Assessment and Rehabilitation in Cervical Radiculopathy—Efficacy of Structured Program with or Without Neurotrophic Agents
Abstract
1. Introduction
2. Patients and Methods
2.1. Study Design
- Study Group (SG): This group included 42 patients who followed a standardized physical rehabilitation program complemented by oral administration of PHSD, a neurotrophic supplement, over a 3-month period.
- Control Group (CG): This group included 40 patients who received the same rehabilitation program without PHSD supplementation.
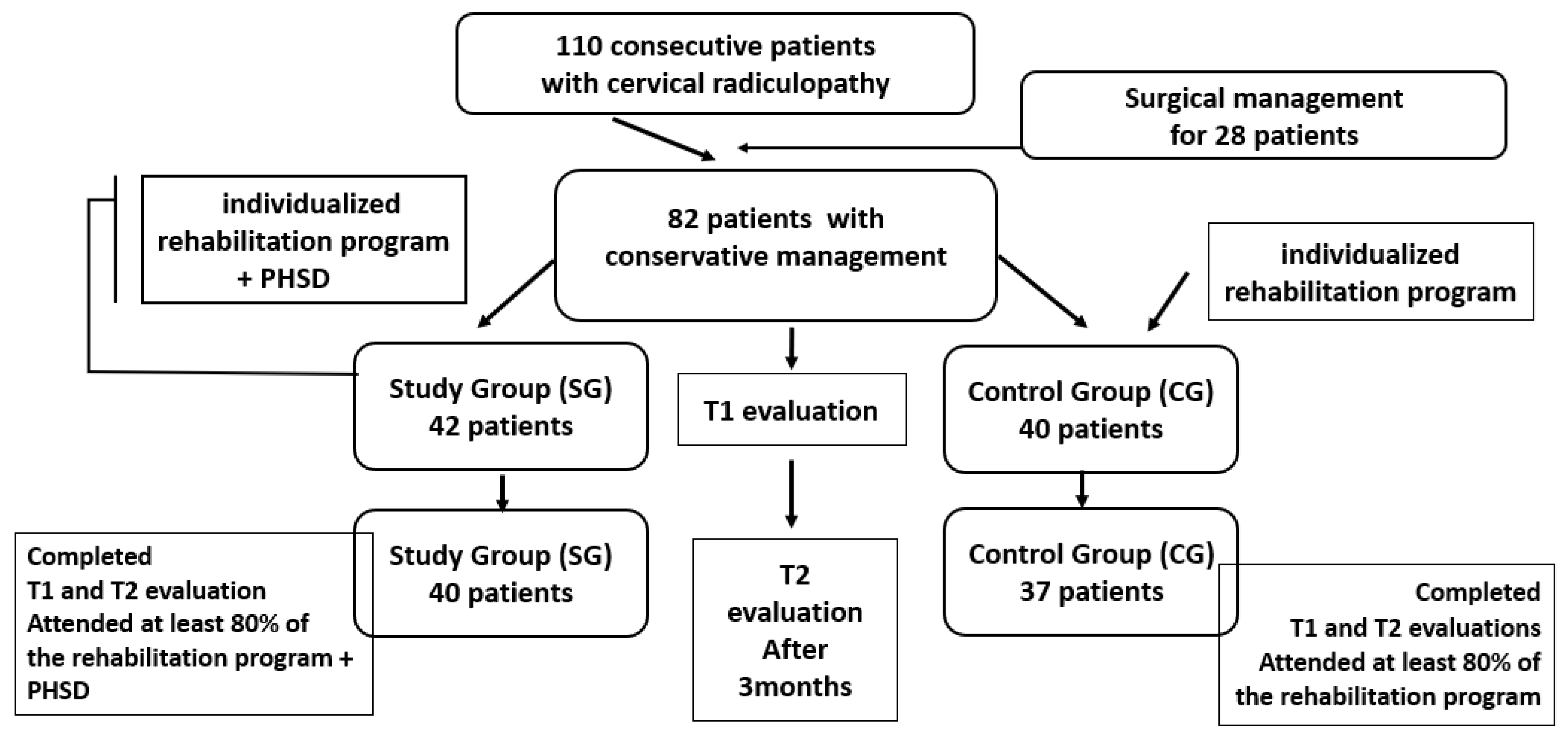
2.2. Participants
2.3. Patients’ Treatment
- Electrotherapy (Electrical Muscle Stimulation—Endomed 482, device series 42.400, Enraf-Nonius, Rotterdam, The Netherlands): 30-min daily sessions using biphasic rectangular pulses at 100 Hz, with intensity titrated to visible, pain-free muscle contractions. Electrodes were placed on neck and upper limb muscles to promote neuromuscular activation.
- Low-Level Laser Therapy (ASTAR PhysioGo 500I/501I, Bielsko-Biala, Poland, PhysioGo series): 20-min sessions targeting cervical paravertebral muscles, upper trapezius, and shoulder girdle, using an 808 nm wavelength laser at a power of 100 mW and energy dose of 7 J/cm2 per point.
- Deep Oscillation Therapy with manual applicator Personal device (DOP1.1.–INDIVID–Physiomed, device series—2442007, Schnaittach, Germany, Physiomed Elektromedizin AG): 30-min sessions applying high-frequency oscillations (100 Hz) for analgesic and muscle-relaxing effects, followed by low-frequency oscillations (5–25 Hz) to enhance local metabolism. A 5-cm manual applicator was used to treat cervical, trapezius, and shoulder muscles bilaterally. The physiotherapy program was administered daily, 5 days per week, for 2 weeks.
- Warm-Up (5–10 min): neck stretches, shoulder rolls;
- Strengthening Exercises (15 min): isometric neck stabilization, shoulder blade squeezes, upper limb resistance exercises using 0.5–1 kg weights;
- Aerobic Conditioning (15 min): low-intensity walking or stationary cycling to promote circulation and reduce pain sensitivity;
- Mobility and Flexibility Drills (10 min): dynamic neck movements within pain-free range, shoulder girdle mobilizations, upper body stretches;
- Cool-Down (5 min): repetition of stretches and deep breathing exercises for muscle relaxation.
