Effect of Early Extracorporeal Shockwave Therapy on Postoperative Pain and Functional Recovery After Intramedullary Nailing: An Open-Label Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Ethical Approval
2.2. Participants
- -
- Age ≥ 50 years;
- -
- Unilateral proximal femoral fracture treated with IM nailing;
- -
- Able to provide informed consent.
- -
- Non-ambulatory status preoperatively (Koval Grade 6);
- -
- Cognitive impairment preventing informed consent or outcome assessment;
- -
- Active infection at the surgical site at the time of enrollment;
- -
- Severe cardiac arrhythmia or hemodynamic instability requiring ICU care;
- -
- Pregnancy or refusal to participate;
- -
- Prior ESWT within 3 months;
- -
- For the ESWT group specifically: inability to complete all three scheduled ESWT sessions.
2.3. Randomization and Blinding
2.4. Interventions
2.4.1. Standard Postoperative Care (Control Group)
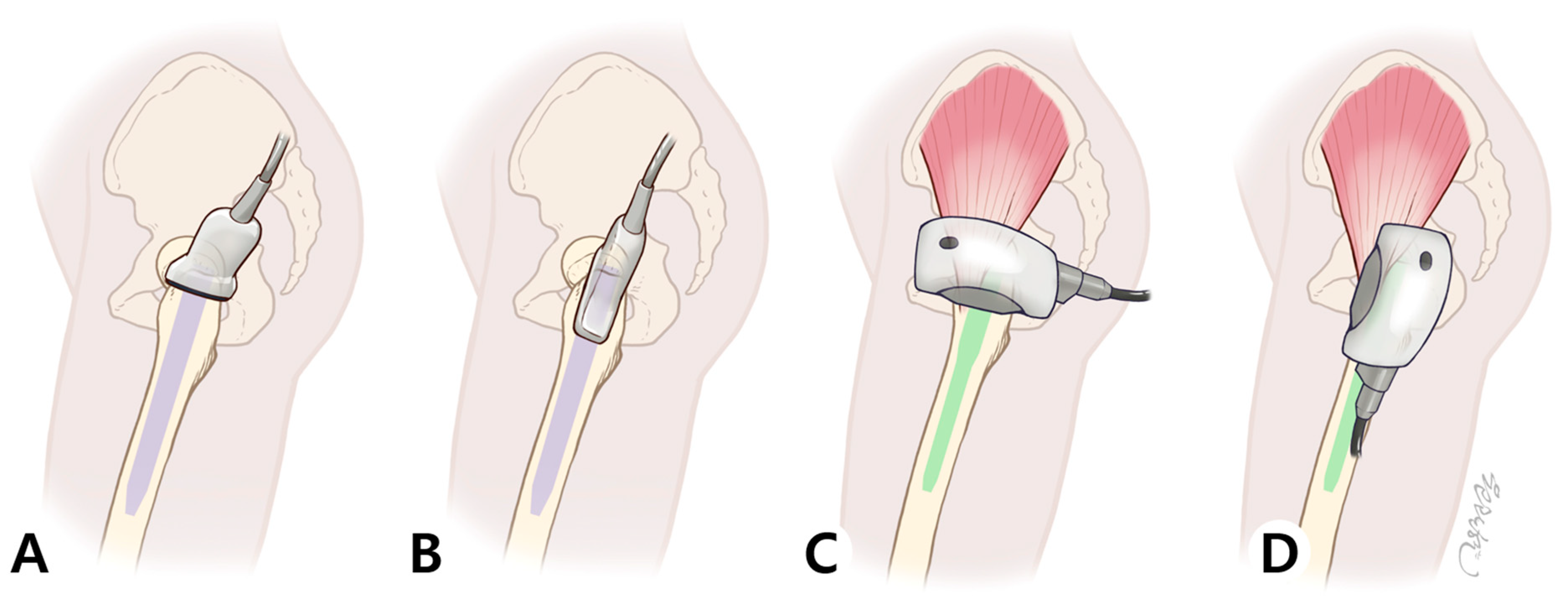
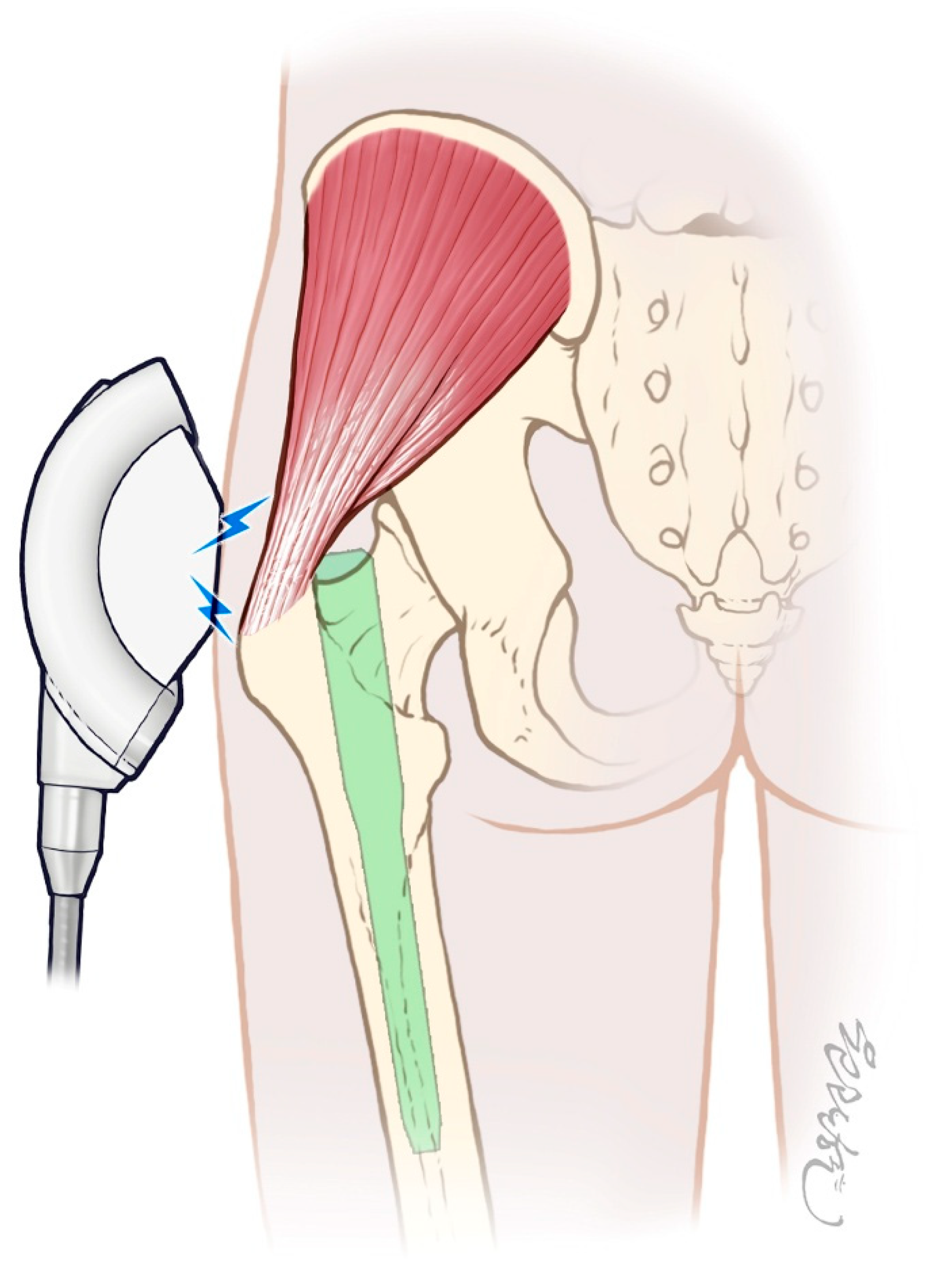
2.4.2. ESWT Protocol (Intervention Group)
2.5. Outcome Measures
2.6. Statistical Analysis
