Assessment of Elbow Joint Incongruity as a Primary Cause of Canine Elbow Dysplasia: Comparative Imaging Analysis Using CT and Radiography in 108 Dogs—A Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Anesthesia Protocol
2.3. Radiographic Technique
2.4. CT Technique
2.5. Measurement Techniques
2.6. Statistical Analysis
- Student’s t-test for independent samples when comparing two groups;
- One-way analysis of variance (ANOVA) when comparing more than two groups;
- Tukey’s HSD (honestly significant difference) post hoc test for multiple pairwise comparisons, applied following rejection of the hypothesis of equality of all means, to determine between which groups the differences occurred;
- Fisher’s exact test to assess differences in the frequency of occurrence of phenomena between groups (differences between proportions).
- All analyses were conducted at a 5% level of significance using the STATISTICA data analysis software system, version 12 (StatSoft, Inc., Tulsa, OK, USA, 2014), except for Fisher’s exact test, which was performed using the PQStat software, version 1.6.2 (PQStat Software, Poznań, Poland, 2016).
3. Results
3.1. Test Results of Dogs from Group I
3.1.1. X-Ray Results for the Elbow Joints
3.1.2. CT Results of the Elbow Joints
3.2. Test Results of Dogs from Group II
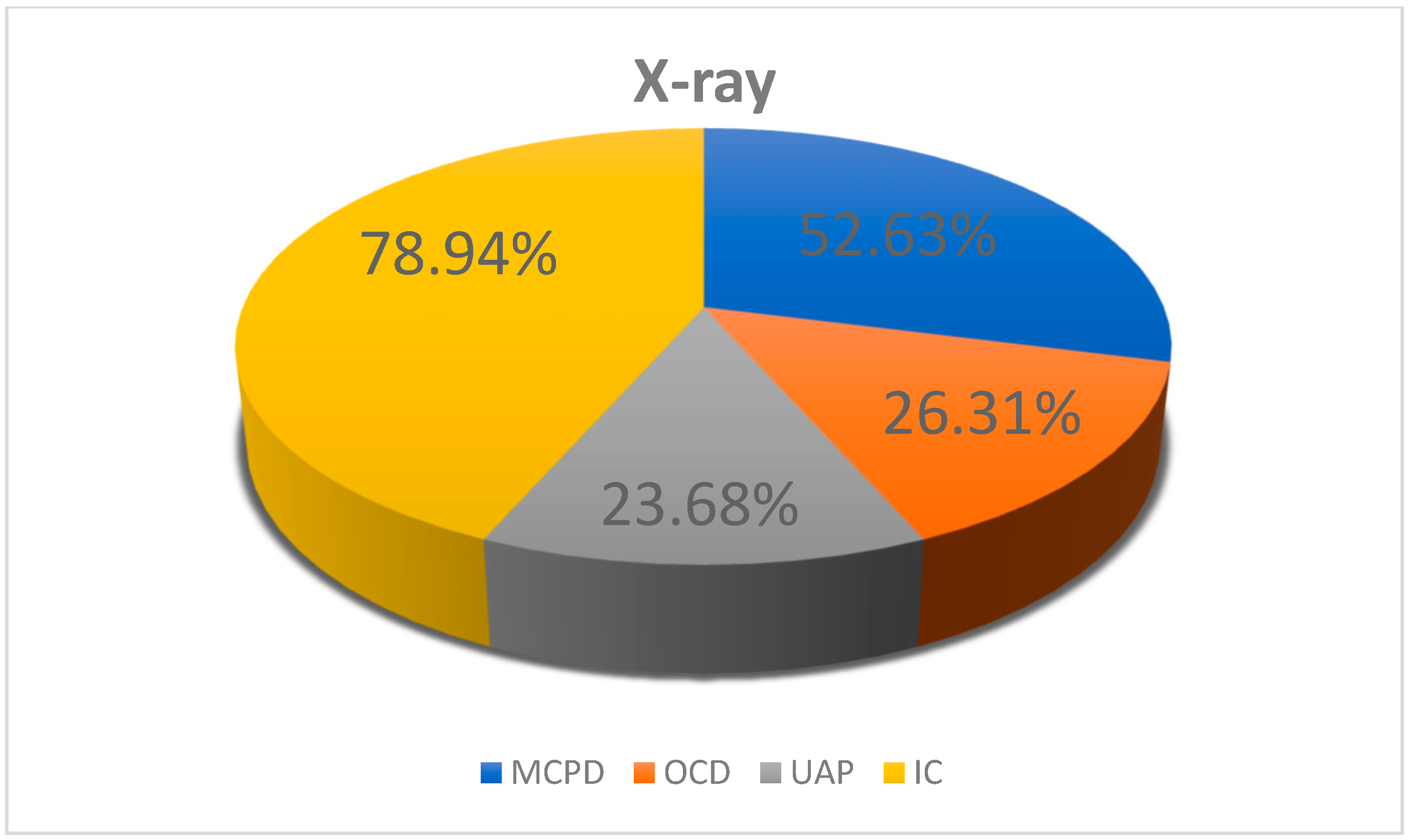
3.2.1. X-Ray Results of the Elbow Joints
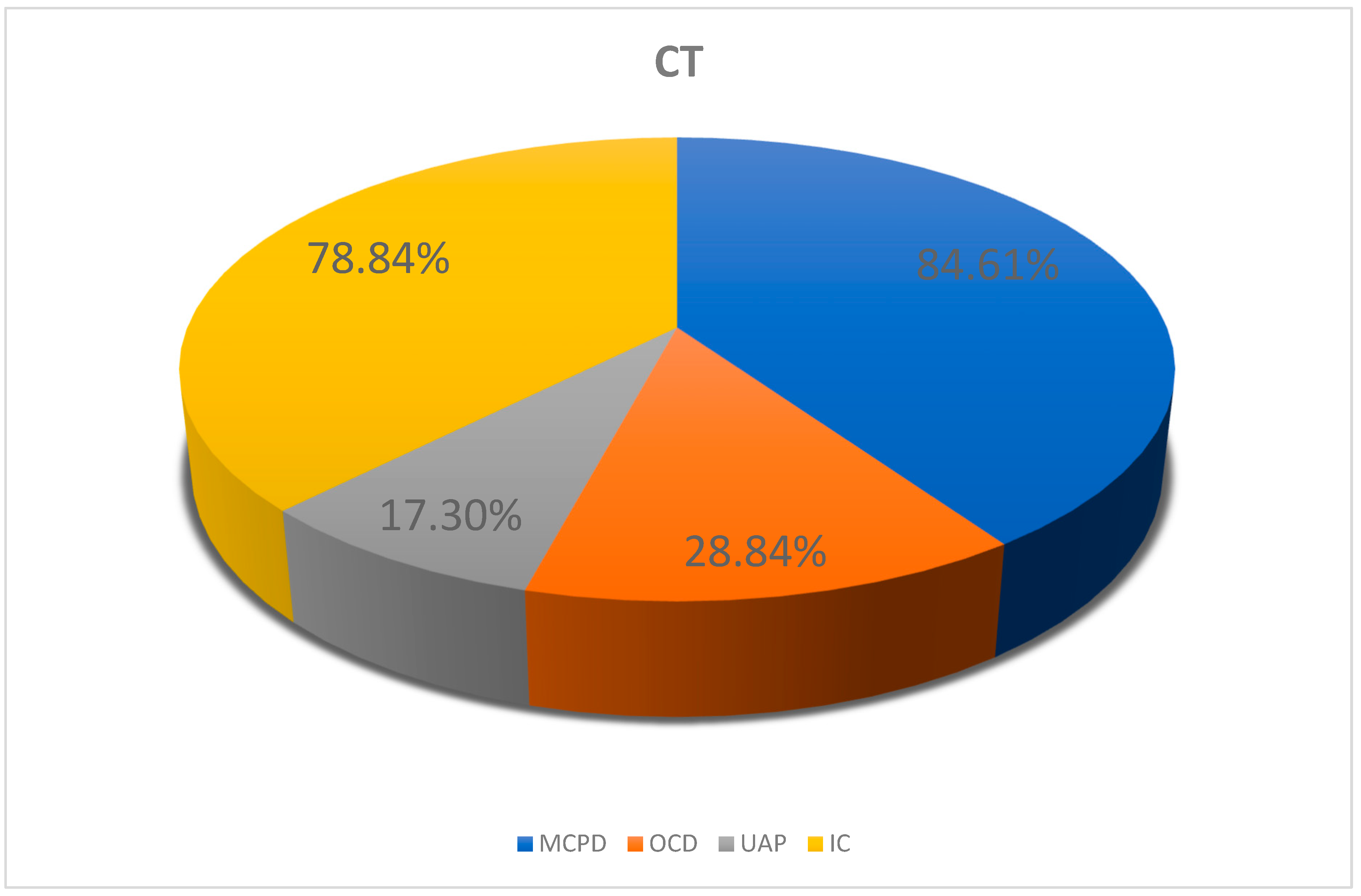
3.2.2. CT Results for the Elbow Joints
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ED | Elbow dysplasia |
| DJD | Degenerative joint disease |
| UAP | Ununited anconeal process |
| OCD | Osteochondritis dissecans |
| MCPD | Medial coronoid process disease |
| FMCP | Fragmented medial coronoid process |
| INC H-U | Humeroulnar incongruity |
| INC R-U | Radioulnar incongruity |
| INC H-R | Humeroradial incongruity |
