Hybrid Open and Endovascular Repair in Pararenal Abdominal Aortic Pseudoaneurysm—Literature Review and Case Presentation
Abstract
1. Introduction
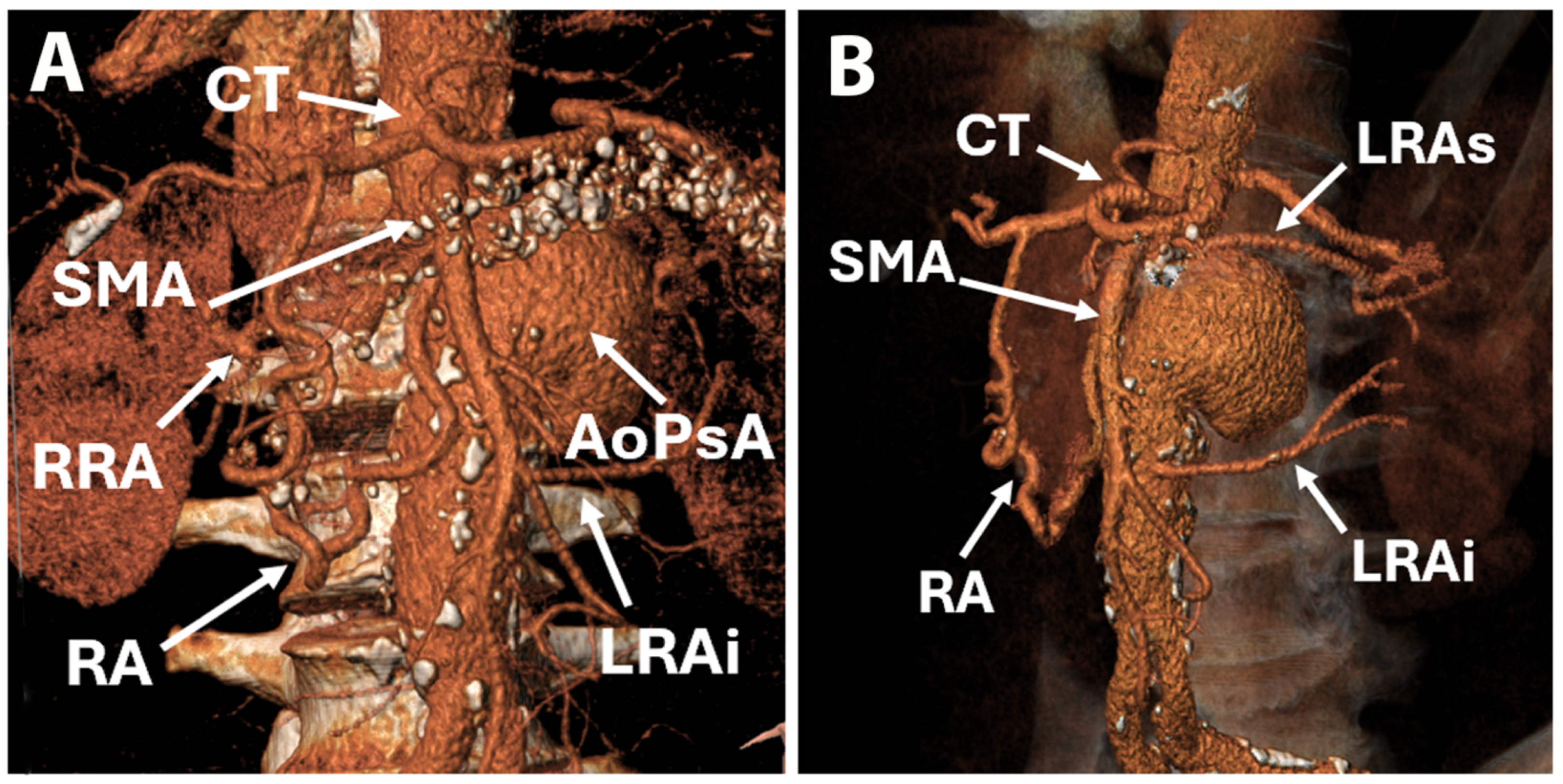
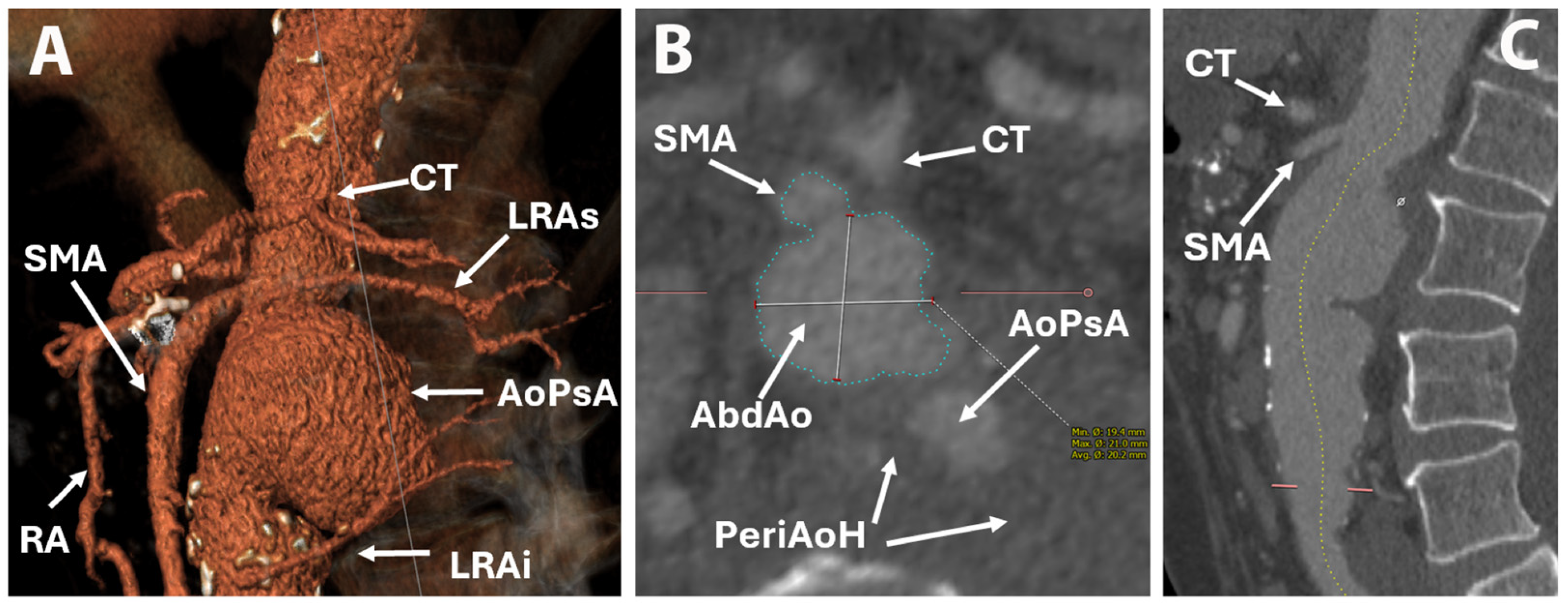
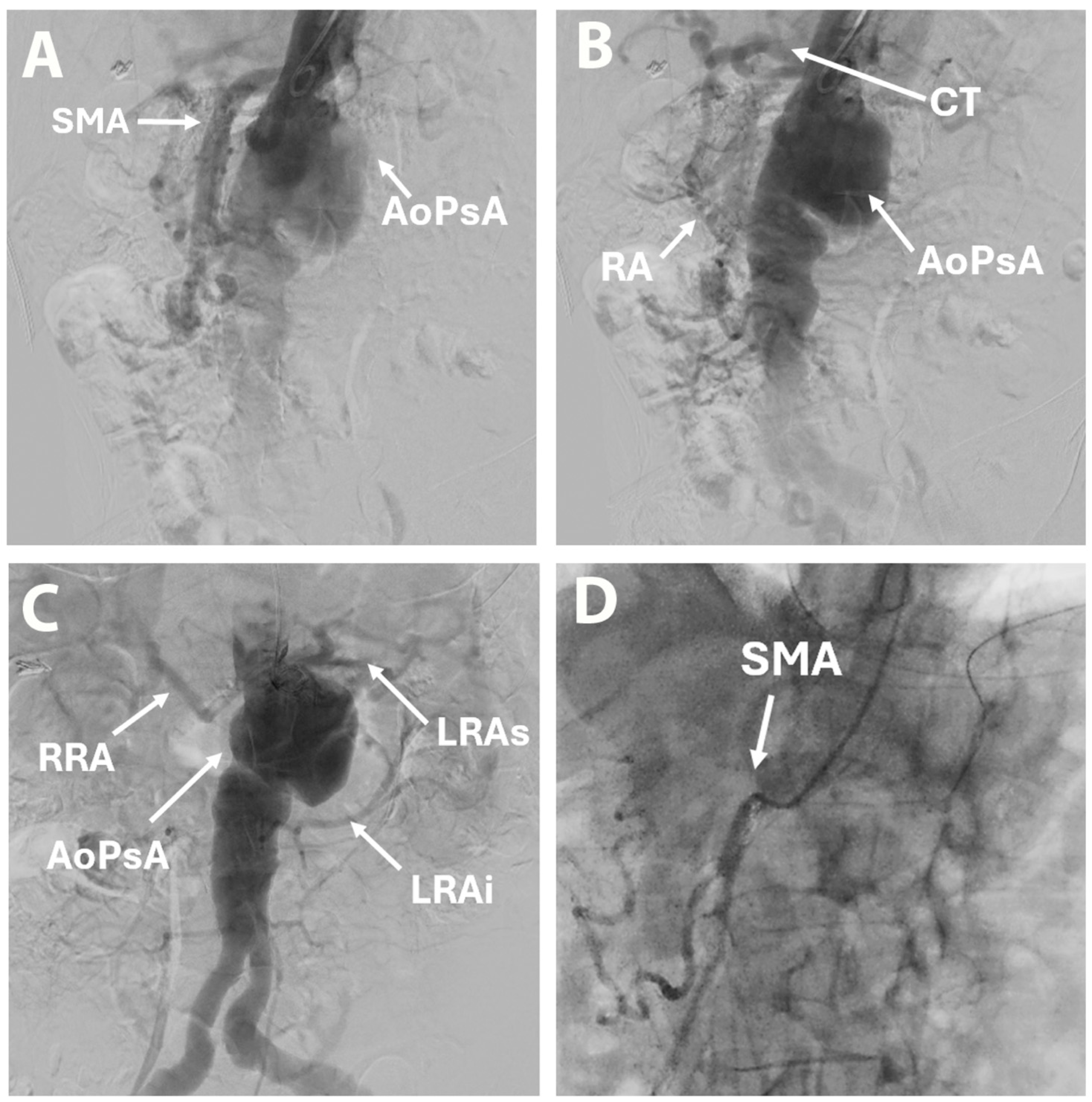
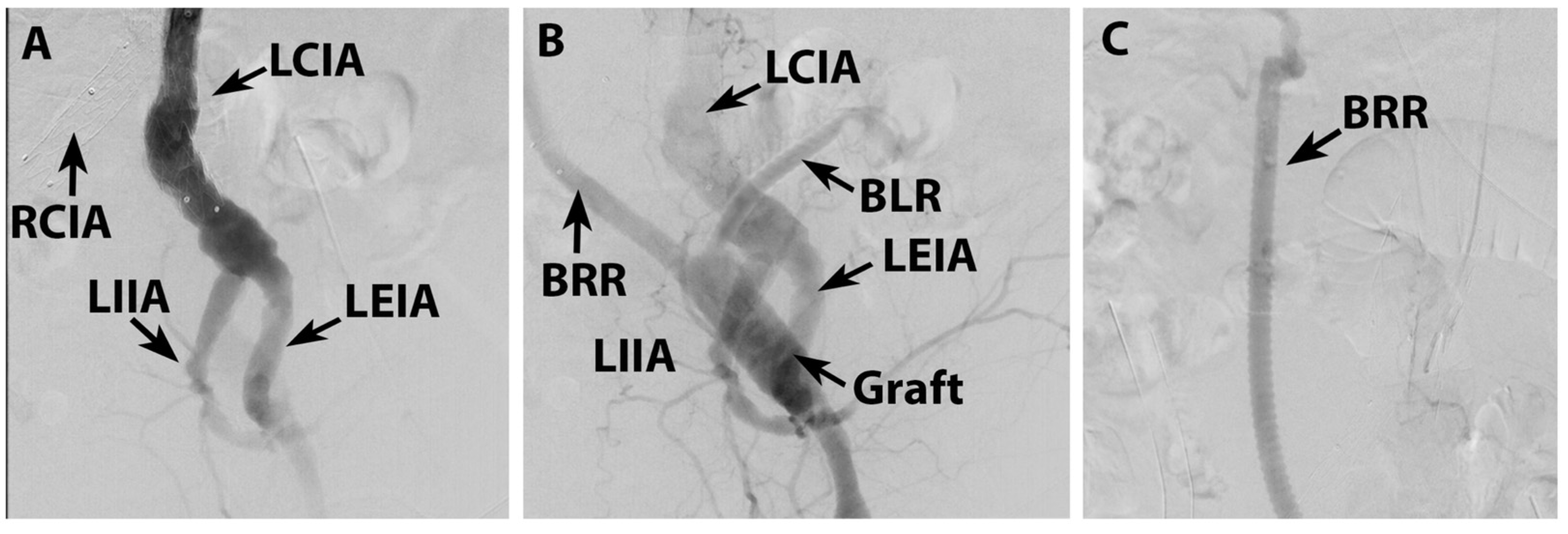
2. Case Report
3. Discussions and Review of the Literature
4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wanhainen, A.; Van Herzeele, I.; Goncalves, F.B.; Montoya, S.B.; Berard, X.; Boyle, J.R.; D’oRia, M.; Prendes, C.F.; Karkos, C.D.; Kazimierczak, A.; et al. Editor’s Choice—European Society for Vascular Surgery (ESVS) 2024 Clinical Practice Guidelines on the Management of Abdominal Aorto-Iliac Artery Aneurysms. Eur. J. Vasc. Endovasc. Surg. 2024, 67, 192–331. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.H.Y.; Asciutto, G.; Dias, N.; Wanhainen, A.; Karelis, A.; Sonesson, B.; Mani, K. Outcomes of elective open surgical repair or fenestrated endovascular aneurysm repair for juxtarenal abdominal aortic aneurysms in Sweden. Br. J. Surg. 2024, 111, znae279. [Google Scholar] [CrossRef]
- Quinones-Baldrich, W.; Jimenez, J.C.; DeRubertis, B.; Moore, W.S. Combined endovascular and surgical approach (CESA) to thoracoabdominal aortic pathology: A 10-year experience. J. Vasc. Surg. 2009, 49, 1125–1134. [Google Scholar] [CrossRef]
- Escobar, G.A.; Oderich, G.S.; Farber, M.A.; de Souza, L.R.; Quinones-Baldrich, W.J.; Patel, H.J.; Eliason, J.L.; Upchurch, G.R.; Timaran, C.H.; Black, J.H.; et al. Results of the North American Complex Abdominal Aortic Debranching (NACAAD) Registry. Circulation 2022, 146, 1149–1158. [Google Scholar] [CrossRef]
- Zlatanovic, P.; Mascia, D.; Ancetti, S.; Yeung, K.K.; Graumans, M.J.; Jongkind, V.; Viitala, H.; Venermo, M.; Chiesa, R.; Gargiulo, M.; et al. Short Term and Long Term Clinical Outcomes of Endovascular versus Open Repair for Juxtarenal and Pararenal Abdominal Aortic Aneurysms Using Propensity Score Matching: Results from Juxta- and pararenal aortic Aneurysm Multicentre European Study (JAMES). Eur. J. Vasc. Endovasc. Surg. 2023, 65, 828–836. [Google Scholar] [CrossRef]
- Setacci, F.; Pecoraro, F.; Chaykovska, L.; Mangialardi, N.; Shingaki, M.; Veith, F.J.; Rancic, Z.; Lachat, M. The Gore Hybrid Vascular Graft in renovisceral debranching for complex aortic aneurysm repair. J. Vasc. Surg. 2016, 64, 33–38. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Moulakakis, K.G.; Mylonas, S.N.; Avgerinos, E.D.; Kakisis, J.D.; Brunkwall, J.; Liapis, C.D. Hybrid open endovascular technique for aortic thoracoabdominal pathologies. Circulation 2011, 124, 2670–2680. [Google Scholar] [CrossRef]
