Therapeutic Impact of Vericiguat on Ventricular Remodeling in a Pressure-Overload Heart Failure Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animal Model and Experimental Design
2.3. Induction of Pressure-Overload Heart Failure
2.4. Drug Administration
2.5. Histological Analysis
2.6. Functional Assessment
2.7. Proteomic Profiling
2.8. Statistical Analysis
3. Results
3.1. Ventricular Wall Stress Assessment
3.2. Left Ventricular Remodeling and Function
3.3. Myocardial Hypertrophy and Fibrosis Assessment
3.4. Fibroblast Proteome Reprogramming
3.5. Myocyte Proteomic Remodeling
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hao, W.R.; Cheng, C.H.; Chen, H.Y.; Liu, J.C.; Cheng, T.H.; Chen, J.J. Exploring Vericiguat in Heart Failure: Mechanistic Insights, Therapeutic Advantages, and Clinical Validation. Curr. Drug Saf. 2025. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Germinal, K.; Milfort, A.; Chen, W.H.; Chang, S.H.; Huang, W.; Li, Y.; Lu, Y.; Ahmed, M.M.; Kimmel, S.E.; et al. The most effective combination of pharmacological therapy for heart failure with reduced ejection fraction: A network meta-analysis of randomized controlled trials. BMC Cardiovasc. Disord. 2024, 24, 666. [Google Scholar] [CrossRef] [PubMed]
- Mentz, R.J.; Butler, J.; McMullan, C.J.; Wojdyla, D.M.; Anstrom, K.J.; Barash, I.; Bonaca, M.P.; Borentain, M.; Corda, S.; Ezekowitz, J.A.; et al. Blood pressure, safety and clinical efficacy of vericiguat in chronic heart failure with reduced ejection fraction: Insights from the VICTOR trial. Eur. J. Heart Fail. 2025. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.; McMullan, C.J.; Anstrom, K.J.; Barash, I.; Bonaca, M.P.; Borentain, M.; Corda, S.; Ezekowitz, J.A.; Felker, G.M.; Gates, D.; et al. Vericiguat in patients with chronic heart failure and reduced ejection fraction (VICTOR): A double-blind, placebo-controlled, randomised, phase 3 trial. Lancet 2025, 406, 1341–1350. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhou, W.; Liu, H.; Zhang, C.; Qi, Y.; Yuan, X.; Yuan, Z.; Sun, L.; She, J.; Lou, B. Initiation of vericiguat and short-term cardiovascular function improvement in heart failure patients with and without worsened renal function. Front. Cardiovasc. Med. 2025, 12, 1628411. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Butler, J.; Young, R.; Lewis, B.S.; Escobedo, J.; Refsgaard, J.; Reyes, E.; Roessig, L.; Blaustein, R.O.; Lam, C.S.P.; et al. Vericiguat and Cardiovascular Outcomes in Heart Failure by Baseline Diabetes Status: Insights from the VICTORIA Trial. JACC Heart Fail. 2024, 12, 1750–1759. [Google Scholar] [CrossRef] [PubMed]