| Components | Description |
|---|---|
| Start doing these exercises for 5 repetitions, three times a day. Gradually increase the number of repetitions to about 10 to 20 repetitions. Aims: reduce pain; prepare muscle for reactivation; improve mobility of the shoulders and upper limb: improve grip strength and postural awareness. | |
| Warm-Up (5–10 min) | Neck stretches. Gentle stretching exercises including neck flexion, extension, lateral flexion, and rotation. Hold each stretch for about 15–20 s. Shoulder rolls. Slowly roll the shoulders forward and backward in a circular motion to reduce tension. |
| Strengthening Exercises (15 min) | Isometric neck exercises. Press the palm against the forehead and push while resisting with the neck and hold for 5 s. Repeat on each side of the head as well as the back. Shoulder blade squeeze. Sit or stand with arms at the sides and squeeze the shoulder blades together, holding for 5 s. Upper limb strengthening. Sit or stand holding hands on chest with ½ to 1 kg weights in patient’s hands. Alternating arms lift the weights from chest straight up and bring back down. Repeat on each side |
| Aerobic Conditioning (15 min) | Walking/Stationary cycling. Engage in a light aerobic activity to increase heart rate and blood flow to the muscles, which aids in recovery and pain reduction. |
| Mobility and Flexibility Drills (10 min) | Dynamic neck movements. Perform controlled neck movements in all directions but within a pain-free range. Shoulder girdle and scapula exercises. Lift shoulders and hold for 1 to 2 s. Then relax the shoulders again. To make this harder, shrug shoulders by keeping both hands on the waist. Move shoulder blades gently back and up (small movement). Hold the contraction for 5 to 10 s. Upper body stretches. Include stretches for the upper back, arms, and chest to improve overall flexibility and reduce muscle imbalances |
| Cool Down (5 min) | Gentle stretching. Repeat the neck and shoulder stretches from the warm-up to gently relax and lengthen the muscles, especially upper trapezius and scalene. Deep breathing exercises. Focus on slow, deep breaths to relax the entire body and reduce any residual muscle tension. |
| Education and Self-Management | Posture training. Educate on the importance of good posture to avoid additional nerve compression. Activity modification. Teach how to avoid positions and activities that exacerbate symptoms. |
2.4. Parameters and Measurements
- -
- Anthropometric measures: Body mass index (BMI) calculated as weight in kilograms divided by height in meters squared (kg/m2).
- -
- Cervical mobility indexes: Chin-Sternum Index (CSI): distance between the chin and sternum in maximal cervical flexion; Occiput-Wall Index (OWI): distance between the occiput and wall in maximal cervical extension; Tragus-Acromion Index (TAI): distance measured with the head in neutral position for lateral flexion assessment. Each parameter was recorded in centimeters using a flexible metric tape. These indexes provide quantitative assessment of cervical spine mobility limitations.
- -
- Pain Assessment: Visual Analogue Scale (VAS): patients rated their pain intensity on a 0–10 scale (0: no pain; 10: worst imaginable pain). A minimal clinically important difference (MCID) of 2 points was considered significant improvement, based on validated recommendations for chronic musculoskeletal pain [17].
- -
- Functional Disability Assessment: Neck Disability Index (NDI): a 10-item questionnaire evaluating neck-related disability in daily activities. Each item scored 0–5; total score multiplied by 2 yields percentage disability (0–100%), with higher scores indicating greater impairment. An MCID of 7.5 points and minimally detectable change (MDC) of 10 points were used to interpret clinically relevant change [18,19]. The validated online NDI form was applied to all participants.
- -
- Activities of Daily Living: Katz Index of Independence in Activities of Daily Living (Katz ADL): assesses six basic functions (bathing, dressing, toileting, transferring, continence, feeding), each scored yes/no. Scores: 6 = full function; 4 = moderate impairment; ≤2 = severe functional impairment [20,21,22].
- -
- Magnetic Resonance Imaging (MRI): performed within 6 months prior to inclusion to confirm cervical discopathy and excludes other causes of cervical radiculopathy such as neoplasms or spinal cord compression.
- -
- Radiographic Evaluation: cervical spine radiographs analyzed for spinal alignment, intervertebral space narrowing, osteophytes, vertebral morphology, and calcifications of cervical ligaments.
2.5. Ethics Approval
2.6. Statistical Analysis
3. Results
3.1. Baseline Patient Characteristics
3.2. Time-Evolution of Clinical and Functional Parameters in the Study Group
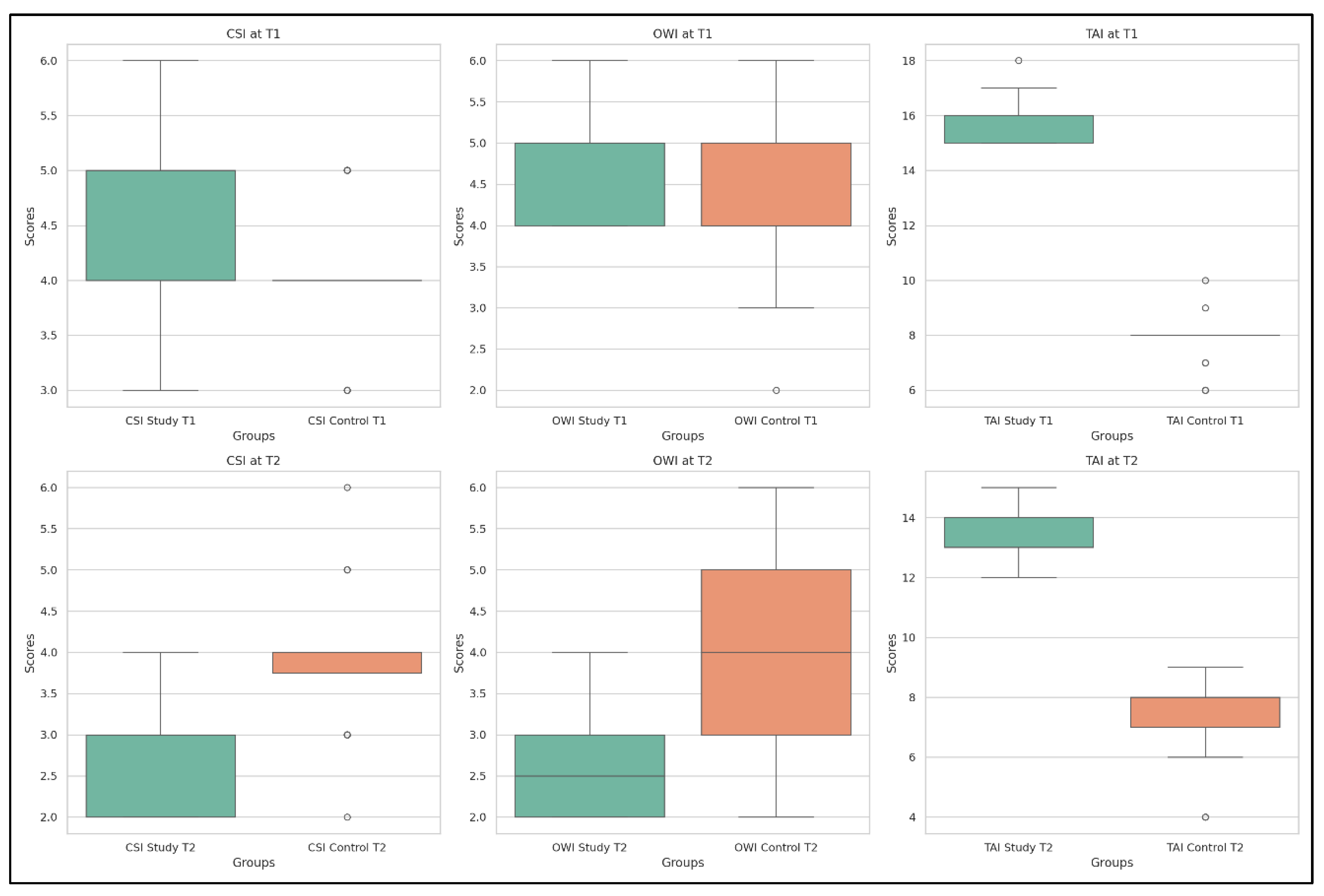
- Chin-Stern Index (CSI) values decreased significantly from T1 to T2 (p = 4.55 × 10−13), indicating a reduction in CR pain following the complete therapeutic measures.