- Titarenko, V.; Beer, A.; Schonefeld-Siepmann, E.; Muschal, F.; Beyer, J.K. Giant Symptomatic Unruptured Juxtarenal Abdominal Aortic Aneurysm. Vasc. Spéc. Int. 2022, 38, 23. [Google Scholar] [CrossRef]
- Scali, S.T.; Arnaoutakis, D.J.; Neal, D.; Giles, K.A.; Goodney, P.P.; Suckow, B.D.; Powell, R.J.; Columbo, J.A.; Back, M.R.; Berceli, S.A.; et al. Association between surgeon case volume and years of practice experience with open abdominal aortic aneurysm repair outcomes. J. Vasc. Surg. 2021, 73, 1213–1226.e2. [Google Scholar] [CrossRef] [PubMed]
- Zlatanovic, P.; Jovanovic, A.; Tripodi, P.; Davidovic, L. Chimney vs. Fenestrated Endovascular vs. Open Repair for Juxta/Pararenal Abdominal Aortic Aneurysms: Systematic Review and Network Meta-Analysis of the Medium-Term Results. J. Clin. Med. 2022, 11, 6779. [Google Scholar] [CrossRef]
- Oderich, G.S.; Forbes, T.L.; Chaer, R.; Davies, M.G.; Lindsay, T.F.; Mastracci, T.; Singh, M.J.; Timaran, C.; Woo, E.Y. Reporting standards for endovascular aortic repair of aneurysms involving the renal-mesenteric arteries. J. Vasc. Surg. 2021, 73, 4S–52S. [Google Scholar] [CrossRef] [PubMed]
- Michel, M.; Becquemin, J.P.; Marzelle, J.; Quelen, C.; Durand-Zaleski, I.; WINDOW Trial participants. Editor’s Choice—A Study of the Cost-effectiveness of Fenestrated/branched EVAR Compared with Open Surgery for Patients with Complex Aortic Aneurysms at 2 Years. Eur. J. Vasc. Endovasc. Surg. 2018, 56, 15–21. [Google Scholar] [CrossRef]
- Donas, K.P.; Lee, J.T.; Lachat, M.; Torsello, G.; Veith, F.J.; PERICLES investigators. Collected world experience about the performance of the snorkel/chimney endovascular technique in the treatment of complex aortic pathologies: The PERICLES registry. Ann. Surg. 2015, 262, 546–553. [Google Scholar] [CrossRef]
- Kansal, N.; LoGerfo, F.W.; Belfield, A.K.; Pomposelli, F.B.; Hamdan, A.D.; Angle, N.; Campbell, D.R.; Sridhar, A.; Freischlag, J.A.; Quiñones-Baldrich, W. A Comparison of antegrade and retrograde mesenteric bypass. Ann. Vasc. Surg. 2002, 16, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Stiru, O.; Geana, R.C.; Pavel, P.; Croitoru, M.; Boros, C.; Iovu, I.; Iliescu, V.A. Descending Thoracic Aortic Aneurysm Rupture Treated with Thoracic Endovascular Aortic Repair in a Patient with Peripheral Artery Disease. Hear. Surg. Forum 2018, 21, E112–E116. [Google Scholar] [CrossRef] [PubMed]
- Jongkind, V.; Yeung, K.K.; Akkersdijk, G.J.; Heidsieck, D.; Reitsma, J.B.; Tangelder, G.J.; Wisselink, W. Juxtarenal aortic aneurysm repair. J. Vasc. Surg. 2010, 52, 760–767. [Google Scholar] [CrossRef]
- Stiru, O.; Nayyerani, R.; Robu, M.; Geana, R.C.; Dragulescu, P.R.; Blibie, O.A.; Bubenek-Turconi, S.-I.; Iliescu, V.A.; Parasca, C. Combined Endovascular and Endoscopic Management of a Secondary Aortoesophageal Fistula after Open Surgical Aortic Repair in a Giant Descending Thoracic Aortic Pseudoaneurysm: Case Report and Review of Literature. J. Pers. Med. 2024, 14, 625. [Google Scholar] [CrossRef]
