Abstract
Background and Aims: Decreased cerebrovascular reactivity (CVR) in patients with significant internal carotid artery stenosis (ICAS ≥ 70%) is an independent risk factor for cerebral infarction. To evaluate CVR, changes in cerebral perfusion pressure and blood flow velocity (BFV) of the middle cerebral artery (MCA) can be estimated by CO2- (hyperventilation—HV and breath-holding—BH) and pressure–flow-based (Common Carotid Artery Compression—CCC and Valsalva Maneuver—VM) stimuli. We used a multimodal approach to characterize CVR in patients before carotid endarterectomy (CEA). Methods: HV, BH, CCC, and VM tests were performed on 31, 26, and 34 patients. BFV of MCAs was monitored by transcranial Doppler, and continuous arterial blood pressure was registered non-invasively. CVR was compared between the operated significantly stenotic and the contralateral sides. Results: The extent of HV- and BH-induced CVR was similar, but the time to the lowest HV-induced BFV was shorter on the side with significant ICAS. The response to CCC was sensitive to hemodynamic asymmetry in the transient hyperemic response ratio and in the cumulative change in the (mean arterial blood pressure)/(mean BFV) ratio. In VM, the slope of BFV increased in the ascending (2b) phase, and the time to overshoot correlated with the side of the stenosis. Conclusions: These results suggest that in patients with significant ICAS, in addition to CO2 reactivity measurements, a more complex estimation of CVR, by using hemodynamic tests (CCC and VM), should also be used to better quantify cerebral ischemic risk.
1. Introduction
To maintain a proper and adequate cerebral blood supply, cerebral vasoreactivity (CVR) is essential for the brain. This means rapid and significant reactions of the cerebral arterial vessels, also known as resistance vessels, which protect the brain tissue from hyper- and hypoperfusion by altering their diameter [1,2]. CVR is controlled by several different mechanisms, often acting simultaneously [3,4]. These vascular responses are elicited by changes in perfusion pressure and flow, blood carbon dioxide partial pressure (pCO2), and increases in local neural activity by neurovascular coupling [1]. Changes in the diameter of the resistance vessels results in changes in blood flow to the brain tissue, which is associated with a proportional change in the blood flow velocity in the large cerebral arteries. Transcranial Doppler ultrasonography (TCD) is an easy-to-use, inexpensive, and non-invasive technique, which is an ideal tool for estimating cerebrovascular reactivity as a function of time, by measuring changes in blood flow velocity in large cerebral arteries to different stimuli [5,6,7,8,9,10,11,12].
Atherosclerosis frequently develops in elderly population, affecting the blood circulation of nearly all organs, and thus the blood supply of the brain as well. Indeed, significant atherosclerotic stenosis in the internal carotid artery (ICAS, ≥70%) is responsible for approximately 10–20% of cerebral ischemia/infarctions [13]. It is known that severe ICAS can lead to impaired CVR [14], which is an independent risk factor for cerebral infarction as described in the 2023 European Society for Vascular Surgery (ESVS) Guidelines [15]. In patients with advanced carotid artery atherosclerosis, reduced cerebral perfusion (pressure and flow) can develop due to exhaustion of the capacity of cerebrovascular reactivity [16,17,18,19].
Thus, the aim of the present study was to characterize cerebrovascular reactivity in a complex manner as a function of the severity of ICAS in patients before undergoing carotid reconstruction surgery, which helps to a better and more comprehensive preoperative estimation of cerebral ischemic risk [20].
The impairment of CVR because of severe ICAS has already been examined in several previous studies using different approaches [14,21,22,23,24,25,26,27]; therefore, we decided to examine a patient population with a complex TCD protocol.
1.1. Reactivity of Cerebrovascular System to Changes in Blood CO2 Concentration
Reactivity of cerebral vessels to CO2 is the phenomenon when arterioles respond to changes in arterial partial pressure of carbon dioxide (pCO2) between 20 and 60 mmHg [28]. An increase results in the dilation of cerebral resistance vessels with consequent increase in cerebral blood flow, whereas a decrease in pCO2 induces vasoconstriction and thus decrease in cerebral perfusion [28,29,30,31,32,33]. In humans, changes in arterial pCO2 can be elicited by moderate hyperventilation (HV) and breath-holding tests (BH), which are self-controlled and therefore safe [7,34]. During hyperventilation, the pCO2 concentration of arterial blood decreases, which causes vasoconstriction of cerebral arterioles [35]. Vice versa, during breath-holding, the arterial pCO2 increases, which leads to the dilation of the cerebral resistance vessels. The subsequent changes in the mean blood flow velocity (MBFV) in the middle cerebral artery (MCA) can be measured by using TCD [7,8,34,36,37,38].
1.2. Pressure–Flow Reactivity
The pressure–flow reactivity of cerebral resistance vessels, which also characterizes cerebral vasoreactivity, can be measured in several ways.
1.2.1. Common Carotid Artery Compression (CCC) Test
One of them is the common carotid artery compression (CCC) test, a vasoactive stimulus that reflects the autoregulatory integrity of cerebral vessels [9,10,11,39,40]. During the CCC test, a manual compression on the common carotid artery is performed for several seconds, which results in a temporary decrease in perfusion in the ipsilateral MCA. Because of the compression, the decreased MCA perfusion results in the dilation of small cerebral vessels of that MCA territory to maintain an adequate blood supply to the brain tissue. After carotid compression, BFV transiently increases, indicating reactive hyperemia (THR) and is likely the result of a decrease in blood flow resistance induced by cerebral hypoperfusion during carotid compression. The CCC test allows for calculating the transient hyperemic response ratio (THRR), which indicates the “normalized” changes in blood flow velocity to changes in the cerebral perfusion pressure [11]. The CCC test is also an accepted test for modeling the effect of carotid clamping during surgical interventions; thus, this test is relevant to quantify CVR in these patient populations [24,41].
1.2.2. Valsalva Maneuver (VM)
Valsalva Maneuver (VM) is another standardized test providing physiological stimuli evoking considerable changes in the central arterial blood pressure during forced exhalation against a resistance (due to closed glottis or a valve). The analysis of cerebral blood flow velocity response to mean arterial blood pressure (MABP) changes is suitable for estimating cerebrovascular reactivity, whereas changes in blood pressure and heart rate indicate the regulation of cardiovascular functions by the autonomic nervous system [42,43,44,45,46,47,48,49]. During VM, characteristic changes occur in cerebral MBFV, MABP, and HR, which can be divided into four phases as a function of time [42].
Phase I: At the onset of the VM, the increase in intrathoracic pressure is transmitted to the vascular system, thereby increasing central venous pressure and MABP.
Phase IIa (early): The maintained increased intrathoracic pressure reduces venous return to the heart, resulting in a decrease in the right and then in the left ventricular stroke volume, and thus MABP progressively decreases.
Phase IIb (late): A decrease in MABP activates the sympathetic nervous system, which regulates the cardiovascular system, which causes an increase in peripheral vascular resistance and heart rate, leading to an increase in MABP.