| CT | Computed tomography |
| MPR | Multiplanar reconstruction |
| SD | Standard deviation |
References
- Kirberger, R.M.; Fourie, S.L. Elbow dysplasia in the dog: Pathophysiology, diagnosis and control. J. S. Afr. Vet. Assoc. 1998, 69, 43–54. [Google Scholar] [CrossRef]
- Lavrijsen, I.C.M.; Heuven, H.C.M.; Voorhout, G.; Meij, B.P.; Theyse, L.F.H.; Leegwater, P.A.J.; Hazewinkel, H.A.W. Phenotypic and genetic evaluation of elbow dysplasia in Dutch Labrador Retrievers, Golden Retrievers, and Bernese Mountain Dogs. Vet. J. 2012, 193, 486–492. [Google Scholar] [CrossRef]
- Robins, G.M. Some aspects of the radiographic examination of the canine elbow joint. J. Small Anim. Prac. 1980, 21, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Lindberg, A.; Fehr, M.; Nolte, I. Co-existence of ununited anconeal process and fragmented medial coronoid process of the ulna in the dog. J. Small Anim. Prac. 2006, 47, 61–65. [Google Scholar] [CrossRef]
- Samoy, Y.; Van Ryssen, B.; Gielen, I.; Walschot, N.; Van Bree, H. Review of the literature: Elbow incongruity in the dog. Vet. Comp. Orthop. Traumatol. 2006, 19, 1–8. [Google Scholar]
- Gemmill, T.J.; Clements, D.N. Fragmented coronoid process in the dog: Is there a role for incongruency? J. Small Anim. Prac. 2007, 48, 361–368. [Google Scholar] [CrossRef]
- Janutta, V.; Hamann, H.; Klein, S.; Tellhelm, B.; Distl, O. Genetic analysis of three different classification protocols for the evaluation of elbow dysplasia in German Shepherd Dogs. J. Small Anim. Pract. 2006, 47, 75–82. [Google Scholar] [CrossRef]
- Samoy, Y.; Gielen, I.; Van Bree, H.; Van Ryssen, B. Dysplastic elbow diseases in dog. Vlaams Diergeneeskd. Tijdschr. 2011, 80, 327–338. [Google Scholar] [CrossRef]
