Li, H.; Wu, Y. The role of NMDA receptors in Alzheimer’s disease. Front. Neurosci. 2019, 13, 43. [Google Scholar] [CrossRef] [PubMed]
- Ambrosi, G.; Cerri, S.; Blandini, F. A further update on the role of excitotoxicity in the pathogenesis of Parkinson’s disease. J. Neural Transm. 2014, 121, 849–859. [Google Scholar] [CrossRef]
- Helton, T.D.; Otsuka, T.; Lee, M.C.; Mu, Y.Y.; Ehlers, M.D. Pruning and loss of excitatory synapses by the Parkin ubiquitin ligase. Proc. Natl. Acad. Sci. USA 2008, 105, 19492–19497. [Google Scholar] [CrossRef]
- Lang, A.E.; Lozano, A.M. Parkinson’s disease-first of two parts. N. Engl. J. Med. 1998, 339, 1044–1053. [Google Scholar] [CrossRef]
- Aviner, R.; Lee, T.-T.; Masto, V.B.; Li, K.H.; Andino, R.; Frydman, J. Polyglutamine-mediated ribotoxicity disrupts proteostasis and stress responses in Huntington’s disease. Nat. Cell Biol. 2024, 26, 892–902. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, H.B.; Raymond, L.A. NMDA Receptors and Huntington’s Disease. In Biology of the NMDA Receptor; Van Dongen, A.M., Ed.; Chapter 2; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2009. [Google Scholar]
- Coyle, J.T.; Schwarcz, R. Lesion of striatal neurons with kainic acid provides a model for Huntington’s chorea. Nature 1976, 263, 244–246. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.Q.; Wang, X.X.; Wang, Y.; Chuang, D.M.; DiFiglia, M.; Chase, T.N.; Qin, Z.H. Susceptibility of striatal neurons to excitotoxic injury correlates with basal levels of Bcl-2 and the induction of P53 and c-Myc immunoreactivity. Neurobiol. Dis. 2005, 20, 562–573. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Geim, A.K. Graphene: Status and prospects. Science 2009, 324, 1530–1534. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, V.C.; Jachak, A.; Hurt, R.H.; Kane, A.B. Biological interactions of graphene-family nanomaterials: An interdisciplinary review. Chem. Res. Toxicol. 2012, 25, 15–34. [Google Scholar] [CrossRef]
- Kostarelos, K.; Novoselov, K.S. Materials science. Exploring the interface of graphene and biology. Science 2014, 344, 261–263. [Google Scholar] [CrossRef]
- Bramini, M.; Alberini, G.; Colombo, E.; Chiacchiaretta, M.; DiFrancesco, M.L.; Maya-Vetencourt, J.F.; Maragliano, L.; Benfenati, F.; Cesca, F. Interfacing graphene-based materials with neural cells. Front. Syst. Neurosci. 2018, 12, 358913. [Google Scholar] [CrossRef]
- Kitko, K.E.; Zhang, Q. Graphene-based nanomaterials: From production to integration with modern tools in neuroscience. Front. Syst. Neurosci. 2019, 13, 26. [Google Scholar] [CrossRef]
- Cellot, G.; Franceschi Biagioni, A.; Ballerini, L. Nanomedicine and graphene-based materials: Advanced technologies for potential treatments of diseases in the developing nervous system. Pediatr. Res. 2022, 92, 71–79. [Google Scholar] [CrossRef]
- Reina, G.; Gonzalez-Dominguez, J.M.; Criado, A.; Vázquez, E.; Bianco, A.; Prato, M. Promises, facts and challenges for graphene in biomedical applications. Chem. Soc. Rev. 2017, 46, 4400–4416. [Google Scholar] [CrossRef] [PubMed]