Phase III: When forced exhalation against resistance is stopped, there is a sudden drop in the intrathoracic pressure, resulting in a rapid and temporary decrease in MABP.
Phase IV: A fast and significant rise in the MABP and HR in several seconds is observed due to the previously elevated peripheral vascular resistance and the increased venous return (overshoot; OS). Following the OS, reflex bradycardia occurs due to the activation of the baroreflex, and finally, MABP and HR return to baseline.
2. Materials and Methods
This cross-sectional, single-center, exploratory study was approved by the Semmelweis University Regional and Institutional Committee of Science and Research Ethics (SE RKEB, Number: 256/2018) and registered at ClinicalTrials.gov (Identifier: NCT03840265). The study was conducted according to the guidelines of the Declaration of Helsinki. Patients admitted to the Department of Vascular Surgery of Semmelweis University for reconstruction of ICAS were enrolled consecutively between 15 January 2019 and 30 September 2021 after providing written consent. The severity of ICAS was determined by Computer Tomography Angiography according to the North American Symptomatic Carotid Endarterectomy Trial (NASCET) criteria, with a stenosis of 70% or greater considered hemodynamically significant (i.e., flow-limiting stenosis) [50,51].
In the present study, patients were enrolled with significant (≥70%) and tend-to-be-operated ICAS—ICAop, regardless of the condition of the contralateral side—not-to-be-operated—ICAnonop, which was <70% or ≥70%. Exclusion criteria were suboptimal transtemporal insonation window, atrial fibrillation, pacemaker therapy, and extensive atherosclerotic plaques in the CCA. The examinations were performed at the Doppler Laboratory of the Department of Neurology at Semmelweis University. Patients were continuously monitored clinically during the investigations. Demographic and medical history data were collected. To achieve reproducibility and best signal-to-noise ratio, the CCC and VM were performed three times, and the largest response was evaluated for each patient. The study group consisted overall of 48 eligible patients (male: 36 mean age ± SD: 68.08 ± 7.23 years; female: 12 mean age ± SD: 70.16 ± 7.35 years). The risk comorbidities of the patients were as follows: 44 patients had hypertension (91.7%), 21 of them had diabetes mellitus (43.8%), 16 were smokers (33.3%), and 14 patients had ischemic heart disease (29.2%). The patients were asymptomatic regarding the ICAS and received antiplatelet therapy in addition to antihypertensives and oral statin medication. The series of vasoactive tests used took an average of one hour. All stimuli were safe, none of the patients tested reported any discomfort, and no neurological complications were experienced. The patients’ cooperation was excellent in all tests, and the VM was performed well after 1–2 attempts.
2.1. Transcranial Doppler (TCD) Study Protocol
As previously described [25,26], the blood flow velocity (BFV, cm/s) in both MCAs was recorded by TCD probes (2 MHz, DWL Multi-Dop T2, Sipplingen, Germany) in a semi-sitting position through the transtemporal window at a depth of 45–55 mm. During TCD measurements, continuous, non-invasive, beat-to-beat arterial blood pressure (ABP) monitoring was performed by radial artery applanation tonometry (Colin-BP508, Hayashi Komaki Aichi, Japan). Heart rate was continuously monitored with electrocardiogram (ECG).
During the examination of patients, 3 vasoactive stimuli were applied in the following order:
- HV/BH test
- CCC test
- VM
2.1.1. HV/BH Test
We used a combined stimulus to assess the CO2 reactivity of cerebral vessels, with the aim of evaluating the maximal CO2-dependent cerebral vascular response; therefore, we chose voluntary hyperventilation and breath-holding as a simple, safe, and well-tolerated maneuver [7,34,37]. In basal conditions (baseline, BL), parameters measured were recorded after 15 min adaptation period. After BL, patients performed the HV test for 30 s in a semi-sitting position and were asked to inhale deeply and exhale dynamically. At the beginning of the HV test, MCA MBFV values started to decrease until they reached their minimum value. Then, 30 s later, patients were asked to inhale and hold their breath as long as they could. During the BH test, MBFV values started to increase and reached their maximum. Patients raised their hands when they finished the BH test. Each patient had a different BH test time (average 42.3 s, SD: ± 21.67). A total of 31 patients met the best signal-to-noise ratio requirements (74.2% male, age: 68 ± 5.11 years, and 25.8% female, age: 71.5 ± 4.89 years).
2.1.2. CCC Test
Before defining the study protocol, we carefully reviewed previous publications regarding CCC tests and noticed that no clinical complications have been reported [10,11,52,53]. Since previous publications confirmed that 10 s were required to achieve the maximal CCC BFV response, we used 10 s of manual compression of the CCA [10]. During the maneuver, bilateral MCA blood flow velocity values were measured with TCD. The CCC test was performed on both sides, one by one, after the CCA status was previously checked with duplex Doppler ultrasound. In case of extensive CCA atherosclerosis, the carotid compression test was omitted due to the risk of embolism. Compression was performed 3 times on both CCAs, with a 2 min interval between each maneuver. For the statistical analysis, the technically best and largest amplitude reactions were selected. A total of 26 patients met the best signal-to-noise ratio criteria (76.9% male, age: 68.67 ± 8,6 years, and 23.07% female, age: 71.17 ± 0.22 years).
2.1.3. VM
The duration of the VM was standardized to 15 s. VM was performed with forced exhalation against a sphygmomanometer by maintaining their expiratory pressure at 40 mmHg, which was controlled by the patient and the assistant as well. Recordings of those patients were used, which had an adequate signal-to-noise ratio and were thus suitable for complex signal analysis. The 34 patients’ VM was evaluated (76.5% male, age: 66.96 ± 7.34 years and 23.5% female, age: 70.62 ± 5.59 years).
2.2. Data Processing
Digitization of analog signals was performed in parallel on four channels (TCD1, TCD2, ABP tonometry, ECG), with a sampling rate frequency of 500 Hz. Raw data were stored in the European Data Format files. The files were imported, digitally filtered, and segmented using the LabChart software (ADInstruments, LabChart ver. 8, Colorado Springs, CO, USA). The pre-processed data segments describing each cardiac cycle were stored in separate text files. These files were further processed by custom-written Python code (Python version 3.12.2). The program script interpolated cardiac cycle data linearly into 0.5 s equidistant intervals. The interpolated data were exported to Microsoft Excel, and the changes in systolic, mean, and diastolic BFV, ABP, HR, and various vascular reactivity parameters were calculated.
2.3. Variables
2.3.1. HV and BH Tests
Variables of the two stimuli are summarized in Table 1. Figure 1. shows the flow chart of the HV and BH tests.

Table 1.
Summary of the variables of the hyperventilation and breath-holding tests.