- Geana, R.C.; Pavel, P.; Nayyerani, R.; Kulcsar, I.; Tulin, A.; Honciuc, O.; Balescu, I.; Bacalbasa, N.; Stiru, O.; Iliescu, V.A.; et al. Successfully superior mesenteric artery stenting in operated type A aortic dissection complicated with delayed mesenteric malperfusion. SAGE Open Med. Case Rep. 2021, 9, 2050313X211021184. [Google Scholar] [CrossRef]
- van Lammeren, G.W.; Ünlü, Ç.; Verschoor, S.; van Dongen, E.P.; Wille, J.; van de Pavoordt, E.D.; de Vries-Werson, D.A.; De Vries, J.-P.P. Results of open pararenal abdominal aortic aneurysm repair: Single centre series and pooled analysis of literature. Vascular 2017, 25, 234–241. [Google Scholar] [CrossRef]
- Reyes, A.; Donas, K.P.; Pitoulias, G.; Austermann, M.; Gandarias, C.; Torsello, G. Complementary Role of Fenestrated/Branched Endografting and the Chimney Technique in the Treatment of Pararenal Aneurysms After Open Abdominal Aortic Repair. J. Endovasc. Ther. 2016, 23, 599–605. [Google Scholar] [CrossRef]
- Mirza, A.K.; Oderich, G.S.; Sandri, G.A.; Tenorio, E.R.; Davila, V.J.; Kärkkäinen, J.M.; Hofer, J.; Cha, S. Outcomes of upper extremity access during fenestrated-branched endovascular aortic repair. J. Vasc. Surg. 2019, 69, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Werlin, E.C.; Kaushik, S.; Gasper, W.J.; Hoffman, M.; Reilly, L.M.; Chuter, T.A.; Hiramoto, J.S. Multibranched endovascular aortic aneurysm repair in patients with and without chronic aortic dissections. J. Vasc. Surg. 2019, 70, 1419–1426. [Google Scholar] [CrossRef] [PubMed]
- Scali, S.T.; Neal, D.; Sollanek, V.; Martin, T.; Sablik, J.; Huber, T.S.; Beck, A.W. Outcomes of surgeon-modified fenestrated-branched endograft repair for acute aortic pathology. J. Vasc. Surg. 2015, 62, 1148–1159.e2. [Google Scholar] [CrossRef]
- Tshomba, Y.; Sica, S.; Minelli, F.; Ferraresi, M.; de Waure, C.; Donati, T.; De Nigris, F.; Vincenzoni, C.; Snider, F.; Tinelli, G. Long-Term Results of Complex Abdominal Aortic Aneurysm Open Repair. J. Pers. Med. 2022, 12, 1630. [Google Scholar] [CrossRef]
- Wang, M.; Yao, C.; Yin, H.-H.; Wang, J.-S.; Liao, B.-Y.; Li, Z.-L.; Wu, R.-D.; Peng, G.-Y.; Chang, G.-Q. Endovascular Treatment of Ruptured or Symptomatic Thoracoabdominal and Pararenal Aortic Aneurysms Using Octopus Endograft Technique: Mid-Term Clinical Outcomes. J. Endovasc. Ther. 2023, 30, 163–175. [Google Scholar] [CrossRef]
- Gallitto, E.; Faggioli, G.; Austermann, M.; Kölbel, T.; Tsilimparis, N.; Dias, N.; Melissano, G.; Simonte, G.; Katsargyris, A.; Oikonomou, K.; et al. Urgent endovascular repair of juxtarenal/pararenal aneurysm by off-the-shelf multibranched endograft. J. Vasc. Surg. 2024, 80, 1336–1349.e4. [Google Scholar] [CrossRef]