- Khounphinith, E.; Zhou, Y.; Yi, Z.; Li, T.; Li, L. Vericiguat reduces pyroptosis in rats with coronary microembolization by inhibiting the AMPK/Nrf2/NLRP3 signaling pathway. Korean J. Physiol. Pharmacol. 2025, 29, 755–767. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Sheng, Y.; Xu, P.; Peng, Q.; Ruan, Z. Vericiguat reduces atrial fibrillation recurrence by alleviating myocardial fibrosis via the TGF-β1/Smad2/3 pathway. PLoS ONE 2025, 20, e0328272. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Abuín, X.; Moraña-Fernández, S.; Aragón-Herrera, A.; Sandner, P.; Thomitzek, K.; García-Seara, J.; Bravo, S.B.; Otero-Santiago, M.; de la Fuente-López, P.; Tilves-Bellas, C.; et al. Soluble guanylate cyclase stimulation improves cardiac function and mitochondrial activity in a rat model of early-stage heart failure with preserved ejection fraction. Biomed. Pharmacother. 2025, 191, 118439. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wu, Y.; Li, W.; Song, M.; Xu, K.; Wu, M.; Lin, L. Vericiguat improves cardiac remodelling and function in rats with doxorubicin-induced cardiomyopathy. ESC Heart Fail. 2025, 12, 1807–1817. [Google Scholar] [CrossRef] [PubMed]
- Hao, W.R.; Cheng, C.H.; Chen, H.Y.; Cheng, T.H.; Liu, J.C.; Chen, J.J. Fucoidan Attenuates Cardiac Remodeling by Inhibiting Galectin-3 Secretion, Fibrosis, and Inflammation in a Mouse Model of Pressure Overload. Biomedicines 2024, 12, 2847. [Google Scholar] [CrossRef] [PubMed]
- Harada, T.; Kondo, H.; Nakamura, K.; He, Y.; Goto, S.; Takahashi, M.; Yamasaki, H.; Matsuda, N.; Takano, M.; Abe, I.; et al. Soluble Guanylate Cyclase Stimulator Vericiguat Attenuates Angiotensin II-Induced Oxidative Stress and Cardiac Remodeling. Circ. J. 2025, 89, 982–991. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.S.P.; Mulder, H.; Lopatin, Y.; Vazquez-Tanus, J.B.; Siu, D.; Ezekowitz, J.; Pieske, B.; O’Connor, C.M.; Roessig, L.; Patel, M.J.; et al. Blood Pressure and Safety Events with Vericiguat in the VICTORIA Trial. J. Am. Heart Assoc. 2021, 10, e021094. [Google Scholar] [CrossRef] [PubMed]
- Mentz, R.J.; Stebbins, A.; Butler, J.; Chiang, C.E.; Ezekowitz, J.A.; Hernandez, A.F.; Hilkert, R.; Lam, C.S.P.; McDonald, K.; O’Connor, C.M.; et al. Recurrent Hospitalizations and Response to Vericiguat in Heart Failure and Reduced Ejection Fraction. JACC Heart Fail. 2024, 12, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Zhang, H.; Xu, T.; Mei, X.; Wang, X.; Yang, Q.; Luo, Z.; Zeng, Q.; Xu, D.; Ren, H. Vericiguat attenuates doxorubicin-induced cardiotoxicity through the PRKG1/PINK1/STING axis. Transl. Res. 2024, 273, 90–103. [Google Scholar] [CrossRef] [PubMed]
