1. Introduction
The use of acidifiers in poultry nutrition has become an essential strategy to improve production performance and animal health. Organic acids, such as malic, citric, formic, lactic, and butyric acids, have demonstrated positive effects on broiler performance, enhancing feed conversion ratios, nutrient digestibility, and growth metrics [
1,
2,
3,
4]. The mechanisms underlying these performance improvements are closely linked to the acidifiers’ profound effects on the intestinal microbiota composition, as demonstrated by sodium diformate supplementation modulating beneficial bacteria populations while reducing pathogenic species [
5] and phosphoric and lactic acids significantly decreasing E. coli and Salmonella counts in broiler cecum [
6]. Drinking water acidification has emerged as an effective delivery method, with studies reporting that water acidification improved bone metabolism through altered serum biochemical indicators including increased phosphorus concentrations and reduced inflammatory markers [
7] while demonstrating significant gastrointestinal pH reductions and improved feed conversion efficiency [
8]. The effects of acidifiers extend to egg quality parameters, with recent studies showing enhanced Haugh unit scores and shell thickness in acidifier-supplemented laying hens [
9], supported by documented improved egg yolk composition with combined probiotic–acidifier treatments [
10]. Furthermore, recent studies have shown that acidifiers also demonstrate excellent effects in regulating the intestinal microbial flora and controlling pathogens in laying hens [
11,
12,
13,
14]. Contemporary research has increasingly focused on innovative formulations, including microencapsulated organic acid systems that provide targeted intestinal delivery [
15] and synergistic combinations with probiotics and botanicals that enhance overall efficacy while maintaining intestinal homeostasis [
16,
17].
Recent advances in antibiotic-free poultry production have intensified research on feed additives that enhance growth performance and gut health, with 2-hydroxy-4-methylsulfonylbutyric acid (HMTBa), lactic acid, and phosphoric acid emerging as promising alternatives [
18]. Phosphoric acid presents a unique dual functionality as both an acidifier and a phosphorus source, with studies showing 93% relative bioavailability and optimal inclusion at 0.1–0.2 g/kg for improved feed conversion and intestinal enzyme activities [
18]. Meta-analyses encompassing 93 studies confirmed lactic acid bacteria’s positive effects on growth performance and immune responses [
19], with recent trials demonstrating effectiveness in mitigating high-stocking-density stress [
20]. Lactic acid has shown consistent antimicrobial benefits, with encapsulated forms at 0.6% inclusion significantly reducing Salmonella counts and enhancing beneficial bacteria populations [
21], while strain-specific Lactobacillus plantarum applications increased beneficial microbiota and decreased pathogenic Desulfovibrio [
22]. Multiple investigations have established HMTBa’s superior performance at higher inclusion levels with reduced negative impact on feed intake [
23,
24]. HMTBa has demonstrated 60–80% relative bioavailability compared to DL-methionine while offering unique metabolic advantages through concentration-dependent absorption mechanisms [
25,
26], with studies confirming its dual functionality in promoting beneficial butyrate-producing bacteria while reducing pathogens [
27]. Despite these advances, significant research gaps persist, particularly regarding phosphoric acid applications in modern production systems and the synergistic effects of combined acidifier strategies under current high-density, fast-growth conditions.
The BIAN chicken, an indigenous breed in Shanxi Province, China, is notable for its moderate body size, distinctive feather pattern, and adaptability to the semi-intensive production system typical of traditional poultry breeding in the region [
28]. Based on the documented benefits of organic acids in poultry nutrition, we hypothesized that supplementation with composite acidifying agents would enhance both production performance and gut health in 300-day-old BIAN chickens through improved nutrient utilization and beneficial microbiota modulation. This study therefore evaluated the effects of two concentrations of composite acidifiers (containing 2-hydroxy-4-methylthiobutyric acid, lactic acid, and phosphoric acid) on egg production, quality parameters, serum biochemistry, intestinal function, and cecal microbiota composition. By examining these integrated physiological responses, we sought to determine the optimal acidifier dosage and elucidate the mechanisms underlying performance improvements, providing evidence for implementing acidifier-based feeding strategies as sustainable alternatives to antibiotics in indigenous chicken production.
