Abstract
This study examines whether white matter integrity mediates the link between psychological stress and skin aging. This cross-sectional study included 92 healthy Japanese adults (aged 22–62 years) who underwent diffusion tensor imaging to obtain Fractional Anisotropy Brain Healthcare Quotients (FA-BHQs) for major white matter tracts, while skin aging was assessed using Motion Scan Technology. Correlation analyses revealed significant associations among stress, skin aging, and FA-BHQ in the corpus callosum (CC) and internal capsule (IC). Mediation analyses suggested, at the statistical level, a potential that the CC fully mediates the association between stress and skin aging. These findings suggest a relationship between interhemispheric white matter integrity, psychological stress, and skin aging in line with the concept of the brain–skin axis.
1. Introduction
Skin aging manifests in various forms, with wrinkles and unwanted pigmentation being its most prominent characteristics [1]. During the aging process, skin undergoes a contraction of its surface, leading to the widening of the sulcus cutis and cristae cutis. The cristae cutis are the inward projections of the epidermis into the dermis at the dermal-epidermal junction, as seen histologically in vertical sections [2]. This interdigitated organization of cristae cutis and dermal papillae at the dermoepidermal interface increases the contact surface area between the epidermis and dermis, thereby enhancing dermal–epidermal adhesion. This process of widened cristae cutis thus also results in the formation of wrinkles [3]. Although photo-aging caused by UV radiation accounts for the majority of visible skin changes, recent evidence also links psychological stress to skin conditions [4,5,6].
In the brain, stress responses are mediated mainly through the hypothalamic–pituitary–adrenal (HPA) axis. Corticotropin-releasing hormone (CRH), adrenocorticotropin (ACTH), cortisol, and glucocorticoids (GC) are primarily produced as part of the stress response through the activation of the HPA axis. These stress hormones could influence skin conditions in several ways. Specifically, cortisol primarily affects the immune system by acting as an immunosuppressant [7]. CRH acts on epidermal melanocytes as a survival factor under starvation stress (anti-apoptotic) and as an inhibitor of growth factor-induced cell proliferation [8] and has been shown to exhibit pro-inflammatory effects in mast cells and keratinocytes [9,10]. ACTH also shows a pro-inflammatory effect in keratinocytes and induces differentiation in sebocytes [11,12].
Given the central role of the brain in regulating stress responses and their systemic effects on the skin, it is crucial to consider the neural pathways involved in stress processing. White matter (WM) tracts play a critical role in neural communication and in the integration of stress-related signals. Based on the existing evidence, we select seven regions of white matter [13] as regions of interest that have been implicated in the pathophysiology of anxiety, stress, and emotional disorders, including the internal capsule (IC), corpus callosum (CC), fornix, anterior corona radiata (ACR), cingulum, uncinate fasciculus (UF), and superior longitudinal fasciculus (SLF). To measure the integrity of white matter, we used the Fractional-Anisotropy Brain Healthcare Quotient (FA-BHQ), which is calculated using the FA-BHQ method developed by Nemoto et al. (2017) [14].
We selected these regions because previous studies have shown that their microstructure is associated with several emotion-related disorders, including major depressive disorder (MDD) and schizophrenia [15,16]. Specifically, the fornix, being part of the limbic system, is involved in the regulation of emotions by higher order frontal cortical brain regions [17,18]. Similarly, the UF, which connects the orbitofrontal cortex to the temporal lobe, is involved in emotional regulation, and its reduced integrity has been associated with heightened anxiety and major depressive disorder [18,19]. The cingulum, another limbic white matter tract, has also been associated with emotional processing, with structural abnormalities linked to anxiety disorders and attentional bias toward negative interpersonal stimuli [20,21]. The CC, a critical structure for interhemispheric communication, integrates cognitive and emotional processes. Alterations in its integrity have been reported in anxiety and stress-related disorders, as well as aggressive behavior in bipolar disorder [22,23]. Additionally, the anterior limb of the IC serves as a site of convergence for thalamic radiations involved in emotional processing [24]. The ACR, which connects thalamic and prefrontal regions, has been implicated in worry prediction and emotional control [25]. Also, the limbic-thalamo-cortical circuit includes thalamic projections from the IC to the prefrontal cortex. ACR functions as a part of emotion regulation systems, as a decrease in FA in ACR has been shown to be linked to bipolar I patients [26]. Finally, the SLF, a major association tract linking the parietal and frontal lobes, plays a role in attention, executive function, and emotion regulation, with reduced integrity observed in PTSD, OCD, and depression [27,28]. Table 1 summarizes the functions of regions of interest in stress and emotion regulation.

Table 1.
Functions of regions of interest in stress and emotion regulation.
Taken together, although previous studies have highlighted the roles of stress and white matter integrity in emotional processes, it remains unclear how these factors relate to skin aging. The present study aims to address this gap by examining the associations among psychological stress, skin aging, and white matter integrity, with a focus on key tracts. By investigating the potential mediating role of white matter integrity, this study provides novel insights into the brain–skin axis, emphasizes the importance of considering neural pathways when exploring the impact of stress on skin health, and also explores possible underlying mechanisms.
To our knowledge, this is the first study to investigate the role of key white matter tracts in the relationship between skin condition—indicated by the number of cristae cutis—and stress. To examine this, we conduct an analysis to explore the mediating effect of FA-BHQ in the skin aging process.
2. Materials and Methods
2.1. Subjects
Using G*Power 3.1.9.7, the required sample size for a correlation analysis with a medium effect size (r = 0.3), 5% significance level, and 80% power was calculated as 82. This follows Cohen’s (1988) guidelines [29]. To account for a potential dropout rate of about 5% and the reduction in degrees of freedom caused by inclusion of control variables, we aimed to collect data from 90 participants. Between 24 May and 17 June 2022, 95 healthy Japanese adults were recruited from Tokyo through a smartphone application developed by an IT company for businesspeople.