- Occiput-Wall Index (OWI) scores improved markedly (p = 3.98 × 10−13), suggesting better functional integration in occupational activities and reduced disability.
- Tragus-Acromion Index (TAI) scores were significantly reduced (p = 4.15 × 10−13), reflecting lower fear avoidance behaviors (the patient’s fear of mobilizing the head to avoid exacerbating symptoms) and increased confidence in movement.
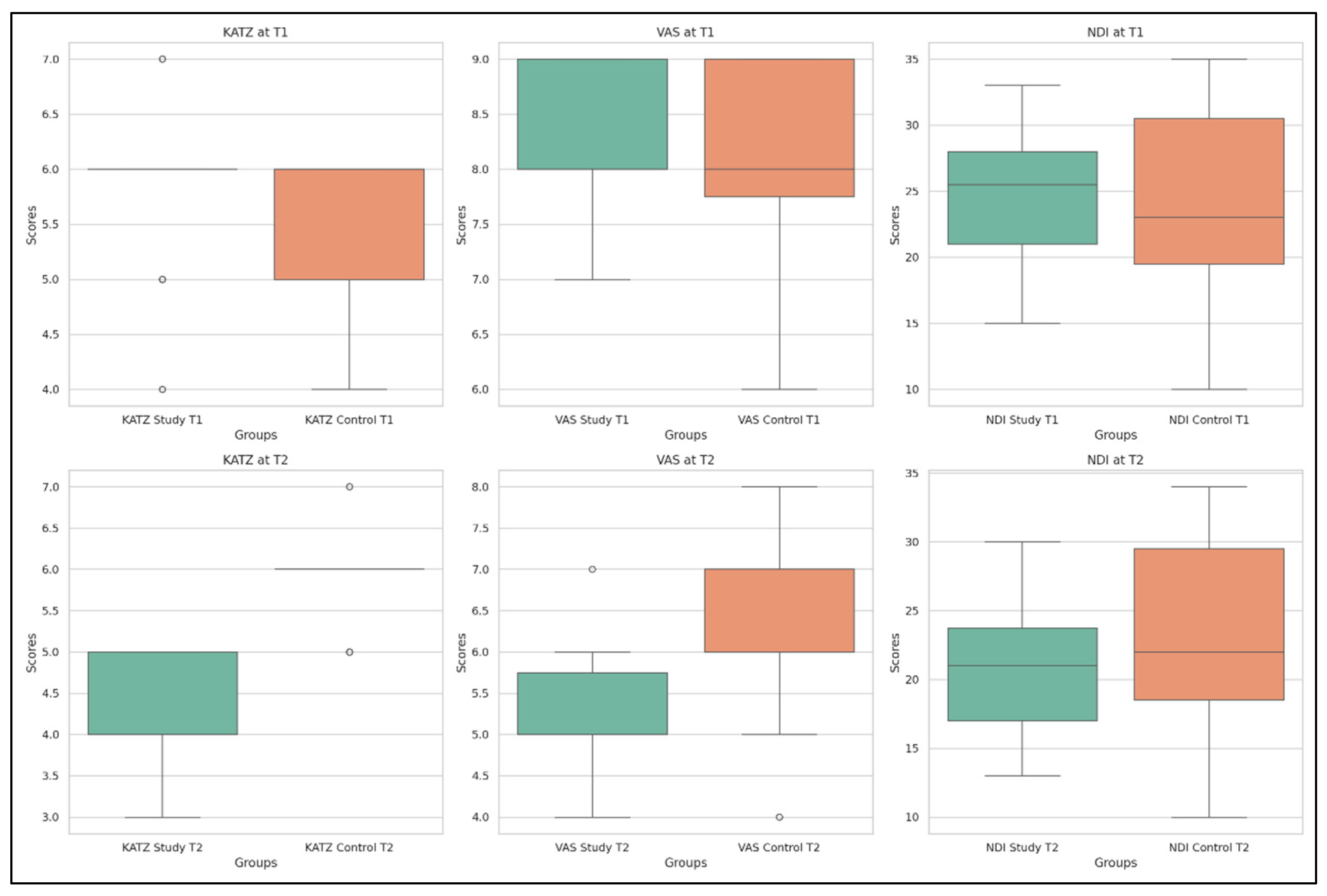
- Visual Analogue Scale (VAS) pain scores decreased substantially (p = 3.78 × 10−13), confirming pain relief after the rehabilitation protocol.
- KATZ Index scores showed significant improvement (p = 2.42 × 10−8), indicating a better ability to perform activities of daily living independently.
