Oropouche Virus: An Emerging Arboviral Threat and Its Implications for Europe
Abstract
1. Introduction
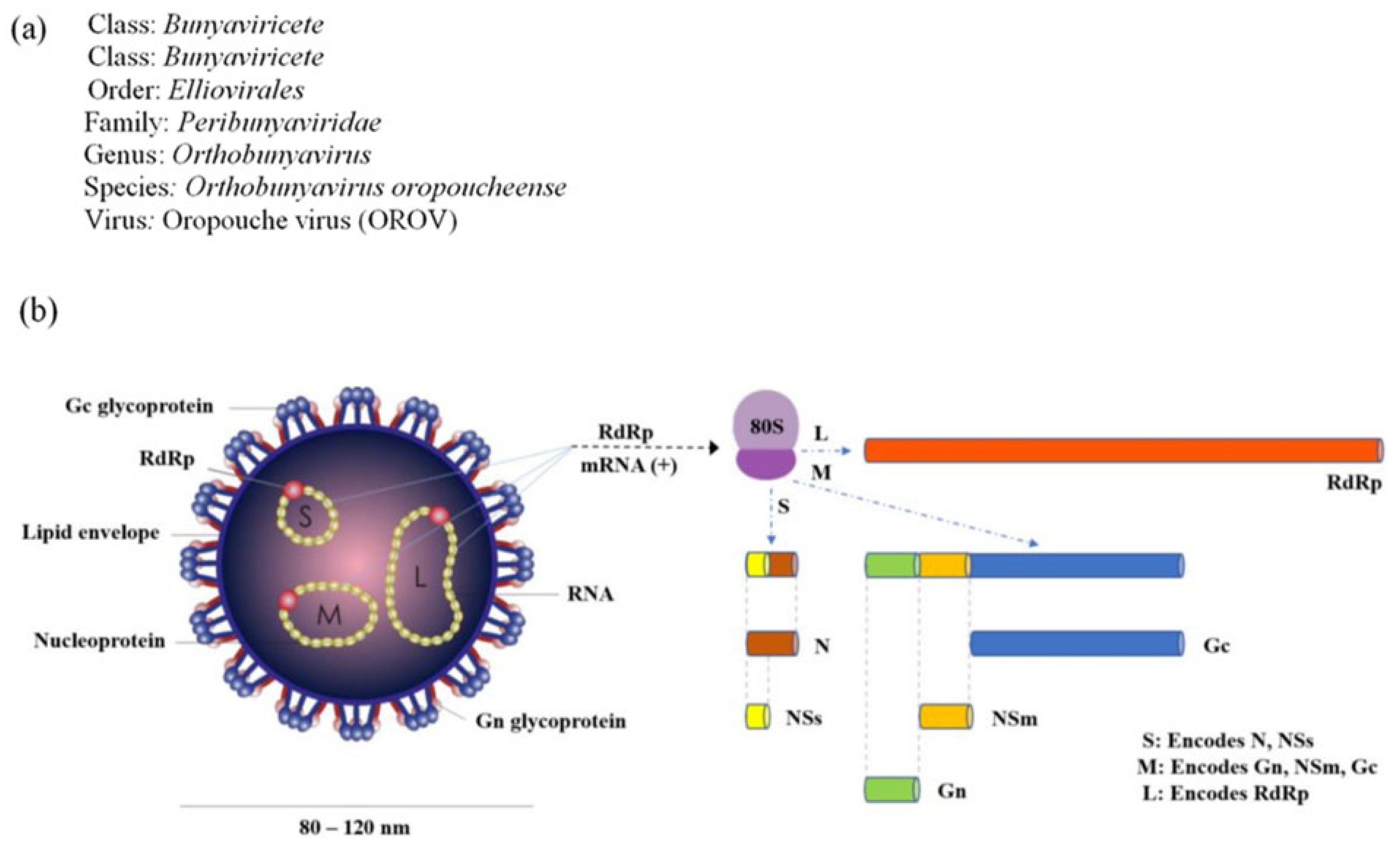
2. Virological and Genetic Characteristics
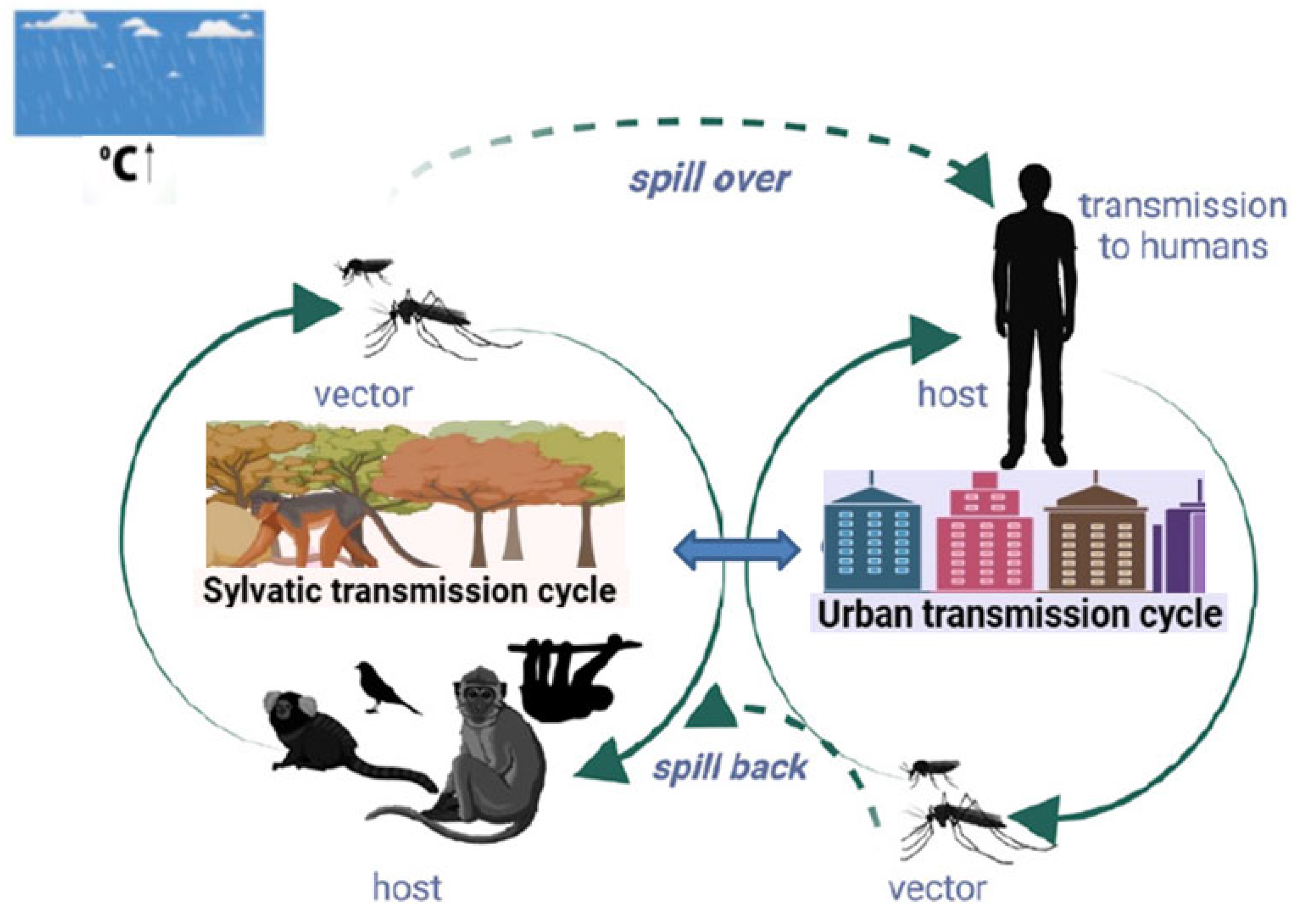
3. Transmission Cycles and Epidemiology
4. Clinical Manifestations
5. Diagnosis, Treatment, and Vaccine Development
6. Recent Outbreaks: 2024–2025
7. Drivers of the 2024 Epidemic
8. Congenital Infections and Vertical/Sexual Transmission
9. OROV in Europe: Epidemiological Risk and Vector Competence Assessment
10. Italy and the Jubilee 2025
11. Future Surveillance and Preparedness Recommendations
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weaver, S.C.; Reisen, W.K. Present and future arboviral threats. Antivir. Res. 2010, 85, 328–345. [Google Scholar] [CrossRef]
- Chala, B.; Hamde, F. Emerging and Re-emerging Vector-Borne Infectious Diseases and the Challenges for Control: A Review. Front. Public Health 2021, 9, 715759. [Google Scholar] [CrossRef]
- Lanciotti, R.S.; Roehrig, J.T.; Deubel, V.; Smith, J.; Parker, M.; Steele, K.; Crise, B.; Volpe, K.E.; Crabtree, M.B.; Scherret, J.H.; et al. Origin of the West Nile virus responsible for an outbreak of encephalitis in the northeastern United States. Science 1999, 286, 2333–2337. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.H.; Wilson, M.E. Yellow fever control: Current epidemiology and vaccination strategies. Trop. Dis. Travel. Med. Vaccines 2020, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Zhan, J.; Chen, L.; Chen, H.; Cheng, S. Global, regional, and national dengue burden from1990 to 2017: A systematic analysis based on the global burden of disease study. eClinicalMedicine 2021, 32, 100712. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Guo, Y.; Gao, J.; Tang, H.; Xu, K.; Liu, Q.; Xu, L. Climate Change Drives the Transmission and Spread of Vector-Borne Diseases: An Ecological Perspective. Biology 2022, 11, 1628. [Google Scholar] [CrossRef]
- Ortiz, D.I.; Piche-Ovares, M.; Romero-Vega, L.M.; Wagman, J.; Troyo, A. The Impact of Deforestation, Urbanization, and Changing Land Use Patterns on the Ecology of Mosquito and Tick-Borne Diseases in Central America. Insects 2022, 13, 20. [Google Scholar] [CrossRef]
- Zavaleta-Monestel, E.; Rojas-Chinchilla, C.; Molina-Sojo, P.; Murillo-Castro, M.F.; Rojas-Molina, J.P.; Martínez-Vargas, E. Impact of Climate Change on the Global Dynamics of Vector-Borne Infectious Diseases: A Narrative Review. Cureus 2025, 17, e77972. [Google Scholar] [CrossRef]
- Findlater, A.; Bogoch, I.I. Human Mobility and the Global Spread of Infectious Diseases: A Focus on Air Travel. Trends Parasitol. 2018, 34, 772–783. [Google Scholar] [CrossRef]