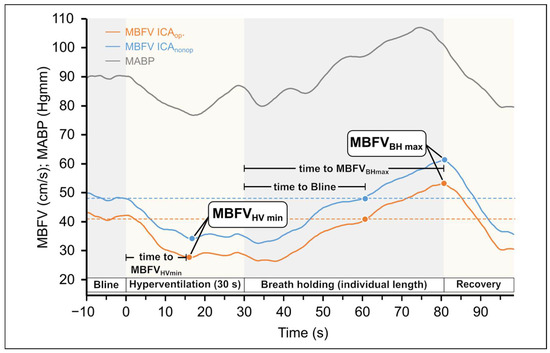
Figure 1.
Original records of changes in the mean blood flow velocities (MBFV) measured in the middle cerebral artery (MCA) in patients with unilateral carotid stenosis during hyperventilation and breath-holding tests. The MBFV decreased during hyperventilation, whereas it increased during breath holding test, indicating that changes in blood CO2 level elicited the expected changes in cerebrovascular resistance. The blue line indicates the side of ICAnonop, whereas the yellow line indicates the side ICAop. The minimum and maximum values of MBFV are indicated together with time characteristics. It can be seen that during the whole response, the MBFV was substantially lower (with about 10 mm/s) in the severe stenotic side compared to the other side and followed the changes in blood pressure. The changes in mean blood pressure are indicated with a gray line.
2.3.2. Cerebral Arterial Resistance (CAR) Values During HV/BH Test
Cerebral arterial resistance was defined by the ratio of systemic mean arterial blood pressure (MABP) and mean cerebral blood flow velocity in the middle cerebral artery (MBFV) according to the formula [25,54,55] (see Figure 2):
CAR = MABP/MBFV.
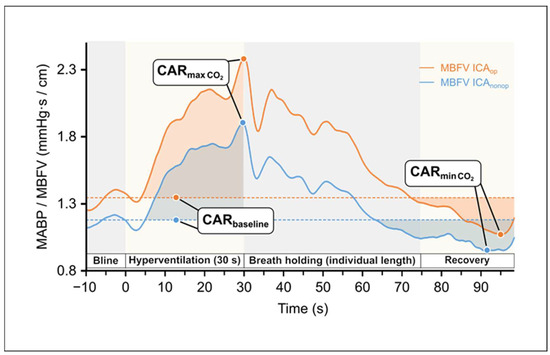
Figure 2.
Changes in the calculated cerebral arterial resistance (CAR: MAPB/MBFV) of both hemispheres during hyperventilation test as a function of time indicate cerebral vasoconstriction and during breath-holding test indicate cerebral vasodilation, respectively. The areas with darker colors represent the area under the curve (Data used from Figure 1).
The variables regarding CAR in HV/BH tests are summarized in Table 2.

Table 2.
Summary of the Cerebral Arterial Resistance values of the Hyperventilation and Breath-Holding test.
2.3.3. Cerebral Arterial Resistance Area Under the Curve (CAR-AUC) Calculations During HV/BH
The baseline CAR for HV/BH tests was determined by averaging the CAR values over the 10 s preceding the stimulus. Actual CAR values were subtracted from baseline (CARbl—CARactual), and the cumulative change in CAR until return to baseline CAR was determined by calculating the area under the curve (AUC) using the trapezoidal method (see Figure 2).
2.3.4. CCC Test
The defined variables of the CCC test are summarized in Table 3.

Table 3.
Summary of the variables defining common carotid artery compression test.
CARCCCAUC: The baseline CAR for CCC tests was determined by averaging the CAR values over the 10 s preceding the stimulus. CAR could not be calculated during carotid compression because the perfusion pressure required to maintain MCA MBFV could not be measured. After carotid compression was released, actual CAR values were subtracted from the baseline (CARBL—CARactual), and the cumulative change in CAR until return to baseline CAR was determined by calculating the area under the curve (AUC) using the so-called trapezoidal method mentioned before. The CAR AUC of the ICAop and ICAnonop sides was compared statistically (see Figure 3).
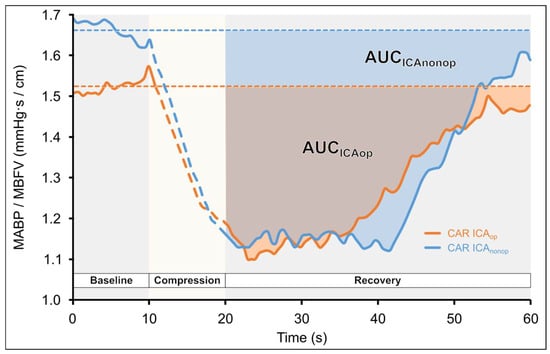
Figure 3.
Changes in the calculated cerebral arterial resistance (CAR: MAPB/MBFV) during common carotid artery compression (CCC) test in a patient with unilateral carotid stenosis as a function of time. Carotid compression resulted in cerebral hypoperfusion, which elicited dilation in the arterial vessels supplied by the middle cerebral artery (MCA); thus, CAR decreases. After the compression is released, the CAR gradually returns to baseline. Area under the curve (AUC) was calculated by the trapezoid method until the baseline-subtracted CAR values returned to zero. The areas with darker colors represent the area under the curve. It can be seen that AUC of ICAop is smaller than the contralateral one.
2.3.5. VM
The variables of the VM are summarized in Table 4. Figure 4. shows the changes in the MBFV and MABP elicited by VM (see the detailed description in Figure 4. legend).

Table 4.
Summary of the variables defining the Valsalva Maneuver.
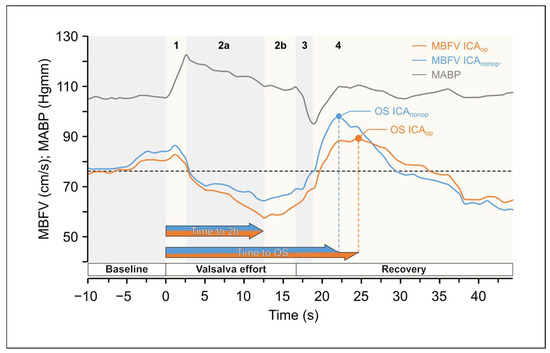
Figure 4.
Original records of changes in the mean blood flow velocities (MBFV) measured in the middle cerebral artery (MCA) in patients with unilateral carotid stenosis during Valsalva Maneuver (VM) as a function of time. There were considerable changes in the mean arte-rial blood pressure (MABP). yet Yet changes in MBFV in the middle cerebral arteries (MCAs) were different in the two sides. Changes were divided into four phases: there was side difference in MBFVdifference of MBFV values during phase 2a and 2b and a time shift in maximalshift of maximal values between MBFV overshoot (OS) in phase 4. The changes in mean blood pressure are indicated with graygrey line.
2.4. Statistical Analysis
Statistical analysis was performed with TIBCO Statistica® 13.5.0 program and the R programming language version 4.4.1. Data were visualized using Inkscape 1.4. Software and the R programming language version 4.4.1. The significance level was defined as p < 0.05 for all tests.