- Gawrys, O.; Husková, Z.; Škaroupková, P.; Honetschlägerová, Z.; Vaňourková, Z.; Kikerlová, S.; Melenovský, V.; Bačová, B.S.; Sykora, M.; Táborský, M.; et al. The treatment with sGC stimulator improves survival of hypertensive rats in response to volume-overload induced by aorto-caval fistula. Naunyn Schmiedebergs Arch. Pharmacol. 2023, 396, 3757–3773. [Google Scholar] [CrossRef] [PubMed]
- Mira Hernandez, J.; Shen, E.Y.; Ko, C.Y.; Hourani, Z.; Spencer, E.R.; Smoliarchuk, D.; Bossuyt, J.; Granzier, H.; Bers, D.M.; Hegyi, B. Differential sex-dependent susceptibility to diastolic dysfunction and arrhythmia in cardiomyocytes from obese diabetic heart failure with preserved ejection fraction model. Cardiovasc. Res. 2025, 121, 254–266. [Google Scholar] [CrossRef] [PubMed]
- deFilippi, C.R.; Shah, P.; Shah, S.J.; Alemayehu, W.; Lam, C.S.P.; Butler, J.; Roessig, L.; O’Connor, C.M.; Westerhout, C.M.; Armstrong, P.W. Proteomics Identify Clinical Phenotypes and Predict Functional Outcomes in Heart Failure with Preserved Ejection Fraction: Insights From VITALITY-HFpEF. Circ. Heart Fail. 2024, 17, e011792. [Google Scholar] [CrossRef] [PubMed]
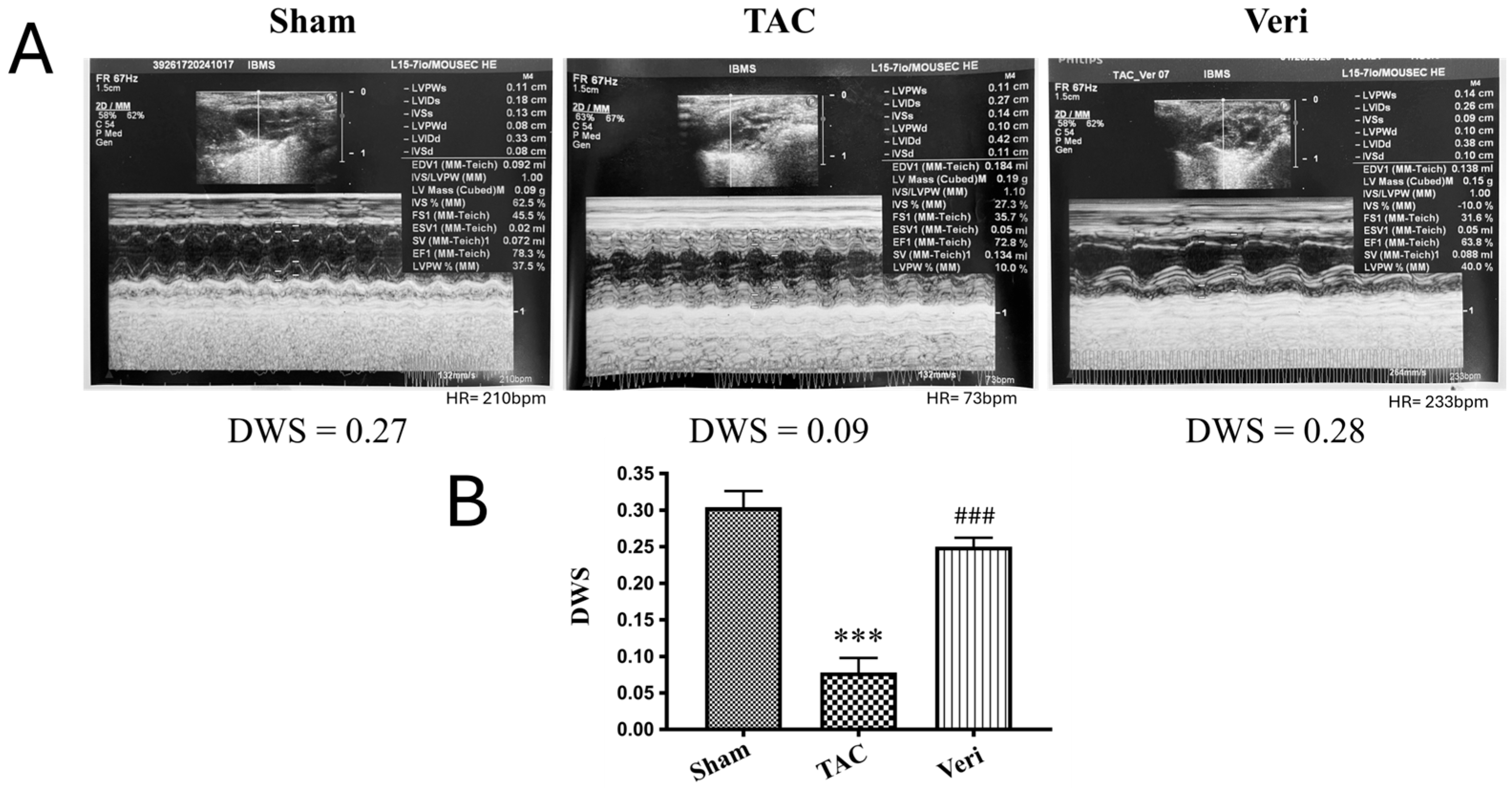

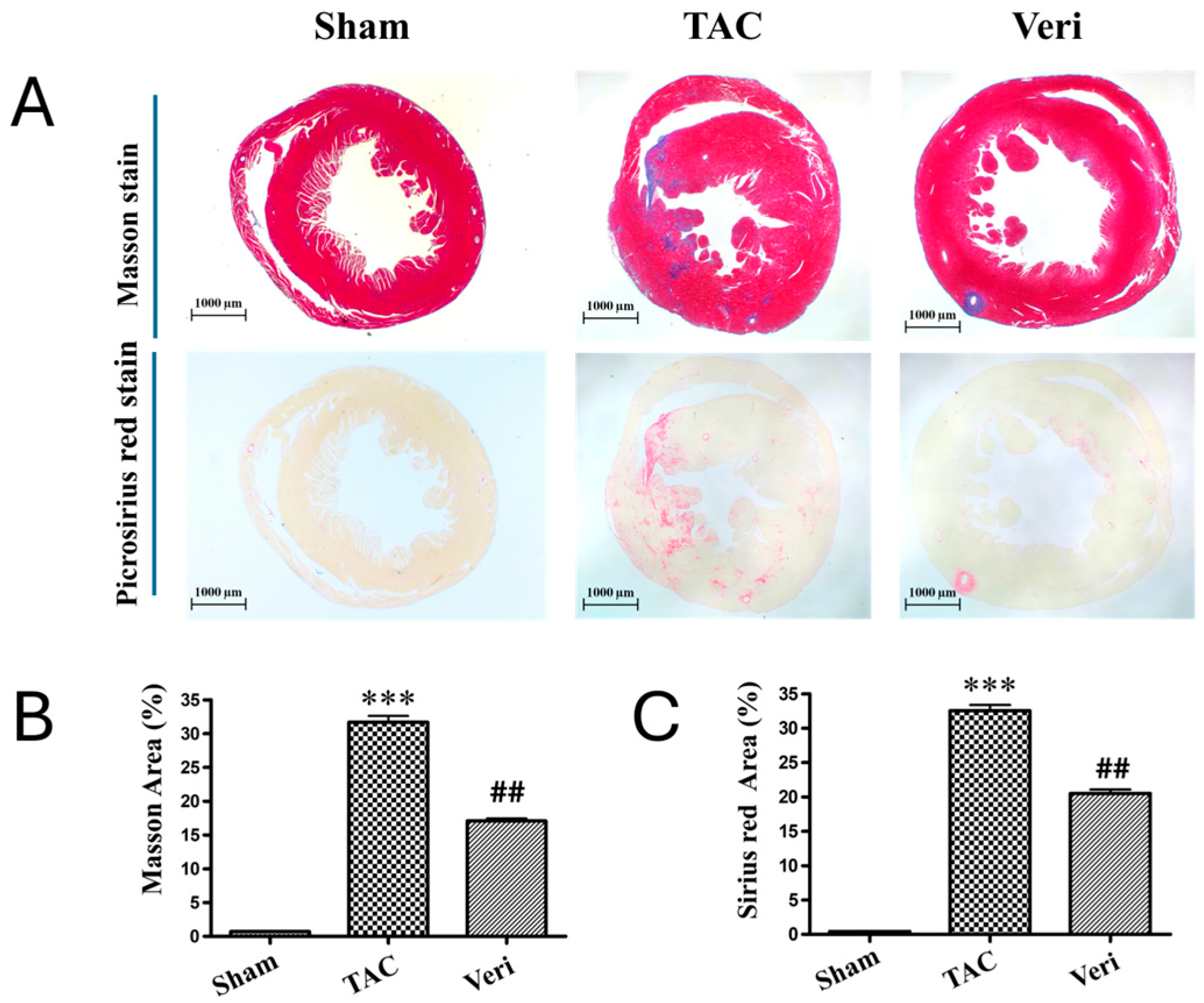
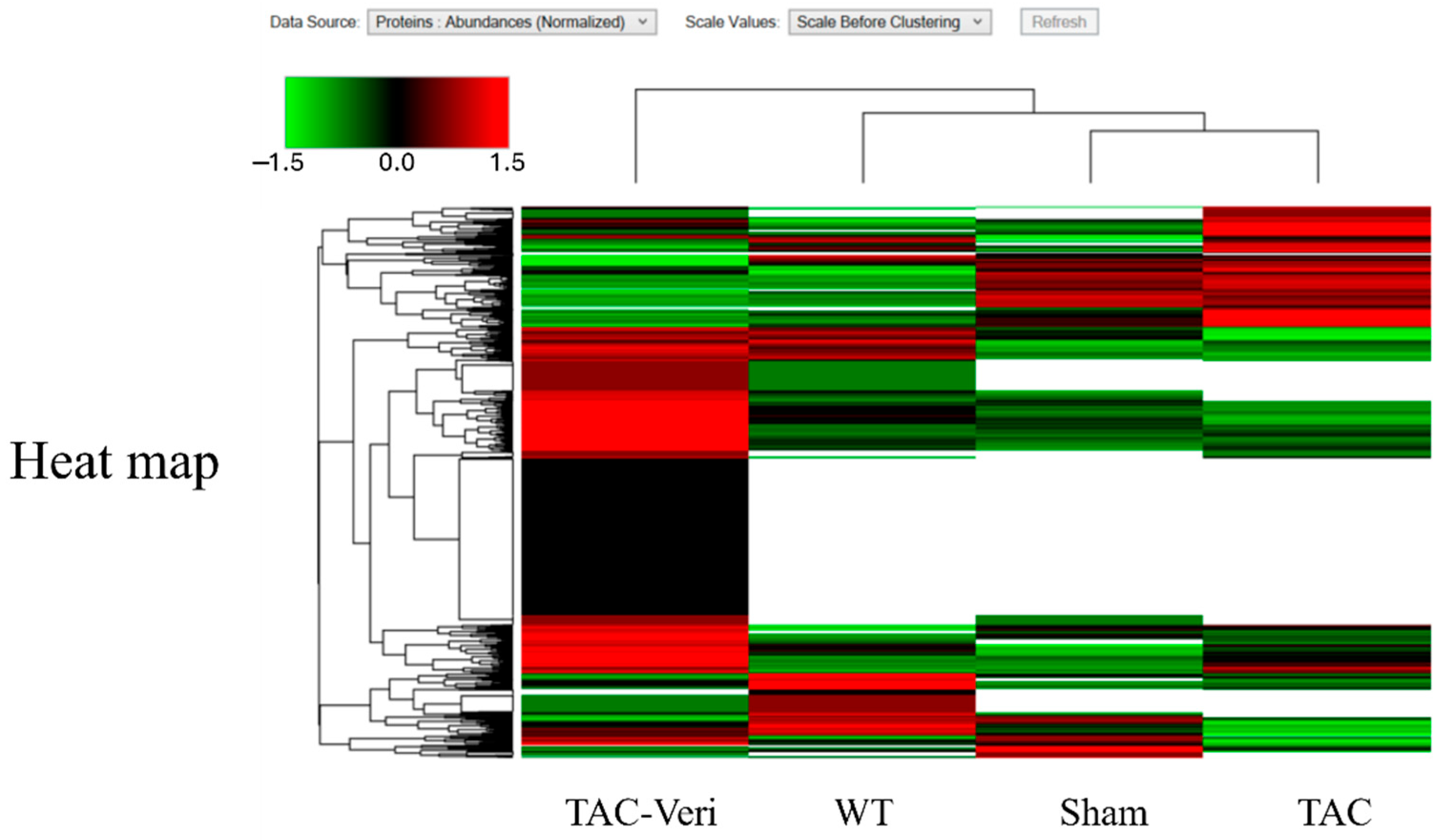
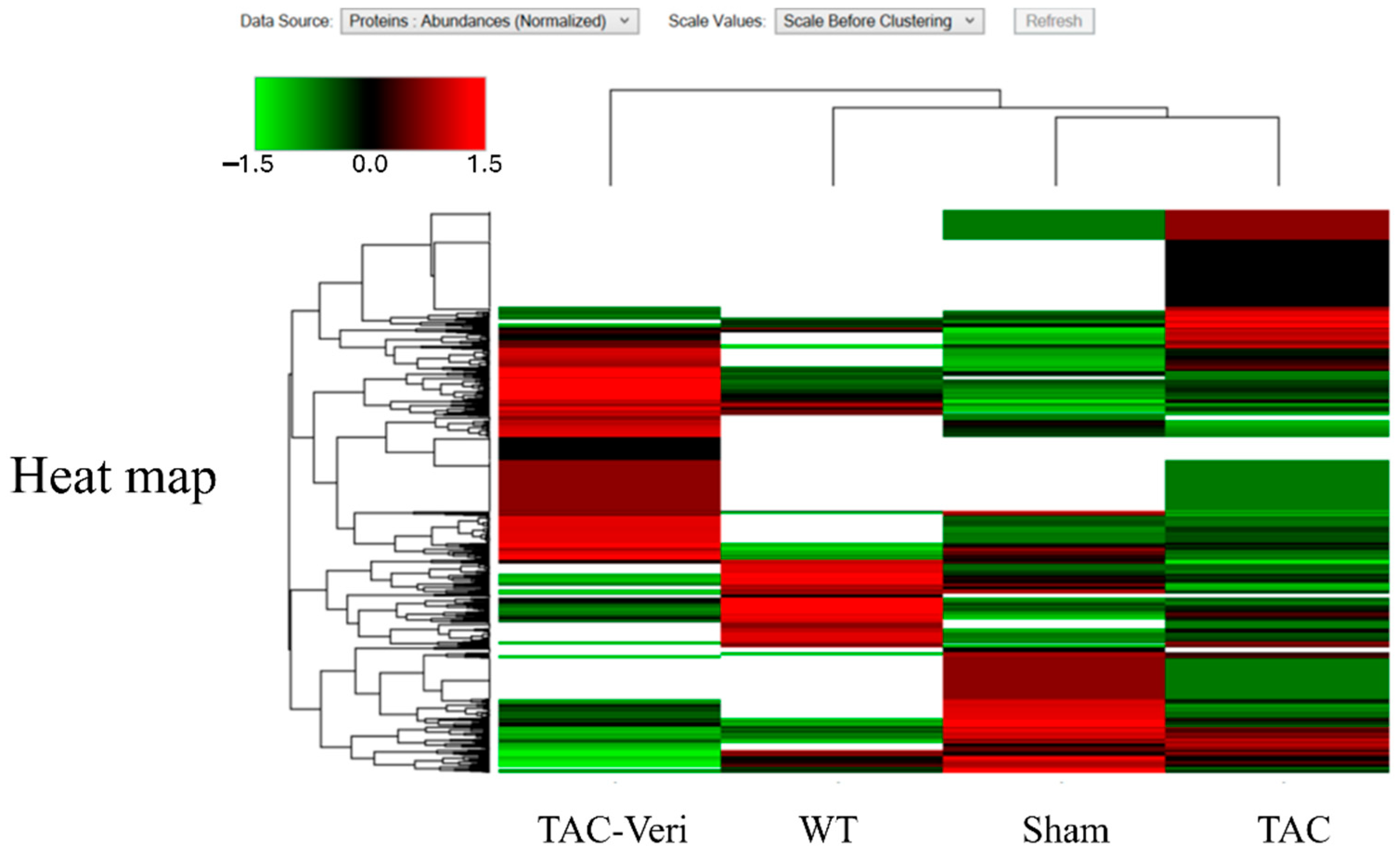
| Sham | TAC | Veri | |
|---|---|---|---|
| LVEDV (mL) | 0.131 ± 0.023 | 0.241 ± 0.086 * | 0.124 ± 0.037 # |
| LVESV (mL) | 0.034 ± 0.012 | 0.086 ± 0.037 * | 0.038 ± 0.019 # |
| SV (mL) | 0.097 ± 0.019 | 0.155 ± 0.050 * | 0.086 ± 0.019 # |
| LVd Mass | 0.104 ± 0.010 | 0.204 ± 0.031 *** | 0.150 ± 0.018 ## |
| LVIDs | 0.226 ± 0.031 | 0.318 ± 0.045 ** | 0.234 ± 0.041 ## |
| LVIDd | 0.372 ± 0.024 | 0.456 ± 0.055 * | 0.362 ± 0.038 # |
| LVPWd | 0.080 ± 0.000 | 0.096 ± 0.010 ** | 0.100 ± 0.000 # |
| IVSs | 0.116 ± 0.010 | 0.122 ± 0.012 | 0.138 ± 0.028 |
| IVSd | 0.080 ± 0.000 | 0.108 ± 0.007 *** | 0.114 ± 0.012 |
| Accession ID | Protein Name | WTC Abundance Ratio | STC Abundance Ratio | TTVeri Abundance Ratio | p-Value |
|---|---|---|---|---|---|
| Metabolism and Energy-Related | |||||
| P53395 | Lipoamide acyltransferase component of branched-chain alpha-keto acid dehydrogenase complex, mitochondrial | 0.01 | 0.01 | 100 | 1 × 10−17 |
| Q9CXJ1 | Nondiscriminating glutamyl-tRNA synthetase EARS2, mitochondrial | 0.01 | 0.01 | 100 | 1 × 10−17 |