- Reichle, J.K.; Park, R.D.; Bahr, A.M. Computed tomographic findings of dogs with cubital joint lameness. Vet. Radiol. Ultrasound 2000, 41, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Janutta, V.; Hamann, H.; Klein, S.; Tellhelm, B.; Distl, O. Genetic evaluation of elbow angles as predictors of elbow dysplasia in German Shepherd Dogs. J. Vet. Med. A Physiol. Pathol. Clin. Med. 2005, 52, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Hazewinkel, H.A.W. Elbow Dysplasia; definitions and clinical diagnoses. In Proceedings of the 23rd Annual Meeting of the International Elbow Working Group, Dublin, Ireland, 20 August 2008; pp. 8–12. [Google Scholar]
- Dallago, M.; De Bakker, E.; Coppieters, E.; Saunders, J.; Gielen, I.; Van Ryssen, B. Medial coronoid disease in an eleven-year-old Labrador Retriever. Vlaams Diergeneeskd. Tijdschr. 2015, 84, 257–263. [Google Scholar] [CrossRef]
- How, K.L. Etiology of ununited anconeal process (UAP), osteochondritis dissecans (OCD) and elbow incongruity (EI). In Proceedings of the 33rd Annual Meeting of the International Elbow Working Group, Singapore, 24 September 2018; pp. 11–13. [Google Scholar]
- Narojek, T.; Fiszdon, K.; Hanysz, E. Canine elbow dysplasia in different breeds. Bull. Vet. Inst. Pulawy 2008, 52, 169–173. [Google Scholar]
- Hebel, M.; Panek, W.K.; Ruszkowski, J.J.; Nabzdyk, M.; Niedzielski, D.; Pituch, K.C.; Jackson, A.M.; Kiełbowicz, M.; Pomorska-Mól, M. Computed tomography findings in a cohort of 169 dogs with elbow dysplasia—A retrospective study. BMC Vet. Res. 2021, 17, 296. [Google Scholar] [CrossRef]
- Tromblee, T.C.; Jones, J.C.; Bahr, A.M.; Shires, P.K.; Aref, S. Effect of computed tomography display window and image plane on diagnostic certainty for characteristics of dysplastic elbow joints in dogs. Am. J. Vet. Res. 2007, 68, 858–871. [Google Scholar] [CrossRef]