- Primary Analysis
- Baseline Imbalance and Sensitivity Analysis
- Clinical Significance
- Model Assumptions and Missing Data
3. Results
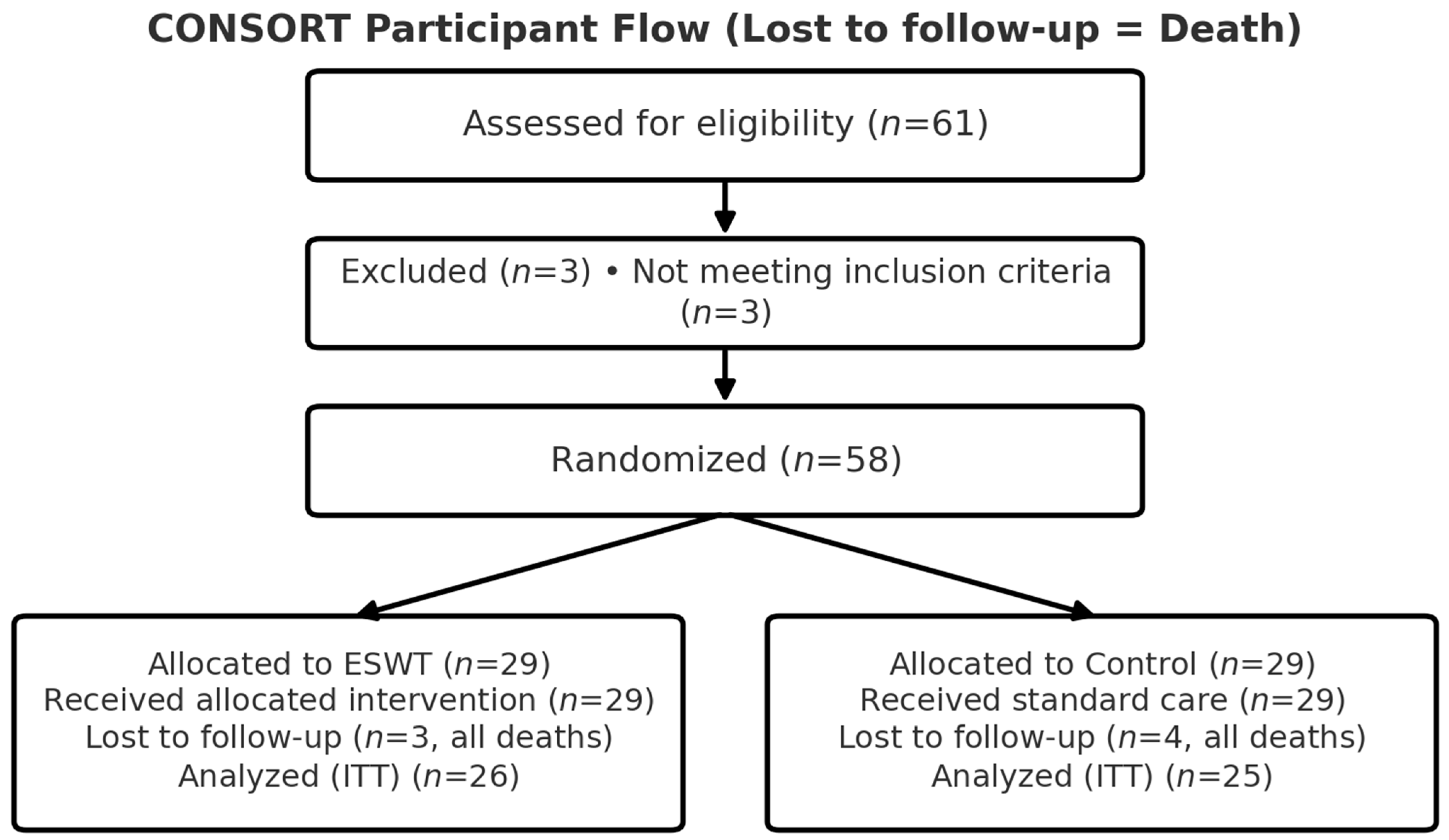
3.1. Participant Flow and Baseline Characteristics
3.2. Pain Outcomes (VAS)
3.3. Sensitivity Analysis: Accounting for Baseline Imbalance
3.4. Functional Outcomes (mHHS)
3.5. Safety and Adverse Events
4. Discussion
4.1. Principal Findings and Deeper Interpretation
4.2. The Pain–Function Dissociation: Mechanisms and Clinical Implications
- Different Recovery Timelines
- 2.