- Ramos-Rincón, J.M. Imported diseases and travel medicine. Med. Clin. 2021, 156, 558–560. [Google Scholar] [CrossRef]
- Anderson, C.R.; Spence, L.; Downs, W.G.; Aitken, T.H.G. Oropouche virus: A new human disease agent from Trinidad, West Indies. Am. J. Trop. Med. Hyg. 1961, 10, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Sakkas, H.; Bozidis, P.; Franks, A.; Papadopoulou, C. Oropouche Fever: A Review. Viruses 2018, 10, 175. [Google Scholar] [CrossRef] [PubMed]
- Files, M.A.; Hansen, C.A.; Herrera, V.C.; Schindewolf, C.; Barrett, A.D.T.; Beasley, D.W.C.; Bourne, N.; Milligan, G.N. Baseline mapping of Oropouche virology, epidemiology, therapeutics, and vaccine research and development. NPJ Vaccines 2022, 7, 38. [Google Scholar] [CrossRef] [PubMed]
- Bai, F.; Denyoh, P.M.D.; Urquhart, C.; Shrestha, S.; Yee, D.A. A Comprehensive Review of the Neglected and Emerging Oropouche Virus. Viruses 2025, 17, 439. [Google Scholar] [CrossRef]
- PAHO/WHO (Pan American Health Organization/World Health Organization). Epidemiological Alert: Oropouche in the Region of the Americas, 1 August 2024; PAHO/WHO: Washington, DC, USA, 2024. [Google Scholar]
- Lorenz, C.; de Azevedo, T.S.; Sallum, M.A.M.; Chiaravalloti-Neto, F. Oropouche fever outbreak in Brazil: Key factors behind the largest epidemic in history. PLoS ONE 2025, 20, e0327845. [Google Scholar] [CrossRef]
- Travassos da Rosa, J.F.; de Souza, W.M.; Pinheiro, F.P.; Figueiredo, M.L.; Cardoso, J.F.; Acrani, G.O.; Nunes, M.R.T. Oropouche Virus: Clinical, Epidemiological, and Molecular Aspects of a Neglected Orthobunyavirus. Am. J. Trop. Med. Hyg. 2017, 96, 1019–1030. [Google Scholar] [CrossRef]
- The Lancet Infectious Diseases. Oropouche fever, the mysterious threat. Lancet Infect. Dis. 2024, 24, 935. [Google Scholar] [CrossRef]
- Elliott, R.M. Orthobunyaviruses: Recent genetic and structural insights. Nat. Rev. Microbiol. 2014, 12, 673–685. [Google Scholar] [CrossRef]
- de Souza, W.M.; Calisher, C.H.; Carrera, J.P.; Hughes, H.R.; Nunes, M.R.T.; Russell, B.; Tilson-Lunel, N.L.; Venter, M.; Xia, H. ICTV Virus Taxonomy Profile: Peribunyaviridae 2024. J. Gen. Virol. 2024, 105, 002034. [Google Scholar] [CrossRef]
- Nunes, M.R.T.; Martins, L.C.; Rodrigues, S.G.; Chiang, J.O.; Azevedo, R.D.S.d.S.; da Rosa, A.P.T.; Vasconcelos, P.F.d.C. Oropouche virus isolation, southeast Brazil. Emerg. Infect. Dis. 2005, 11, 1610–1613. [Google Scholar] [CrossRef]
- Tilston-Lunel, N.L.; Acrani, G.O.; Randall, R.E.; Elliott, R.M. Generation of recombinant oropouche viruses lacking the nonstructural protein NSm or NSs. J. Virol. 2016, 90, 2616–2627. [Google Scholar] [CrossRef]