- Moores, A.P.; Benigni, L.; Lamb, C.R. Computed tomography versus arthroscopy for detection of canine elbow dysplasia lesions. Vet. Surg. 2008, 37, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Samoy, Y.; Gielen, I.; Van Caelenberg, A.; Van Bree, H.; Duchateau, L.; Van Ryssen, B. Computed tomography findings in 32 joints affected with severe elbow incongruity and fragmented medial coronoid process. Vet. Surg. 2012, 41, 486–494. [Google Scholar] [CrossRef]
- Wind, A.P. Elbow incongruity and developmental elbow diseases in the dog: Part 1. J. Am. Anim. Hosp. Assoc. 1986, 22, 711–724. [Google Scholar]
- Brunnberg, L.; Viehman, B.; Waibl, H. Computergestütze auswertung von röntgenbildern zur erfassung von parametern der ellbogengelenkdysplasie teil 2: Stufenbildungen im gelenk. Kleintierpraxis 1999, 44, 637–646. [Google Scholar]
- Hazewinkel, H.A.W. Clinical diagnosis and surgical treatment of Elbow Dysplasia. In Proceedings of the 15th Annual Meeting of the International Elbow Working Group, Bangkok, Thailand, 24 October 2003. [Google Scholar]
- Proks, P.; Stehlík, L.; Irová, K.; Dvořák, M.; Srnec, R.; Nečas, A. Relationship between radioulnar incongruity of elbow joints and the type of fragmented processus coronoideus medialis. Acta Vet. Brno 2010, 79, 307–312. [Google Scholar] [CrossRef]
- Komsta, R.; Dębiak, P.; Twardowski, P. Radiographic evaluation of joints in dogs with elbow dysplasia—Clinical observations. Bull. Vet. Inst. Pulawy. 2008, 52, 179–183. [Google Scholar]
- Remy, D.; Neuhart, L.; Fau, D.; Genevois, J.P. Canine elbow dysplasia and primary lesions in German Shepherd Dogs in France. J. Small Anim. Pract. 2004, 45, 244–248. [Google Scholar] [CrossRef]