- Outcome Measure Sensitivity
- 3.
- Ceiling Effects
- 4.
- Time-Course Mismatch
- 5.
- Mechanistic Mismatch
4.3. Clinical Implications and Comparison with Literature
4.4. Limitations and Future Directions
- Retrospective Trial Registration. The trial was registered retrospectively (CRIS PRE20250926-004) due to administrative delays at enrollment. While this may introduce a reporting bias risk, this is partially mitigated by: (a) the protocol and primary outcomes were established before patient enrollment; (b) outcome assessors were blinded to group allocation; (c) all pre-specified outcomes (VAS, mHHS) were consistently measured at all scheduled time points.
- Open-Label Design and Placebo Effects. The open-label design and lack of sham control prevent definitively ruling out placebo effects. This is a critical limitation for ESWT evaluation. Future double-blind, sham-controlled trials using matched transducers are necessary to isolate ESWT-specific effects from psychological and contextual factors.
- Limited Sample Size and Statistical Power. With n = 51 (25 control, 26 ESWT), statistical power for subgroup analyses is limited. Findings from stratified analysis—particularly for right-sided fractures (ESWT n = 4)—should be interpreted with appropriate caution due to the very small subsample size. Large-scale multicenter trials are needed to confirm efficacy, evaluate generalizability across populations, and provide sufficient power for clinically meaningful subgroup analyses.
- Single-Center Design and Generalizability. Conduct at a single tertiary academic medical center in Korea may limit generalizability. Results may not reflect outcomes in community hospitals, different healthcare systems, different cultural contexts, or populations with different demographic characteristics or comorbidity profiles.
- Baseline Imbalance in Fracture Laterality (NOW ADDRESSED). A notable baseline imbalance in fracture laterality was observed between groups (left-sided: 40.0% in control vs. 86.2% in ESWT; χ2 = 8.756, p = 0.003). However, rather than representing an unresolved confounding problem, this was comprehensively addressed through: (a) sensitivity analysis including laterality as a fixed covariate in the mixed-effects model (Supplementary Table S1), with results unchanged; and (b) stratified analysis for left-sided and right-sided fractures separately (Supplementary Table S2). These additional analyses demonstrated that primary findings were robust to the baseline imbalance, suggesting it did not confound treatment effects.
- Unmeasured Confounding Variables. We did not systematically assess psychological status, patient motivation, family support, socioeconomic factors, or cognitive status—all of which influence postoperative recovery and rehabilitation engagement. Future comprehensive studies should incorporate validated instruments for these psychosocial and economic dimensions.
- Limited Sensitivity of Functional Outcome Measure. While the mHHS is psychometrically sound, it may lack sensitivity for detecting early, abductor-specific functional improvements in the immediate postoperative period. Performance-based measures (gait speed, Trendelenburg test, abductor strength via dynamometry) might reveal functional benefits not captured by this composite score.