- Murillo, J.L.; Cabral, A.D.; Uehara, M.; da Silva, V.M.; dos Santos, J.V.; Muniz, J.R.C.; Estrozi, L.F.; Fenel, D.; Garcia, W.; Sperança, M.A. Nucleoprotein from the unique human infecting Orthobunyavirus of Simbu serogroup (Oropouche virus) forms higher order oligomers in complex with nucleic acids in vitro. Amino Acids 2018, 50, 711–721. [Google Scholar] [CrossRef]
- Hellert, J.; Aebischer, A.; Wernike, K.; Haouz, A.; Brocchi, E.; Reiche, S.; Guardado-Calvo, P.; Beer, M.; Rey, F.A. Orthobunyavirus spike architecture and recognition by neutralizing antibodies. Nat. Commun. 2019, 10, 879. [Google Scholar] [CrossRef] [PubMed]
- Aquino, V.H.; Moreli, M.L.; Moraes Figueiredo, L.T. Analysis of Oropouche virus L. protein amino acid sequence showed the presence of an additional conserved region that could harbour an important role for the polymerase activity. Arch. Virol. 2003, 148, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, P.V.; Barrett, A.D.; Saeed, M.F.; Watts, D.M.; Russell, K.; Guevara, C.; Ampuero, J.S.; Suarez, L.; Cespedes, M.; Montgomery, J.M.; et al. Iquitos virus: A novel reassortant Orthobunyavirus associated with human illness in Peru. PLoS Negl. Trop. Dis. 2011, 5, e1315. [Google Scholar] [CrossRef] [PubMed]
- Nunes, M.R.; de Souza, W.M.; Savji, N.; Figueiredo, M.L.; Cardoso, J.F.; da Silva, S.P.; da Silva de Lima, C.P.; Vasconcelos, H.B.; Rodrigues, S.G.; Lipkin, W.I.; et al. Oropouche Orthobunyavirus: Genetic characterization of full-length genomes and development of molecular methods to discriminate natural reassortments. Infect. Genet. Evol. 2019, 68, 16–22. [Google Scholar] [CrossRef]
- Naveca, F.G.; de Almeida, T.A.P.; Souza, V.; Nascimento, V.; Silva, D.; Nascimento, F.; Mejía, M.; de Oliveira, Y.S.; Rocha, L.; Xavier, N.; et al. Human outbreaks of a novel reassortant Oropouche virus in the Brazilian Amazon region. Nat. Med. 2024, 30, 3509–3521. [Google Scholar] [CrossRef]
- Schwartz, D.A. Novel Reassortants of Oropouche Virus (OROV) are Causing Maternal-Fetal Infection During Pregnancy, Stillbirth, Congenital Microcephaly and Malformation Syndromes. Genes 2025, 16, 87. [Google Scholar] [CrossRef]
- Pinheiro, F.P.; Da Rosa, A.P.T.; Da Rosa, J.F.T.; Ishak, R.; Freitas, R.B.; Gomes, M.L.; LeDuc, J.W.; Oliva, O.F. Oropouche virus. I. A review of clinical, epidemiological, and ecological findings. Am. J. Trop. Med. Hyg. 1981, 30, 149–160. [Google Scholar] [CrossRef]