4. Discussion
The present study demonstrated that dietary supplementation with a composite acidifier significantly increased the average egg weight in 300-day-old BIAN chickens, with test group B showing a remarkable 4.6% improvement compared to the control group. This finding is consistent with previous research showing that acidifiers enhance egg weight through improved nutrient digestibility and intestinal morphology [
29,
30]. The mechanism underlying this improvement involves acidifiers’ ability to lower the gastrointestinal pH, which enhances digestive enzyme activities and mineral absorption, particularly calcium and phosphorus utilization, crucial for egg formation [
31,
32]. Although the egg production rate showed no significant improvement in our study, the enhanced egg weight suggests that acidifiers primarily influence egg quality parameters rather than quantity in 300-day-old hens. At the laying stage, ovulation patterns are physiologically established, limiting the potential for increased egg numbers. However, the improved digestive enzyme activities and enhanced intestinal architecture enabled more efficient nutrient absorption, which birds preferentially allocated toward increasing individual egg mass rather than egg frequency. This nutrient partitioning pattern is consistent with findings in Japanese quails where acidifiers improved the egg weight and shell quality without significantly affecting the laying rate [
33]. The lack of significant differences in the feed intake and feed-to-egg ratio between groups indicates that the improved egg weight resulted from enhanced nutrient utilization efficiency rather than increased consumption [
34]. These results support the potential of composite acidifiers as effective alternatives to antibiotic growth promoters in laying hen production, offering a sustainable approach to improving egg quality while maintaining production efficiency [
35]. The significant improvements in the Haugh unit and eggshell strength observed in test group A align with findings that composite acidifiers enhance calcium utilization and protein digestibility in laying poultry [
36]. Enhanced Haugh unit values in both treatment groups corroborate recent evidence that organic acids improve albumen quality through increased nutrient absorption and antioxidant capacity [
37]. The differential responses between groups suggest dose-dependent effects of acidifiers on mineral metabolism and shell gland function in BIAN chickens [
38].
The present study demonstrated that dietary supplementation with composite acidifying agents significantly enhanced serum biochemical indicators in 300-day-old BIAN chickens, with test group B (65.49% globulin increase, 61.76% T-AOC elevation) showing superior effects compared to test group A (28.33% globulin increase). These findings align with recent studies reporting that organic acids influence serum biochemical parameters and the health status in poultry [
39,
40]. The marked elevation in serum globulin levels reflects enhanced immunoglobulin production, as globulins primarily consist of immunoglobulins (IgG, IgM, IgA) that constitute the humoral immune response. The mechanism by which composite acidifiers enhance globulin production likely involves multiple pathways. Acidifiers reduce the intestinal pH, creating conditions that favor beneficial bacteria like
Akkermansia and Lachnospiraceae, which produce immunomodulatory metabolites including short-chain fatty acids (SCFAs). These SCFAs, particularly butyrate, activate G-protein-coupled receptors on intestinal epithelial cells and dendritic cells, triggering downstream signaling [
41,
42]. Additionally, the improved intestinal barrier function (evidenced by the increased villus-to-crypt ratio) reduces pathogenic bacterial translocation, decreasing inflammatory cytokine production while promoting regulatory T-cell responses that support optimal antibody production. The concurrent increase in total antioxidant capacity may further support immune function by reducing oxidative stress on immune cells, thereby preserving their capacity for antibody production [
43,
44]. The superior performance of test group B suggests that optimal acidifier dosage is crucial for maximizing immunomodulatory effects, as higher concentrations promote better intestinal pH regulation and nutrient absorption [
45]. The enhanced antioxidant status observed in our study, as evidenced by the significant increase in total antioxidant capacity (T-AOC), demonstrates the beneficial effects of acidifiers on oxidative stress resistance. Previous research has suggested various pathways through which organic acids may enhance antioxidant capacity, including modulation of cellular signaling pathways and direct scavenging activities [
46]. These improvements in immune and antioxidant parameters collectively contribute to a better health status and potentially enhanced production performance in BIAN chickens, supporting acidifiers as effective antibiotic alternatives in poultry production.
The composite acidifying agent significantly enhanced intestinal digestive enzyme activities in 300-day-old BIAN chickens, with test group A demonstrating a superior enzymatic response compared to test group B. The marked increases in lipase activity (61.73% and 48.60% in duodenal and jejunal contents, respectively) and trypsin activity (24.74% in duodenal content) in test group A align with studies showing that organic acids stimulate pancreatic secretion through pH-mediated secretin release [
47]. The elevated amylase activity (37.43% in jejunal content) corroborates previous findings that acidifiers enhance carbohydrate digestion by promoting enzyme secretion [
48]. Organic acids likely exert their effects by lowering the gastrointestinal pH, thereby activating pepsinogen to pepsin and triggering the release of cholecystokinin and gastrin, which subsequently stimulate pancreatic enzyme production [
49]. The differential enzyme responses between test groups suggest dose-dependent effects on pancreatic function, with optimal acidifier concentrations maximizing proteolytic and lipolytic activities [
50]. These enzymatic improvements facilitate nutrient digestion and absorption, potentially explaining the enhanced growth performance commonly observed with acidifier supplementation [
51], thereby supporting acidifiers as effective alternatives to antibiotic growth promoters in BIAN chicken production. The composite acidifying agent demonstrated dose-dependent effects on intestinal morphology in 300-day-old BIAN chickens, with test group B showing significant improvements in duodenal architecture. The marked reduction in the crypt depth (26.02% decrease) and substantial increase in the villus-to-crypt ratio (44.53% elevation) in test group B align with studies demonstrating that organic acids enhance intestinal structure through pH modulation and antimicrobial activity [
52]. The improved V:C ratio indicates enhanced absorptive efficiency and reduced epithelial cell turnover, consistent with findings that acidifiers promote intestinal maturation and nutrient absorption capacity [
53]. The absence of morphological changes in test group A suggests that an optimal acidifier concentration is crucial for structural modifications, corroborating the dose-dependent effects reported in previous studies [
54]. The decreased crypt depth likely reflects reduced pathogenic bacterial colonization and diminished inflammatory responses, as organic acids create an unfavorable environment for harmful microorganisms [
55]. These morphological improvements are indicative of enhanced intestinal health and function, potentially explaining the superior performance outcomes commonly associated with acidifier supplementation [
56].