- Blond, L.; Dupis, J.; Beauregard, G.; Breton, L.; Moreau, M. Sensitivity of radiographic detection of canine elbow incongruence in an in vitro model. Vet. Radiol. Ultrasound. 2005, 46, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Mason, D.R.; Schulz, K.S.; Samii, V.F.; Fujita, Y.; Hornof, W.J.; Herrgesell, E.J.; Long, C.D.; Morgan, J.P.; Kass, P.H. Sensitivity of radiographic evaluation of radio-ulnar incongruence in the dog in vitro. Vet. Surg. 2002, 31, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.T.; Lewis, D.D.; Shiroma, J.T.; Neuwirth, L.A.; Parker, R.B.; Kubilis, P.S. Effect of radiographic positioning on interpretation of cubital joint incongruity in dogs. Am. J. Vet. Res. 1998, 11, 1351–1357. [Google Scholar] [CrossRef]
- Wagner, K.; Griffon, D.J.; Thomas, M.W.; Schaeffer, D.J.; Schulz, K.; Samii, V.F.; Necas, A. Radiographic, computed tomographic, and arthroscopic evaluation of experimental radio-ulnar incongruence in the dog. Vet. Surg. 2007, 36, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Griffon, D.J.; Mostafa, A.A.; Blond, L.; Schaeffer, D.J. Radiographic, computed tomographic, and arthroscopic diagnosis of radioulnar incongruence in dogs with medial coronoid disease. Vet. Surg. 2018, 47, 333–342. [Google Scholar] [CrossRef]
- Remy, D.; Maitre, P.; Cachon, T. Elbow dysplasia in German Shepherds: Sex-related differences, characteristics of unilateral and bilateral dysplasia associated osteoarthrosis. In Proceedings of the 23rd Annual Meeting of the International Elbow Working Group, Dublin, Ireland, 20 August 2008; pp. 29–30. [Google Scholar]
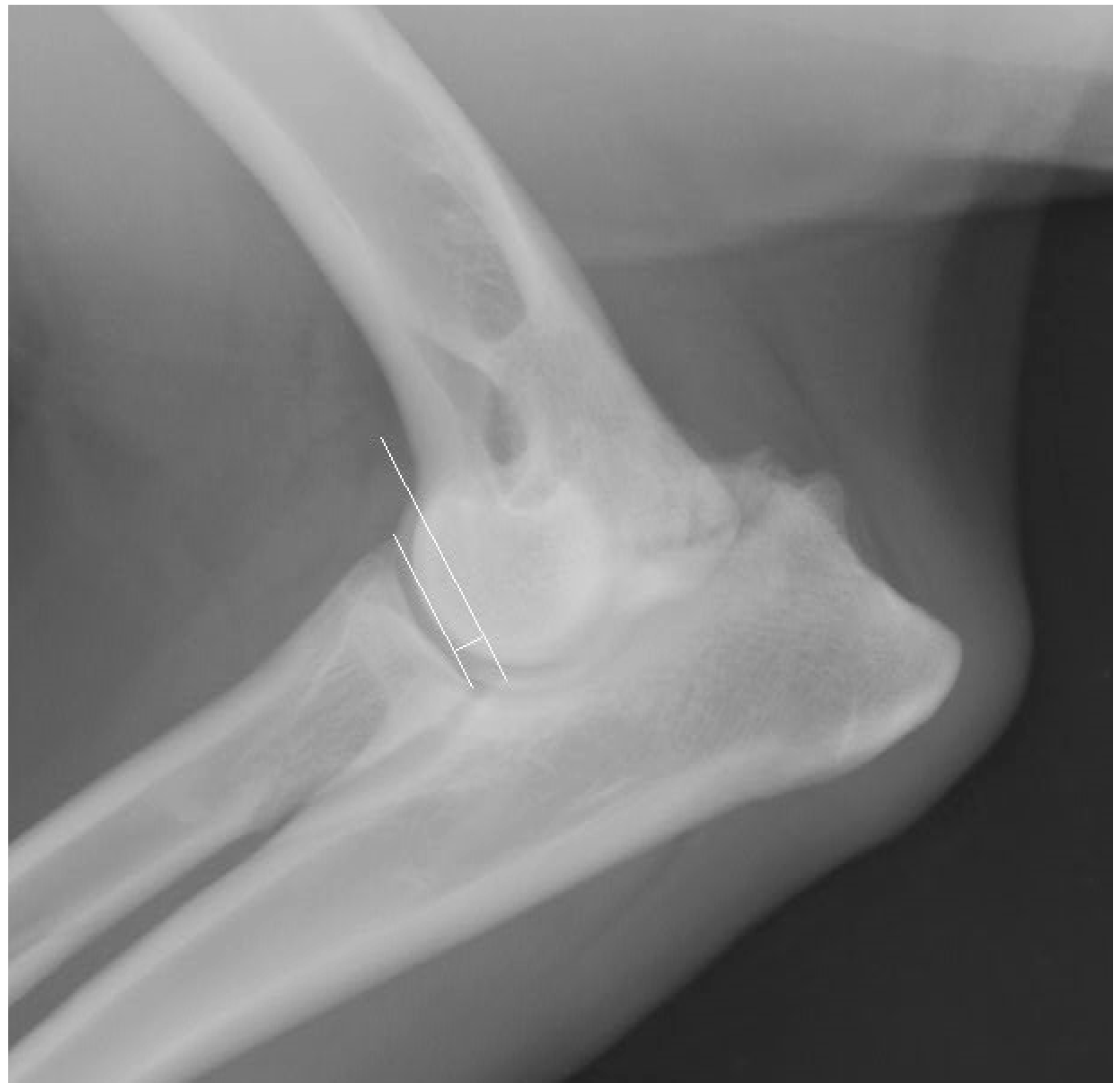
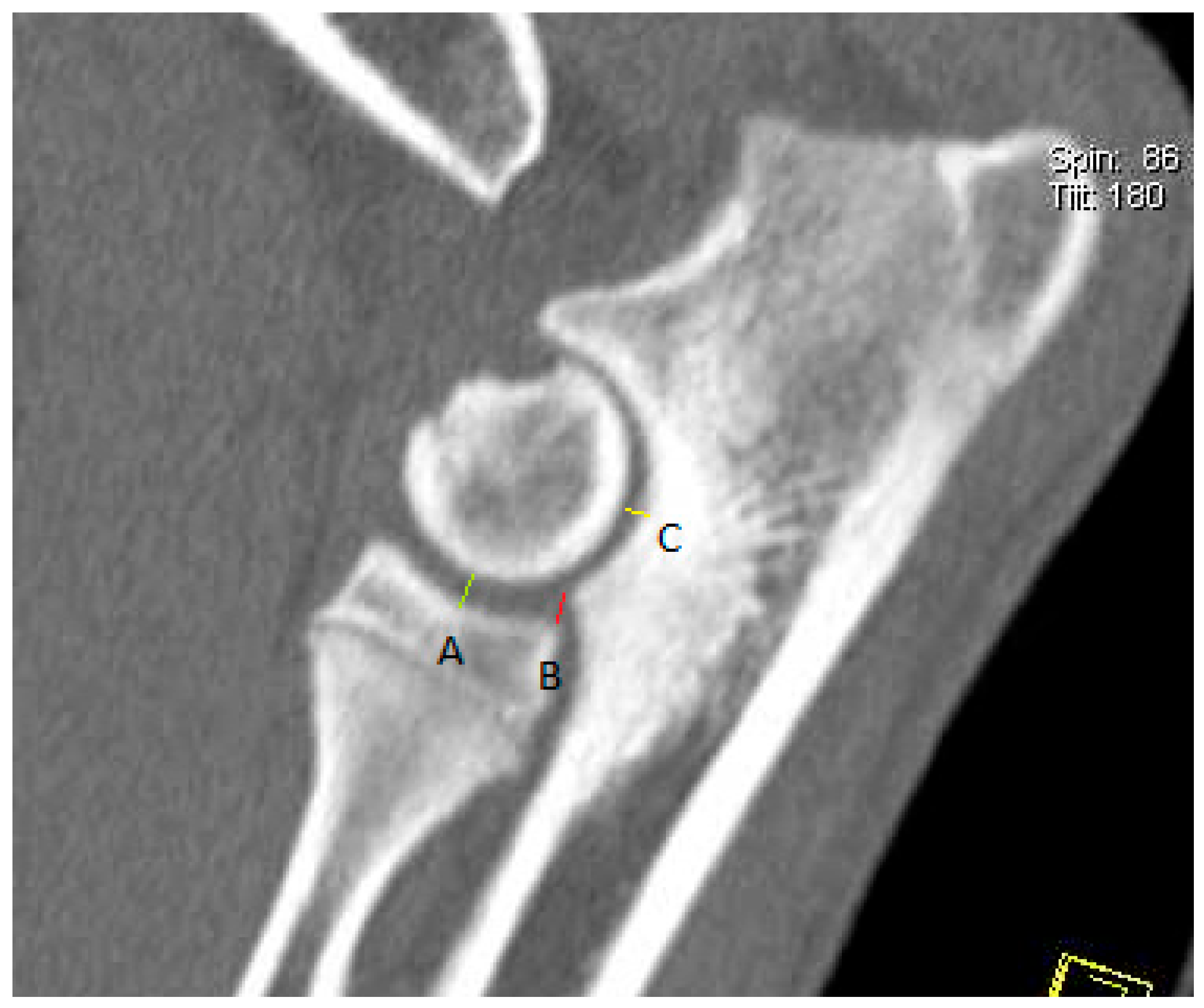

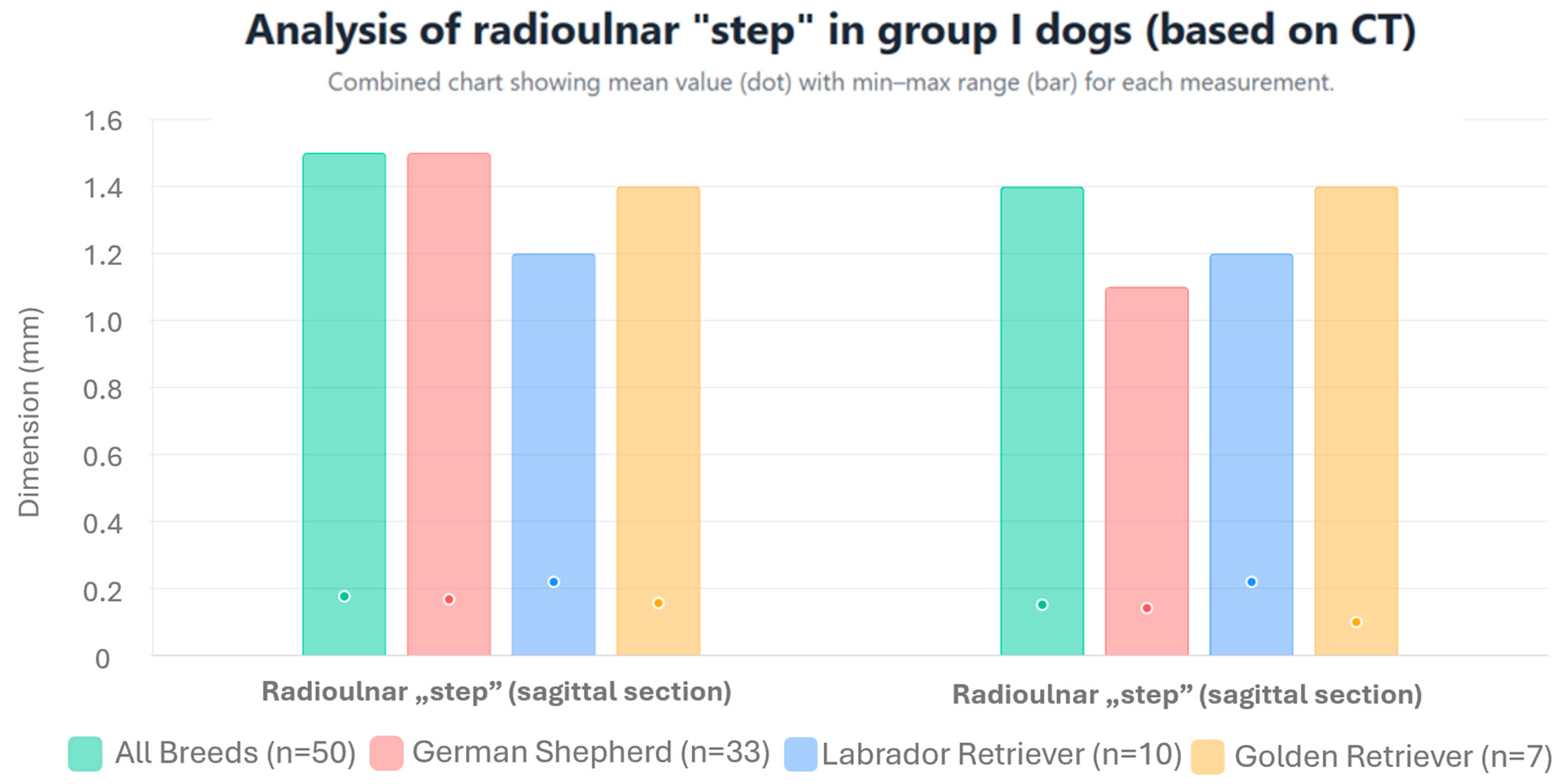
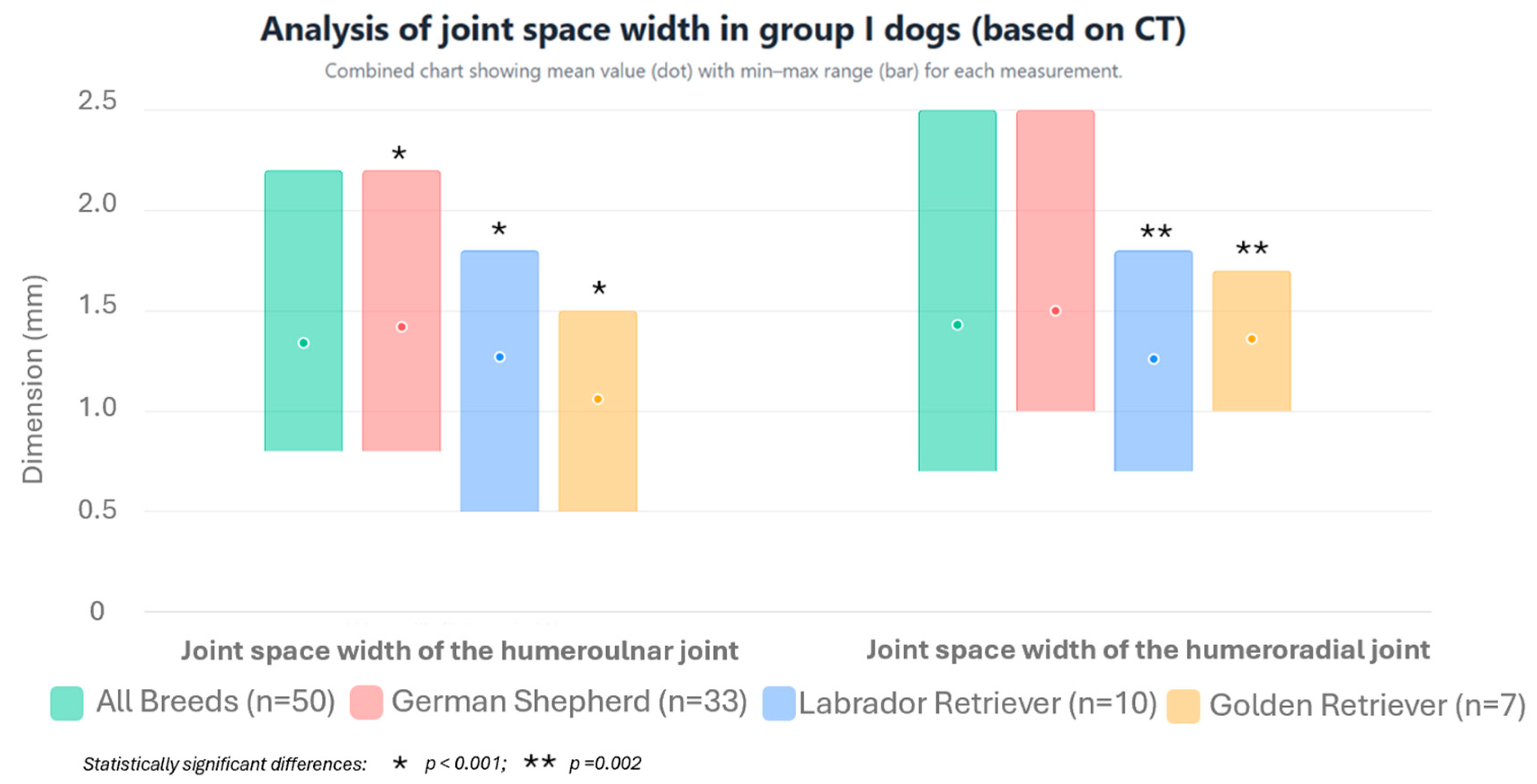

| MPR | Parameter | Unit | Group I | Group II | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Maximum | Minimum | Mean | SD | Maximum | Minimum | ||||
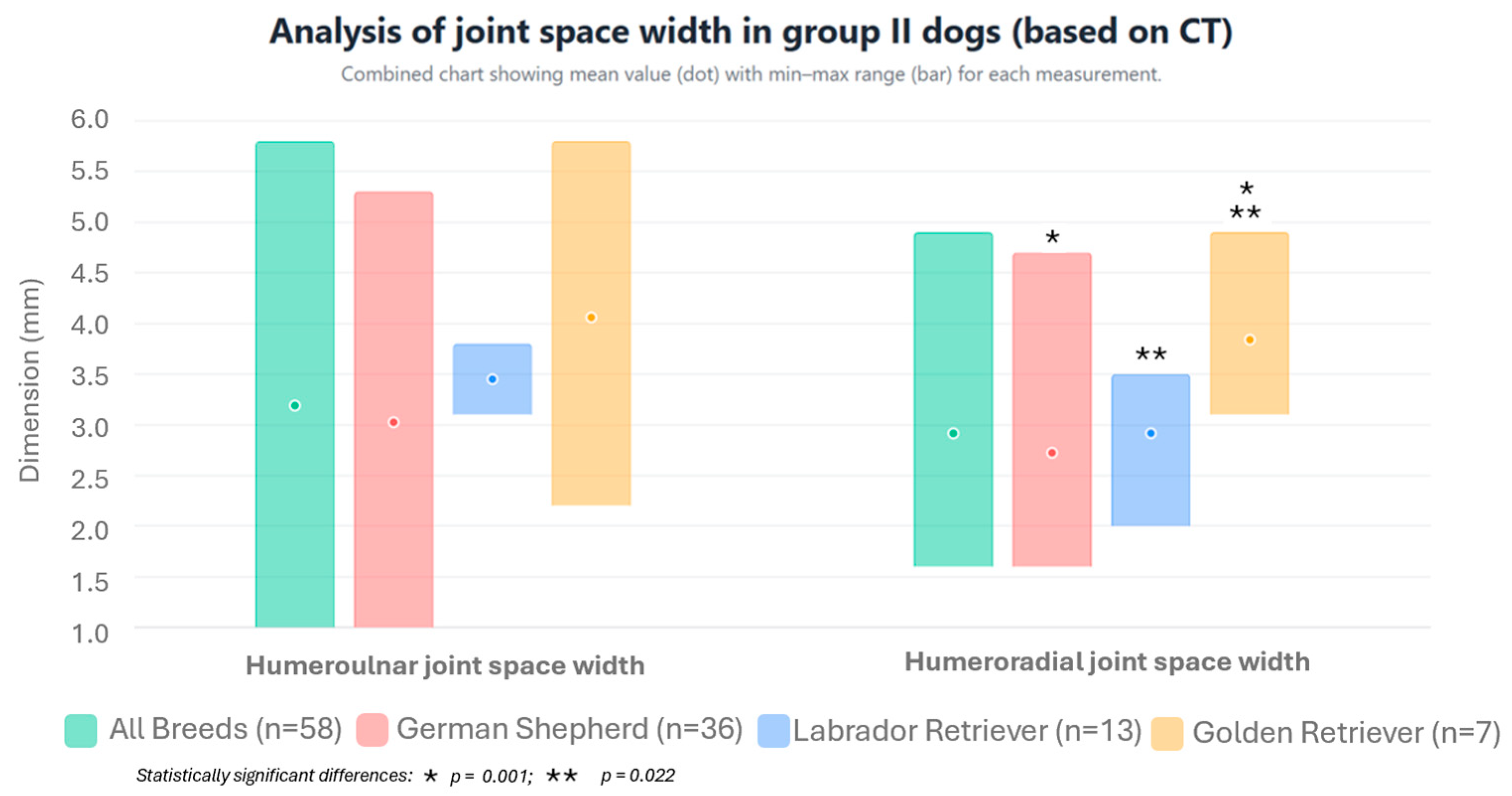
| All breeds | Sagittal section | Joint space of the humeroulnar joint | mm | 1.34 * | ±0.34 | 2.2 | 0.8 | 3.189 * | ±1.03 | 5.8 | 1.0 |