- Absence of Economic Analysis. This study did not include formal cost-effectiveness or economic analysis. ESWT equipment acquisition costs, maintenance, and personnel time must be weighed against potential savings from reduced analgesic consumption, shorter rehabilitation duration, and earlier return to work or activity. Comprehensive health economic evaluation is essential before clinical implementation and resource allocation decisions can be confidently recommended.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Loggers, S.A.I.; Van Lieshout, E.M.M.; Joosse, P.; Verhofstad, M.H.J.; Willems, H.C. Prognosis of nonoperative treatment in elderly patients with a hip fracture: A systematic review and meta-analysis. Injury 2020, 51, 2407–2413. [Google Scholar] [CrossRef]
- Kim, S.M.; Moon, Y.W.; Lim, S.J.; Yoon, B.K.; Min, Y.K.; Lee, D.Y.; Park, Y.S. Prediction of survival, second fracture, and functional recovery following the first hip fracture surgery in elderly patients. Bone 2012, 50, 1343–1350. [Google Scholar] [CrossRef]
- Choi, J.Y.; Park, J.W.; Kim, K.I.; Lee, Y.K.; Kim, C.H. Prediction of 5-year survival rate after hip fracture surgery using a comprehensive geriatric assessment-based frailty score model. J. Korean Med. Sci. 2025, 40, e40. [Google Scholar] [CrossRef]
- Palm, H. Hip fracture: The choice of surgery. In Orthogeriatrics: The Management of Older Patients with Fragility Fractures; Falaschi, P., Marsh, D., Eds.; Springer: Cham, Switzerland, 2021; pp. 125–141. [Google Scholar]
- Joglekar, S.B.; Lindvall, E.M.; Martirosian, A. Contemporary management of subtrochanteric fractures. Orthop. Clin. N. Am. 2015, 46, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Panteli, M.; Mauffrey, C.; Giannoudis, P.V. Subtrochanteric fractures: Issues and challenges. Injury 2017, 48, 2023–2026. [Google Scholar] [CrossRef] [PubMed]
- Çölbe, S.A.; Çiftdemir, M.; Ustabaşıoğlu, F.E.; Özgür, C. Iatrogenic gluteus medius muscle insertion injury while trochanteric entry nailing due to trochanteric fractures: A comparative study in forty patients with gray-scale ultrasound and shear-wave elastography. Int. Orthop. 2021, 45, 3253–3261. [Google Scholar] [CrossRef]
- Yoon, Y.; Lee, H.; Lam, K.H.S.; Lee, M.; Lee, J.; Hwang, J. The sonographic evaluation of abductor injury after intramedullary nailing for the hip fractures. J. Clin. Med. 2025, 14, 5498. [Google Scholar] [CrossRef] [PubMed]
- McConnell, T.; Tornetta, P., 3rd; Benson, E.; Manuel, J. Gluteus medius tendon injury during reaming for gamma nail insertion. Clin. Orthop. Relat. Res. 2003, 407, 199–202. [Google Scholar] [CrossRef]
- Noda, M.; Saegusa, Y.; Takahashi, M.; Takada, Y.; Fujita, M.; Shinohara, I. Decreased postoperative gluteus medius muscle cross-sectional area measured by computed tomography scan in patients with intertrochanteric fractures nailing. J. Orthop. Surg. 2017, 25, 2309499017727943. [Google Scholar] [CrossRef]
- Wang, H.; Shi, Y. Extracorporeal shock wave treatment for post-surgical fracture nonunion: Insight into its mechanism, efficacy, safety and prognostic factors (Review). Exp. Ther. Med. 2023, 26, 332. [Google Scholar] [CrossRef]