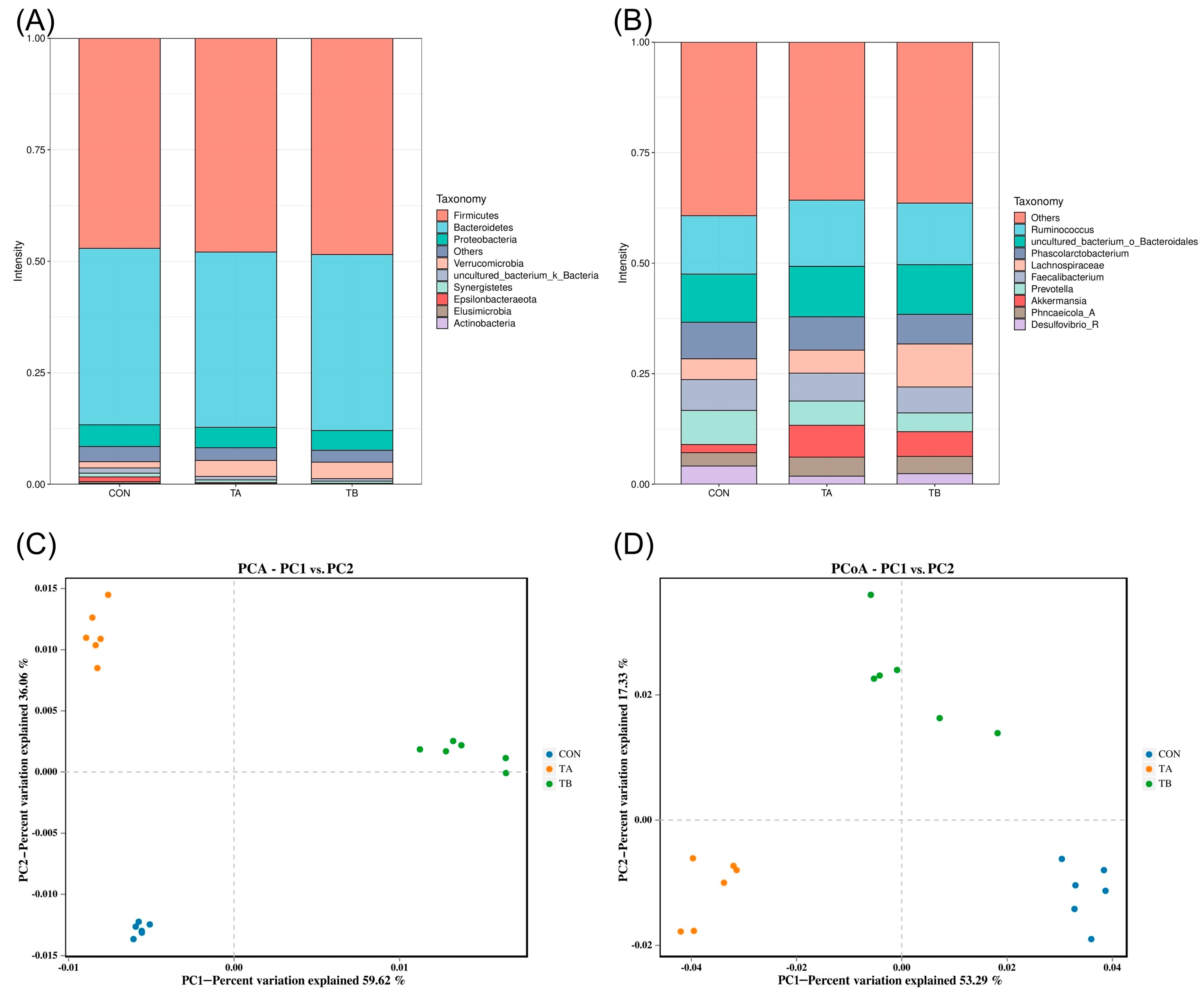
The composite acidifying agent induced significant modulation of cecal microbiota composition through multiple interconnected mechanisms. The acidifiers directly lower the intestinal pH, which inhibits acid-sensitive pathogens through disruption of their cytoplasmic pH homeostasis as organic acids penetrate bacterial membranes in an undissociated form and dissociate intracellularly, causing metabolic dysfunction. This pH reduction selectively favors acid-tolerant beneficial bacteria like Lactobacillus and promotes
Akkermansia growth, which thrives in mildly acidic mucin-rich environments. The increased
Akkermansia abundance (from 1.8% to 7.2%) likely enhanced mucin turnover, producing acetate and propionate that serve as cross-feeding substrates for the enhanced Lachnospiraceae population (9.7% vs. 4.7% in control). The butyrate produced by Lachnospiraceae upregulates intestinal tight junction proteins and stimulates antimicrobial peptide secretion from Paneth cells, explaining the improved V:C ratio (44.53% increase), contributing to the observed 61.76% increase in antioxidant capacity [
57]. This pattern contrasts with previous findings where higher-performing birds exhibited greater alpha diversity, suggesting that acidifiers specifically target certain bacterial populations rather than broadly increasing diversity [
58]. The marked increase in
Akkermansia abundance from 1.8% in the control to 7.2% in test group A and 5.6% in test group B was particularly noteworthy, as this genus has been associated with improved intestinal barrier function and metabolic health through mucin degradation and cross-feeding interactions with butyrate-producing bacteria [
59]. The increased
Akkermansia abundance observed in our study (from 1.8% in the control to 7.2% in test group A and 5.6% in test group B) may contribute to the improved egg production performance and intestinal health observed in BIAN chickens.
Akkermansia species are known for their mucin-degrading capabilities and have been associated with enhanced intestinal barrier function and metabolic health through cross-feeding interactions with butyrate-producing bacteria [
60]. The substantial enhancement in Lachnospiraceae abundance, particularly in test group B (9.7% vs. 4.7% in control), indicated improved fiber fermentation capacity and short-chain fatty acid production, as these bacteria are primary degraders of complex polysaccharides and major butyrate producers [