To examine the impact of ICAS on CVR, the variables of the four stimuli of the side to be operated on (ICAop) and not to be operated on (ICAnonop) were compared. Additionally, grouped comparisons were also performed by dividing the side not to be operated into two groups:
Subgroup 1: Where the stenosis of the ICA was estimated to be equal to or above 70%.
Subgroup 2: A group where the stenosis was less than 70%.
The Wilcoxon rank test was used as the main tool due to the low sample size and non-normal distribution of variables. Since almost all time-to-event variables were free from censoring, they were also compared using the above tests; however, they were evaluated using clustered Cox proportional hazards models, too.
3. Results
3.1. HV and BH Tests
Original records of variables and the hyperventilation protocol are shown in Figure 1. Figure 1. illustrates the changes in the MBVF and the MABP during the HV and BH tests. During HV in both MCA, a decrease in MBFV can be observed due to the vasoconstriction of the cerebral vessels triggered by the consequently reduced arterial CO2 levels. In contrast, the MCA MBFV values increase during the breath holding because of vasodilation.
Figure 2 presents the CAR changes elicited by the mentioned vasoconstriction and vasodilation; CAR values increase during HV and decrease during BH. Summary data of this test are shown in Table 5.

Table 5.
Summary of differences in the measured parameters and calculated indices of the hyperventilation and breath-holding test of 31 patients.
The data show that the time to MBFVHVmin was significantly shorter on the ICAop side (Wilcoxon ranked test p = 0.012) compared to the contralateral side. According to subgroup analysis, the difference was only justified if the stenosis on the ICAnonop side did not reach 70% (Wilcoxon ranked test p = 0.0088).
In response to the BH test, original record shows no differences in the response of the two MCA sides. Data in Table 5 indicate that no significant differences were found between the defined indices, except in the subgroup 2 analysis regarding ΔMBFVBH (p = 0.042).
3.2. CCC Test
During CCC, a reduction in MBFV is observed in the ipsilateral MCA due to the compression, which is followed by a consequent transient hyperemic response after the release of the compression. Figure 3. presents the CAR-AUC calculations during the CCC test.
The statistical data obtained in this test are included in Table 6. The BL values of the PSV were significantly different between the two sides; thus, relative values were quantified. The value of THRRPSV was significantly higher on the ICAnonop side (Wilcoxon ranked test p = 0.0046 and p = 0.0033 according to the subgroup 2 analysis). It has also been verified previously that the PSV values might be more sensitive when quantifying the CCC test [52].

Table 6.
Summary of differences in the measured parameters and calculated indices in case of 26 patients of the common carotid artery compression test.
The calculated arterial resistance, CARCCCAUC, was significantly lower on the ICAop side (Wilcoxon ranked test p = 0.021 and p = 0.039 according to the subgroup 2 analysis) compared to the ICAnonop side.
Values are mean ± SD using Wilcoxon ranked test (p < 0.05). Significant differences are indicated in bold letter.
3.3. VM Test
The statistical essences of the VM are the following:
- (1)
- CVAR had a significantly lower value on the ICAop side. (Wilcoxon ranked test p = 0.002 and p = 0.014 according to the subgroup 2 analysis).
- (2)
- OS time was significantly longer on the ICAop side (both according to Wilcoxon ranked test with p = 0.015; p = 0.038 according to the subgroup-2 analysis) and the Wald test of the Cox proportional hazards model with p < 0.0001.
- (3)
- Evaluating the autonomic values, we found the following:
- 15 patients had a VHRR value less than 1.35;
- 8 patients had a longer PRT than 4 sec;
- 23 patients had an SI underneath 0.
The statistical data obtained in this test are included in Table 7.

Table 7.
Summary of differences in the measured parameters and calculated indices of the Valsalva Maneuver (VM) in case of 34 patients.
4. Discussion
The novel aspect of our study was the use of a comprehensive protocol to assess cerebrovascular regulatory impairments in patients with significant internal carotid artery stenosis. This protocol combined evaluations of changes in blood pCO2, hemodynamic forces, and autonomic nervous system, using transcranial Doppler method to measure middle cerebral artery blood flow velocity responses to several physiological tests.
The present study provided several important findings in ICA stenotic patients:
- CO2 changes induced by hyperventilation and breath-holding elicited similar responses in MBFV on both carotid/MCA sides (i.e., ICAop and ICAnonop). The only significant differences were found between the two sides in the time to reach the minimum MBFV of the ipsilateral MCA induced by hyperventilation and ΔMBFVBH in subgroup 2.
- CCC elicited different reactive hyperemic blood flow velocity responses in the two carotid/MCA vascular regions. Two indices of response to CCC were different: a) the THRR index calculated from MCA peak systolic blood flow velocity values and b) the cumulative change in cerebral arterial resistance (CAR-AUC) after cessation of common carotid artery compression. The two mentioned variables were significantly lower on the planned operated side (ICAop) compared to the other side (ICAnonop).
- Valsalva Maneuver induced different flow changes in the two carotid/MCA sides. Calculated resistance index, CVAR, and the time to OS were significantly reduced on the side to be operated on (ICAop) compared to the other side (ICAnonop).
- Changes in systemic arterial blood pressure and heart rate in response to VM indicated that most patients also had severe cardiovascular autonomic dysfunction.
4.1. Clinical Importance of Our Findings
4.1.1. CO2 Reactivity (Hyperventilation/Breath-Holding)
In the cerebral vasoreactivity studies included in a meta-analysis, CVR was determined based on changes in cerebral blood flow velocity induced by increasing blood CO2 concentration or intravenous administration of acetazolamide, evaluating the maximal cerebral vasodilation capacity [27].
In the protocol of the present study, using the hyperventilation (HV) and breath-holding (BH) tests, the total CO2 reactivity was calculated in addition to the previously recommended indices. This represents our novel approach for evaluating the overall CO2 reactivity of cerebral resistance vessels.
In the patient population studied, no difference was found in the magnitude of CO2-decrease–induced reactivity between the ICAop and ICAnonop sides, but the minimum of the MBFV occurred earlier on the ICAop side. This difference can indicate reduced cerebral vasoconstriction capacity on the ICAop side.
It is of note that the BH index calculated from the MBFV data of patients showed a higher value than that of healthy individuals of the same age reported in a previous publication [8]. This is probably due to the fact that during the BH test, the arterial blood pressure of patients involved in our study significantly increased, and in parallel, their cerebral blood flow velocity increased partially, as a function of systemic blood pressure, indicating an impairment of the CVR. Thus, we recommend that the simultaneous change in arterial blood pressure should be considered when establishing the BH index and that the CO2 vasodilation reactivity be characterized by the resistance index calculated in this way (Figure 2).
4.1.2. Pressure–Flow Cerebral Vasoreactivity
The response of cerebral arterial vessels to changes in cerebral perfusion pressure/flow was assessed by the CCC test and the Valsalva Maneuver.