- Neck Disability Index (NDI) scores improved significantly (p = 3.17 × 10−7), pointing to a decrease in functional limitations associated with cervical spine dysfunction.
| Parameters | Mean Value | SD | Min Value | 25th Percentile | Median Value | 75th Percentile | Max Value | |
|---|---|---|---|---|---|---|---|---|
| CSI (cm) | T1 | 4.85 | 0.84 | 3 | 4 | 5 | 5 | 6 |
| T2 | 2.61 | 0.58 | 2 | 2 | 3 | 3 | 4 | |
| OWI (cm) | T1 | 4.71 | 0.59 | 4 | 4 | 5 | 5 | 6 |
| T2 | 2.54 | 0.58 | 2 | 2 | 2.5 | 3 | 4 | |
| TAI (cm) | T1 | 15.88 | 0.73 | 15 | 15 | 16 | 16 | 18 |
| T2 | 13.21 | 0.84 | 12 | 13 | 13 | 14 | 15 | |
| VAS | T1 | 8.16 | 0.72 | 7 | 8 | 8 | 9 | 9 |
| T2 | 5.11 | 0.70 | 4 | 5 | 5 | 5.75 | 7 | |
| KATZ | T1 | 4.26 | 0.62 | 3 | 4 | 4 | 5 | 5 |
| T2 | 5.76 | 0.69 | 4 | 6 | 6 | 6 | 7 | |
| NDI % | T1 | 24.71 | 5.13 | 15 | 21 | 25.5 | 28 | 33 |
| T2 | 20.90 | 4.49 | 13 | 17 | 21 | 23.75 | 30 | |
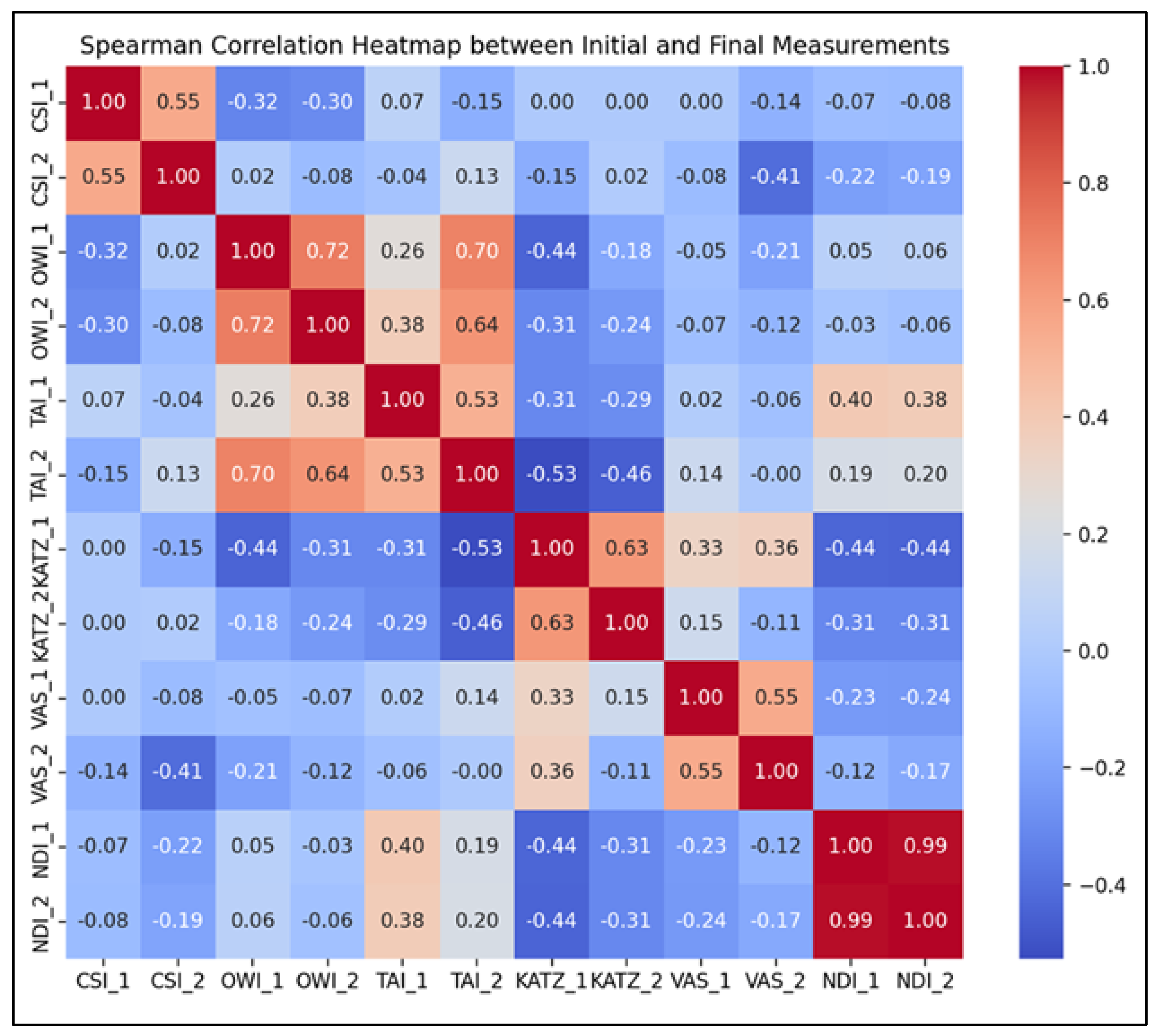
- Chin-Stern Index (CSI): A moderate positive correlation was observed (r = 0.55, p < 0.001), suggesting that baseline levels of central sensitization were moderately predictive of post-intervention outcomes.
- Occiput-Wall Index (OWI): Displayed a strong positive correlation (r = 0.72, p < 0.001), indicating a robust link between initial and final levels of perceived occupational functionality and general well-being.
- Tragus-Acromion Index (TAI): Showed a moderate positive correlation (r = 0.53, p < 0.001), implying consistent therapeutic responsiveness and a predictable reduction in kinesiophobia.
- KATZ Index: Revealed a strong positive correlation (r = 0.63, p < 0.001), supporting the idea that individuals with greater initial independence retained or improved that status post-intervention.
- Visual Analogue Scale (VAS): Exhibited a moderate positive correlation (r = 0.55, p < 0.001), suggesting that individuals with higher baseline pain tended to maintain relatively higher levels of discomfort post-treatment, albeit reduced overall.
- Neck Disability Index (NDI): Demonstrated an almost perfect positive correlation (r = 0.99, p < 0.001), indicating that initial neck disability scores were highly predictive of final scores, potentially reflecting consistent response patterns to targeted cervical rehabilitation.