- Wang, C.J. Extracorporeal shockwave therapy in musculoskeletal disorders. J. Orthop. Surg. Res. 2012, 7, 11. [Google Scholar] [CrossRef]
- d’Agostino, M.C.; Craig, K.; Tibalt, E.; Respizzi, S. Shock wave as biological therapeutic tool: From mechanical stimulation to recovery and healing, through mechanotransduction. Int. J. Surg. 2015, 24, 147–153. [Google Scholar] [CrossRef]
- Rhim, H.C.; Shin, J.; Beling, A.; Guo, R.; Pan, X.; Afunugo, W.; Ruiz, J.; Andrew, M.N.; Kim, J.; Tenforde, A.S. Extracorporeal Shockwave Therapy for Greater Trochanteric Pain Syndrome: A Systematic Review with Meta-Analysis of Randomized Clinical Trials. JBJS Rev. 2024, 12, e24. [Google Scholar] [CrossRef]
- Patel, R.; Sokhal, B.S.; Singh, R.; Nandra, R.; Heaver, C.; Banerjee, R. A systematic review and meta-analysis of randomized controlled trials of the effectiveness of focused extracorporeal shockwave therapy in the management of greater trochanteric pain syndrome. Orthop. Proc. 2025, 107, 6. [Google Scholar] [CrossRef]
- Rau, O.R.; Cheng, J.; Jivanelli, B.; Tenforde, A.S.; Wyss, J.F. Extracorporeal shockwave therapy for tendinopathies around the hip and pelvis: A systematic review. HSS J. 2025, 15563316251332189. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.T.; Cheng, J.H.; Jhan, S.W.; Chen, P.C.; Wang, C.J.; Chou, W.Y. Prognostic factors of extracorporeal shockwave therapy in the treatment of nonunion in long bones: A retrospective study. Int. J. Surg. 2024, 110, 6426–6431. [Google Scholar] [CrossRef] [PubMed]
- Kemp, J.L.; Collins, N.J.; Roos, E.M.; Crossley, K.M. Psychometric properties of patient-reported outcome measures for hip arthroscopic surgery. Am. J. Sports Med. 2013, 41, 2065–2073. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.H.; Jung, Y.J.; Lee, J.Y.; Choi, J.S.; Mun, J.H.; Park, W.Y.; Seo, C.H.; Jang, K.U. The effect of extracorporeal shock wave therapy on myofascial pain syndrome. Ann. Rehabil. Med. 2012, 36, 665–674. [Google Scholar] [CrossRef]
- Ji, H.M.; Kim, H.J.; Han, S.J. Extracorporeal Shock Wave Therapy in Myofascial Pain Syndrome of Upper Trapezius. Ann. Rehabil. Med. 2012, 36, 675–680. [Google Scholar] [CrossRef]
- Yoo, J.I.; Oh, M.K.; Chun, S.W.; Lee, S.U.; Lee, C.H. The effect of focused extracorporeal shock wave therapy on myofascial pain syndrome of trapezius: A systematic review and meta-analysis. Medicine 2020, 99, e19085. [Google Scholar] [CrossRef]
- Moretti, B.; Notarnicola, A.; Moretti, L.; Patella, S.; Tatò, I.; Patella, V. Bone healing induced by ESWT. Clin. Cases Min. Bone Metab. 2009, 6, 155–158. [Google Scholar]
- Wang, C.-J.; Liu, H.-C.; Fu, T.-H. The effects of extracorporeal shockwave on acute high-energy long bone fractures of the lower extremity. Arch. Orthop. Trauma Surg. 2007, 127, 137–142. [Google Scholar] [CrossRef]
- Vishwanathan, K.; Akbari, K.; Patel, A.J. Is the modified Harris hip score valid and responsive instrument for outcome assessment in the Indian population with pertrochanteric fractures? J. Orthop. 2018, 15, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Hansen, C.F.; Jensen, J.; Odgaard, A.; Siersma, V.; Comins, J.D.; Brodersen, J.; Krogsgaard, M.R. Four of five frequently used orthopedic PROMs possess inadequate content validity: A COSMIN evaluation of the mHHS, HAGOS, IKDC-SKF, KOOS and KNEES-ACL. Knee Surg. Sports Traumatol. Arthrosc. 2022, 30, 3602–3615. [Google Scholar] [CrossRef] [PubMed]