- Ferrer, C.; Gallitto, E.; Borghese, O.; Lodato, M.; Cappiello, A.; Cao, P.; Gargiulo, M.; Giudice, R. Long-term results of fenestrated and branched endovascular aneurysm repair for complex abdominal and thoracoabdominal aortic aneurysms in young and fit patients. J. Vasc. Surg. 2024, 80, 1639–1649. [Google Scholar] [CrossRef] [PubMed]
- Yeung, K.K.; Nederhoed, J.H.; Tran, B.L.; Di Gregorio, S.; Pratesi, G.; Bastianon, M.; Melani, C.; Riambau, V.; Bloemert-Tuin, T.; Hazenberg, C.E.V.B.; et al. Endovascular Repair of Juxtarenal and Pararenal Abdominal Aortic Aneurysms Using a Novel Low-Profile Fenestrated Custom-Made Endograft: Technical Details and Short-Term Outcomes. J. Endovasc. Ther. 2024, 32, 1988–1993. [Google Scholar] [CrossRef]
- Sultan, S.; Acharya, Y.; Tawfick, W.; Wijns, W.; Soliman, O. Comparative study of acute kidney injury in pararenal aortic aneurysm: Open surgical versus endovascular repair. Front. Surg. 2024, 11, 1457583. [Google Scholar] [CrossRef]
- Branzan, D.; Geisler, A.; Grunert, R.; Steiner, S.; Bausback, Y.; Gockel, I.; Scheinert, D.; Schmidt, A. The Influence of 3D Printed Aortic Models on the Evolution of Physician Modified Stent Grafts for the Urgent Treatment of Thoraco-abdominal and Pararenal Aortic Pathologies. Eur. J. Vasc. Endovasc. Surg. 2021, 61, 407–412. [Google Scholar] [CrossRef]
- Zhang, H.P.; Ge, Y.Y.; Wang, J.B.; Fan, T.T.; Guo, W. Off the Shelf Multibranched Endograft for Thoraco-Abdominal and Pararenal Abdominal Aortic Aneurysms: A Prospective, Single Centre Study of the G-Branch Endograft. Eur. J. Vasc. Endovasc. Surg. 2024, 67, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, T.F.; Quintas, A.; Soares Ferreira, R.; Ferreira, M.E.; Bastos Gonçalves, F.; Principal Investigators of the Portuguese National Registry of Vascular Procedures. Nationwide Outcomes of Open versus Endovascular Repair for Complex Infrarenal Neck, Juxtarenal, and Pararenal Abdominal Aortic Aneurysm in Portugal. Eur. J. Vasc. Endovasc. Surg. 2025, 70, 336–343. [Google Scholar] [CrossRef] [PubMed]
- D’Oria, M.; Wanhainen, A.; Lindström, D.; Tegler, G.; Mani, K. Editor’s Choice—Pre-Operative Moderate to Severe Chronic Kidney Disease is Associated with Worse Short-Term and Mid-Term Outcomes in Patients Undergoing Fenestrated-Branched Endovascular Aortic Repair. Eur. J. Vasc. Endovasc. Surg. 2021, 62, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Yazar, O.; Huysmans, M.; Lacquet, M.; Salemans, P.B.; Wong, C.Y.; Bouwman, L.H. Single-Center Experience with Inner-Branched Endograft for the Treatment of Pararenal Abdominal Aortic Aneurysms. J. Endovasc. Ther. 2025, 32, 1119–1126. [Google Scholar] [CrossRef]