| P32020 | Sterol carrier protein 2 | 0.01 | 0.01 | 100 | 1 × 10−17 |
| A2APY7 | rginine-hydroxylase NDUFAF5, mitochondrial | 0.01 | 0.01 | 100 | 1 × 10−17 |
| P17182 | Alpha-enolase | 0.01 | 0.01 | 100 | 1 × 10−17 |
| P06745 | Glucose-6-phosphate isomerase | 0.01 | 0.01 | 100 | 1 × 10−17 |
| Q8R3F5 | Malonyl-CoA-acyl carrier protein transacylase, mitochondrial | 0.01 | 0.01 | 100 | 1 × 10−17 |
| Q9CQ43 | Deoxyuridine 5′-triphosphate nucleotidohydrolase | 0.01 | 0.01 | 100 | 1 × 10−17 |
| Q8K009 | Mitochondrial 10-formyltetrahydrofolate dehydrogenase | 0.01 | 0.01 | 100 | 1 × 10−17 |
| O88986 | 2-amino-3-ketobutyrate coenzyme A ligase, mitochondrial | 0.01 | 0.01 | 100 | 1 × 10−17 |
| Q3UUI3 | Acyl-coenzyme A thioesterase THEM4 | 0.01 | 0.01 | 100 | 1 × 10−17 |
| Q9D009 | Octanoyl-[acyl-carrier-protein]:protein N-octanoyltransferase LIPT2, mitochondrial | 0.01 | 0.01 | 100 | 1 × 10−17 |
| P17563 | Methanethiol oxidase | 0.01 | 0.01 | 100 | 1 × 10−17 |
| Q9EQ06 | Estradiol 17-beta-dehydrogenase 11 | 0.01 | 0.01 | 100 | 1 × 10−17 |
| Q9CQX8 | Alpha-ketoglutarate dehydrogenase component 4 | 0.01 | 0.01 | 100 | 1 × 10−17 |
| Q9QZF2 | Glypican-1 | 0.01 | 0.01 | 100 | 1 × 10−17 |
| A0A1B0GR11 | Transaldolase | 0.01 | 0.01 | 100 | 1 × 10−17 |
| P98192 | Dihydroxyacetone phosphate acyltransferase | 0.01 | 0.01 | 100 | 1 × 10−17 |
| P11152 | Lipoprotein lipase | 0.01 | 0.01 | 100 | 1 × 10−17 |
| Protein Synthesis and Modification | |||||
| P68040 | Small ribosomal subunit protein RACK1 | 0.01 | 0.01 | 100 | 1 × 10−17 |
| Q9DAT5 | Mitochondrial tRNA-specific 2-thiouridylase 1 | 0.01 | 0.01 | 100 | 1 × 10−17 |
| Q9CQ40 | Large ribosomal subunit protein mL49 | 0.01 | 0.01 | 100 | 1 × 10−17 |
| Q9DCI9 | Large ribosomal subunit protein bL32m | 0.01 | 0.01 | 100 | 1 × 10−17 |
| Q9D2R8 | Small ribosomal subunit protein mS33 | 0.01 | 0.01 | 100 | 1 × 10−17 |
| P83882 | Large ribosomal subunit protein eL42 | 0.01 | 0.01 | 100 | 1 × 10−17 |
| P62264 | Small ribosomal subunit protein uS11 | 0.01 | 0.01 | 100 | 1 × 10−17 |
| P15532 | Nucleoside diphosphate kinase A | 0.01 | 0.01 | 100 | 1 × 10−17 |
| Structural and Cytoskeletal | |||||
| Q8BFZ3 | Beta-actin-like protein 2 | 0.01 | 0.01 | 100 | 1 × 10−17 |
| D3YUI7 | Myosin regulatory light chain 2, ventricular/cardiac muscle isoform (Fragment) | 0.01 | 0.01 | 100 | 1 × 10−17 |
| F8VQJ3 | Laminin, gamma 1 | 0.01 | 0.01 | 100 | 1 × 10−17 |
| P82348 | Gamma-sarcoglycan | 100 | 100 | 0.01 | 1 × 10−17 |
| Q02788 | Collagen alpha-2(VI) chain | 0.01 | 0.01 | 100 | 1 × 10−17 |
| P47754 | F-actin-capping protein subunit alpha-2 | 100 | 100 | 0.01 | 1 × 10−17 |