- Sciancalepore, S.; Schneider, M.C.; Kim, J.; Galan, D.I.; Riviere-Cinnamond, A. Presence and multi- species spatial distribution of Oropouche virus in Brazil within the one health framework. Trop. Med. Infect. Dis. 2022, 7, 111. [Google Scholar] [CrossRef]
- Tilston-Lunel, N.L. Oropouche Virus: An Emerging Orthobunyavirus. J. Gen. Virol. 2024, 105, 002027. [Google Scholar] [CrossRef]
- Pinheiro, F.P.; Rocha, A.G.; Freitas, R.B. Meningitis associated with Oropouche virus infection. Rev. Inst. Med. Trop. Sao Paulo 1982, 24, 246–251. [Google Scholar]
- Silva-Caso, W.; Aguilar-Luis, M.A.; Palomares-Reyes, C.; Mazulis, F.; Weilg, C.; del Valle, L.J.; Espejo-Evaristo, J.; Soto-Febres, F.; Martins-Luna, J.; del Valle-Mendoza, J. First outbreak of Oropouche Fever reported in a non-endemic western region of the Peruvian Amazon: Molecular diagnosis and clinical characteristics. Int. J. Infect. Dis. 2019, 83, 139–144. [Google Scholar] [CrossRef]
- Martins-Luna, J.; Del Valle-Mendoza, J.; Silva-Caso, W.; del Valle, L.J.; Palomares-Reyes, C.; Carrillo-Ng, H.; Peña-Tuesta, I.; Aguilar-Luis, M.A. Oropouche infection a neglected arbovirus in patients with acute febrile illness from the Peruvian coast. BMC Res. Notes 2020, 13, 67. [Google Scholar] [CrossRef] [PubMed]
- Vernal, S.; Martini, C.C.R.; da Fonseca, B.A.L. Oropouche Virus Associated Aseptic Meningoencephalitis, Southeastern Brazil. Emerg. Infect. Dis. 2019, 25, 380–382. [Google Scholar] [CrossRef] [PubMed]
- Almeida, G.M.; Souza, J.P.; Mendes, N.D.; Pontelli, M.C.; Pinheiro, N.R.; Nogueira, G.O.; Cardoso, R.S.; Paiva, I.M.; Ferrari, G.D.; Veras, F.P.; et al. Neural Infection by Oropouche Virus in Adult Human Brain Slices Induces an Inflammatory and Toxic Response. Front. Neurosci. 2021, 15, 674576. [Google Scholar] [CrossRef] [PubMed]
- de Souza Bastos, M.; Figueiredo, L.T.; Naveca, F.G.; Monte, R.L.; Lessa, N.; de Figueiredo, R.M.; de Lima Gimaque, J.B.; Joao, G.P.; Ramasawmy, R.; Mourao, M.P. Identification of Oropouche Orthobunyavirus in the cerebrospinal fluid of three patients in the Amazonas, Brazil. Am. J. Trop. Med. Hyg. 2012, 86, 732–735. [Google Scholar] [CrossRef]
- Chiang, J.O.; Azevedo, R.S.; Justino, M.C.A.; Matos, H.J.; Cabeça, H.L.S.; Silva, S.P.; Henriques, D.F.; Silva, E.V.P.; Andrade, G.S.S.; Vasconcelos, P.F.; et al. Neurological disease caused by Oropouche virus in northern Brazil: Should it be included in the scope of clinical neurological diseases? J. Neurovirol. 2021, 27, 626–630. [Google Scholar] [CrossRef]
- CDC (Center for Disease Control). Clinical Overview of Oropouche Virus Disease. 2025. Available online: https://www.cdc.gov/oropouche/hcp/clinical-overview/index.html (accessed on 20 September 2025).