3.3. Time-Evolution of Clinical and Functional Parameters in the Control Group
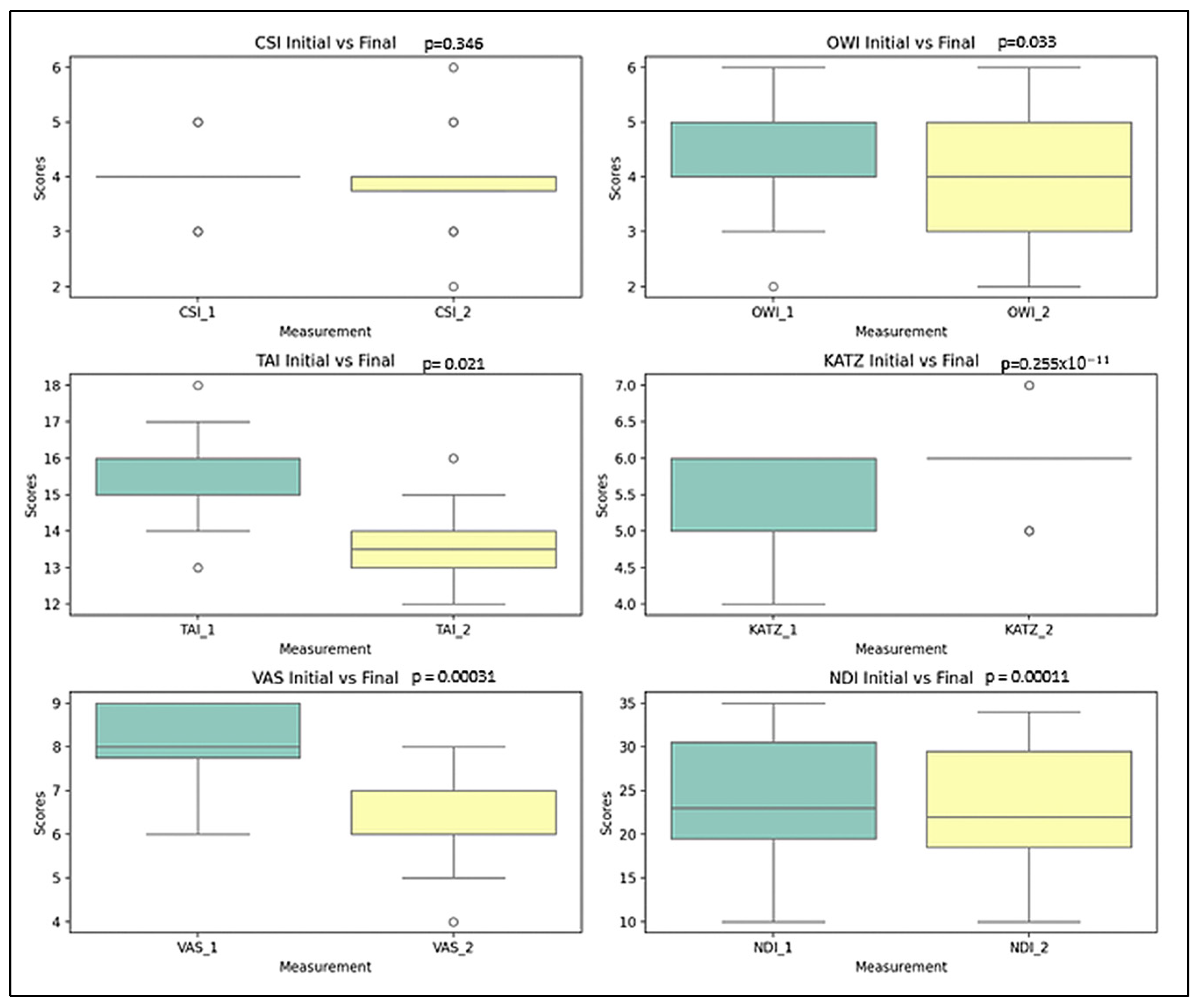
- Chin-Stern Index (CSI): The mean value slightly decreased from 4.00 to 3.90; however, this change was not statistically significant (p = 0.346), indicating stability in central sensitization symptoms.
- Occiput-Wall Index (OWI): Showed a modest decrease in the mean score from 4.25 to 4.00, a change that was statistically significant (p = 0.033), suggesting some spontaneous improvement in functional well-being.
- Tragus-Acromion Index (TAI): The mean value declined from 15.67 to 13.57, reflecting a significant improvement (p = 0.021) in movement-related fear.
- Visual Analogue Scale (VAS): The mean pain score decreased notably from 8.00 to 6.20, with a highly significant change (p ≈ 2.55 × 10−11), indicating substantial pain relief over time.
- KATZ Index: The average score increased from 5.52 to 5.85, which—contrary to expectations—suggests a deterioration in functional independence (p = 0.00031).
- Neck Disability Index (NDI): A small change in the mean value from 23.72% to 23.00% was nonetheless statistically significant (p = 0.00011), reflecting a worsening trend in cervical disability.
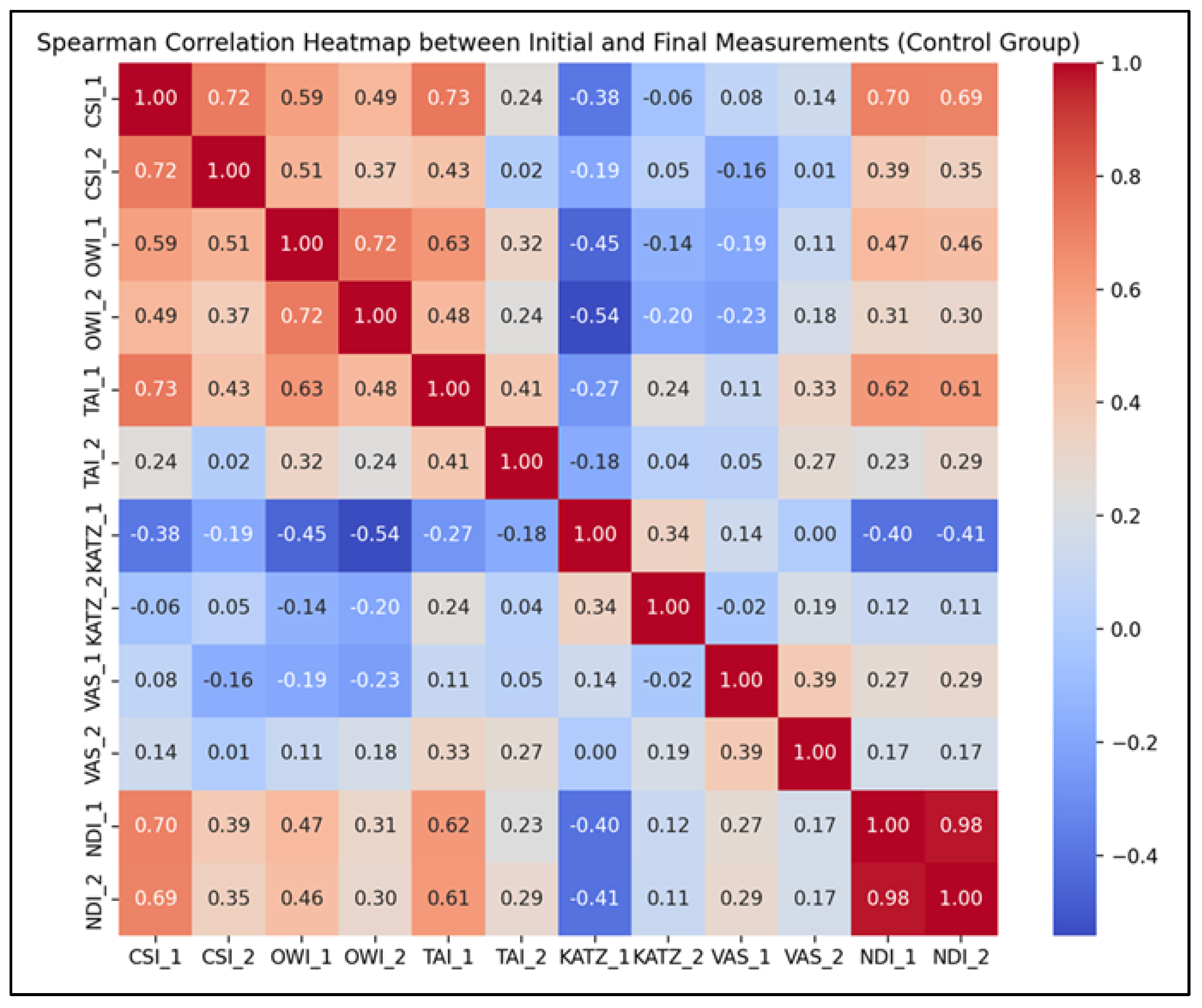
- Strong positive correlations were observed for CSI (r = 0.72, p < 0.001), OWI (r = 0.72, p < 0.001), and NDI (r = 0.98, p < 0.001), indicating that individuals’ initial values for these parameters were highly predictive of their corresponding final scores, even in the absence of a structured rehabilitation program.