- Hung, M.; Hon, S.D.; Cheng, C.; Franklin, J.D.; Aoki, S.K.; Anderson, M.B.; Kapron, A.L.; Peters, C.L.; Pelt, C.E. Psychometric evaluation of the lower extremity computerized adaptive test, the modified Harris hip score, and the hip outcome score. Orthop. J. Sports Med. 2014, 2, 2325967114562191. [Google Scholar] [CrossRef]
- Li, D.J.; Clohisy, J.C.; Schwabe, M.T.; Yanik, E.L.; Pascual-Garrido, C. PROMIS versus legacy patient-reported outcome measures in patients undergoing surgical treatment for symptomatic acetabular dysplasia. Am. J. Sports Med. 2020, 48, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Zissler, A.; Stoiber, W.; Pittner, S.; Sänger, A.M. Extracorporeal shock wave therapy in acute injury care: A systematic review. Rehabil. Process. Outcome 2018, 7, 1179572718765138. [Google Scholar] [CrossRef]
- Kimmel, L.A.; Liew, S.M.; Sayer, J.M.; Holland, A.E. HIP4Hips (High Intensity Physiotherapy for Hip fractures in the acute hospital setting): A randomised controlled trial. Med. J. Aust. 2016, 205, 73–78. [Google Scholar] [CrossRef]
- Latham, N.K.; Harris, B.A.; Bean, J.F.; Heeren, T.; Goodyear, C.; Zawacki, S.; Heislein, D.M.; Mustafa, J.; Pardasaney, P.; Giorgetti, M.; et al. Effect of a home-based exercise program on functional recovery following rehabilitation after hip fracture: A randomized clinical trial. Jama 2014, 311, 700–708. [Google Scholar] [CrossRef]
- Harding, D.; Cameron, L.; Monga, A.; Winter, S. Is shockwave therapy effective in the management of greater trochanteric pain syndrome? A systematic review and meta-analysis. Musculoskelet. Care 2024, 22, e1892. [Google Scholar] [CrossRef]
- Mazin, Y.; Lemos, C.; Paiva, C.; Amaral Oliveira, L.; Borges, A.; Lopes, T. The role of extracorporeal shock wave therapy in the treatment of muscle injuries: A systematic review. Cureus 2023, 15, e44196. [Google Scholar] [CrossRef] [PubMed]
- Morrison, R.S.; Dickman, E.; Hwang, U.; Akhtar, S.; Ferguson, T.; Huang, J.; Jeng, C.L.; Nelson, B.P.; Rosenblatt, M.A.; Silverstein, J.H.; et al. Regional nerve blocks improve pain and functional outcomes in hip fracture: A randomized controlled trial. J. Am. Geriatr. Soc. 2016, 64, 2433–2439. [Google Scholar] [CrossRef] [PubMed]
- Oldmeadow, L.B.; Edwards, E.R.; Kimmel, L.A.; Kipen, E.; Robertson, V.J.; Bailey, M.J. No rest for the wounded: Early ambulation after hip surgery accelerates recovery. ANZ J. Surg. 2006, 76, 607–611. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | ESWT | p-Value | ||
|---|---|---|---|---|
| Control (n = 25) | ESWT (n = 26) | |||
| Age | 79.84 ± 7.88 | 81.19 ± 8.86 | 0.567 | |
| Sex (%) | Female | 17 (68.0) | 23 (88.5) | 0.151 |
| Male | 8 (32.0) | 3 (11.5) | ||
| Site (%) | Lt | 10 (40.0) | 22 (84.6) | 0.003 |
| Rt | 15 (60.0) | 4 (15.4) | ||
| Diagnosis (%) | femur neck stress fracture | 0 (0.0) | 1 (3.8) | 1.000 |
| intertrochanteric fracture | 20 (80.0) | 21 (80.8) | ||