| Joint space of the humeroradial joint | mm | 1.43 ** | ±0.31 | 2.5 | 0.7 | 2.916 ** | ±0.702 | 4.9 | 1.6 | ||
| Radioulnar “step” | mm | 0.177 | ±0.333 | 1.5 | 0 | 2.33 | ±0.739 | 5.2 | 1.6 | ||
| Dorsal section | Radioulnar “step” | mm | 0.152 *** | ±0.312 | 1.4 | 0 | 2.255 *** | ±0.758 | 4.6 | 1.5 | |
| German Shepherds | Sagittal section | Joint space of the humeroulnar joint | mm | 1.42 ● | ±0.30 | 2.2 | 0.8 | 3.025 | ±0.975 | 5.3 | 1.0 |
| Joint space of the humeroradial joint | mm | 1.50 ●● | ±0.30 | 2.5 | 1.0 | 2.725 ◊ | ±0.620 | 4.7 | 1.6 | ||
| Radioulnar “step” | mm | 0.168 | ±0.321 | 1.5 | 0 | 1.967 | ±0.673 | 4.6 | 1.6 | ||
| Dorsal section | Radioulnar “step” | mm | 0.142 | ±0.294 | 1.1 | 0 | 2.148 | ±0.740 | 4.6 | 1.5 | |
| Labrador Retrievers | Sagittal section | Joint space of the humeroulnar joint | mm | 1.27 ● | ±0.31 | 1.8 | 0.5 | 3.45 | ±0.243 | 3.8 | 3.1 |
| Joint space of the humeroradial joint | mm | 1.26 ●● | ±0.31 | 1.8 | 0.7 | 2.917 ●●● | ±0.611 | 3.5 | 2.0 | ||
| Radioulnar “step” | mm | 0.22 | ±0.323 | 1.2 | 0 | 2.158 | ±0.472 | 3.0 | 1.7 | ||
| Dorsal section | Radioulnar “step” | mm | 0.22 | ±0.322 | 1.2 | 0 | 2.27 | ±0.472 | 3.1 | 1.7 | |
| Golden Retrievers | Sagittal section | Joint space of the humeroulnar joint | mm | 1.06 ● | ±0.40 | 1.5 | 0.5 | 4.06 | ±1.590 | 5.8 | 2.2 |
| Joint space of the humeroradial joint | mm | 1.36 | ±0.25 | 1.7 | 1.0 | 3.84 ●●●,◊ | ±0.713 | 4.9 | 3.1 | ||
| Radioulnar “step” | mm | 0.157 | ±0.416 | 1.4 | 0 | 3.0 | ±1.4 | 5.2 | 2.0 | ||
| Dorsal section | Radioulnar “step” | mm | 0.1 | ±0.374 | 1.4 | 0 | 2.72 | ±0.68 | 3.6 | 2.4 | |
| Breed | Radioulnar “Step” | Joint Space Width of the H–U Joint | Altered Shape of the Trocheal Notch of the Ulnar Bone | Altered Shape of the Humeral Trochlea |
|---|---|---|---|---|
| German Shepherd Dog | 36% | 15% | 11% | 2% |