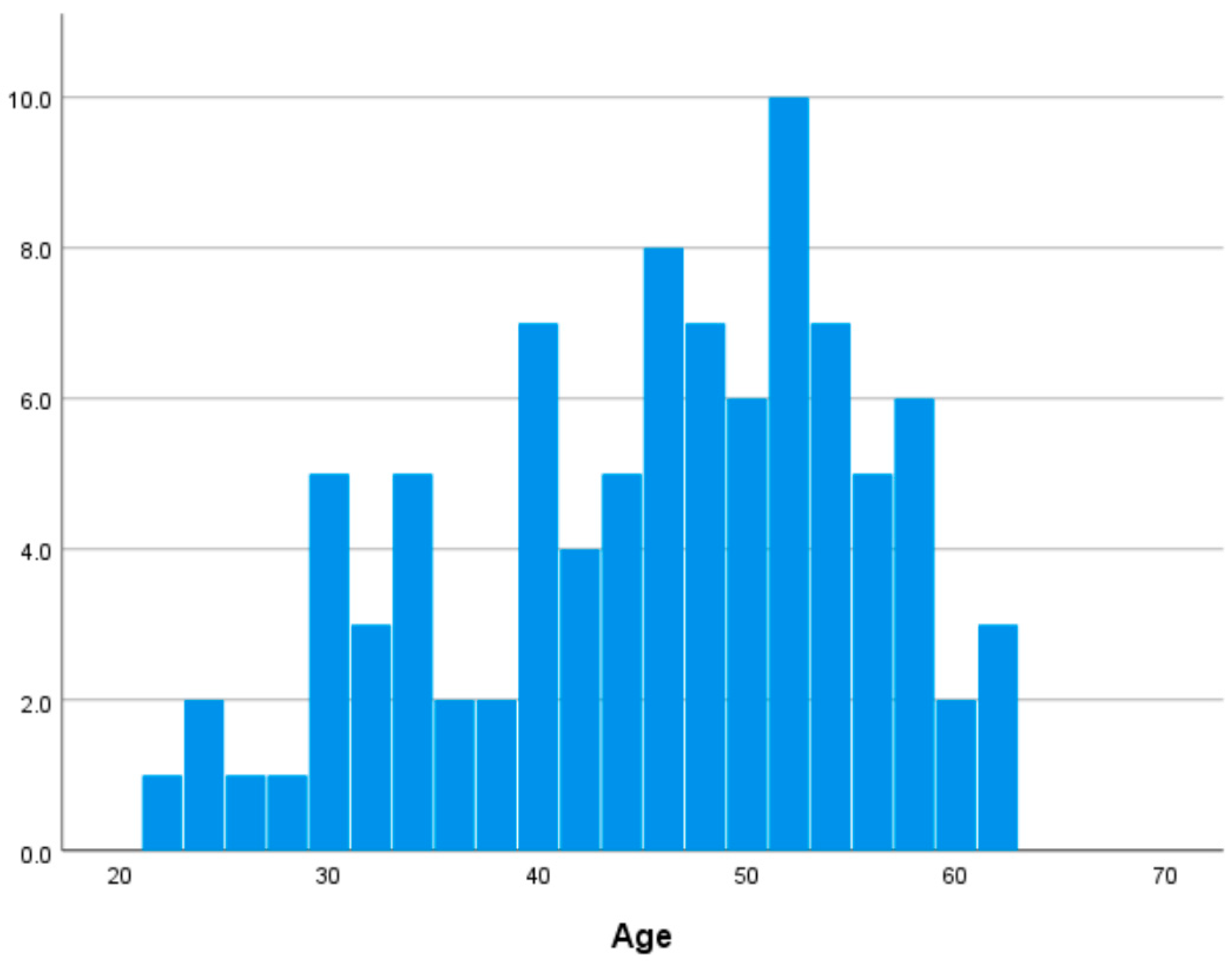
In this study, 92 participants (62 females and 30 males, aged 22–62 years, 45.076 ± 9.965 years) were included in the final analysis, excluding three individuals due to missing brain imaging data. Self-reported medical histories indicated that none of the participants had neurological, psychiatric, or other medical conditions that could influence the central nervous system. Table S3 in Supplementary Materials shows descriptive statistics of major variables. Figure 1 shows the distribution of age in our dataset.
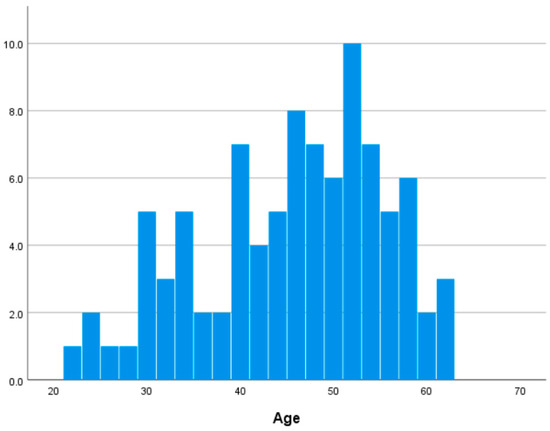
Figure 1.
Distribution of age.
This study was approved by the Ethics Committee of the Institute of Science Tokyo (Approval Numbers: 2022130). All procedures were conducted in accordance with relevant guidelines and regulations and the principles outlined in the Declaration of Helsinki. Written informed consent was obtained from all participants prior to their involvement, and anonymity was rigorously maintained throughout the study.
2.2. Skin Data
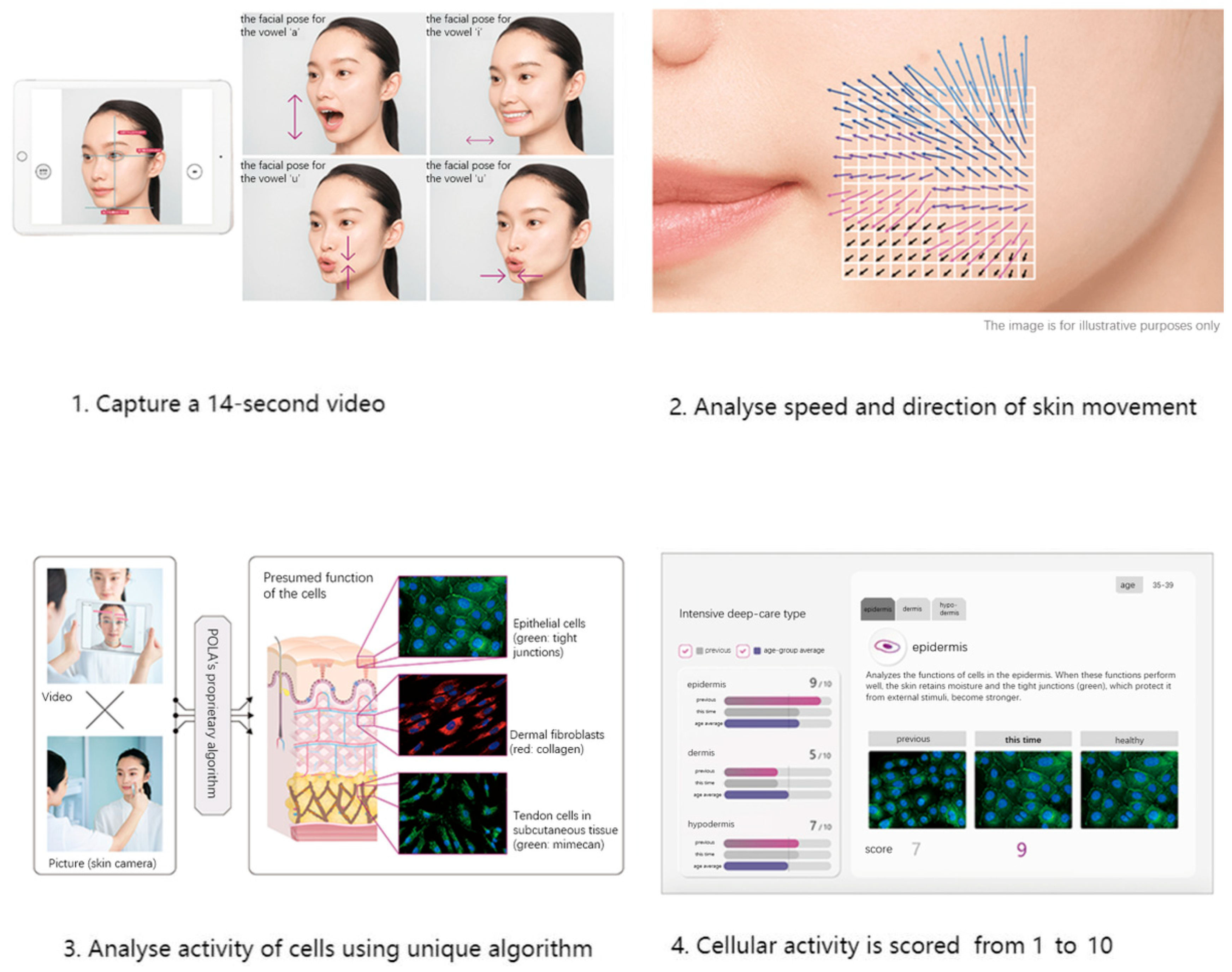
We used skin data provided by POLA Inc. (2-2-3, Nishi-Gotanda, Shinagawa-ku, Tokyo 141-8523, Japan) [30] using Motion Scan Technology developed by POLA Inc. Figure 2 shows the detailed process of this technology. This technology has been extensively applied in research on skin aging and in the field of dermatology [31,32].