- Moderate positive correlations were recorded for TAI (r = 0.41, p = 0.0075), VAS (r = 0.39, p = 0.011), and KATZ (r = 0.34, p = 0.026), suggesting that although trends were preserved, natural variability influenced the progression of these indicators.
3.4. Study Group Versus Control Group
- Cervical Indexes: CSI (p = 1.07 × 10−5), OWI (p = 0.0167), and TAI (p = 4.18 × 10−16); these results indicate notable disparities in cervical spine alignment and mobility between the two groups prior to any therapeutic intervention.
- Functional Symptom-related parameters: KATZ Index (p = 0.598), VAS (p = 0.056), and NDI (p = 0.360 and 0.791 depending on component); no statistically significant differences were found for these variables, indicating comparable levels of functional independence, pain, and disability at baseline between the groups.
- Cervical Indexes: CSI (p = 7.92 × 10−11), OWI (p = 3.67 × 10−10), and TAI (p = 1.12 × 10−15); these highly significant changes favor the study group and support the beneficial impact of the therapeutic intervention on cervical posture and mobility.
- Functional Symptom-related parameters: KATZ Index (p = 0.000167) and VAS (p = 8.91 × 10−8); these outcomes confirm the intervention’s positive effect on daily functioning and pain levels. No significant difference was found at T2 (p = 0.162) for NDI, suggesting comparable levels of neck disability across both groups despite improvements in other domains.
4. Discussions
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Corey, D.L.; Comeau, D. Cervical radiculopathy. Med. Clin. N. Am. 2014, 98, 791–799. [Google Scholar] [CrossRef]
- Yonenobu, K. Cervical radiculopathy and myelopathy: When and what can surgery contribute to treatment? Eur. Spine J. 2000, 9, 1–7. [Google Scholar] [CrossRef]
- Fehlings, M.G.; Tetreault, L.A.; Riew, K.D.; Middleton, J.W.; Aarabi, B.; Arnold, P.M.; Brodke, D.S.; Burns, A.S.; Carette, S.; Chen, R.; et al. A clinical practice guideline for the management of patients with degenerative cervical myelopathy. Global Spine J. 2017, 7 (Suppl. 3), 70S–83S. [Google Scholar] [CrossRef]
- Doughty, C.T.; Bowley, M.P. Entrapment neuropathies of the upper extremity. Med. Clin. N. Am. 2019, 103, 357–370. [Google Scholar] [CrossRef]
- Ament, J.D.; Karnati, T.; Kulubya, E.; Kim, K.D.; Johnson, J.P. Treatment of cervical radiculopathy: A review of the evolution and economics. Surg. Neurol. Int. 2018, 9, 35. [Google Scholar] [CrossRef]
- Magnus, W.; Viswanath, O.; Viswanathan, V.K.; Meskin, F.B. Cervical radiculopathy. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- McCartney, S.; Baskerville, R.; Blagg, S.; McCartney, D. Cervical radiculopathy and cervical myelopathy: Diagnosis and management in primary care. Br. J. Gen. Pract. 2018, 68, 44–46. [Google Scholar] [CrossRef] [PubMed]
- Thoomes, E.J. Effectiveness of manual therapy for cervical radiculopathy, a review. Chiropr. Man. Therap. 2016, 24, 45. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.; Oh, D.; Lee, B.; Lee, H.; Ko, M.; Moon, S.; Park, Y.; Kim, S.; Kim, S. Neuropathic pain component in patients with cervical radicular pain: A single-center retrospective study. Medicina 2022, 58, 1191. [Google Scholar] [CrossRef] [PubMed]
- Koszela, K. A three-stage concept of spine pathology treatment—A different perspective. Reumatologia 2024, 62, 58–63. [Google Scholar] [CrossRef]
- Kjaer, P.; Kongsted, A.; Hartvigsen, J.; Isenberg-Jørgensen, A.; Boyle, E.; Hestbæk, L. National clinical guidelines for non-surgical treatment of patients with recent onset neck pain or cervical radiculopathy. Eur. Spine J. 2017, 26, 2242–2257. [Google Scholar] [CrossRef]
- North American Spine Society (NASS). Evidence-Based Clinical Guidelines for Multidisciplinary Spine Care: Diagnosis and Treatment of Cervical Radiculopathy from Degenerative Disorders. NASS. 2021. Available online: https://www.spine.org/Research-Clinical-Care/Quality-Improvement/Clinical-Guidelines (accessed on 25 August 2025).