| Labrador Retriever | 27% | 23% | 19% | 4% |
| Golden Retriever | 0% | 27% | 27% | 11% |
| Combination of Primary Causes of Elbow Dysplasia | Bilateral Elbow Dysplasia | Unilateral Elbow Dysplasia | Number of Cases | ||
|---|---|---|---|---|---|
| Left Elbow Joint | Right Elbow Joint | Left Elbow Joint | Right Elbow Joint | ||
| OCD + IC | - | - | - | OCD + IC | 1 (4.16%) |
| OCD + UAP | OCD + UAP | OCD + UAP | - | - | 2 (8.33%) |
| MCPD + UAP | MCPD + UAP | MCPD + UAP | - | - | 1 (4.16%) |
| UAP + IC | UAP + IC | UAP | - | - | 2 (8.32%) |
| - | - | UAP + IC | - | ||
| MCPD + IC | MCPD + IC (4) | MCPD + IC (4) | MCPD + IC (3) | 11 (45.89%) | |
| MCPD + UAP + IC | MCPD + UAP + IC (1) | MCPD + UAP + IC (1) | MCPD + UAP + IC (1) | 3 (12.5%) | |
| MCPD + OCD + IC | MCPD + OCD + IC | MCPD + IC | - | - | 4 (16.64%) |
| MCPD + OCD + IC | MCPD + OCD + IC | - | - | ||
| - | - | MCPD + OCD + IC | - | ||
| - | - | - | MCPD + OCD + IC | ||
| Breed | INC H-U | INC H-R | INC R-U |
|---|---|---|---|
| German Shepherd | 46% | 30% | 36% |
| Labrador Retriever | 23% | 19% | 42% |
| Golden Retriever | 22% | 27% | 27% |
| Combination of Primary Causes of Elbow Dysplasia | Bilateral Elbow Dysplasia | Unilateral Elbow Dysplasia | Number of Cases | ||
|---|---|---|---|---|---|
| Left Elbow Joint | Right Elbow Joint | Left Elbow Joint | Right Elbow Joint | ||
| MCPD + IC | MCPD + IC | IC | - | - | 22 (55%) |
| IC | MCPD + IC | - | - | ||
| MCPD + IC | MCPD + IC | - | - | ||
| MCPD | MCPD + IC | - | - | ||
| - | - | - | MCPD + IC | ||
| - | - | MCPD + IC | - | ||
| MCPD + OCD + IC | MCPD + OCD + IC | MCPD + IC | - | - | 9 (22.5%) |
| MCPD + IC | MCPD + OCD + IC | - | - | ||
| MCPD + OCD + IC | MCPD + OCD + IC | - | - | ||
| MCPD + IC | OCD | - | - | ||
| - | - | - | MCPD + OCD + IC | ||
| - | - | MCPD + OCD + IC | - | ||