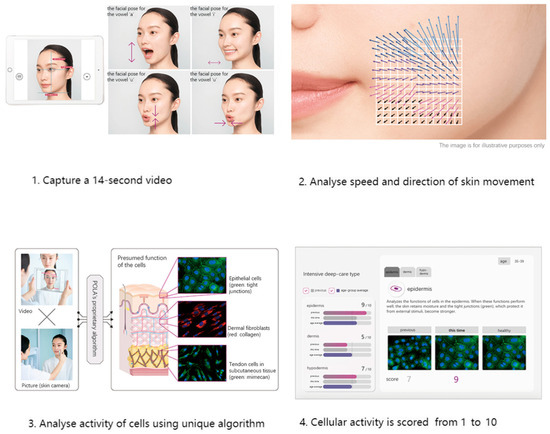
Figure 2.
Workflow of Motion Scan Technology developed by POLA Inc.
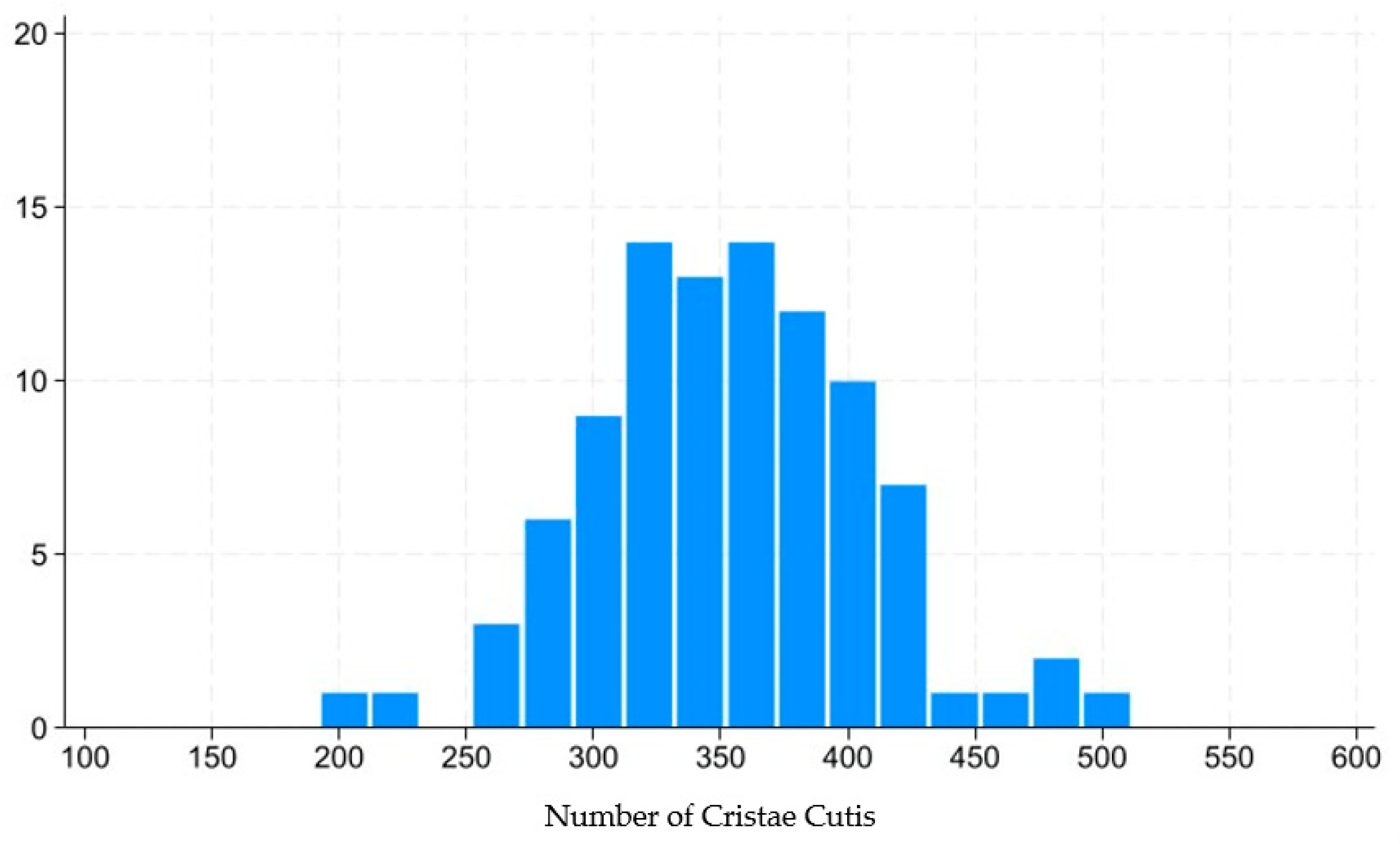
This technology captures the dynamic motion of the skin’s surface to estimate the condition of the underlying skin layers, including the epidermis, dermis, and subcutaneous tissue. The system analyzes various characteristics of skin movement, such as speed and direction, extracting approximately 1.7 million data points from a 14 s video sequence. Initially, the camera aligned with the subject’s face to capture a video, which is equivalent to approximately 840 still frames. For each frame, the speed and direction of skin movement were segmented and analyzed, revealing distinctive features. POLA’s proprietary algorithm was then employed to estimate the cellular activity within the three skin layers (epidermis, dermis, and subcutaneous tissue) within approximately three minutes. The outcomes were presented on an analysis screen, where cellular activity in each layer was rated on a scale from 1 to 10. Figure 3 shows distribution of skin scale, which in this paper stands for number of cristae cutis. A higher number of cristae cutis indicates smoother skin, whereas a lower count is associated with increased wrinkles and a greater degree of skin aging. The number of cristae cutis has been shown to be closely related to skin texture and aging [33,34]. Such a method for assessing facial skin aging has also been widely employed in studies evaluating the efficacy of cosmetic formulations, as well as in investigations of skin aging and its associated environmental factors [35,36].
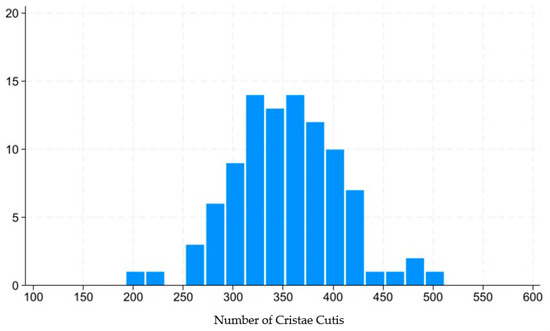
Figure 3.
Distribution of number of cristae cutis.
2.3. Demographic Scales
To assess demographic factors, we assessed eight variables: body mass index (BMI), sex, sleep status, sleep time, alcohol consumption, exercise, UV exposure, and stress.
BMI in kg/m2 was calculated from height and weight measurements. Sleep status was categorized into 4 levels, ranging from “completely unsatisfied” to “perfectly satisfied.” Sleep time was derived from the Pittsburgh Sleep Quality Index Japanese Version (PSQI-J), based on questions about usual wake-up time, average nightly sleep duration, and difficulty falling asleep within 30 min [37,38,39]. Alcohol consumption was measured on seven levels, from “0 mL per day” to “more than 900 mL per day.” Exercise was measured using the IPAQ met-light indicator, which measures low levels of physical activity and was conducted using the International Physical Activity Questionnaire (IPAQ) Japanese Version. It was measured using MET-minute/week units, which is equivalent to kilocalories for a 60 kg person. Kilocalories were computed from MET-minutes using the following equation: MET-min x (weight in kilograms/60 kg) [40,41]. For example, the official IPAQ guidelines (IPAQ group (2005)) define physical activity intensity as 3.3, 4.0, and 8.0 METs for walking, moderate intensity, and vigorous intensity, respectively [42]. UV exposure was used to measure the extent to which participants were exposed to ultraviolet rays in their daily lives, and it was assessed with the question “Do you often get exposed to ultraviolet (UV) rays?” and categorized into four levels: “Strongly agree,” “Agree,” “Slightly agree,” and “Disagree.” Stress was assessed using the Japanese version of the Profile of Mood States 2nd Edition (POMS2), a validated instrument designed to evaluate individual mood states and generate a comprehensive mood profile [43,44,45]. POMS2 contains a summary scale called Total Mood Disturbance (TMD), which has been widely used in previous studies as an indicator of stress levels and was also employed in our research for this purpose [46,47].