- Biggs, J.H.; Tenorio, E.R.; DeMartino, R.R.; Oderich, G.S.; Mendes, B.C. Outcomes Following Urgent Fenestrated-Branched Endovascular Repair for Pararenal and Thoracoabdominal Aortic Aneurysms. Ann. Vasc. Surg. 2022, 85, 87–95. [Google Scholar] [CrossRef]
- Wang, C.; Gao, J.; Zhang, H.; Zhang, X.; Jiang, J.; Dai, X.; Fu, W.; Wang, W.; Li, Z.; Chen, Z.; et al. Early Outcome of WeFlow-JAAA Off-the-Shelf Endograft in the Treatment of Juxtarenal/Pararenal Abdominal Aortic Aneurysms. Ann. Vasc. Surg. 2025, 122, 187–193. [Google Scholar] [CrossRef]
- Rastogi, V.; Marcaccio, C.L.; Kim, N.H.; Patel, P.B.; Anjorin, A.C.; Zettervall, S.L.; Patel, V.I.; de Bruin, J.L.; Verhagen, H.J.; Schermerhorn, M.L. The effect of supraceliac versus infraceliac landing zone on outcomes following fenestrated endovascular repair of juxta-/pararenal aortic aneurysms. J. Vasc. Surg. 2023, 77, 9–19.e2. [Google Scholar] [CrossRef]
- Gallitto, E.; Faggioli, G.; Pini, R.; Logiacco, A.; Mascoli, C.; Fenelli, C.; Abualhin, M.; Gargiulo, M. Reinterventions after fenestrated and branched endografting for degenerative aortic aneurysms. J. Vasc. Surg. 2021, 74, 1808–1816.e4. [Google Scholar] [CrossRef]
- Shibata, T.; Mitsuoka, H.; Iba, Y.; Hashizume, K.; Hongo, N.; Yasuhara, K.; Kuwada, N.; Katada, Y.; Hashiguchi, H.; Uzuka, T.; et al. Mid-term outcomes of physician-modified endograft therapy for complex aortic aneurysms. Interdiscip. Cardiovasc. Thorac. Surg. (ICVTS) 2024, 38, ivae044. [Google Scholar] [CrossRef]
- Bisdas, T.; Zambas, N.; Zertalis, M.; Theodorides, P.; Iatrou, N.; Dimopoulos, C.; Charalambous, N. Real-World Evaluation of the Off-the-Shelf Precannulated Inner-Branched Endograft for Pararenal Aortic Aneurysms. J. Endovasc. Ther. 2024, 32, 2127–2136. [Google Scholar] [CrossRef]
- Pitoulias, G.A.; Fazzini, S.; Donas, K.P.; Scali, S.T.; D’oRia, M.; Torsello, G.; Veith, F.J.; Puchner, S.B. Multicenter Mid-Term Outcomes of the Chimney Technique in the Elective Treatment of Degenerative Pararenal Aortic Aneurysms. J. Endovasc. Ther. 2022, 29, 226–239. [Google Scholar] [CrossRef]
- van der Riet, C.; Schuurmann, R.C.L.; Verhoeven, E.L.G.; Zeebregts, C.J.; Tielliu, I.F.J.; Bokkers, R.P.H.; Katsargyris, A.; de Vries, J.-P.P.M. Outcomes of Advanta V12 Covered Stents After Fenestrated Endovascular Aneurysm Repair. J. Endovasc. Ther. 2021, 28, 700–706. [Google Scholar] [CrossRef]
- Piazza, M.; Squizzato, F.; Pratesi, G.; Tshomba, Y.; Gaggiano, A.; Gatta, E.; Simonte, G.; Piffaretti, G.; Frigatti, P.; Veraldi, G.F.; et al. Editor’s Choice—Early Outcomes of a Novel Off the Shelf Preloaded Inner Branch Endograft for the Treatment of Complex Aortic Pathologies in the ItaliaN Branched Registry of E-nside EnDograft (INBREED). Eur. J. Vasc. Endovasc. Surg. 2023, 65, 811–817. [Google Scholar] [CrossRef]