- Rauti, R.; Medelin, M.; Newman, L.; Vranic, S.; Reina, G.; Bianco, A.; Prato, M.; Kostarelos, K.; Ballerini, L. Graphene oxide flakes tune excitatory neurotransmission in vivo by targeting hippocampal synapses. Nano Lett. 2019, 19, 2858–2870. [Google Scholar] [CrossRef]
- Zhou, Y.; Morris, G.H.B.; Nair, M. Current and emerging strategies for biocompatible materials for implantable electronics. Cell Rep. Phys. Sci. 2024, 5, 101852. [Google Scholar] [CrossRef]
- Xu, B.; Pei, J.; Feng, L.; Zhang, X.-D. Graphene and graphene-related materials as brain electrodes. J. Mater. Chem. B 2021, 9, 9485–9496. [Google Scholar] [CrossRef] [PubMed]
- Kuzum, D.; Takano, H.; Shim, E.; Reed, J.C.; Juul, H.; Richardson, A.G.; de Vries, J.; Bink, H.; Dichter, M.A.; Lucas, T.H.; et al. Transparent and flexible low noise graphene electrodes for simultaneous electrophysiology and neuroimaging. Nat. Commun. 2014, 5, 5259. [Google Scholar] [CrossRef]
- Masvidal-Codina, E.; Smith, T.M.; Rathore, D.; Gao, Y.; Illa, X.; Prats-Alfonso, E.; Corro, E.D.; Calia, A.B.; Rius, G.; Martin-Fernandez, I.; et al. Characterization of optogenetically-induced cortical spreading depression in awake mice using graphene micro-transistor arrays. J. Neural Eng. 2021, 18, 056005. [Google Scholar] [CrossRef]
- Ria, N.; Eladly, A.; Masvidal-Codina, E.; Illa, X.; Guimerà, A.; Hills, K.; Garcia-Cortadella, R.; Duvan, F.T.; Flaherty, S.M.; Prokop, M.; et al. Flexible graphene-based neurotechnology for deep brain mapping and neuromodulation in Parkinsonian rats. Nat. Commun. 2025, 16, 2891. [Google Scholar] [CrossRef]
- Guo, W.; Qiu, J.; Liu, J.; Liu, H. Graphene microfiber as a scaffold for regulation of neural stem cells differentiation. Sci. Rep. 2017, 7, 5678. [Google Scholar] [CrossRef]
- Rauti, R.; Secomandi, N.; Martín, C.; Bosi, S.; Severino, F.P.U.; Scaini, D.; Prato, M.; Vázquez, E.; Ballerini, L. Tuning neuronal circuit formation in 3D polymeric scaffolds by introducing graphene at the bio/material interface. Adv. Biosyst. 2020, 4, e1900233. [Google Scholar] [CrossRef] [PubMed]
- Bressan, E.; Ferroni, L.; Gardin, C.; Sbricoli, L.; Gobbato, L.; Ludovichetti, F.S.; Tocco, I.; Carraro, A.; Piattelli, A.; Zavan, B. Graphene based scaffolds effects on stem cells commitment. J. Transl. Med. 2014, 12, 296. [Google Scholar] [CrossRef]
- Girão, A.F.; Barroca, N.; Hernández-Martín, Y.; Completo, A.; Marques, P.A.A.P.; Serrano, M.C. 3D nanofibrous frameworks with on-demand engineered gray and white matters for reconstructing the injured spinal cord. Biomater. Adv. 2025, 170, 214200. [Google Scholar] [CrossRef]
- Zhu, R.; Shi, J.; Zhou, H.; Ge, L.; Yin, W.; Zeng, H.; Wang, X. Biological applications of graphene-based nanomaterials in neurodegenerative diseases. Mater. Today Bio 2025, 33, 102064. [Google Scholar] [CrossRef]
- Guo, Z.; Chakraborty, S.; Monikh, F.A.; Varsou, D.-D.; Chetwynd, A.J.; Afantitis, A.; Lynch, I.; Zhang, P. Surface functionalization of graphene-based materials: Biological behavior, toxicology, and safe-by-design aspects. Adv. Biol. 2021, 5, e2100637. [Google Scholar] [CrossRef]