CCC Test
The CCC test estimates the effectiveness of cerebral pressure–flow regulation by temporarily reducing the ipsilateral MCA perfusion [9]. If, during CCC, the 10 s hemispheric cerebral low perfusion reaches a critical level, focal deficit symptoms may appear [52], but we did not observe this in the present study, confirming the safety of the CCC test.
In response to CCC, the calculated THRRPSV index was significantly lower on the ICAop side. During compression, ipsilateral MCA perfusion was reduced which was indicated by the decreased MBFV. This elicited (passive and active) vasomotor response of cerebral resistance vessels, indicated by the cerebral arterial resistance change. After the compression was released, a transient increase in MCA MBFV was observed, and then CAR was lower than the baseline value preceding CCC, which may be due to the dilation of cerebral resistance vessels of MCA territory. The cumulative change in the cerebral arterial resistance (CARCCCAUC) was significantly smaller on the side of the ICAop side, suggesting that the dilation capacity of resistance vessels was reduced. Thus, the index which was introduced in the present study seems to be sensitive to hemodynamic disturbances resulting from severe carotid stenosis (Figure 3).
VM Test
To assess the VM-test–induced cerebrovascular reactivity, several indices have been published previously [17,46,49,56]. In the present study, CVAR (rate of increase in MCA MBFV in phase 2b) was significantly lower on the ICAop side. In addition, there was a difference in the OS time, because OS occurred later the ICAop than on the contralateral side; thus, this new temporal variable may also be a suitable index for characterizing the dynamics of cerebrovascular reactivity (Figure 4).
The Valsalva Maneuver allows to assess not only cerebral pressure–dependent vasoreactivity but also the regulation of arterial blood pressure and heart rate by the autonomic nervous system. The values of specific cardiovascular autonomic variables of the Valsalva Maneuver suggest that the majority of patients studied also had cardiovascular autonomic dysfunction, which, in addition to impaired cerebral vasomotor regulation, may further increase the risk of cerebral ischemia, especially during potential hypotension during carotid reconstruction surgery/intervention [58]. In these cases, we thus consider VM as an important, overall comprehensive hemodynamic and cardiovascular autonomic test. In these cases, we recommend expanding intraoperative arterial blood pressure and heart rate monitoring with simultaneous and continuous middle cerebral artery TCD recording.
4.2. Why Is It Necessary to Use Several Tests and a More Complex TCD Protocol?
In clinical practice, the most widely used and proven methods for determining cerebrovascular reactivity are the CO2 reactivity tests and the breath-holding index. However, when this index is calculated, changes in arterial blood pressure are not taken into consideration, since, based on the prevailing assumption, no significant hemodynamic changes occur during the test. However, the data of the present study showed that changes in arterial blood pressure are not negligible during these tests. Thus, changes in cerebral blood flow velocity must be “normalized” to changes in arterial blood pressure to obtain a correct evaluation of changes in CVR, which would be very important to develop a more proper preoperative ischemic risk assessment.
The patients involved in the present study had severe carotid artery stenosis, which can cause reduced cerebral vasoreactivity, and were awaiting reconstructive carotid vascular surgery. During the operation, the carotid arteries are clamped, eliciting reduction in brain perfusion (intraoperative cerebral hypoperfusion). That is why we added hemodynamic stimuli to the preoperative assessment of cerebrovascular reactivity tests.
The results of the measurements and detailed analysis of data confirmed that the CCC and VM tests, which induce significant changes in pressure/flow in the cerebral arteries, were different between the two sides, providing important information regarding the preoperative cerebral ischemic risk.
The VM and CCC tests are eliciting fast and considerable hemodynamic changes, i.e., the levels of blood pressure and flow to the brain. In contrast, during the HV/BH test, cerebral hemodynamic conditions remain relatively stable; thus, changes in cerebrovascular reactivity are predominantly due to changes in arterial CO2 concentration. As we have shown, almost all members of our patient population were hypertensive; in addition, they had other diseases, such as diabetes, and harmful habits like smoking, which may have also impaired cerebrovascular function. In the present study, according to the data of comparative statistical analysis, we conclude that CO2 sensitivity of cerebral vessels is maintained even in severe ICA stenosis; thus, an assessment based only on CO2 tests does not provide sufficient information about the hemodynamic effects of severe carotid stenosis.
In other words, the HV and BH tests do not provide sufficient information regarding the effects of carotid stenosis on the vasomotor capacity of cerebral circulation regulation in a significant proportion of patients. In the present study, however, the responses to hemodynamic changes elicited by CCC and VM tests were prolonged on the ICAop side compared to the ICAnonop side. Thus, we propose that inclusion of CCC and VM tests in the evaluation of patients is important to provide a more complex assessment of cerebrovascular reactivity in patients with ICA stenosis. Overall, a complex testing protocol that includes tests based on pressure–flow changes in addition to CO2 reactivity tests might be beneficial.
4.3. Study Limitations
One of the limitations of our study was the relatively low number of patients mainly due to the lack of adequate insonation window. In addition to proper insonation window requirements, we only used the bilateral TCD measurements for the statistical analysis, which also limited the number of patients examined. Moreover, due to our strict set of criteria, we found the quality of many measurements to be inappropriate, and despite the initial relatively high numbers of patients examined, the statistical measurements were performed on a relatively smaller number of measurements. Another disadvantage is that the duration of the BH test cannot be fully standardized, because not all patients were able to complete the task in accordance with the instructions. In the future, we will consider standardizing the duration of the BH test. We were unable to calculate the CAR AUC value of the HV test in one patient due to movement artifact, and similarly, in 11 cases, the CAR AUC value of the BH test could not be calculated because the CAR value did not exceed the BL level. Our method for assessing cerebral vascular CO2 reactivity lacked end-tidal CO2 measurement, which would have facilitated comparative analysis of the data despite different breath-holding times. This may explain why, in contrast to previous publications, we did not observe a significant decrease in CO2 reactivity on the side of carotid stenosis.
Furthermore, in an ideal situation, one could have checked the hemodynamic and cerebral vascular reactivity consequences of the ICAS by Single Photon Emission Computer Tomography; however, at the time of the present study, it was not available to us due to the restrictions during the COVID-19 pandemic.
4.4. Strength of the Study
Our study summarizes the tests previously considered suitable for assessing CVR and presents it on a relevant patient population. The combination of the vasoreactivity tests utilized allows complex hemodynamic characterization and even more personalized decision-making. This aspect could have implications for ICA-stenotic patients’ preoperative risk assessment in future studies.
4.5. Translational Aspects
The findings of the present study have high translational values as they were obtained in humans; thus, in clinical settings measuring, these parameters would allow personalized diagnosis regarding cerebrovascular status and systemic hemodynamics and provide evidence to further develop the guidelines.