Our control variables included BMI, sex, Gray Matter Brain Healthcare Quotient (GM-BHQ), sleep status, sleep time, alcohol consumption, exercise, and UV exposure. BMI was included as a control variable due to its established correlation with WM FA, as demonstrated in numerous studies [48,49]. Additionally, BMI was positively correlated with loss of skin elasticity after controlling for age [50]. Therefore, controlling for BMI is essential when examining the relationship between WM FA as well as the relationship between age and skin scale. Moreover, studies have highlighted the importance of lifestyle—such as sleep quality and duration, alcohol consumption, and physical activity or exercise—on the skin aging process [51,52]. Studies have shown that low sleep quality is associated with increased signs of intrinsic aging, and also that it is associated with being perceived as having hanging eyelids, more wrinkles, and more droopy corners of the mouth [53,54]. Alcohol consumption has also been shown to be connected to skin aging, as chronic alcohol consumption may lead to tolerance of HPA axis-activating effects and, like prolonged glucocorticoid exposure, contribute to premature or exaggerated aging [52,55].
It has also been pointed out that physical activity could on one hand improve skin health by enhancing collagen, circulation, and lowering cortisol, but on the other hand may also cause barrier damage, excess oil, and infections [56].
In addition to the variables noted above, we also included sex and UV exposure as control variables, as sex steroids and UV rays may also significantly influence skin aging patterns [57,58,59]. Additionally, given that prior research has identified a positive correlation between gray matter volume, behavioral activation, and social factors [14,60], Gray Matter GM-BHQ [13], which represents gray matter volume as a standardized score, taking into account that the degree of atrophy in gray matter varies by region, was also included as a covariate.
2.4. MRI Data Acquisition
MRI data were acquired on a 3-T Siemens scanner (MAGNETOM Prisma, Siemens, Munich, Germany) with a 32-channel head coil. A high-resolution structural image was obtained using a 3D T1-weighted magnetization-prepared rapid-acquisition gradient echo pulse sequence, with the following parameters: TR = 1900 ms, TE = 2.52 ms, TI = 900 ms, flip angle = 9°, matrix = 256 × 256, FOV = 256 mm, and slice thickness = 1 mm. DTI data were collected using SE-EPI with GRAPPA, aligned to the orbitomeatal line. DTI parameters included: TR = 14,100 ms, TE = 81 ms, flip angle = 90°, matrix = 114 × 114, FOV = 224 mm, slice thickness = 2 mm, a baseline image (b = 0 s/mm2), and 30 diffusion directions with b = 1000 s/mm2.
2.5. FA-BHQ and GM-BHQ
FA was calculated using the FA-BHQ method developed by Nemoto et al. (2017) [14]. The FA-BHQ method has been validated through its positive correlations with cognitive function [61], as well as with the personal traits of spiritual growth, happiness, and work engagement [62,63,64]. Additionally, negative correlations have been observed with anxiety [65] and fatigue [66]. The FA-BHQ has also been recognized as an international standard (H.861.1, 2018) [67] and is considered a reliable measure for assessing brain health in healthy adults, as in this population.
T1-weighted images were preprocessed and analyzed using SPM12 on MATLAB R2015b. Each MPRAGE image was segmented into gray matter (GMP), white matter (WM), and cerebrospinal fluid (CSF), with GM images normalized using the exponentiated lie algebra (DARTEL) algorithm. A modulation step was applied during preprocessing to adjust for regional volume and maintain the total GM volume prior to warping. After modulation and spatial normalization, images were smoothed with an 8 mm half-maximum (FWHM) Gaussian kernel. Intracranial volume (ICV) was calculated by summing GM, WM, and CSF volumes. To account for variations in whole-brain volume across participants, proportional GM images were generated by dividing the smoothed GM image by the ICV. These proportional GM images were then used to calculate mean and standard deviation (SD) images for all participants.
The GM-BHQ was calculated as an IQ-like measure, with a mean of 100 and an SD of 15. The formula used was 100 + 15 × (individual proportional GM−mean)/SD. Regional GM quotients were extracted using the AAL atlas and averaged to create participant-specific GM-BHQs.
DTI data were preprocessed using FSL 5.0.11. First, all diffusion images were aligned to the initial b0 image, and eddy current correction was applied to address motion and distortion. Following these corrections, FA images were generated using DTIFit. The FA images were then spatially normalized to MNI space using FLIRT and FNIRT. Mean and SD images were derived from all FA images, and individual FA quotient images were calculated using the formula 100 + 15 × (individual FA−mean)/SD. Regional FA quotients were extracted from the JHU DTI-based WM atlas and averaged across regions to create participant-specific FA-BHQs. For more details, see Nemoto et al. (2017) [14].
2.6. Statistical Analysis
We first conducted correlation analysis to examine the relationship between subscales of FA-BHQ and skin scale, which was measured by number of cristae cutis. A p-value threshold of 0.05 was adopted for statistical significance, and the Benjamini–Hochberg (BH) method was used to control for multiple comparisons. Based on the results of partial correlation, mediation analysis of specific subscales of FA-BHQ in relationship between stress and skin aging was conducted. The covariates included in both the partial correlation and mediation analyses were derived from demographic information, including sex, age, GM-BHQ, BMI, sleep status, sleep time, alcohol consumption, UV exposure, and exercise, as previously noted.
All analyses in this study were conducted using R studio (R version: 4.4.1) and SPSS version 26 (IBM Corporation, Armonk, NY, USA) with PROCESS_V4.2_beta package (models 4), which uses bootstrapping with higher power and more accurate confidence intervals, and STATA 18 SE (StataCorp, College Station, TX, USA).
3. Results
The results of the correlation analysis are presented in Supplementary Materials Table S4 and Supplementary Materials Figure S1, and the results of the partial correlation analysis using control variables noted above are adjusted using the BH method to control for multiple comparisons (see Table 2 and Supplementary Materials Figure S2). Based on these findings, we selected the regions whose FA-BHQ subscales remained significant after BH correction and thus conducted an analysis to explore the mediation effect of those specific subscales of FA-BHQ in the relationship between stress and skin aging, and the results are shown in Figure 4 and Figure 5. The covariates included in both the partial correlation and mediate analyses were derived from demographic information, including sex, age, GM-BHQ, BMI, sleep status, sleep time, alcohol consumption, UV exposure, and exercise, as previously noted.

Table 2.
Results of partial correlation analysis between FA-BHQ subscales, stress and skin scale controlled for GM-BHQ, age, BMI, sex, sleep status, sleep time, alcohol consumption, exercise, and UV exposure.