- Daneman, R.; Prat, A. The blood-brain barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a020412. [Google Scholar] [CrossRef]
- McCallion, C.; Burthem, J.; Rees-Unwin, K.; Golovanov, A.; Pluen, A. Graphene in therapeutics delivery: Problems, solutions and future opportunities. Eur. J. Pharm. Biopharm. 2016, 104, 235–250. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2009, 39, 228–240. [Google Scholar] [CrossRef]
- Tan, X.; Feng, L.; Zhang, J.; Yang, K.; Zhang, S.; Liu, Z.; Peng, R. Functionalization of graphene oxide generates a unique interface for selective serum protein interactions. ACS Appl. Mater. Interfaces 2013, 5, 1370–1377. [Google Scholar] [CrossRef]
- Bao, H.; Pan, Y.; Ping, Y.; Sahoo, N.G.; Wu, T.; Li, L.; Li, J.; Gan, L.H. Chitosan-functionalized graphene oxide as a nanocarrier for drug and gene delivery. Small 2011, 7, 1569–1578. [Google Scholar] [CrossRef]
- Jokar, S.; Pourjavadi, A.; Adeli, M. Albumin–graphene oxide conjugates; carriers for anticancer drugs. RSC Adv. 2014, 4, 33001–33006. [Google Scholar] [CrossRef]
- Wang, K.; Wang, L.; Chen, L.; Peng, C.; Luo, B.; Mo, J.; Chen, W. Intranasal administration of dauricine loaded on graphene oxide: Multi-target therapy for Alzheimer’s disease. Drug Deliv. 2021, 28, 580–593. [Google Scholar] [CrossRef]
- Yang, Z.; Ge, C.; Liu, J.; Chong, Y.; Gu, Z.; Jimenez-Cruz, C.A.; Chai, Z.; Zhou, R. Destruction of amyloid fibrils by graphene through penetration and extraction of peptides. Nanoscale 2015, 7, 18725–18737. [Google Scholar] [CrossRef]
- Yaghoubi, F.; Motlagh, N.S.; Naghib, S.M.; Haghiralsadat, F.; Jaliani, H.Z.; Moradi, A. A functionalized graphene oxide with improved cytocompatibility for stimuli-responsive co-delivery of curcumin and doxorubicin in cancer treatment. Sci. Rep. 2022, 12, 1959. [Google Scholar] [CrossRef]
- Zhang, L.; Lu, Z.; Zhao, Q.; Huang, J.; Shen, H.; Zhang, Z. Enhanced chemotherapy efficacy by sequential delivery of siRNA and anticancer drugs using PEI-grafted graphene oxide. Small 2011, 7, 460–464. [Google Scholar] [CrossRef]
- Liu, G.; Shen, H.; Mao, J.; Zhang, L.; Jiang, Z.; Sun, T.; Lan, Q.; Zhang, Z. Transferrin modified graphene oxide for glioma-targeted drug delivery: In vitro and in vivo evaluations. ACS Appl. Mater. Interfaces 2013, 5, 6909–6914. [Google Scholar] [CrossRef]
- Rauti, R.; Lozano, N.; León, V.; Scaini, D.; Musto, M.; Rago, I.; Ulloa Severino, F.P.; Fabbro, A.; Casalis, L.; Vázquez, E.; et al. Graphene oxide nanosheets reshape synaptic function in cultured brain networks. ACS Nano 2016, 10, 4459–4471. [Google Scholar] [CrossRef]
- Li, Y.; Yuan, H.; von dem Bussche, A.; Creighton, M.; Hurt, R.H.; Kane, A.B.; Gao, H. Graphene microsheets enter cells through spontaneous membrane penetration at edge asperities and corner sites. Proc. Natl. Acad. Sci. USA 2013, 110, 12295–12300. [Google Scholar] [CrossRef]