5. Conclusions
A significant finding of the present study is that in patients with significant carotid artery stenosis the CO2 reactivity tests were not sufficiently sensitive to hemodynamic disturbances caused by significant carotid artery stenosis. Thus, other tests, such as hemodynamic tests (CCC and VM), which elicit changes in cerebral perfusion pressure and flow, should be used to assess cerebrovascular reactivity and quantify cerebral ischemic risk. Thus, it is important to assess cerebrovascular reactivity in a complex manner. However, we recommend further prospective studies to assess whether this complex preoperative risk assessment method can be recommended in clinical practice.
Author Contributions
Conceptualization, R.D. and P.S.; methodology, R.D. and T.H.; software, T.H.; validation, R.D.; formal analysis, R.D.; investigation, H.P., R.M.-S., B.C., A.G., and R.D.; resources, P.S. and A.K.; data curation, H.P. and B.D.; writing—original draft preparation, H.P., R.D., A.K., D.B., and R.M.-S.; writing—review and editing, R.D., H.P., A.K., and D.B.; visualization, T.H. and H.P.; supervision, R.D. and P.S.; project administration, R.D., Z.M., R.M.-S., and Z.C.; funding acquisition, P.S. All authors have read and agreed to the published version of the manuscript.
Funding
The study was supported by the National Office for Research, Development and Innovation (Project no. NKFI-K129277 (“Evaluation of cerebrovascular events in patients with occlusive carotid artery disorders based on morphological and hemodynamic features”) has been implemented with the support provided by the Ministry of Innovation and Technology of Hungary from the National Research, Development, and Innovation Fund; NKFI-1 K OTKA 132596 K¬19, TKP2021-EGA-37 of Ministry of Innovation and Technology of Hungary-NRDI TKP2021-EGA funding and HAS/MTA Post-COVID 2021-34; Ministry of Innovation and Technology of Hungary from the National Research, Development and Innovation Fund (Grant No: TKP2021-EGA/TKP2021-NVA/TKP2021-NKTA). The work was partly supported by grant No: TKP2021-EGA-25.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the Semmelweis University Regional and Institutional Committee of Science and Research Ethics (SE RKEB, Number: 256/2018), date of approval:14 January, 2019, and registered at ClinicalTrials.gov (Identifier: NCT03840265, URL: https://clinicaltrials.gov/study/NCT03840265?term=NCT03840265&rank=1 initial release:12 February 2019.).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| ABP | Arterial Blood Pressure |
| AUC | Area Under the Curve |
| BFV | Blood Flow Velocity |
| BH | Breath-Holding Test |
| BHI | Breath-Holding Index |
| BL | Baseline |
| CAR | Cerebral Arterial Resistance |
| CCA | Common Carotid Artery |
| CAR-AUC | Cerebral Arterial Resistance—Area Under the Curve |
| CCC | Common Carotid Artery Compression Test |
| CEA | Carotid Endarterectomy |
| CPVR | Centro-Peripheral Valsalva Ratio |
| CPOI | Centro-Peripheral Overshoot Index |
| CVAR | Cerebrovascular Valsalva Ratio |
| CVR | Cerebral Vasoreactivity |
| ECG | Electrocardiogram |
| ESVS | European Society for Vascular Surgery |
| HV | Hyperventilation |
| HVI | Hyperventilation Index |
| ICA | Internal Carotid Artery |
| ICAop | Side to be operated |
| ICAnonop | Side no to be operated |
| ICAS | Internal Carotid Artery Stenosis |
| MABP | Mean Arterial Blood Pressure |
| MBFV | Mean Blood Flow Velocity |
| MCA | Middle Cerebral Artery |
| NASCET | North American Symptomatic Carotid Endarterectomy Trial |
| PRT | Pressure Recovery Time |
| PSV | Peak Systolic Velocity |
| RTB | Return to Baseline |
| SI | Sympathetic Index |
| TCD | Transcranial Doppler |
| THR | Transient Hyperemic Response |
| THRR | Transient Hyperemic Response Ratio |
| VM | Valsalva Maneuver |
| VHRR | Valsalva Heart Rate Ratio |
References
- Claassen, J.A.H.R.; Thijssen, D.H.J.; Panerai, R.B.; Faraci, F.M. Regulation of cerebral blood flowin humans: Physiology and clinical implications of autoregulation. Physiol. Rev. 2021, 101, 1487–1559. [Google Scholar] [CrossRef] [PubMed]
- Toth, P.; Szarka, N.; Farkas, E.; Ezer, E.; Czeiter, E.; Amrein, K.; Ungvari, Z.; Hartings, J.A.; Buki, A.; Koller, A. Traumatic brain injury-induced autoregulatory dysfunction and spreading depression-related neurovascular uncoupling: Pathomechanisms, perspectives, and therapeutic implications. Am. J. Physiol. Heart Circ. Physiol. 2016, 311, H1118–H1131. [Google Scholar] [CrossRef]
- Nemeth, Z.; Eros, K.; Munkacsy, G.; Koller, A. Differential Gene and Protein Expressions Responsible for Vasomotor Signaling Provide Mechanistic Bases for the Opposite Flow-Induced Responses of Pre- and Post-Circle of Willis Arteries. Life 2025, 15, 856. [Google Scholar] [CrossRef]
- Toth, P.; Rozsa, B.; Springo, Z.; Doczi, T.; Koller, A. Isolated human and rat cerebral arteries constrict to increases in flow: Role of 20-HETE and TP receptors. J. Cereb. Blood Flow Metab. 2011, 31, 2096–2105. [Google Scholar] [CrossRef]
- Aaslid, R.; Markwalder, T.-M. Noninvasive transcranial Doppler ultrasound recording of flow velocity in basal cerebral arteries. J. Neurosurg. 1982, 57, 769–774. [Google Scholar] [CrossRef]
- Aaslid, R.; Lindegaard, K.-F.; Sorteberg, W.; Nornes, H. Cerebral Autoregulation Dynamics in Humans. Stroke 1989, 20, 45–52. [Google Scholar] [CrossRef]
- Markus, H.S.; Harrison, M.J.G. Estimation of Cerebrovascular Reactivity Using Transcranial Doppler, Including the Use of Breath-Holding as the Vasodilatory Stimulus. Stroke 1992, 23, 668–673. [Google Scholar] [CrossRef]
- Zavoreo, I.; Demarin, V. Breath holding index in the evaluation of cerebral vasoreactivity. Acta Clin. Croat. 2004, 43, 15–19. [Google Scholar]