61]. The synergistic interactions between Akkermansia and Lachnospiraceae create a beneficial metabolic network that enhances the observed improvements in egg weight and antioxidant capacity through improved mineral absorption and reduced intestinal inflammation. The clear separation between treatment groups was confirmed by both ordination methods: principal component analysis (PCA) showed that the first principal component (PC1) explained 59.62% of the variation, while principal coordinate analysis (PCoA) revealed that the first principal coordinate (PCo1) accounted for 53.29% of the variation. The consistency of group separation across these different analytical approaches strengthens the conclusion that acidifier supplementation created distinct microbial ecological niches [
62]. These microbiota shifts aligned with the physiological improvements observed, as the enhanced populations of beneficial bacteria likely contributed to improved nutrient digestibility, immune function, and intestinal health through multiple mechanisms including competitive exclusion of pathogens, production of antimicrobial compounds, and modulation of host immune responses [
63]. The maintenance of Firmicutes and Bacteroidetes dominance while selectively enhancing beneficial genera demonstrated that acidifiers preserved core microbiota stability while optimizing functional capacity [
64]. The differential responses between test groups suggested that an optimal acidifier concentration was crucial for achieving the desired microbiota modulation, with test group B showing more pronounced beneficial changes in Lachnospiraceae abundance [
65]. These microbiota alterations likely contributed to the observed improvements in digestive enzyme activities and intestinal morphology through enhanced production of microbial metabolites, particularly short-chain fatty acids, which serve as energy sources for intestinal epithelial cells and regulate gut homeostasis [
66]. The comprehensive microbiome remodeling induced by composite acidifiers supports their role as effective alternatives to antibiotics in maintaining gut health and optimizing production performance in BIAN chickens.
The study shows that composite acidifiers significantly improve egg production performance and health in BIAN chickens, likely due to the metabolic effects of their primary components, such as organic acids, and their impact on gut health and immune function. HMTBa serves as a methionine source, supporting protein synthesis and related sulfur amino acid pathways in poultry. Lactic acid contributes to lumen acidification and antimicrobial control, which aligns with documented benefits of lactic acid systems in poultry. Phosphoric acid provides bioavailable phosphate in addition to its acidifying action, thereby aiding phosphorus availability for metabolism and eggshell formation. These metabolic processes underline the significant effects of compound acidifiers in enhancing poultry production and health, and further research into the detailed metabolic pathways involved will be valuable.