| midshaft fracture | 2 (8.0) | 2 (7.7) | ||
| midshaft peri-implant fracture | 1 (4.0) | 0 (0.0) | ||
| proximal shaft fracture | 1 (4.0) | 0 (0.0) | ||
| subtrochanteric fracture | 1 (4.0) | 2 (7.7) | ||
| Preop.Koval.Gr (%) | 1 | 15 (60.0) | 11 (42.3) | 0.397 |
| 2 | 5 (20.0) | 3 (11.5) | ||
| 3 | 1 (4.0) | 5 (19.2) | ||
| 4 | 1 (4.0) | 2 (7.7) | ||
| 5 | 3 (12.0) | 4 (15.4) | ||
| 6 | 0 (0.0) | 1 (3.8) | ||
| Control | ESWT | |
|---|---|---|
| n | 25 | 26 |
| Baseline VAS | 6.08 ± 1.59 | 6.31 ± 1.74 |
| 3-month VAS | 3.68 ± 1.55 | 3.46 ± 1.83 |
| 6-month VAS | 2.72 ± 1.73 | 2.16 ± 1.87 |
| 12-month VAS | 1.72 ± 1.80 | 0.89 ± 1.47 |
| 3-month mHHS | 57.72 ± 10.52 | 58.65 ± 16.20 |
| 6-month mHHS | 62.72 ± 11.06 | 64.38 ± 16.68 |
| 12-month mHHS | 67.72 ± 11.06 | 73.27 ± 14.35 |
| VAS_diff(base-3 m) | 1.86 ± 1.98 | 2.04 ± 2.60 |
| VAS_diff(base-6 m) | 2.62 ± 2.25 | 3.39 ± 2.79 |
| VAS_diff(base-12 m) | 3.57 ± 2.29 | 4.35 ± 2.08 |
| Characteristic | Beta | 95% CI | p-Value |
|---|---|---|---|
| 0.337 | 0.289–0.392 | <0.001 | |
| Month (per month) | |||
| Group (ESWT vs. Control, ref = Control) | — | — | |
| 0.125 | −0.659–0.908 | p = 0.755 | |
| Month × ESWT (interaction) | 0.086 | 0.010–0.162 | p = 0.027 |
| Characteristic | Beta | 95% CI | p-Value |
|---|---|---|---|
| Month (per month) | 4.732 | 3.810–5.655 | <0.001 |
| Group (ESWT vs. Control, ref = Control) | — | — | |
| −0.451 | −9.421, 8.519 | p = 0.9 | |
| Month × ESWT (interaction) | |||
| 0.485 | −0.807, 1.777 | 0.462 |
| Interval | Control (n = 25) | ESWT (n = 26) |
|---|---|---|
| 6 m–3 m | 7/25 (28.0 %) | 3/26 (11.5 %) |
| 12 m–6 m | 9/25 (36.0 %) | 7/26 (26.9 %) |
| 12 m–3 m | 16/25 (64.0 %) | 13/26 (50.0 %) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, Y.; Hwang, J.; Lee, J.; Lam, K.H.S.; Castro, J.C.d.; Kim, H.; Sung, D.; Kim, S.; Lee, M.; Park, C. Effect of Early Extracorporeal Shockwave Therapy on Postoperative Pain and Functional Recovery After Intramedullary Nailing: An Open-Label Randomized Controlled Trial. Life 2025, 15, 1704. https://doi.org/10.3390/life15111704
Yoon Y, Hwang J, Lee J, Lam KHS, Castro JCd, Kim H, Sung D, Kim S, Lee M, Park C. Effect of Early Extracorporeal Shockwave Therapy on Postoperative Pain and Functional Recovery After Intramedullary Nailing: An Open-Label Randomized Controlled Trial. Life. 2025; 15(11):1704. https://doi.org/10.3390/life15111704
Chicago/Turabian StyleYoon, Yonghyun, Jihyo Hwang, Jaeyoung Lee, King Hei Stanley Lam, Jeimylo C. de Castro, Hyeongjik Kim, Dongyeun Sung, Seungbeom Kim, MinJae Lee, and Chanwool Park. 2025. "Effect of Early Extracorporeal Shockwave Therapy on Postoperative Pain and Functional Recovery After Intramedullary Nailing: An Open-Label Randomized Controlled Trial" Life 15, no. 11: 1704. https://doi.org/10.3390/life15111704
APA StyleYoon, Y., Hwang, J., Lee, J., Lam, K. H. S., Castro, J. C. d., Kim, H., Sung, D., Kim, S., Lee, M., & Park, C. (2025). Effect of Early Extracorporeal Shockwave Therapy on Postoperative Pain and Functional Recovery After Intramedullary Nailing: An Open-Label Randomized Controlled Trial. Life, 15(11), 1704. https://doi.org/10.3390/life15111704