| Mitochondrial Function | |||||
| A0A0R4J0T0 | HscB iron-sulfur cluster co-chaperone | 0.01 | 0.01 | 100 | 1 × 10−17 |
| O35943 | Frataxin, mitochondrial | 0.01 | 0.01 | 100 | 1 × 10−17 |
| Q99P30 | Peroxisomal coenzyme A diphosphatase NUDT7 | 0.01 | 0.01 | 100 | 1 × 10−17 |
| Q9DCB8 | Iron-sulfur cluster assembly 2 homolog, mitochondrial | 0.01 | 0.01 | 100 | 1 × 10−17 |
| P56501 | Putative mitochondrial transporter UCP3 | 0.01 | 0.01 | 100 | 1 × 10−17 |
| Q8BUY5 | Complex I assembly factor TIMMDC1, mitochondrial | 0.01 | 0.01 | 100 | 1 × 10−17 |
| Signal Transduction and Regulation | |||||
| Q3TL44 | NLR family member X1 | 0.01 | 0.01 | 100 | 1 × 10−17 |
| A0A1B0GRP7 | Pyridoxal phosphate binding protein | 0.01 | 0.01 | 100 | 1 × 10−17 |
| Q76I26 | Methyltransferase hypoxia inducible domain containing 1 | 0.01 | 0.01 | 100 | 1 × 10−17 |
| Q8BXN7 | Protein phosphatase Mn(2+)-dependent 1K | 0.01 | 0.01 | 100 | 1 × 10−17 |
| Q99LX0 | Parkinson disease protein 7 homolog | 0.01 | 0.01 | 100 | 1 × 10−17 |
| P47199 | Quinone oxidoreductase | 0.01 | 0.01 | 100 | 1 × 10−17 |
| Q8VDK1 | Deaminated glutathione amidase | 0.01 | 0.01 | 100 | 1 × 10−17 |
| Q6YI28 | EP1 | 0.01 | 0.01 | 100 | 1 × 10−17 |
| Transport and Translocation | |||||
| Q8BJS4 | SUN domain-containing protein 2 | 0.01 | 0.01 | 100 | 1 × 10−17 |
| Q9D273 | Corrinoid adenosyltransferase MMAB | 0.01 | 0.01 | 100 | 1 × 10−17 |
| Q5SWT3 | Solute carrier family 25 member 35 | 0.01 | 0.01 | 100 | 1 × 10−17 |
| Q3URS9 | Mitochondrial potassium channel | 0.01 | 0.01 | 100 | 1 × 10−17 |
| Accession ID | Protein Name | WTC Abundance Ratio | STC Abundance Ratio | TTVeri Abundance Ratio | p-Value |
|---|---|---|---|---|---|
| Metabolism and Energy-Related | |||||
| A0A338P776 | ATP synthase subunit O | 0.01 | 0.01 | 100 | 1 × 10−17 |
| A0A2R8VJW0 | Aconitase 2, mitochondrial | 0.01 | 0.01 | 100 | 1 × 10−17 |
| O08528 | Hexokinase-2 | 0.01 | 0.01 | 100 | 1 × 10−17 |
| P11152 | Lipoprotein lipase | 0.01 | 0.01 | 100 | 1 × 10−17 |
| Q64516 | Glycerol kinase | 0.01 | 0.01 | 100 | 1 × 10−17 |
| Q9QX60 | Deoxyguanosine kinase, mitochondrial | 0.01 | 0.01 | 100 | 1 × 10−17 |
| Q8R3F5 | Malonyl-CoA-acyl carrier protein transacylase | 0.01 | 0.01 | 100 | 1 × 10−17 |
| Q8BHF7 | CDP-diacylglycerol--glycerol-3-phosphate 3-phosphatidyltransferase, mitochondrial | 0.01 | 0.01 | 100 | 1 × 10−17 |
| A0A0R4J135 | Methanethiol oxidase | 0.01 | 0.01 | 100 | 1 × 10−17 |
| Q9Z0K8 | Pantetheinase | 0.01 | 0.01 | 100 | 1 × 10−17 |