- Farber, M.A.; Han, S.; Makaroun, M.S.; Matsumura, J.S.; Mendes, B.C.; Oderich, G.S.; Sanchez, L.A.; Suckow, B.D.; Timaran, C.H. One-year outcomes from the pivotal trial of a four-branch off-the-shelf solution to treat pararenal and extent IV thoracoabdominal aortic aneurysms. J. Vasc. Surg. 2025, 82, 740–749.e2. [Google Scholar] [CrossRef]
- Latz, C.A.; Boitano, L.T.; Tanious, A.; Wang, L.J.; Schwartz, S.I.; Pendleton, A.A.; DeCarlo, C.; Dua, A.; Conrad, M.F. Endovascular Versus Open Repair for Ruptured Complex Abdominal Aortic Aneurysms: A Propensity Weighted Analysis. Ann. Vasc. Surg. 2020, 68, 34–43. [Google Scholar] [CrossRef]
- Fenelli, C.; Tsilimparis, N.; Faggioli, G.; Stana, J.; Gallitto, E.; Stavroulakis, K.; Prendes, C.F.; Gargiulo, M. Early and Mid-Term Outcomes of the Inverted Limb Configuration Below Fenestrated and Branched Endografts: Experience from Two European Centers. J. Endovasc. Ther. 2024, 31, 410–420. [Google Scholar] [CrossRef]
- Le Houérou, T.; Álvarez-Marcos, F.; Gaudin, A.; Bosse, C.; Costanzo, A.; Vallée, A.; Haulon, S.; Fabre, D. Midterm Outcomes of Antegrade In Situ Laser Fenestration of Polyester Endografts for Urgent Treatment of Aortic Pathologies Involving the Visceral and Renal Arteries. Eur. J. Vasc. Endovasc. Surg. 2023, 65, 720–727. [Google Scholar] [CrossRef] [PubMed]
- D’Oria, M.; Budtz-Lilly, J.; Lindstrom, D.; Lundberg, G.; Jonsson, M.; Wanhainen, A.; Mani, K.; Unosson, J. Comparison of Early and Mid-Term Outcomes After Fenestrated-Branched Endovascular Aortic Repair in Patients With or Without Prior Infrarenal Repair. J. Endovasc. Ther. 2022, 29, 544–554. [Google Scholar] [CrossRef] [PubMed]
- Xodo, A.; Pilon, F.; Gregio, A.; Ongaro, G.; Desole, A.; Barbui, F.; Romagnoni, G.; Milite, D. Technical Considerations and Preliminary Experience of Intraprocedural Aneurysm Sac Embolization During Fenestrated and Branched EVAR (Embo F/BEVAR Technique): A Case Series. J. Clin. Med. 2025, 14, 2709. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Gittinger, M.; Gryzbowski, C.; Patel, S.; Asirwatham, M.; Grundy, S.; Zwiebel, B.; Shames, M.; Arnaoutakis, D.J. One-hundred Consecutive Physician-Modified Fenestrated Endovascular Aneurysm Repair of Pararenal and Thoracoabdominal Aortic Aneurysms Using the Terumo TREO Stent Graft. Ann. Vasc. Surg. 2024, 106, 369–376. [Google Scholar] [CrossRef]




| Authors | Year | Country | Number of Patients | Age (Years) | Type of Aneurysm | Treatment Strategy | Mean Follow-Up (Months) | 30-Day Mortality | Survival |
|---|---|---|---|---|---|---|---|---|---|