- Schwartz, D.A.; Dashraath, P.; Baud, D. Oropouche Virus (OROV) in Pregnancy: An Emerging Cause of Placental and Fetal Infection Associated with Stillbirth and Microcephaly following Vertical Transmission. Viruses 2024, 16, 1435. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Chatterjee, P.; Tilak, R. Possibility of Invasion of Oropouche Virus (OROV) in Asia: A Real-Time Assessment is an Imperative Necessity. J. Commun. Dis. 2025, 57, 197–202. [Google Scholar] [CrossRef]
- Cenci Dietrich, V.; Costa, J.M.C.; Oliveira, M.M.G.L.; Aguiar, C.E.O.; Silva, L.G.O.; Luz, M.S.; Lemos, F.F.B.; de Melo, F.F. Pathogenesis and clinical management of arboviral diseases. World J. Virol. 2025, 14, 100489. [Google Scholar] [CrossRef]
- Wesselmann, K.M.; Postigo-Hidalgo, I.; Pezzi, L.; de Oliveira-Filho, E.F.; Fischer, C.; de Lamballerie, X.; Drexler, J.F. Emergence of Oropouche fever in Latin America: A narrative review. Lancet Infect. Dis. 2024, 24, e439–e452. [Google Scholar] [CrossRef] [PubMed]
- Moreli, M.L.; Aquino, V.H.; Cruz, A.C.R.; Figueiredo, L.T.M. Diagnosis of Oropouche virus infection by RT-nested-PCR. J. Med. Virol. 2002, 66, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Weidmann, M.; Rudaz, V.; Nunes, M.R.T.; Vasconcelos, P.F.C.; Hufert, F.T. Rapid detection of human pathogenic Orthobunyaviruses. J. Clin. Microbiol. 2003, 41, 3299–3305. [Google Scholar] [CrossRef] [PubMed]
- Wise, E.L.; Pullan, S.T.; Márquez, S.; Paz, V.; Mosquera, J.D.; Zapata, S.; Jackson, S.K.; Fejer, G.; Trueba, G.; Logue, C.H. Isolation of Oropouche Virus from Febrile Patient, Ecuador. Emerg. Infect. Dis. 2018, 24, 935–937. [Google Scholar] [CrossRef]
- Nascimento, V.A.D.; Santos, J.H.A.; da Silva Monteiro, D.C.; Pessoa, K.P.; Cardoso, A.J.L.; de Souza, V.C.; Abdalla, L.F.; Naveca, F.G. Oropouche virus detection in saliva and urine. Mem. Inst. Oswaldo Cruz 2020, 115, e190338. [Google Scholar] [CrossRef]
- Gopalsamy, R.G.; Barreto, M.D.S.; Santos, R.S.; Jesus, P.C.; Souza, J.B.; Sena, L.O.C.; Bezerra, G.V.B.; Santos, C.A.D.; Mota Santana, L.A.D.; Hariharan, G.; et al. A View on the Emerging Concern of Oropouche Fever in Brazil and Its Diagnosis. Int. J. Health Plan. Manag. 2025, 40, 519–521. [Google Scholar] [CrossRef]
- Porwal, S.; Malviya, R.; Sridhar, S.B.; Shareef, J.; Wadhwa, T. Mysterious Oropouche virus: Transmission, symptoms, and control. Infect Med. 2025, 4, 100177. [Google Scholar] [CrossRef]
- Livonesi, M.C.; De Sousa, R.L.M.; Badra, S.J.; Figueiredo, L.T.M. In vitro and in vivo studies of ribavirin action on Brazilian Orthobunyavirus. Am. J. Trop. Med. Hyg. 2006, 75, 1011–1016. [Google Scholar] [CrossRef]
- Livonesi, M.C.; de Sousa, R.L.M.; Badra, S.J.; Figueiredo, L.T.M. In vitro and in vivo studies of the interferon-alpha action on distinct Orthobunyavirus. Antivir. Res. 2007, 75, 121–128. [Google Scholar] [CrossRef]
- Furuta, Y.; Komeno, T.; Nakamura, T. Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proceed Jpn. Acad. Ser. B Phys. Biol. Sci. 2017, 93, 449–463. [Google Scholar] [CrossRef] [PubMed]