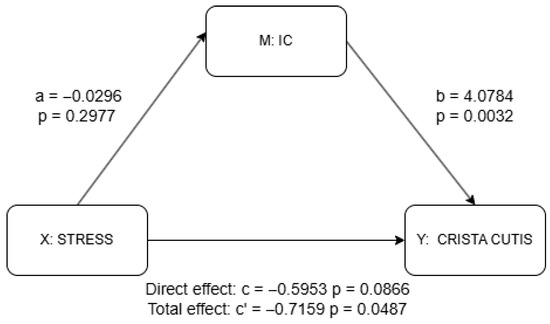
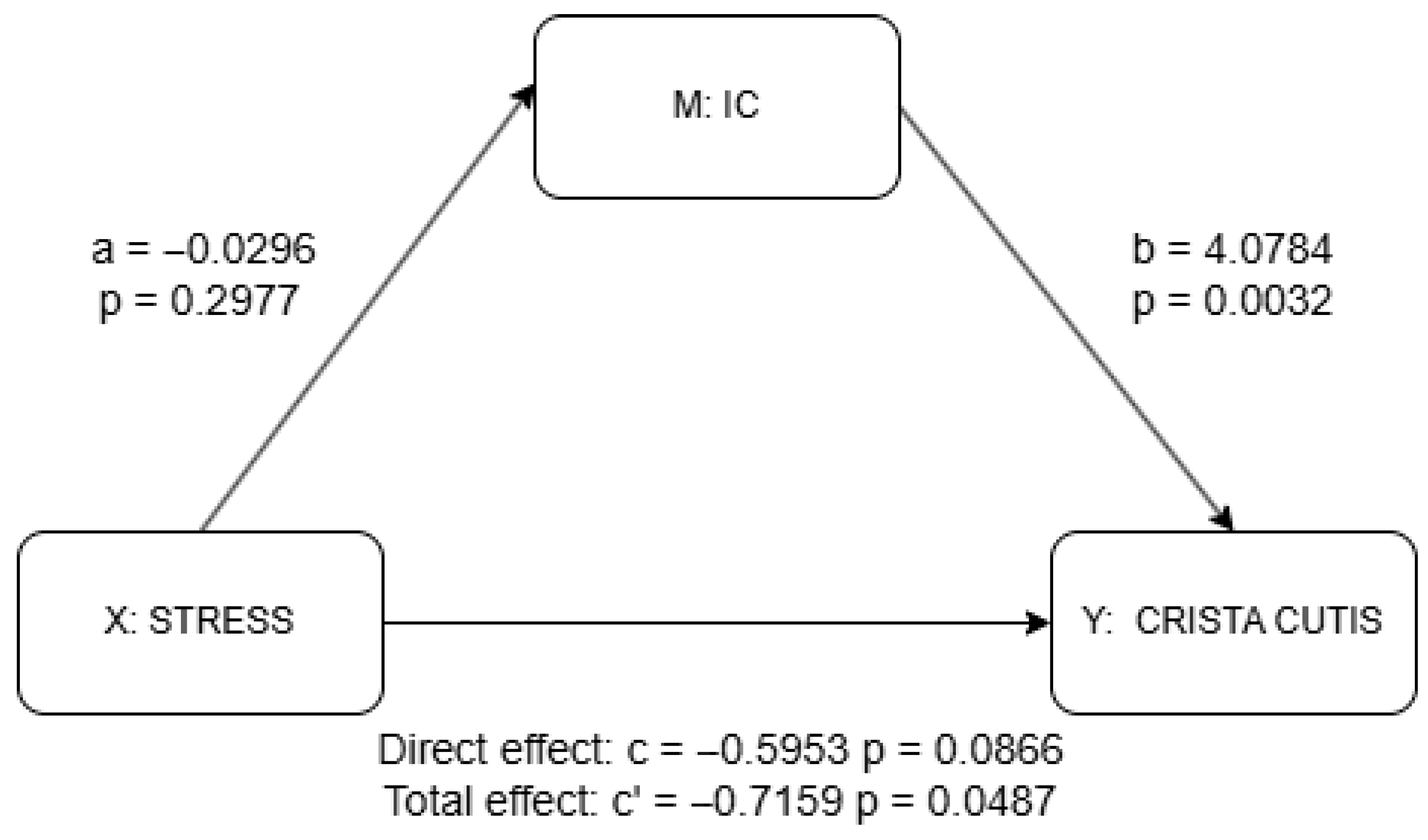
Figure 4.
Mediation analysis results for IC using PROCESS package. a = effect of X (stress) on M (IC); b = effect of M (IC) on Y (skin scale) controlling for X (stress); c’ = total effect of X (stress) on Y (skin scale) without M (IC); c = direct effect of X (stress) on Y (skin scale) controlling for M (IC). Detailed results shown in Supplementary Materials Table S2.
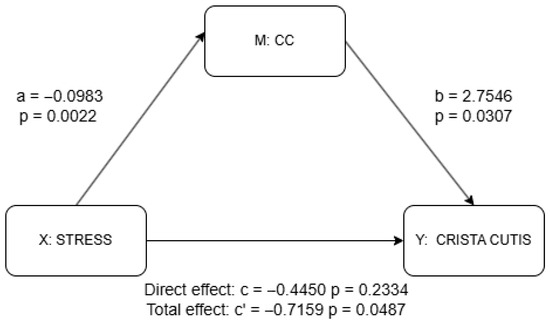
Figure 5.
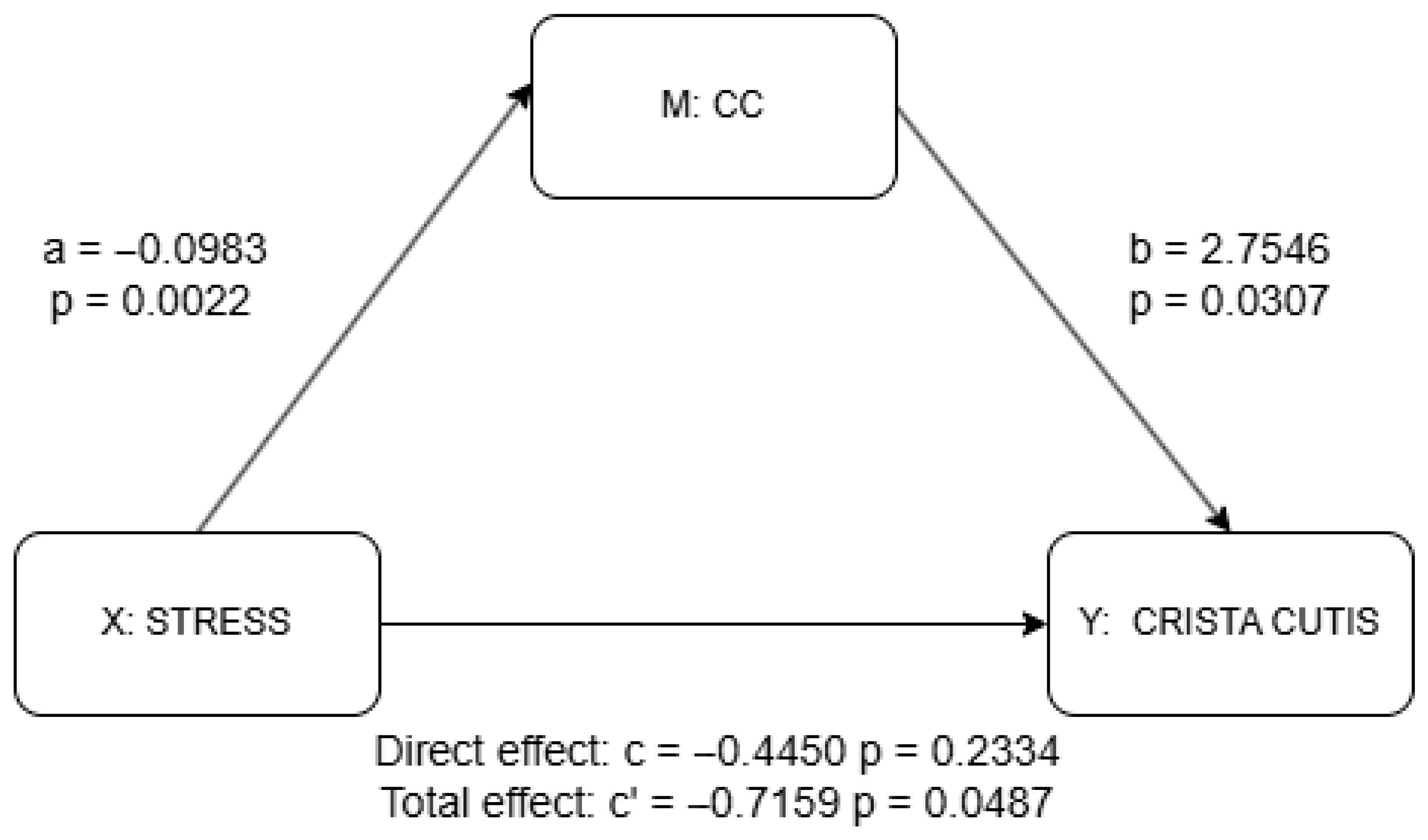
Mediation analysis results for CC using PROCESS package. a = effect of X (stress) on M (CC); b = effect of M (CC) on Y (skin scale) controlling for X (stress); c’ = total effect of X (stress) on Y (skin scale) without M (CC); c = direct effect of X (stress) on Y (skin scale) controlling for M (IC). Detailed results shown in Supplementary Materials Table S1.