- Secomandi, N.; Franceschi Biagioni, A.; Kostarelos, K.; Cellot, G.; Ballerini, L. Thin graphene oxide nanoflakes modulate glutamatergic synapses in the amygdala cultured circuits: Exploiting synaptic approaches to anxiety disorders. Nanomed. Nanotechnol. Biol. Med. 2020, 26, 102174. [Google Scholar] [CrossRef]
- Cellot, G.; Vranic, S.; Shin, Y.; Worsley, R.; Rodrigues, A.F.; Bussy, C.; Casiraghi, C.; Kostarelos, K.; McDearmid, J.R. Graphene oxide nanosheets modulate spinal glutamatergic transmission and modify locomotor behaviour in an in vivo zebrafish model. Nanoscale Horiz. 2020, 5, 1250–1263. [Google Scholar] [CrossRef]
- Pati, E.; Franceschi Biagioni, A.; Casani, R.; Lozano, N.; Kostarelos, K.; Cellot, G.; Ballerini, L. Delivery of graphene oxide nanosheets modulates glutamate release and normalizes amygdala synaptic plasticity to improve anxiety related behavior. Nanoscale 2023, 15, 18581–18591. [Google Scholar] [CrossRef]
- Franceschi Biagioni, A.; Cellot, G.; Pati, E.; Lozano, N.; Ballesteros, B.; Casani, R.; Coimbra, N.C.; Kostarelos, K.; Ballerini, L. Graphene oxide prevents lateral amygdala dysfunctional synaptic plasticity and reverts long lasting anxiety behavior in rats. Biomaterials 2021, 271, 120749. [Google Scholar] [CrossRef]
- Di Mauro, G.A.; Lozano, N.; Carnasciali, A.; Guasti, D.; Becucci, M.; Cellot, G.; Kostarelos, K.; Ballerini, C.; Ballerini, L. Graphene oxide nanosheets reduce astrocyte reactivity to inflammation and ameliorate experimental autoimmune encephalomyelitis. ACS Nano 2023, 17, 1965–1978. [Google Scholar] [CrossRef] [PubMed]
- Tortella, L.; Santini, I.; Lozano, N.; Kostarelos, K.; Cellot, G.; Ballerini, L. Graphene oxide nanosheets hamper glutamate mediated excitotoxicity and protect neuronal survival in an in vitro stroke model. Chemistry 2023, 29, e202301762. [Google Scholar] [CrossRef] [PubMed]
- Bramini, M.; Sacchetti, S.; Armirotti, A.; Rocchi, A.; Vázquez, E.; León Castellanos, V.; Bandiera, T.; Cesca, F.; Benfenati, F. Graphene oxide nanosheets disrupt lipid composition, Ca2+ homeostasis, and synaptic transmission in primary cortical neurons. ACS Nano 2016, 10, 7154–7171. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cellot, G.; Ballerini, L. Interplay Among Synaptic Glutamate Release and Excitotoxicity: Neuronal Damage and Graphene-Based Materials Related Protection. Life 2025, 15, 1776. https://doi.org/10.3390/life15111776
Cellot G, Ballerini L. Interplay Among Synaptic Glutamate Release and Excitotoxicity: Neuronal Damage and Graphene-Based Materials Related Protection. Life. 2025; 15(11):1776. https://doi.org/10.3390/life15111776
Chicago/Turabian StyleCellot, Giada, and Laura Ballerini. 2025. "Interplay Among Synaptic Glutamate Release and Excitotoxicity: Neuronal Damage and Graphene-Based Materials Related Protection" Life 15, no. 11: 1776. https://doi.org/10.3390/life15111776
APA StyleCellot, G., & Ballerini, L. (2025). Interplay Among Synaptic Glutamate Release and Excitotoxicity: Neuronal Damage and Graphene-Based Materials Related Protection. Life, 15(11), 1776. https://doi.org/10.3390/life15111776