- Gowen, B.B.; Hickerson, B.T. Hemorrhagic fever of bunyavirus etiology: Disease models and progress towards new therapies. J. Microbiol. 2017, 55, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Acrani, G.O.; Tilston-Lunel, N.L.; Spiegel, M.; Weidmann, M.; Dilcher, M.; Andrade da Silva, D.E.; Nunes, M.R.T.; Elliott, R.M. Establishment of a minigenome system for Oropouche virus reveals the S genome segment to be significantly longer than reported previously. J. Gen. Virol. 2015, 96 Pt 3, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Stubbs, S.H.; Cornejo Pontelli, M.; Mishra, N.; Zhou, C.; de Paula Souza, J.; Mendes Viana, R.M.; Lipkin, W.I.; Knipe, D.M.; Arruda, E.; Whelan, S.P.J. Vesicular Stomatitis Virus Chimeras Expressing the Oropouche Virus Glycoproteins Elicit Protective Immune Responses in Mice. mBio 2021, 12, e0046321. [Google Scholar] [CrossRef]
- Barbosa, N.S.; Concha, J.O.; daSilva, L.L.P.; Crump, C.M.; Graham, S.C. Oropouche Virus Glycoprotein Topology and Cellular Requirements for Glycoprotein Secretion. J. Virol. 2023, 97, e0133122. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Wu, Z.; Feng, S.; Lu, K.; Zhu, W.; Sun, H.; Niu, G. Oropouche virus: A neglected global arboviral threat. Virus. Res. 2024, 341, 199318. [Google Scholar] [CrossRef]
- Scachetti, G.C.; Forato, J.; Claro, I.M.; Hua, X.; Salgado, B.B.; Vieira, A.; Simeoni, C.L.; Barbosa, A.R.C.; Rosa, I.L.; de Souza, G.F. Re-emergence of Oropouche virus between 2023 and 2024 in Brazil: An observational epidemiological study. Lancet Infect. Dis. 2025, 25, 166–175. [Google Scholar] [CrossRef]
- PAHO/WHO (Pan American Health Organization/World Health Organization). Public Health Risk Assessment Related to Oropouche virus (OROV) in the Region of the Americas General Risk Statement; PAHO/WHO: Washington, DC, USA, 2024. [Google Scholar]
- Naveca, F.G.; Nascimento, V.A.d.; de Souza, V.C.; Nunes, B.T.D.; Rodrigues, D.S.G.; Vasconcelos, P.F.d.C. Multiplexed reverse transcription real-time polymerase chain reaction for simultaneous detection of Mayaro, Oropouche, and Oropouche-like viruses. Mem. Inst. Oswaldo Cruz 2017, 112, 510–513. [Google Scholar] [CrossRef]
- World Health Organization. Disease Outbreak News—Oropouche Virus Disease—Cuba. 11 June 2024. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2024-DON521 (accessed on 29 September 2025).
- Paz, L. Bolivia (the Plurinational State of) International Health Regulations National Focal Point (IHR NFP). 2024. Available online: https://www.paho.org/en/topics/international-health-regulations (accessed on 10 September 2025).
- Bandeira, A.C.; Pereira, F.M.; Leal, A.; Santos, S.P.O.; Barbosa, A.C.; Souza, M.S.P.L.; de Souza, D.R.; Guimaraes, N.; Fonseca, V.; Giovanetti, M.; et al. Fatal Oropouche Virus Infections in Nonendemic Region, Brazil, 2024. Emerg. Infect. Dis. 2024, 30, 2370–2374. [Google Scholar] [CrossRef]
- Morrison, A.; White, J.L.; Hughes, H.R.; Guagliardo, S.A.J.; Velez, J.O.; Fitzpatrick, K.A.; Davis, E.H.; Stanek, D.; Kopp, E.; Dumoulin, P.; et al. Oropouche Virus Disease Among, U.S. Travelers—United States. Morb. Mortal. Wkly. Rep. 2024, 73, 769–773. [Google Scholar] [CrossRef]
- Peiró-Mestres, A.; Riera, E.; Flores Calderón, C.; Navero-Castillejos, J.; Martinez, M.J.; Camprubí-Ferrer, D. Emergence of Oropouche virus among international travelers: A growing concern in non-endemic areas. Enferm. Infecc. Microbiol. Clin. 2025, 43, 523–526. [Google Scholar] [CrossRef]