Partial correlation coefficients between the FA-BHQ subscales and skin scale, controlling for all covariates, are presented in Table 2, while scatter plots illustrating these relationships, including non-significant pairs, are shown in Supplementary Materials Figure S2. Statistically significant correlations at the 5% level were observed for the following pairs: skin scale and corpus callosum (CC) (r = 0.316, p = 0.001), skin scale and internal capsule (IC) (r = 0.365, p = 0.004), anterior corona radiata (ACR) (r = 0.241, p = 0.028), stress (r = −0.231, p = 0.036), and skin scale. However, only CC and IC pairs passed the BH method.
The results of the mediation analysis as discussed previously are shown in Figure 4 and Figure 5 as well as in Supplementary Materials Tables S1 and S2. There was no significant effect of stress on the IC (a = −0.030, p = 0.298), although the IC significantly predicted higher skin scale scores (b = 4.078, p = 0.003). While the total effect of stress on skin scale was statistically significant (c = −0.716, p = 0.049), the direct effect was not (c′ = −0.595, p = 0.087), suggesting a possible indirect pathway through the IC, though the lack of a significant a-path weakens support for mediation. In contrast, stress was significantly negatively associated with the CC (a = −0.098, p = 0.002), and the CC, in turn, significantly predicted skin scale (b = 2.755, p = 0.031). Although the total effect of stress on skin scale remained significant (c = −0.716, p = 0.049), the direct effect was not statistically significant (c′ = −0.445, p = 0.233), indicating that the CC may serve as a mediator. Taken together, only the CC provides statistically significant evidence of mediation. The absence of a significant direct effect further suggests the possibility of full mediation in this pathway.
4. Discussion
The speed of human skin aging is primarily influenced by photoaging; however, recent evidence also indicates that psychological stress plays a contributing role in this process. A reduction in the number of cristae cutis, often also observed as an increase in wrinkles, is one of the key expressions of skin aging. In this study, we aim to evaluate how brain WM may mediate the skin aging process driven by stress using mediation analysis. The results indicate that, across all examined regions with significant correlation with skin scale, the effect of stress on the number of cristae cutis is fully mediated by CC.
When the body experiences stress, the skin can respond through the immune system and inflammation. In the brain, stress primarily influences the skin via the hypothalamic–pituitary–adrenal (HPA) axis. Several previous studies have examined the relationship between hormones released during this process and stress-induced changes in skin condition.
This starts with neurons in the hypothalamus releasing corticotropin-releasing hormone (CRH). The CRH travels to the pituitary gland, where it attaches to CRH receptor type-1 (CRH-R1). This triggers the release of neuropeptides derived from proopiomelanocortin, such as adrenocorticotropin (ACTH). ACTH then moves through the bloodstream to the adrenal cortex, where it binds to MC2 receptors in the outer layer and prompts the production of glucocorticoids, including cortisol and corticosterone [68]. In skin, CRH and ACTH function in various ways. For example, in mast cells, CRH triggers degranulation and increases vascular permeability [9]. Also, CRH has been found to have age-related effects on the skin. In aged skin, there is an upregulation of CRH in sebaceous glands and an increased expression of CRHR1 in hair follicles and the epidermis [69]. ACTH, similarly, functions in skin via immune system. For example, ACTH stimulates IL-18 production in skin keratinocytes. IL-18 is a pro-inflammatory cytokine that enhances T-cell activity and promotes T helper type 2 (Th2) cytokines production [11].
Psychological stress is also associated with negative effects on WM microstructure through immune mediators and stress-related hormones [13,60]. The CC is the largest interhemispheric commissure in the human brain, connecting regions such as the anterior cingulate cortex and orbitofrontal cortex in both hemispheres, which are crucial for mood regulation [70]. Previous studies have found that rhesus monkeys exposed to early maternal maltreatment exhibit reduced FA in the CC, which was associated with elevated cortisol levels during infancy [71]. Additionally, stress has been shown to be related to reductions in FA in the CC, as evidenced in cases of PTSD in adolescents, as well as in patients with MDD and schizophrenia [15,16,72].
Based on the existing evidence, FA values in the CC are closely linked to exposure to psychological stress, particularly during early developmental periods [73,74,75]. Reduced FA in this white matter region may reflect disruptions in neural pathways critical for emotional processing and stress regulation—circuits often impaired in stress-related psychiatric conditions such as post-traumatic stress disorder (PTSD), major depressive disorder, bipolar disorder, and schizophrenia [76,77,78]. While it remains uncertain whether diminished white matter integrity directly contributes to dysregulation of the HPA axis, these structural alterations may indicate underlying vulnerabilities or damage associated with chronic stress or traumatic experiences. Given that activation of the HPA axis influences skin health through inflammatory and immune responses, individuals with lower FA values in CC may be more susceptible to accelerated skin aging via neuroendocrine and immunological mechanisms, or higher FA values in the CC may reflect some forms of stress resilience, as suggested in the previous literature [79].
To our knowledge, this is the first study to demonstrate a relationship between skin aging and stress and how it is associated with brain white matter microstructure. These findings suggest potential implications for the integration of skin anti-aging care, brain health promotion, and stress reduction. Reducing stress may improve mental health and may also slow aspects of skin aging. A preventive approach could combine stress management, brain-function monitoring, and evidence-based skin care to support overall well-being.
5. Limitation
There are three major limitations in this study.
Firstly, the sample size is relatively limited. Although the available dataset allows for statistical analysis, the restricted number of observations and cross-sectional data leaves challenges in ensuring the robustness and generalizability of the findings, particularly regarding the interpretation of full mediation. Future studies should use larger and more comprehensive panel data samples to strengthen the validity and reliability of the results.
Secondly, this study primarily provides correlational evidence regarding the potential stress-related link between WM microstructure and skin aging. However, it is important to note that, although we controlled for several related variables such as addictive habits, other confounding factors still may be involved in this relationship which were not fully accounted for in the present analysis. For example, data on hormonal status, dermatologic conditions, and medication use were not available in the current study. Therefore, causal interpretation of the results remains limited. Future research should aim to control for additional confounding variables and explore more comprehensive mechanisms underlying the association between stress, WM microstructure, and skin aging.
Another limitation of the present study lies in the use of the POMS2 TMD score. While this measure captures short-term, state-dependent emotional conditions, facial skin aging represents a long-term, cumulative biological process. Thus, potential temporal incongruity may exist between the time scale of the psychological predictor and that of the skin scale. This possible mismatch should be considered when interpreting the observed associations in the present study.