| Q9Z1J3 | Cysteine desulfurase | 0.01 | 0.01 | 100 | 1 × 10−17 |
| P32020 | Sterol carrier protein 2 | 0.01 | 0.01 | 100 | 1 × 10−17 |
| A2AL50 | Alkylglycerone-phosphate synthase | 0.01 | 0.01 | 100 | 1 × 10−17 |
| Q8BP40 | Lysophosphatidic acid phosphatase type 6 | 0.01 | 0.01 | 100 | 1 × 10−17 |
| Q9CQ43 | Deoxyuridine 5′-triphosphate nucleotidohydrolase | 0.01 | 0.01 | 100 | 1 × 10−17 |
| Q99L04 | Dehydrogenase/reductase SDR family member 1 | 0.01 | 0.01 | 100 | 1 × 10−17 |
| D3YU39 | Cholinephosphotransferase 1 | 0.01 | 0.01 | 100 | 1 × 10−17 |
| Protein Synthesis and Modification | |||||
| Q8BU88 | Large ribosomal subunit protein uL22m | 0.01 | 0.01 | 100 | 1 × 10−17 |
| Q9D773 | Large ribosomal subunit protein uL2m | 0.01 | 0.01 | 100 | 1 × 10−17 |
| Q9CXW2 | Small ribosomal subunit protein mS22 | 0.01 | 0.01 | 100 | 1 × 10−17 |
| Q9CXJ1 | Nondiscriminating glutamyl-tRNA synthetase EARS2 | 0.01 | 0.01 | 100 | 1 × 10−17 |
| Q9DB15 | Large ribosomal subunit protein bL12m | 0.01 | 0.01 | 100 | 1 × 10−17 |
| P62918 | Large ribosomal subunit protein uL2 | 0.01 | 0.01 | 100 | 1 × 10−17 |
| A0A286YCZ5 | phenylalanine-tRNA ligase | 0.01 | 0.01 | 100 | 1 × 10−17 |
| Q924T2 | Small ribosomal subunit protein uS2m | 0.01 | 0.01 | 100 | 1 × 10−17 |
| Q6ZWV7 | Large ribosomal subunit protein uL29 | 0.01 | 0.01 | 100 | 1 × 10−17 |
| Q9DAT5 | Mitochondrial tRNA-specific 2-thiouridylase 1 | 0.01 | 0.01 | 100 | 1 × 10−17 |
| P61255 | Large ribosomal subunit protein uL24 | 0.01 | 0.01 | 100 | 1 × 10−17 |
| P46978 | Dolichyl-diphosphooligosaccharide--protein glycosyltransferase subunit STT3A | 0.01 | 0.01 | 100 | 1 × 10−17 |
| Q8BL63 | GPI-anchor transamidase | 0.01 | 0.01 | 100 | 1 × 10−17 |
| Q9CPR4 | Large ribosomal subunit protein uL22 | 0.01 | 0.01 | 100 | 1 × 10−17 |
| P27659 | Large ribosomal subunit protein uL3 | 0.01 | 0.01 | 100 | 1 × 10−17 |
| P62852 | Small ribosomal subunit protein eS25 | 0.01 | 0.01 | 100 | 1 × 10−17 |
| O08832 | Polypeptide N-acetylgalactosaminyltransferase 4 | 0.01 | 0.01 | 100 | 1 × 10−17 |
| Structural and Cytoskeletal | |||||
| Q5SX40 | Myosin-1 | 0.01 | 0.01 | 100 | 1 × 10−17 |
| Q9Z2T6 | Keratin, type II cuticular Hb5 | 0.01 | 0.01 | 100 | 1 × 10−17 |
| Q8BFZ3 | Beta-actin-like protein 2 | 0.01 | 0.01 | 100 | 1 × 10−17 |
| A2AQP0 | Myosin-7B | 0.01 | 0.01 | 100 | 1 × 10−17 |
| Q8K0Y2 | Keratin, type I cuticular Ha3-I | 0.01 | 0.01 | 100 | 1 × 10−17 |
| P05977 | Myosin light chain 1/3, skeletal muscle isoform | 0.01 | 0.01 | 100 | 1 × 10−17 |
| Q61554 | Fibrillin-1 | 0.01 | 0.01 | 100 | 1 × 10−17 |