- Castilletti, C.; Mori, A.; Matucci, A.; Ronzoni, N.; Van Duffel, L.; Rossini, G.; Sponga, P.; D’Errico, M.L.; Rodari, P.; Cristini, F.; et al. Oropouche fever cases diagnosed in Italy in two epidemiologically non-related travellers from Cuba, late May to early. Euro Surveill. 2024, 29, 2400362. [Google Scholar] [CrossRef] [PubMed]
- Maier, M.A.; Neurohr, E.M.; Barreto-Miranda, I.; Gabriel, M.; Günther, S.; Bélard, S. Imported Oropouche fever to Germany in a returning traveller from Cuba. Infection 2025, 53, 753–754. [Google Scholar] [CrossRef] [PubMed]
- Gourjault, C.; Pezzi, L.; Doudier, B.; Minodier, P.; Klitting, R.; Cano, P.; Ayhan, N.; Touret, F.; Grard, G.; Durand, G.A.; et al. Persistence of Oropouche virus in body fluids among imported cases in France, 2024. Lancet Infect Dis. 2025, 25, e64–e65. [Google Scholar] [CrossRef] [PubMed]
- Bandeira, A.C.; da Silva, A.C.F.N.; Souza, M.; da Costa Saavedra, R.; Pereira, F.M.; de Oliveira Santos, S.P.; de Mello, A.L.E.S.; da Purificação, S.M.O.; de Souza, D.R.; de Almeida Lessa, A.A.; et al. Clinical profile of Oropouche Fever in Bahia, Brazil: Unexpected fatal cases. SciELO Prepr. 2024. [Google Scholar] [CrossRef]
- das Neves Martins, F.E.; Chiang, J.O.; Nunes, B.T.D.; Ribeiro, B.F.R.; Martins, L.C.; Casseb, L.M.N.; Henriques, D.F.; de Oliveira, C.S.; Maciel, E.L.N.; Azevedo, R.D.S.; et al. Newborns with microcephaly in Brazil and potential vertical transmission of Oropouche virus: A case series. Lancet Infect. Dis. 2025, 25, 155–165. [Google Scholar] [CrossRef]
- Ribeiro, B.F.R.; Barreto, A.R.F.; Pessoa, A.; Azevedo, R.d.S.d.S.; Rodrigues, F.d.F.; Borges, B.d.C.B.; Mantilla, N.P.M.; Muniz, D.D.; Chiang, J.O.; Fraga, L.R.; et al. Congenital Oropouche in Humans: Clinical Characterization of a Possible New Teratogenic Syndrome. Viruses 2025, 17, 397. [Google Scholar] [CrossRef]
- Gunter, K.B.; Bowen, J.M.; Clarke, A.T.; McFarlane, M.; Omoga, D.C.A.; Pozuelos, S.; Rogers, L.M.; Aronoff, D.M.; Vornhagen, J.; Brennan, B.; et al. From prototype to outbreak: Conserved pathogenesis of Oropouche virus in a novel murine pregnancy model highlights its public health implications. bioRxiv 2025, 2, 668287. [Google Scholar] [CrossRef]
- Castilletti, C.; Huits, R.; Mantovani, R.P.; Accordini, S.; Alladio, F.; Gobbi, F. Replication-Competent Oropouche Virus in Semen of Traveler Returning to Italy from Cuba. Emerg. Infect. Dis. 2024, 30, 2684–2686. [Google Scholar] [CrossRef]
- Iglói, Z.; Soochit, W.; Munnink, B.B.O.; Anas, A.A.; von Eije, K.J.; van der Linden, A.; Mandigers, M.; Wijnans, K.; Voermans, J.; Chandler, F.D.; et al. Oropouche Virus Genome in Semen and Other Body Fluids from Traveler. Emerg. Infect. Dis. 2025, 31, 205–206. [Google Scholar] [CrossRef]
- ECDC (European Centre for Disease Prevention and Control). Communicable Disease Threats Report, 2–8 August 2025, Week 32: Imported Oropouche Virus Disease Cases–EU/EEA and UK–2024/2025. Available online: https://www.ecdc.europa.eu/en/publications-data/communicable-disease-threats-report-2-8-august-2025-week-32 (accessed on 1 October 2025).