- Gross, A.; Kay, T.M.; Paquin, J.-P.; Blanchette, S.; Lalonde, P.; Christie, T.; Dupont, G.; Graham, N.; Burnie, S.J.; Gelley, G.; et al. Exercises for mechanical neck disorders. Cochrane Database Syst. Rev. 2016, 2016, CD004250. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, B.; McDevitt, A.; Kelly, J.; Mintken, P.; Clewley, D. Manual physical therapy for neck disorders: An umbrella review. J. Man. Manip. Ther. 2025, 33, 18–35. [Google Scholar] [CrossRef] [PubMed]
- García-Juez, S.; Navarro-Santana, M.J.; Valera-Calero, J.A.; Albert-Lucena, D.; Varas-De-La-Fuente, A.B.; Plaza-Manzano, G. Effectiveness of articular and neural mobilization for cervical radiculopathy: A meta-analysis. J. Orthop. Sports Phys. Ther. 2025, 55, 468–481. [Google Scholar] [CrossRef] [PubMed]
- Fejer, R.; Kyvik, K.O.; Hartvigsen, J. The prevalence of neck pain in the world population: A systematic critical review. Eur. Spine J. 2006, 15, 834–848. [Google Scholar] [CrossRef]
- Eubanks, J.D. Cervical radiculopathy: Nonoperative management of neck pain and radicular symptoms. Am. Fam. Physician 2010, 81, 33–40. [Google Scholar]
- Delgado, D.A.; Lambert, B.S.; Boutris, N.; McCulloch, P.C.; Robbins, A.B.; Moreno, M.R.; Harris, J.D. Validation of digital visual analog scale pain scoring. J. Am. Acad. Orthop. Surg. Glob. Res. Rev. 2018, 2, e088. [Google Scholar] [CrossRef]
- Vernon, H. The Neck Disability Index: State-of-the-art, 1991–2008. J. Manip. Physiol. Ther. 2008, 31, 491–502. [Google Scholar] [CrossRef]
- Dennison, B.S.; Leal, M.H. Mechanical neck pain. In Neck and Arm Pain Syndromes; Fernández de las Peñas, C., Cleland, J.A., Huijbregts, P.A., Eds.; Churchill Livingstone: London, UK, 2011; pp. 94–111. [Google Scholar]
- Katz, S. Assessing self-maintenance: ADL, mobility and IADL. J. Am. Geriatr. Soc. 1983, 31, 721–726. [Google Scholar] [CrossRef]
- Hartigan, I. A comparative review of the Katz ADL and the Barthel Index. Int. J. Older People Nurs. 2007, 2, 204–212. [Google Scholar] [CrossRef]
- Michaleff, Z.A.; Maher, C.G.; Lin, C.W.; Rebbeck, T.; Jull, G.; Latimer, J. Prognosis of acute and persistent neck pain: A systematic review and meta-analysis. Pain 2012, 153, 770–781. [Google Scholar] [CrossRef]
- Thoomes, E.J.; Scholten-Peeters, G.G.; de Boer, A.J.; Olsthoorn, R.A.; Verkerk, K.; Lin, C.; Verhagen, A.P. Lack of uniform diagnostic criteria for cervical radiculopathy in conservative intervention trials: A systematic review. Eur. Spine J. 2012, 21, 1459–1470. [Google Scholar] [CrossRef]
- Cohen, S.P.; Hooten, W.M. Advances in the diagnosis and management of neck pain. BMJ 2017, 358, j3221. [Google Scholar] [CrossRef]
- Childs, J.D.; Cleland, J.A.; Elliott, J.M.; Teyhen, D.S.; Wainner, R.S.; Whitman, J.M.; Sopky, B.J.; Godges, J.J.; Flynn, T.W.; Delitto, A.; et al. Neck pain: Clinical practice guidelines. J. Orthop. Sports Phys. Ther. 2008, 38, A1–A34. [Google Scholar] [CrossRef] [PubMed]
- Engebretsen, K.B.; Taso, M.; Bjorland, S.; Jenssen, H.K.; Skaara, H.E.; Brox, J.I. A functional intervention within a cognitive approach to chronic cervical radiculopathy: Study protocol. BMC Musculoskelet. Disord. 2024, 25, 629. [Google Scholar] [CrossRef] [PubMed]
- Kuligowski, T.; Skrzek, A.; Cieślik, B. Manual Therapy in Cervical and Lumbar Radiculopathy: A systematic review of the literature. Int. J. Environ. Res. Public Health 2021, 18, 6176. [Google Scholar] [CrossRef] [PubMed]
- Rissardo, J.P.; Fornari Caprara, A.L. Management of cervical radiculopathy: Current systematic reviews. Indian J. Health Sci. Biomed. Res. 2023, 16, 441–442. [Google Scholar] [CrossRef]
- Negrão, L.; Nunes, P. Uridine monophosphate, folic acid and vitamin B12 in symptomatic peripheral entrapment neuropathies. Pain Manag. 2016, 6, 25–29. [Google Scholar] [CrossRef]
- Goldberg, H.; Mibielli, M.A.; Nunes, C.P.; Goldberg, S.W.; Buchman, L.; Mezitis, S.G.E.; Rzetelna, H.; Oliveira, L.; Geller, M.; Wajnsztajn, F. A double-blind randomized study of uridine triphosphate, cytidine monophosphate and hydroxocobalamin. J. Pain Res. 2017, 10, 397–404. [Google Scholar] [CrossRef]
- Kamal, D.; Trăistaru, R.; O Alexandru, D.; Kamal, C.K.; Pirici, D.; Pop, O.T.; Mălăescu, D.G. Morphometric findings in avascular necrosis of the femoral head. Rom. J. Morphol. Embryol. 2012, 53 (Suppl. S3), 763–767. [Google Scholar]
- Trăistaru, R.; Alexandru, D.O.; Kamal, D.; Kamal, C.K.; Rogoveanu, O.C.; Postolache, P. Boswellia derivates and rehabilitation program in knee osteoarthritis patients. Rev. Chim. 2018, 69, 4105–4108. [Google Scholar] [CrossRef]
- Trăistaru, R.; Alexandru, D.O.; Kamal, D.; Kamal, C.K.; Rogoveanu, O. The role of herbal extracts in knee osteoarthritis females rehabilitation. Farmacia 2018, 66, 507–513. [Google Scholar] [CrossRef]
- Woldeamanuel, Y.W.; Oliveira, C.M.; Brannick, B. The placebo and nocebo effects in neuropathic pain clinical trials: Systematic review and meta-analysis. Pain 2022, 163, 1265–1276. [Google Scholar] [CrossRef]