- Giller, C.A. A bedside test for cerebral autoregulation using transcranial Doppler ultrasound. Acta Neurochir. 1991, 108, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Cavill, G.; Simpson, E.J.; Mahajan, R.P. Factors affecting assessment of cerebral autoregulation using the transient hyperaemic response test. Br. J. Anaesth. 1998, 81, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Smielewski, P.; Czosnyka, M.; Kirkpatrick, P.; McEroy, H.; Rutkowska, H.; Pickard, J.D. Assessment of cerebral autoregulation using carotid artery compression. Stroke 1996, 27, 2197–2203. [Google Scholar] [CrossRef]
- Tiecks, F.P.; Lam, A.M.; Matta, B.F.; Strebel, S.; Douville, C.; Newell, D.W. Effects of the Valsalva Maneuver on Cerebral Circulation in Healthy Adults: A Transcranial Doppler Study. Stroke 1995, 26, 1386–1392. [Google Scholar] [CrossRef]
- Flaherty, M.L.; Kissela, B.; Khoury, J.C.; Alwell, K.; Moomaw, C.J.; Woo, D.; Khatri, P.; Ferioli, S.; Adeoye, O.; Broderick, J.P.; et al. Carotid artery stenosis as a cause of stroke. Neuroepidemiology 2012, 40, 36–41. [Google Scholar] [CrossRef]
- Markus, H.; Cullinane, M. Severely impaired cerebrovascular reactivity predicts stroke and TIA risk in patients with carotid artery stenosis and occlusion. Brain 2001, 124, 457–467. [Google Scholar] [CrossRef]
- Naylor, R.; Rantner, B.; Ancetti, S.; de Borst, G.J.; De Carlo, M.; Halliday, A.; Kakkos, S.K.; Markus, H.S.; McCabe, D.J.H.; Sillesen, H.; et al. Editor’s Choice—European Society for Vascular Surgery (ESVS) 2023 Clinical Practice Guidelines on the Management of Atherosclerotic Carotid and Vertebral Artery Disease. Eur. J. Vasc. Endovasc. Surg. 2023, 65, 7–111. [Google Scholar] [CrossRef]
- Xiong, L.; Leung, H.W.; Chen, X.Y.; Han, J.H.; Leung, W.H.; Soo, O.Y.; Lau, Y.L.; Wong, K.S. Autonomic dysfunction in ischemic stroke with carotid stenosis. Acta Neurol. Scand. 2012, 126, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Vogel, E.R.; Sandroni, P.; Low, P.A. Blood pressure recovery from Valsalva maneuver in patients with autonomic failure. Neurology 2005, 65, 1533–1537. [Google Scholar] [CrossRef] [PubMed]
- Robinson, T.G.; Dawson, S.L.; Eames, P.J.; Panerai, R.B.; Potter, J.F. Cardiac baroreceptor sensitivity predicts long-term outcome after acute ischemic stroke. Stroke 2003, 34, 705–712. [Google Scholar] [CrossRef]
- King, A.; Serena, J.; Bornstein, N.M.; Markus, H.S. Does impaired cerebrovascular reactivity predict stroke risk in asymptomatic carotid stenosis?: A prospective substudy of the asymptomatic carotid emboli study. Stroke 2011, 42, 1550–1555. [Google Scholar] [CrossRef]
- Lengyel, B.; Magyar-Stang, R.; Pál, H.; Debreczeni, R.; Sándor, Á.D.; Székely, A.; Gyürki, D.; Csippa, B.; István, L.; Kovács, I.; et al. Non-Invasive Tools in Perioperative Stroke Risk Assessment for Asymptomatic Carotid Artery Stenosis with a Focus on the Circle of Willis. J. Clin. Med. 2024, 13, 2487. [Google Scholar] [CrossRef] [PubMed]
- Reinhard, M.; Schwarzer, G.; Briel, M.; Altamura, C.; Palazzo, P.; King, A.; Bornstein, N.M.; Petersen, N.; Motschall, E.; Hetzel, A.; et al. Cerebrovascular reactivity predicts stroke in high-grade carotid artery disease. Neurology 2014, 83, 1424–1431. [Google Scholar] [CrossRef]
- Reinhard, M.; Roth, M.; Müller, T.; Guschlbauer, B.; Timmer, J.; Czosnyka, M.; Hetzel, A. Effect of carotid endarterectomy or stenting on impairment of dynamic cerebral autoregulation. Stroke 2004, 35, 1381–1387. [Google Scholar] [CrossRef] [PubMed]
- Kleiser, B.; Widder, B. Course of Carotid Artery Occlusions with impaired cerebrovascular reactivity. Stroke 1992, 23, 171–174. [Google Scholar] [CrossRef]
- Visser, G.H.; Wieneke, G.H.; Van Huffelen, A.C.; Eikelboom, B.C. The use of preoperative transcranial Doppler variables to predict which patients do not need a shunt during carotid endarterectomy. Eur. J. Vasc. Endovasc. Surg. 2000, 19, 226–232. [Google Scholar] [CrossRef][Green Version]
- Magyar-Stang, R.; István, L.; Pál, H.; Csányi, B.; Gaál, A.; Mihály, Z.; Czinege, Z.; Sótonyi, P.; Tamás, H.; Koller, A.; et al. Impaired cerebrovascular reactivity correlates with reduced retinal vessel density in patients with carotid artery stenosis: Cross-sectional, single center study. PLoS ONE 2023, 18, e0291521. [Google Scholar] [CrossRef]
- Magyar-Stang, R.; Pál, H.; Csányi, B.; Gaál, A.; Mihály, Z.; Czinege, Z.; Csipo, T.; Ungvari, Z.; Sótonyi, P.; Varga, A.; et al. Assessment of cerebral autoregulatory function and inter-hemispheric blood flow in older adults with internal carotid artery stenosis using transcranial Doppler sonography-based measurement of transient hyperemic response after carotid artery compression. GeroScience 2023, 45, 3333–3357. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Chazen, J.L.; Hartman, M.; Delgado, D.; Anumula, N.; Shao, H.; Mazumdar, M.; Segal, A.Z.; Kamel, H.; Leifer, D.; et al. Cerebrovascular reserve and stroke risk in patients with carotid stenosis or occlusion: A systematic review and meta-analysis. Stroke 2012, 43, 2884–2891. [Google Scholar] [CrossRef] [PubMed]
- Reivich, M. Arterial Pco2 and Cerebral Hemodynamics. Am. J. Physiol. 1964, 206, 25–35. [Google Scholar] [CrossRef]
- Hoiland, R.L.; Fisher, J.A.; Ainslie, P.N. Regulation of the cerebral circulation by arterial carbon dioxide. Compr. Physiol. 2019, 9, 1101–1154. [Google Scholar] [CrossRef]
- Raichle, M.E.; Plum, F. Hyperventilation and cerebra blood flow. Stroke 1972, 3, 566–575. [Google Scholar] [CrossRef]
- Brian, J.E.J. Carbon dioxide and the cerebral circulation. Anesthesiology 1998, 88, 1365–1386. [Google Scholar] [CrossRef]
- Valdueza, J.M.; Balzer, J.O.; Villringer, A.; Vogl, T.J.; Kutter, R.; Einhäupl, K.M. Changes in Blood Flow Velocity and Diameter of the Middle Cerebral Artery during Hyperventilation: Assessment with MR and Transcranial Doppler Sonography. AJNR Am. J. Neuroradiol. 1997, 18, 1929–1934. [Google Scholar]