| MCPD + OCD | MCPD + OCD | MCPD | - | - | 1 (2.5%) |
| MCPD + UAP + IC | MCPD + UAP + IC | MCPD | - | - | 4 (10%) |
| MCPD + UAP + IC | MCPD + IC | - | - | ||
| - | - | MCPD + UAP + IC | - | ||
| - | - | - | MCPD + UAP + IC | ||
| OCD + UAP | OCD + UAP | OCD + UAP | - | - | 1 (2.5%) |
| MCPD + UAP | MCPD + UAP | UAP | - | - | 1 (2.5%) |
| MCPD + UAP + OCD + IC | MCPD + UAP + OCD + IC | MCPD + UAP + OCD + IC | - | - | 2 (5%) |
| - | - | MCPD + UAP + OCD + IC | - | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kubiak-Nowak, D.; Kiełbowicz, Z.; Hebel, M.; Prządka, P.; Antończyk, A.; Jankowski, M. Assessment of Elbow Joint Incongruity as a Primary Cause of Canine Elbow Dysplasia: Comparative Imaging Analysis Using CT and Radiography in 108 Dogs—A Retrospective Study. Life 2025, 15, 1673. https://doi.org/10.3390/life15111673
Kubiak-Nowak D, Kiełbowicz Z, Hebel M, Prządka P, Antończyk A, Jankowski M. Assessment of Elbow Joint Incongruity as a Primary Cause of Canine Elbow Dysplasia: Comparative Imaging Analysis Using CT and Radiography in 108 Dogs—A Retrospective Study. Life. 2025; 15(11):1673. https://doi.org/10.3390/life15111673
Chicago/Turabian StyleKubiak-Nowak, Dominika, Zdzisław Kiełbowicz, Mateusz Hebel, Przemysław Prządka, Agnieszka Antończyk, and Marcin Jankowski. 2025. "Assessment of Elbow Joint Incongruity as a Primary Cause of Canine Elbow Dysplasia: Comparative Imaging Analysis Using CT and Radiography in 108 Dogs—A Retrospective Study" Life 15, no. 11: 1673. https://doi.org/10.3390/life15111673
APA StyleKubiak-Nowak, D., Kiełbowicz, Z., Hebel, M., Prządka, P., Antończyk, A., & Jankowski, M. (2025). Assessment of Elbow Joint Incongruity as a Primary Cause of Canine Elbow Dysplasia: Comparative Imaging Analysis Using CT and Radiography in 108 Dogs—A Retrospective Study. Life, 15(11), 1673. https://doi.org/10.3390/life15111673