| P26231 | Catenin alpha-1 | 0.01 | 0.01 | 100 | 1 × 10−17 |
| Q64291 | Keratin, type I cytoskeletal 12 | 0.01 | 0.01 | 100 | 1 × 10−17 |
| Q02788 | Collagen alpha-2(VI) chain | 0.01 | 0.01 | 100 | 1 × 10−17 |
| Q9R1Q7 | Proteolipid protein 2 | 0.01 | 0.01 | 100 | 1 × 10−17 |
| Mitochondrial Function | |||||
| Q6PE15 | Palmitoyl-protein thioesterase ABHD10 | 0.01 | 0.01 | 100 | 1 × 10−17 |
| Q3U276 | Succinate dehydrogenase assembly factor 1 | 0.01 | 0.01 | 100 | 1 × 10−17 |
| O88696 | ATP-dependent Clp protease proteolytic subunit | 0.01 | 0.01 | 100 | 1 × 10−17 |
| I3ITR1 | Iron-sulfur cluster assembly 1 homolog | 0.01 | 0.01 | 100 | 1 × 10−17 |
| Q99JB2 | Stomatin-like protein 2 | 0.01 | 0.01 | 100 | 1 × 10−17 |
| Q8BGX2 | Mitochondrial import inner membrane translocase subunit Tim29 | 0.01 | 0.01 | 100 | 1 × 10−17 |
| Q59J78 | NADH dehydrogenase [ubiquinone] 1 alpha subcomplex assembly factor 2 | 0.01 | 0.01 | 100 | 1 × 10−17 |
| Q8BG51 | Mitochondrial Rho GTPase 1 | 0.01 | 0.01 | 100 | 1 × 10−17 |
| Signal Transduction and Regulation | |||||
| Q02819 | Nucleobindin-1 | 0.01 | 0.01 | 100 | 1 × 10−17 |
| P63101 | 14-3-3 protein zeta/delta | 0.01 | 0.01 | 100 | 1 × 10−17 |
| O08795 | Glucosidase 2 subunit beta | 0.01 | 0.01 | 100 | 1 × 10−17 |
| A0A1B0GRP7 | Pyridoxal phosphate binding protein | 0.01 | 0.01 | 100 | 1 × 10−17 |
| Q9QZF2 | Glypican-1 | 0.01 | 0.01 | 100 | 1 × 10−17 |
| P97443 | Histone-lysine N-methyltransferase Smyd1 | 0.01 | 0.01 | 100 | 1 × 10−17 |
| Q8BTE5 | Protein CEBPZOS | 0.01 | 0.01 | 100 | 1 × 10−17 |
| Q99LX0 | Parkinson disease protein 7 homolog | 0.01 | 0.01 | 100 | 1 × 10−17 |
| Q8R0J2 | Mannose-P-dolichol utilization defect 1 protein | 0.01 | 0.01 | 100 | 1 × 10−17 |
| P17426 | AP-2 complex subunit alpha-1 | 0.01 | 0.01 | 100 | 1 × 10−17 |
| Q8BTJ4 | Bis(5′-adenosyl)-triphosphatase enpp4 | 0.01 | 0.01 | 100 | 1 × 10−17 |
| Q9Z2L6 | Multiple inositol polyphosphate phosphatase 1 | 0.01 | 0.01 | 100 | 1 × 10−17 |
| P15532 | Nucleoside diphosphate kinase A | 0.01 | 0.01 | 100 | 1 × 10−17 |
| P01027 | Complement C3 | 0.01 | 0.01 | 100 | 1 × 10−17 |
| A0A0M3HEQ0 | thioredoxin-disulfide reductase | 0.01 | 0.01 | 100 | 1 × 10−17 |
| P11352 | Glutathione peroxidase 1 | 0.01 | 0.01 | 100 | 1 × 10−17 |
| P21460 | Cystatin-C | 0.01 | 0.01 | 100 | 1 × 10−17 |
| Q91X72 | Hemopexin | 0.01 | 0.01 | 100 | 1 × 10−17 |
| Q9CRY7 | Lysophospholipase D GDPD1 | 0.01 | 0.01 | 100 | 1 × 10−17 |
| Q8C0L0 | Thioredoxin-related transmembrane protein 4 | 0.01 | 0.01 | 100 | 1 × 10−17 |