- Baltrusch, S.; Skopp, G.; Ufer, M.; Gleiter, C.H. B-vitamins in the treatment of neuropathic pain: A systematic review and meta-analysis. Pain Physician 2023, 26, 45–58. [Google Scholar]
- Apfel, S.C. Nerve growth factor for the treatment of diabetic neuropathy. Int. Rev. Neurobiol. 2002, 50, 393–413. [Google Scholar] [PubMed]
- Ji, R.R.; Nackley, A.; Huh, Y.; Terrando, N.; Maixner, W. Neuroinflammation and central sensitization in chronic pain. Anesthesiology 2018, 129, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Grace, P.M.; Hutchinson, M.R.; Maier, S.F.; Watkins, L.R. Pathological pain and the neuroimmune interface. Nat. Rev. Immunol. 2014, 14, 217–231. [Google Scholar] [CrossRef]
- Costigan, M.; Scholz, J.; Woolf, C.J. Neuropathic pain: A maladaptive response of the nervous system to damage. Annu. Rev. Neurosci. 2009, 32, 1–32. [Google Scholar] [CrossRef]
- MaMatei, D.; Traistaru, R.; Amzolini, A.M.; Ianosi, L.S.; Neagoe, C.D.; Mitrea, A.; Clenciu, D.; Avramescu, T.E. A comparative study on the pain threshold experienced by fibromyalgia patients following acute SARS-CoV-2 infection. Life 2024, 14, 942. [Google Scholar] [CrossRef]
- Kuijper, B.; Tans, J.T.J.; Beelen, A.; Nollet, F.; Visser, M.D. Cervical collar or physiotherapy versus wait-and-see policy for recent onset cervical radiculopathy: Randomized trial. BMJ 2009, 339, b3883. [Google Scholar] [CrossRef]
- Engquist, M.; Löfgren, H.; Öberg, B.; Holtz, A.; Peolsson, A.; Soderlund, A.; Vavruch, L.; Lind, B. Surgery versus nonsurgical treatment of cervical radiculopathy: A prospective, randomized study comparing surgery plus physiotherapy with physiotherapy alonewith a 2-year follow-up. Spine 2013, 38, 1715–1722. [Google Scholar] [CrossRef]
- Ylinen, J.; Takala, E.-P.; Nykänen, M.; Häkkinen, A.; Mälkiä, E.; Pohjolainen, T.; Karppi, S.-L.; Kautiainen, H.; Airaksinen, O. Active neck muscle training in chronic neck pain in women: A randomized controlled trial. JAMA 2003, 289, 2509–2516. [Google Scholar] [CrossRef]
- Carrasco-Uribarren, A.; Ceballos-Laita, L.; Pérez-Guillén, S.; Jiménez-Del-Barrio, S.; Rodríguez-Rubio, P.R.; Pantaleón-Hernández, D.; Cabanillas-Barea, S. Is manual therapy effective for cervical dizziness? A systematic review and meta-analysis of randomized controlled trials. BMC Musculoskelet. Disord. 2025, 26, 659. [Google Scholar] [CrossRef]

| GENDER | OWN PLACE | PROFESSION (JOB) | PREVIOUS REHABILITATION | TOTAL | |||||
|---|---|---|---|---|---|---|---|---|---|
| F | M | U | R | A | R | Yes | No | ||
| SG | 28 66% | 14 34% | 27 64% | 15 36% | 20 48% | 22 52% | 25 60% | 17 40% | 42 100.00% |
| CG | 25 62% | 15 38% | 25 62% | 15 38% | 16 40% | 24 60% | 24 60% | 16 40% | 40 100.00% |
| Total | 53 65% | 29 35% | 52 63% | 30 37% | 36 44% | 46 56% | 49 60% | 33 40% | 82 100.00% |
| p-value = 0.6075 | p-value = 0.3376 | p-value = 0.6202 | p-value = 0.6044 | ||||||
| Age (years) | Height (meters) | Weight (kg) | BMI (kg/m2) | TOTAL | |||||
| SG | 61.71 ± 5.66 | 1.66 ± 11.24 | 81.80 ± 16.94 | 29.70 ± 5.76 | 42 | ||||
| CG | 60.4 ± 6.31 | 1.63 ± 5.97 | 80.57 ± 13.76 | 30.02 ± 5.19 | 40 | ||||
| p-value = 0.3576 | p-value = 0.1366 | p-value = 0.6693 | p-value = 0.5905 | ||||||
| Parameters | Mean Value | SD | Min Value | 25th Percentile | Median Value | 75th Percentile | Max Value | |
|---|---|---|---|---|---|---|---|---|
| CSI (cm) | T1 | 4 | 0.67 | 3 | 4 | 4 | 4 | 5 |
| T2 | 3.9 | 0.74 | 2 | 3.75 | 4 | 4 | 6 | |
| OWI (cm) | T1 | 4.25 | 0.86 | 2 | 4 | 4 | 5 | 6 |
| T2 | 4 | 0.96 | 2 | 3 | 4 | 5 | 6 | |
| TAI (cm) | T1 | 15.67 | 0.99 | 13 | 15 | 16 | 16 | 18 |
| T2 | 13.57 | 1.01 | 12 | 13 | 13.5 | 14 | 16 | |
| VAS | T1 | 8 | 0.72 | 6 | 7.75 | 8 | 9 | 9 |
| T2 | 6.2 | 0.85 | 4 | 6 | 6 | 7 | 8 | |
| KATZ | T1 | 5.52 | 0.59 | 4 | 5 | 6 | 6 | 6 |
| T2 | 5.85 | 0.48 | 5 | 6 | 6 | 6 | 7 | |
| NDI % | T1 | 23.72 | 7.81 | 10 | 19.5 | 23 | 30.5 | 35 |
| T2 | 23 | 7.66 | 10 | 18.5 | 22 | 29.5 | 34 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trăistaru, R.M.; Kamal, K.C.; Kamal, D.; Tache-Codreanu, D.-L.; Kamal, A.M. Assessment and Rehabilitation in Cervical Radiculopathy—Efficacy of Structured Program with or Without Neurotrophic Agents. Life 2025, 15, 1690. https://doi.org/10.3390/life15111690
Trăistaru RM, Kamal KC, Kamal D, Tache-Codreanu D-L, Kamal AM. Assessment and Rehabilitation in Cervical Radiculopathy—Efficacy of Structured Program with or Without Neurotrophic Agents. Life. 2025; 15(11):1690. https://doi.org/10.3390/life15111690
Chicago/Turabian StyleTrăistaru, Rodica Magdalena, Kamal Constantin Kamal, Diana Kamal, Diana-Lidia Tache-Codreanu, and Adina Maria Kamal. 2025. "Assessment and Rehabilitation in Cervical Radiculopathy—Efficacy of Structured Program with or Without Neurotrophic Agents" Life 15, no. 11: 1690. https://doi.org/10.3390/life15111690
APA StyleTrăistaru, R. M., Kamal, K. C., Kamal, D., Tache-Codreanu, D.-L., & Kamal, A. M. (2025). Assessment and Rehabilitation in Cervical Radiculopathy—Efficacy of Structured Program with or Without Neurotrophic Agents. Life, 15(11), 1690. https://doi.org/10.3390/life15111690