6. Conclusions
This study aims to examine whether white matter integrity mediates the link between psychological stress and skin aging, which is shown by the number of cristae cutis. In 92 healthy Japanese adults, we combined POLA Motion Scan skin measures with DTI-derived FA-BHQ subscales of seven selected tracts and controlled for age, sex, BMI, GM-BHQ, sleep status and time, alcohol use, exercise, and UV exposure. The mediation analysis suggested that CC integrity may serve as an important neural correlate associated with the link between stress and skin aging, which is consistent with the concept of a brain–skin axis in which reduced CC integrity is related to emotional regulation and skin aging.
These findings suggest important implications for the integration of skin anti-aging care, brain health promotion, and stress reduction. Alleviating stress may support not only mental well-being but also the prevention of skin aging, highlighting the potential to combine psychological care with dermatological care as part of a comprehensive health program. Reducing stress may improve mental health and may also slow aspects of skin aging. A preventive approach could combine stress management, brain-function monitoring, and evidence-based skin care to support overall well-being.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/life15111664/s1, Table S1. Detailed results of the mediation analysis of CC using the PROCESS package. Table S2. Detailed results of the mediation analysis of IC using the PROCESS package. Table S3. Descriptive Statistics of Regression Variables. Table S4. Results of correlation analysis between major variables, including subscales of FA-BHQ, control variables, stress, and skin scale. p-value in parentheses. Figure S1. Heatmap of correlation analysis between major variables, including subscales of FA-BHQ, control variables, stress, and skin scale. Figure S2. Scatter plot between subscales of FA-BHQ and skin scale.
Author Contributions
D.Y. contributed to conceptualization, formal analysis, and writing of the original draft; K.K., K.N., and Y.Y. contributed to methodological development, data curation, funding acquisition, investigation, administration, supervision, writing—review, and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the ImPACT Program of the Council for Science, Technology and Innovation (Cabinet Office, Government of Japan) and supported by JSPS KAKENHI (Grant Number JP17H06151; JP25K15384).
Institutional Review Board Statement
This study was approved by the Ethics Committee of Institute of Science Tokyo (Approval Number 2022130, 15 July 2022) and was conducted following the institutes guidelines and regulations.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. All participants provided written informed consent before participation, and their anonymity was maintained.
Data Availability Statement
The datasets generated during the current study are not publicly available but are available from the corresponding author upon reasonable request. The data presented in this study are available on request from the corresponding author due to the need to protect the privacy of participants.
Acknowledgments
The authors would like to thank the staff at Kyoto University, Tokyo Institute of Technology, and Tsukuba University for their support of this project.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Baumann, L. Skin ageing and its treatment. J. Pathol. 2007, 211, 241–251. [Google Scholar] [CrossRef]
- Dorland. Dorland’s Illustrated Medical Dictionary; Saunders: Philadelphia, PA, USA, 2015. [Google Scholar]
- Hiramoto, K.; Sugiyama, D.; Takahashi, Y.; Mafune, E. The amelioration effect of tranexamic acid in wrinkles induced by skin dryness. Biomed. Pharmacother. 2016, 80, 16–22. [Google Scholar] [CrossRef]
- Tobin, D.J. Introduction to skin aging. J. Tissue Viability 2017, 26, 37–46. [Google Scholar] [CrossRef]
- Chen, Y.; Lyga, J. Brain-skin connection: Stress, inflammation and skin aging: Ingenta connect. In Inflammation & Allergy-Drug Targets (Formerly Current Drug Targets—Inflammation & Allergy) (Discontinued); Bentham Science Publishers: Sharjah, United Arab Emirates, 2014; Available online: https://www.ingentaconnect.com/content/ben/iadt/2014/00000013/00000003/art00005 (accessed on 25 July 2025).
- Dunn, J.H.; Koo, J. Psychological stress and skin aging: A review of possible mechanisms and potential therapies. Dermatol. Online J. 2013, 19, 18561. [Google Scholar] [CrossRef] [PubMed]
- Elenkov, I.J.; Webster, E.L.; Torpy, D.J.; Chrousos, G.P. Stress, corticotropin-releasing hormone, glucocorticoids, and the immune/inflammatory response: Acute and chronic effects. Ann. N. Y. Acad. Sci. 1999, 876, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Zbytek, B.; Pisarchik, A.; Slominski, R.; Zmijewski, M.; Wortsman, J. CRH functions as a growth factor/cytokine in the skin. J. Cell. Physiol. 2006, 206, 780–791. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Singh, L.K.; Boucher, W.; Pang, X.; Letourneau, R.; Webster, E.; Chrousos, G. Corticotropin-releasing hormone induces skin mast cell degranulation and increased vascular permeability, a possible explanation for its proinflammatory effects. Endocrinology 1998, 139, 403–413. [Google Scholar] [CrossRef]
- Zbytek, B.; Mysliwski, A.; Slominski, A.; Wortsman, J.; Wei, E.T.; Mysliwska, J. Corticotropin-releasing hormone affects cytokine production in human HaCaT keratinocytes. Life Sci. 2002, 70, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Kim, H.J.; Lee, J.Y.; Cho, B.K.; Gallo, R.L.; Cho, D.H. Adrenocorticotropin hormone stimulates interleukin-18 expression in human HaCaT keratinocytes. J. Investig. Dermatol. 2007, 127, 1210–1216. [Google Scholar] [CrossRef]
- Melanocortin-5 Receptor: A Marker of Human Sebocyte Differentiation—ScienceDirect. Available online: https://www-sciencedirect-com.kyoto-u.idm.oclc.org/science/article/pii/S0196978105004547 (accessed on 25 July 2025).