- Raichle, M.E.; Posner, J.B.; Plum, F. Cerebral Blood Flow During. Arch. Neurol. 1970, 23, 394–403. [Google Scholar] [CrossRef]
- Settakis, G.; Páll, D.; Molnár, C.; Katona, E.; Bereczki, D.; Fülesdi, B. Hyperventilation-induced cerebrovascular reactivity among hypertensive and healthy adolescents. Kidney Blood Press. Res. 2006, 29, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Bogossian, E.G.; Peluso, L.; Creteur, J.; Taccone, F.S. Hyperventilation in Adult TBI Patients: How to Approach It? Front. Neurol. 2021, 11, 580859. [Google Scholar] [CrossRef]
- Müller, M.; Voges, M.; Piepgras, U.; Schimrigk, K. Assessment of cerebral vasomotor reactivity by transcranial Doppler ultrasound and breath-holding. A comparison with acetazolamide as vasodilatory stimulus. Stroke 1995, 26, 96–100. [Google Scholar] [CrossRef]
- Prakash, K.; Chandran, D.S.; Khadgawat, R.; Jaryal, A.K.; Deepak, K.K. Correction for blood pressure improves correlation between cerebrovascular reactivity assessed by breath holding and 6% CO2 breathing. J. Stroke Cerebrovasc. Dis. 2014, 23, 630–635. [Google Scholar] [CrossRef]
- Jung, K.O.; Lee, S.J.; Lee, T.K. A Breath-Holding Index Applied to the Internal Carotid Artery Siphon in Transcranial Doppler Studies. J. Neuroimaging 2020, 30, 862–866. [Google Scholar] [CrossRef] [PubMed]
- Smielewski, P.; Czosnyka, M.; Iyer, V.; Piechnik, S.; Whitehouse, H.; Pickard, J. Computerised transient hyperaemic response test--a method for the assessment of cerebral autoregulation. Ultrasound Med. Biol. 1995, 21, 599–611. [Google Scholar] [CrossRef] [PubMed]
- Czosnyka, M.; Pickard, J.; Whitehouse, H.; Piechnik, S. The hyperaemic response to a transient reduction in cerebral perfusion pressure—A modelling study. Acta Neurochir. 1992, 115, 90–97. [Google Scholar] [CrossRef]
- Anzola, G.P.; Limoni, P.; Cavrini, G. Predictors of carotid clamping intolerance during endarterectomy that would be wise to apply to stenting procedures. Cerebrovasc. Dis. 2008, 26, 494–501. [Google Scholar] [CrossRef]
- Pstras, L.; Thomaseth, K.; Waniewski, J.; Balzani, I.; Bellavere, F. The Valsalva manoeuvre: Physiology and clinical examples. Acta Physiol. 2016, 217, 103–119. [Google Scholar] [CrossRef] [PubMed]
- Castro, P.M.; Santos, R.; Freitas, J.; Panerai, R.B.; Azevedo, E. Autonomic dysfunction affects dynamic cerebral autoregulation during Valsalva maneuver: Comparison between healthy and autonomic dysfunction subjects. J. Appl. Physiol. 2014, 117, 205–213. [Google Scholar] [CrossRef]
- Novak, P. Assessment of sympathetic index from the Valsalva maneuver. Neurology 2011, 76, 2010–2016. [Google Scholar] [CrossRef]
- Švigelj, V.; Šinkovec, M.; Avbelj, V.; Trobec, R.; Gaspar, L.; Petrovič, D.; Kruzliak, P. Cardiovagal and adrenergic function tests in unilateral carotid artery stenosis patients—A Valsalva manoeuvre tool to show an autonomic dysfunction? Wien. Klin. Wochenschr. 2016, 128, 504–512. [Google Scholar] [CrossRef]
- Wallasch, T.M.; Kropp, P. Cerebrovascular response to valsalva maneuver: Methodology, normal values, and retest reliability. J. Clin. Ultrasound 2012, 40, 540–546. [Google Scholar] [CrossRef]
- Sharpey-Schafer, E.P. Effects of Valsalva’s Maneuvre on the normal and failing circulation. Br. Med. J. 1955, 1, 693–695. [Google Scholar] [CrossRef]
- Sharpey-Schafer, E.P.; Taylor, P.J. Absent circulatory reflexes in diabetic neuritis. Lancet 1960, 275, 559–562. [Google Scholar] [CrossRef]
- Zöllei, É.; Paprika, D.; Csillik, A.; Rudas, L. Valsalva maneuver, Müller maneuver: Hemodynamic and reflex mechanisms, relevances. Orvosi Hetil. 2007, 148, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Barnett, H.J.; Taylor, D.W.; Eliasziw, M.; Fox, A.J.; Ferguson, G.G.; Haynes, R.B.; Rankin, R.N.; Clagett, G.P.; Hachinski, V.C.; Sackett, D.L.; et al. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N. Engl. J. Med. 1998, 339, 1415–1425. [Google Scholar] [CrossRef] [PubMed]
- Barnett, H.J.M. Original contributions north american symptomatic carotid endarterectomy trial: Methods, patient characteristics, and progress. Stroke 1991, 22, 711–720. [Google Scholar]
- Hoksbergen, A.W.J.; Legemate, D.A.; Ubbink, D.T.; De Vos, H.J.; Jacobs, M.J.H.M. Influence of the collateral function of the circle of Willis on hemispherical perfusion during carotid occlusion as assessed by transcranial colour-coded duplex ultrasonography. Eur. J. Vasc. Endovasc. Surg. 1999, 17, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Hoksbergen, A.W.J.; Legemate, D.A.; Ubbink, D.T.; Jacobs, M.J.H.M. Collateral variations in circle of willis in atherosclerotic population assessed by means of transcranial color-coded duplex ultrasonography. Stroke 2000, 31, 1656–1660. [Google Scholar] [CrossRef]
- Kuo, T.B.J.; Chern, C.M.; Yang, C.C.H.; Hsu, H.Y.; Wong, W.J.; Sheng, W.Y.; Hu, H.H. Mechanisms underlying phase lag between systemic arterial blood pressure and cerebral blood flow velocity. Cerebrovasc. Dis. 2003, 16, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Dawson, S.L.; Panerai, R.B.; Potter, J.F. Critical closing pressure explains cerebral hemodynamics during the valsalva maneuver. J. Appl. Physiol. 1999, 86, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Wallasch, T.M.; Beckmann, P.; Kropp, P. Cerebrovascular reactivity during the valsalva maneuver in migraine, tension-type headache and medication overuse headache. Funct. Neurol. 2011, 26, 223–227. [Google Scholar]
- Novak, P. Quantitative autonomic testing. J. Vis. Exp. 2011, 2502. [Google Scholar] [CrossRef]
- Mense, L.; Reimann, M.; Rüdiger, H.; Gahn, G.; Reichmann, H.; Hentschel, H.; Ziemssen, T. Autonomic function and cerebral autoregulation in patients undergoing carotid endarterectomy. Circ. J. 2010, 74, 2139–2145. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).