- Mancuso, E.; Severini, F.; Toma, L.; Marsili, G.; Casale, F.; Castilletti, C.; Gobbi, F.G.; Amendola, A.; Merakou, C.; Del Manso, M.; et al. Assessing the vector competence of Italian Culex pipiens and Aedes albopictus mosquitoes for the re-emerging Oropouche virus. Parasites Vectors 2025, 18, 268. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, S.; Groschup, M.H.; Garros, C.; Felippe-Bauer, M.L.; Purse, B.V. Culicoides biting midges, arboviruses and public health in Europe. Antivir. Res. 2013, 100, 102–113. [Google Scholar] [CrossRef]
- Wernike, K.; Eschbaumer, M.; Breithaupt, A.; Hoffmann, B.; Beer, M. Experimental infection of European mosquitoes with OROV. Vet. Microbiol. 2022, 159, 80–86. [Google Scholar]
- Payne, A.F.; Stout, J.; Dumoulin, P.; Locksmith, T.; Heberlein, L.A.; Mitchell, M.; Rodriguez-Hilario, A.; Dupuis, A.P.; Ciota, A.T. Lack of competence of US mosquito species for circulating Oropouche virus. Emerg. Infect. Dis. 2025, 31, 619–621. [Google Scholar] [CrossRef] [PubMed]
- Gallichotte, E.N.; Ebel, G.D.; Carlson, C.J. Vector competence for Oropouche virus: A systematic review of pre-2024 experiments. PLoS Negl. Trop. Dis. 2025, 19, e0013014. [Google Scholar] [CrossRef]
- Pinheiro, F.P.; Travassos da Rosa, A.P.A.; Gomes, M.L.C.; LeDuc, J.W.; Hoch, A.L. Transmission of Oropouche virus from man to hamster by the midge Culicoides paraensis. Science 1982, 215, 1251–1253. [Google Scholar] [CrossRef]
- Elliott, R.M.; Blakqori, G.; van Knippenberg, I.C.; Koudriakova, E.; Li, P.; McLees, A.; Shi, X.; Szemiel, A.M. Establishment of a reverse genetics system for Schmallenberg virus, a newly emerged Orthobunyavirus in Europe. J. Gen. Virol. 2013, 94, 851–859. [Google Scholar] [CrossRef]
- de Mendonça, S.F.; Rocha, M.N.; Ferreira, F.V.; Leite, T.H.J.F.; Amadou, S.C.G.; Sucupira, P.H.F.; Marques, J.T.; Ferreira, A.G.A.; Moreira, L.A. Evaluation of Aedes aegypti, Aedes albopictus, and Culex quinquefasciatus mosquitoes competence to Oropouche virus Infection. Viruses 2021, 13, 755. [Google Scholar] [CrossRef]
- McGregor, B.L.; Connelly, C.R.; Kenney, J.L. Infection, dissemination, and transmission potential of North American Culex quinquefasciatus, Culex tarsalis, and Culicoides sonorensis for Oropouche Virus. Viruses 2021, 3, 226. [Google Scholar] [CrossRef]
- Cardoso, B.F.; Serra, O.P.; Heinen, L.B.; Zuchi, N.; Souza, V.C.; Naveca, F.G.; Santos, M.A.; Slhessarenko, R.D. Detection of Oropouche virus segment S in patients and in Culex quinquefasciatus in the state of Mato Grosso, Brazil. Mem. Inst. Oswaldo Cruz 2015, 110, 745–754. [Google Scholar] [CrossRef]
- Azevedo, E.A.N.; Silva, A.F.D.; Silva, V.G.D.; Machado, L.C.; de Lima, G.B.; Ishigami, B.I.M.; Silva, K.M.P.E.; de O M da Costa, M.M.; Falcão, D.A.; Vasconcelos, A.P.; et al. Genomic and phenotypic characterization of the Oropouche virus strain implicated in the 2022–24 large-scale outbreak in Brazil. J. Med. Virol. 2024, 96, e70012. [Google Scholar] [CrossRef] [PubMed]
- Anonymous. Dicastery for Evangelisation. Jubilee 2025 Official Website. Holy See: Vatican City. 2025. Available online: https://www.iubilaeum2025.va/en.html (accessed on 10 August 2025).
- WHO (World Health Organization). Oropouche Virus Disease—Region of the Americas. Disease Outbreak News. 2024. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2024-DON545 (accessed on 20 September 2025).
- Capobianchi, M.R.; Castilletti, C.; Gobbi, F.G. Potential risks of Oropouche virus importation into Europe. J. Travel. Med. 2024, 31, taae109. [Google Scholar] [CrossRef] [PubMed]
- WHO (World Health Organization). WHO Rapid Risk Assessment–Oropouche Virus Disease, Region of the Americas v.2. WHO: Geneva, Switzerland. 2025. Available online: https://www.who.int/publications/m/item/who-rapid-risk-assessment---oropouche-virus-disease--region-of-the-americas-v.2 (accessed on 1 October 2025).
- Giorgi Rossi, P.; Sangalli, M.; Faustini, A.; Forestiere, F.; Perucci, C.A. Infectious diseases in Rome during the Millennium Year. Euro Surveill. 2003, 8, 181–185. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scotto, G.; Fazio, V.; Massa, S. Oropouche Virus: An Emerging Arboviral Threat and Its Implications for Europe. Life 2025, 15, 1674. https://doi.org/10.3390/life15111674
Scotto G, Fazio V, Massa S. Oropouche Virus: An Emerging Arboviral Threat and Its Implications for Europe. Life. 2025; 15(11):1674. https://doi.org/10.3390/life15111674
Chicago/Turabian StyleScotto, Gaetano, Vincenzina Fazio, and Salvatore Massa. 2025. "Oropouche Virus: An Emerging Arboviral Threat and Its Implications for Europe" Life 15, no. 11: 1674. https://doi.org/10.3390/life15111674
APA StyleScotto, G., Fazio, V., & Massa, S. (2025). Oropouche Virus: An Emerging Arboviral Threat and Its Implications for Europe. Life, 15(11), 1674. https://doi.org/10.3390/life15111674