| Q8K1N1 | Calcium-independent phospholipase A2-gamma | 0.01 | 0.01 | 100 | 1 × 10−17 |
| Q9CPS6 | Adenosine 5′-monophosphoramidase HINT3 | 0.01 | 0.01 | 100 | 1 × 10−17 |
| O88342 | WD repeat-containing protein 1 | 0.01 | 0.01 | 100 | 1 × 10−17 |
| A0A0R4J0Z1 | Protein disulfide-isomerase A4 | 0.01 | 0.01 | 100 | 1 × 10−17 |
| Q9CYW4 | Haloacid dehalogenase-like hydrolase domain-containing protein 3 | 0.01 | 0.01 | 100 | 1 × 10−17 |
| Q61171 | Peroxiredoxin-2 | 0.01 | 0.01 | 100 | 1 × 10−17 |
| Transport and Translocation | |||||
| P53810 | Phosphatidylinositol transfer protein alpha isoform | 0.01 | 0.01 | 100 | 1 × 10−17 |
| Q99KF1 | Transmembrane emp24 domain-containing protein 9 | 0.01 | 0.01 | 100 | 1 × 10−17 |
| P58021 | Transmembrane 9 superfamily member 2 | 0.01 | 0.01 | 100 | 1 × 10−17 |
| Q9EQH3 | Vacuolar protein sorting-associated protein 35 | 0.01 | 0.01 | 100 | 1 × 10−17 |
| Q8R1V4 | Transmembrane emp24 domain-containing protein 4 | 0.01 | 0.01 | 100 | 1 × 10−17 |
| Q8BPE4 | Transmembrane protein 177 | 0.01 | 0.01 | 100 | 1 × 10−17 |
| Q9ET30 | Transmembrane 9 superfamily member 3 | 0.01 | 0.01 | 100 | 1 × 10−17 |
| Q99P72 | Reticulon-4 | 0.01 | 0.01 | 100 | 1 × 10−17 |
| Q9CXE7 | Transmembrane emp24 domain-containing protein 5 | 0.01 | 0.01 | 100 | 1 × 10−17 |
| O08579 | Emerin | 0.01 | 0.01 | 100 | 1 × 10−17 |
| Q3U3G8 | Serine hydrolase-like | 0.01 | 0.01 | 100 | 1 × 10−17 |
| P17047 | Lysosome-associated membrane glycoprotein 2 | 0.01 | 0.01 | 100 | 1 × 10−17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, W.-R.; Chen, C.-C.; Li, F.-A.; Chen, H.-Y.; Liu, J.-C.; Cheng, T.-H.; Chen, J.-J. Therapeutic Impact of Vericiguat on Ventricular Remodeling in a Pressure-Overload Heart Failure Model. Life 2025, 15, 1763. https://doi.org/10.3390/life15111763
Hao W-R, Chen C-C, Li F-A, Chen H-Y, Liu J-C, Cheng T-H, Chen J-J. Therapeutic Impact of Vericiguat on Ventricular Remodeling in a Pressure-Overload Heart Failure Model. Life. 2025; 15(11):1763. https://doi.org/10.3390/life15111763
Chicago/Turabian StyleHao, Wen-Rui, Chun-Chao Chen, Fu-An Li, Huan-Yuan Chen, Ju-Chi Liu, Tzu-Hurng Cheng, and Jin-Jer Chen. 2025. "Therapeutic Impact of Vericiguat on Ventricular Remodeling in a Pressure-Overload Heart Failure Model" Life 15, no. 11: 1763. https://doi.org/10.3390/life15111763
APA StyleHao, W.-R., Chen, C.-C., Li, F.-A., Chen, H.-Y., Liu, J.-C., Cheng, T.-H., & Chen, J.-J. (2025). Therapeutic Impact of Vericiguat on Ventricular Remodeling in a Pressure-Overload Heart Failure Model. Life, 15(11), 1763. https://doi.org/10.3390/life15111763