| van Lammeren GW et al. [20] | 2017 | Netherlands | 214 | 69.8 ± 7.1 | PAAA | OSR | 20 | 3.4% | 5 years—74.2% |
| Reyes et al. [21] | 2016 | Spain | 34 | 74 | PAAA after OSR for AAA (22 PAAP) | Endovascular 17 FEVAR 11 BEVAR 1 FEVAR + BEVAR 4 ChEVAR 1 “sandwich” | 23.2 ± 16.6 | 3% | 1 year—93.9% 2 years—90.9% |
| Mirza et al. [22] | 2019 | USA | 243 | 75 ± 8 | TAAA 147 PAAA 96 | F-BEVAR | 38 ± 15 | 2.5% | N/A |
| Werlin et al. [23] | 2019 | USA | 162 | 73 ± 8 | TAAA 73 PAAA 89 | Endovascular | 28 | 3.08% | N/A |
| Scali et al. [24] | 2015 | USA | 37 | 67 ± 10 | TAAA 24 PAAA 6 Pseudoaneurysm 3 Dissection 2 PAU 2 | F-BEVAR | 10.3 | 19% | 1 year—70% ± 8% 4 years—67% ± 8% |
| Tshomba et al. [25] | 2022 | Italy | 119 | 71.7 ± 6.8 | JAAA 37 PAAA 57 SAAA 18 Type IV TAAA 7 | OSR | 76 | 1.7% | 3 years-83.2% ± 3.4% 5 years-73.1% ± 4.1% 8 years-54.7% ± 6.2% |
| Zlatanovic et al. [5] | 2023 | Italy Serbia Netherlands Finland | 834 | 73 ± 6.6 EVAR 69.5 ± 7.1 OSR | JAAA 483 PAAA 351 | Endovascular 234 OSR 600 | 87 | 4.1% endovascular 5.5% OSR | 38.6% EVAR 42.1% OSR |
| Wang et al. [26] | 2022 | China | 10 | 54.5 ± 14.2 | TAAA PAAA | Endovascular | 30 | 0% | 90% |
| Gallitto et al. [27] | 2024 | Italy Germany Sweden Greece Portugal France | 197 | 75 ± 8 | JAAA 64 PAAA 95 | Endovascular | 19 ± 5 | 11% | 3 years 58% |
| Ferrer et al. [28] | 2024 | Italy | 183 | 64.5 ± 5.7 | JAAA 44 PAAA 33 TAAA 106 | F/BEVAR | 65.7 ± 39.6 | 2.2% | 1 year 94.0% 5 years 85.1% 10 years 72.2% |
| Yeung et al. [29] | 2024 | Netherlands Italy Spain | 42 | 76 ± 6 | IAAA 6 JAAA 33 PAAA 3 | Endovascular | 3 | 2.4% | N/A |
| Sultan et al. [30] | 2024 | Ireland | 99 | 74.7 ± 9.3 EVAR 73.2 ± 7.3 OSR | PAAA | EVAR 63 OSR 36 | 42.17 ± 32.38 EVAR 50.96 ± 38.8 OSR | 0% EVAR 2.78% OSR | N/A |
| Branzan et al. [31] | 2021 | Germany | 17 | 70 ± 9 | PAAA TAAA | Endovascular | 14.4 | 0% | N/A |
| Escobar et al. [4] | 2022 | USA | 208 | 71 ± 8 | TAAA 163 PAAA 45 | Hybrid | 21 | 14.4% | 1 year 77 ± 3% 5 years 61 ± 5% |
| Zhang et al. [32] | 2024 | China | 15 | 63.4 ± 10.7 | TAAA 9 PAAA 6 | Endovascular | 31.4 | 0% | 93% |
| Ribeiro et al. [33] | 2025 | Portugal | 293 | N/A | cAAA | EVAR 35.2% F/BEVAR 32.7% OSR 32.1% | N/A | N/A | N/A |
| D’Oria et al. [34] | 2021 | Sweden | 202 | 72 ± 8 | PAAA TAAA | F-BEVAR | 32.8 | 2% | 3 years 75.2% |
| Yazar et al. [35] | 2025 | Netherlands | 23 | 72.3 ± 7.2 | PAAA | iBEVAR | 15 | 8.3% | 78.3% |
| Biggs et al. [36] | 2022 | USA | 32 | 74 ± 9 | PAAA 10 TAAA 22 | F-BEVAR | 24 ± 22 | 6% | 1 year 70% ± 8% |
| Wang et al. [37] | 2025 | China | 115 | 69.1 | JAAA PAAA | Endovascular | 1 | 0.86% | N/A |
| Rastogi et al. [38] | 2022 | USA | 1486 | N/A | JAAA 575 PAAA 911 | FEVAR | N/A | 2.4% | 3 years 89.5% |
| Gallitto et al. [39] | 2021 | Italy | 221 | N/A | TAAA 110 J/PAAA 111 | F/BEVAR | 27 | 4% | 1 year 89% 3 years 75% 5 years 65% |
| Shibata et al. [40] | 2024 | Japan | 121 | 75.6 ± 7.6 | PAAA 62 TAAA 59 | Endovascular | 24.2 | 5.8% | 3 years 83.3% PAAA 54.1% TAAA |
| Bisdas et al. [41] | 2024 | Greece Cyprus | 21 | 71 | PAAA | Endovascular | 14 ± 7.7 | 0% | 95% |
| Pitoulias et al. [42] | 2022 | Greece Germany Italy USA Austria | 267 | N/A | PAAA | ChEVAR | 25.5 ± 13.3 | 1.9% | 3 years 81.0% |
| van der Riet et al. [43] | 2021 | Netherlands Germany | 194 | 72.2 ± 8.0 | PAAA | FEVAR | 24.6 | 3% | 3 years 77% |
| Piazza et al. [44] | 2023 | Italy | 116 | 73 ± 8 | TAAA PAAA/P | Endovascular | 3 | N/A | 94.8% |
| Farber et al. [45] | 2025 | USA | 102 | N/A | TAAA 59 PAAA 43 | Endovascular | 12 | 0% | 1 year 94.1% |
| Latz et al. [46] | 2020 | USA | 443 | N/A | JAAA 253 PAAA 59 SAAA 100 Type IV 31 | EVAR 23.7% OSR 76.3% | N/A | 30.5% EVAR 23.8% OSR 32.5% | N/A |
| Fenelli et al. [47] | 2024 | Italy Germany | 41 | 71±10 | J/PAAA 8 TAAA 33 | F-BEVAR | 21±16 | 0% | 1 year 90% 2 years 84% |
| Le Houerou et al. [48] | 2023 | France Spain | 42 | 75.1 ± 10 | TAAA PAAA | Endovascular | 24.7 | 4.5% | 2 years 73% |
| D’Oria et al. [49] | 2022 | Sweden Italy Denmark | 222 | 71.6 | PAAA TAAA | F-BEVAR | N/A | N/A | 5 years 61.6% |
| Xodo et al. [50] | 2025 | Italy | 5 | 71 ± 9 | JAAA PAAA | F/BEVAR | 12.4 ± 3.6 | 0% | N/A |
| Nguyen et al. [51] | 2024 | USA | 100 | 73.7 ± 7.0 | TAAA 42 PAAA 58 | Endovascular | 24 | 2% | 2 years 87% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iliescu, V.A.; Nayyerani, R.; Parasca, C.A.; Platon, P.; Baston, C.; Morosanu, B.; Stiru, O. Hybrid Open and Endovascular Repair in Pararenal Abdominal Aortic Pseudoaneurysm—Literature Review and Case Presentation. Life 2025, 15, 1765. https://doi.org/10.3390/life15111765
Iliescu VA, Nayyerani R, Parasca CA, Platon P, Baston C, Morosanu B, Stiru O. Hybrid Open and Endovascular Repair in Pararenal Abdominal Aortic Pseudoaneurysm—Literature Review and Case Presentation. Life. 2025; 15(11):1765. https://doi.org/10.3390/life15111765
Chicago/Turabian StyleIliescu, Vlad Anton, Reza Nayyerani, Catalina Andreea Parasca, Pavel Platon, Catalin Baston, Bianca Morosanu, and Ovidiu Stiru. 2025. "Hybrid Open and Endovascular Repair in Pararenal Abdominal Aortic Pseudoaneurysm—Literature Review and Case Presentation" Life 15, no. 11: 1765. https://doi.org/10.3390/life15111765
APA StyleIliescu, V. A., Nayyerani, R., Parasca, C. A., Platon, P., Baston, C., Morosanu, B., & Stiru, O. (2025). Hybrid Open and Endovascular Repair in Pararenal Abdominal Aortic Pseudoaneurysm—Literature Review and Case Presentation. Life, 15(11), 1765. https://doi.org/10.3390/life15111765