- Yoshida, K.; Nemoto, K.; Hamano, A.; Kawamori, M.; Arai, T.; Yamakawa, Y. Brain healthcare quotient as a tool for standardized approach in brain healthcare interventions. Life 2024, 14, 560. [Google Scholar] [CrossRef]
- Nemoto, K.; Oka, H.; Fukuda, H.; Yamakawa, Y. MRI-based Brain Healthcare Quotients: A bridge between neural and behavioral analyses for keeping the brain healthy. PLoS ONE 2017, 12, e0187137. [Google Scholar] [CrossRef]
- Kelly, S.; Jahanshad, N.; Zalesky, A.; Kochunov, P.; Agartz, I.; Alloza, C.; A Andreassen, O.; Arango, C.; Banaj, N.; Bouix, S.; et al. Widespread white matter microstructural differences in schizophrenia across 4322 individuals: Results from the ENIGMA schizophrenia DTI working group. Mol. Psychiatry 2018, 23, 1261–1269. [Google Scholar] [CrossRef] [PubMed]
- van Velzen, L.S.; Kelly, S.; Isaev, D.; Aleman, A.; Aftanas, L.I.; Bauer, J.; Baune, B.T.; Brak, I.V.; Carballedo, A.; Connolly, C.G.; et al. White matter disturbances in major depressive disorder: A coordinated analysis across 20 international cohorts in the ENIGMA MDD working group. Mol. Psychiatry 2020, 25, 1511–1525. [Google Scholar] [CrossRef] [PubMed]
- Dalgleish, T. The emotional brain. Nat. Rev. Neurosci. 2004, 5, 583–589. [Google Scholar] [CrossRef]
- Modi, S.; Trivedi, R.; Singh, K.; Kumar, P.; Rathore, R.K.; Tripathi, R.P.; Khushu, S. Individual differences in trait anxiety are associated with white matter tract integrity in fornix and uncinate fasciculus: Preliminary evidence from a DTI based tractography study. Behav. Brain Res. 2013, 238, 188–192. [Google Scholar] [CrossRef]
- Catani, M.; Howard, R.J.; Pajevic, S.; Jones, D.K. Virtual in vivo interactive dissection of white matter fasciculi in the human brain. Neuroimage 2002, 17, 77–94. [Google Scholar] [CrossRef]
- Lee, K.S.; Lee, S.H. White matter-based structural brain network of anxiety. In Anxiety Disorders: Rethinking and Understanding Recent Discoveries; Kim, Y.-K., Ed.; Springer: Singapore, 2020; pp. 61–70. [Google Scholar] [CrossRef]
- Keedwell, P.A.; Doidge, A.N.; Meyer, M.; Lawrence, N.; Lawrence, A.D.; Jones, D.K. Subgenual cingulum microstructure supports control of emotional conflict. Cereb. Cortex 2016, 26, 2850–2862. [Google Scholar] [CrossRef]
- Saar-Ashkenazy, R.; Guez, J.; Jacob, Y.; Veksler, R.; Cohen, J.E.; Shelef, I.; Friedman, A.; Benifla, M. White-matter correlates of anxiety: The contribution of the corpus-callosum to the study of anxiety and stress-related disorders. Int. J. Methods Psychiatr. Res. 2023, 32, e1955. [Google Scholar] [CrossRef]
- Saxena, K.; Tamm, L.; Walley, A.; Simmons, A.; Rollins, N.; Chia, J.; Soares, J.C.; Emslie, G.J.; Fan, X.; Huang, H. A preliminary investigation of corpus callosum and anterior commissure aberrations in aggressive youth with bipolar disorders. J. Child Adolesc. Psychopharmacol. 2012, 22, 112–119. [Google Scholar] [CrossRef]
- Mithani, K.; Davison, B.; Meng, Y.; Lipsman, N. The anterior limb of the internal capsule: Anatomy, function, and dysfunction. Behav. Brain Res. 2020, 387, 112588. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Cui, Z.; Cheng, D.; Xu, R.; Gu, R. Individualized prediction of dispositional worry using white matter connectivity. Psychol. Med. 2019, 49, 1999–2008. [Google Scholar] [CrossRef]
- Karababa, I.F.; Bayazıt, H.; Kılıçaslan, N.; Celik, M.; Cece, H.; Karakas, E.; Selek, S. Microstructural changes of anterior corona radiata in bipolar depression. Psychiatry Investig. 2015, 12, 367. [Google Scholar] [CrossRef]
- Makris, N.; Kennedy, D.N.; McInerney, S.; Sorensen, A.G.; Wang, R.; Caviness, V.S.; Pandya, D.N. Segmentation of subcomponents within the superior longitudinal fascicle in humans: A quantitative, in vivo, DT-MRI study. Cereb. Cortex 2005, 15, 854–869. [Google Scholar] [CrossRef]
- Murphy, M.L.; Carballedo, A.; Fagan, A.J.; Morris, D.; Fahey, C.; Meaney, J.; Frodl, T. Neurotrophic tyrosine kinase polymorphism impacts white matter connections in patients with major depressive disorder. Biol. Psychiatry 2012, 72, 663–670. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Routledge: London, UK, 2013. [Google Scholar]
- Skin Analysis | POLA Official: Anti-Aging Care, Whitening, and Cosmetics. Available online: https://www.pola.co.jp/skinanalysis/index.html (accessed on 25 July 2025).
- Kurosumi, M.; Mizukoshi, K.; Hongo, M.; Kamachi, M.G. The effect of observation angles on facial age perceptions: A case study of japanese women. PLoS ONE 2022, 17, e0279339. [Google Scholar] [CrossRef]
- Kurosumi, M.; Yabuzaki, J.; Kuribayashi, M.; Mizukoshi, K. Age-related changes in cheek skin movement: A case study of japanese women. Skin Res. Technol. 2024, 30, e13768. [Google Scholar] [CrossRef]
- Nitta, S.; Matsumoto, M.; Sugama, J.; Nakagami, G.; Okuwa, M.; Nakatani, T.; Sanada, H. New quantitative indicators of evaluating the skin care regimen for older adults with dry skin by using the digital image analysis. J. Nurs. Sci. Eng. 2016, 3, 93–100. [Google Scholar]
- Matsumoto, M.; Matsuo, J.; Dai, M.; Nishizawa, T.; Matsui, K.; Ichikawa, Y.; Okuwa, M.; Sugama, J.; Sanada, H. Influence of differences in washing methods on skin texture. Int. J. Cosmet. Sci. 2014, 36, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Ichibori, R.; Fujiwara, T.; Tanigawa, T.; Kanazawa, S.; Shingaki, K.; Torii, K.; Tomita, K.; Yano, K.; Osaka Twin Research Group; Sakai, Y.; et al. Objective assessment of facial skin aging and the associated environmental factors in japanese monozygotic twins. J. Cosmet. Dermatol. 2014, 13, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Mungmai, L.; Preedalikit, W.; Aunsri, N.; Amornlerdpison, D. Efficacy of cosmetic formulation containing perilla frutescens leaves extract for irritation and aging skin. Biomed. Pharmacol. J. 2020, 13, 779–787. [Google Scholar] [CrossRef]
- Konomi, M.; Suzuki, M.; Ogawa, T.; Otsuka, K.; Hagiwara, A.; Inagaki, T.; Itani, S.; Saito, Y. Examination of sleep disorders in patients with vertigo using the Japanese version of the Pittsburgh Sleep Quality Index (PSQI-J). Equilib. Res. 2014, 73, 502–511. [Google Scholar] [CrossRef]
- Doi, Y.; Minowa, M.; Uchiyama, M.; Okawa, M.; Kim, K.; Shibui, K.; Kamei, Y. Psychometric assessment of subjective sleep quality using the japanese version of the pittsburgh sleep quality index (PSQI-J) in psychiatric disordered and control subjects. Psychiatry Res. 2000, 97, 165–172. [Google Scholar] [CrossRef]
- Doi, Y. Development of the Japanese version of the Pittsburgh Sleep Quality Index (PSQI-J). Jpn. J. Psychiatr. Treat. 1998, 13, 755–763. [Google Scholar]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.L.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef] [PubMed]
- Murase, N.; Katsumura, T.; Ueda, C. International standardization of physical activity: Evaluation of the reliability and validity of the Japanese version of the International Physical Activity Questionnaire (IPAQ). J. Health Welf. Stat. 2002, 49, 1–9. [Google Scholar]
- IPAQ—Score. Available online: https://sites.google.com/view/ipaq/score (accessed on 25 July 2025).
- Heuchert, J.P.; McNair, D.M. Profile of Mood States 2nd EditionTM. 2012. Available online: https://psycnet.apa.org/doiLanding?doi=10.1037/t05057-000 (accessed on 25 July 2025).
- Konuma, H.; Hirose, H.; Yokoyama, K. Relationship of the Japanese translation of the profile of mood states second edition (POMS 2®) to the first edition (POMS®). Juntendo Med. J. 2015, 61, 517–519. [Google Scholar] [CrossRef]
- Heuchert, J.P.; McNair, D.M.; Yokoyama, K.; Watanabe, K. Profile of Mood States 2 (POMS 2) Japanese Version Manual; Kaneko Shobo: Tokyo, Japan, 2015. [Google Scholar]
- Sasaki, H.; Masutomi, H.; Nakamura, S.; Tanigawa, C.; Cui, Y.; Ishihara, K.; Yanagisawa, M.; Kokubo, T. Granola consumption with multiple prebiotics in japanese participants increases bifidobacterium abundance and improves stress and subjective sleepiness. Front. Nutr. 2025, 12, 1551313. [Google Scholar] [CrossRef] [PubMed]
- Nogimura, D.; Moriyasu, K.; Ishida, S.; Kohda, M.; Yazawa, T.; Morita, M. SNP associations in the L-citrulline metabolic pathway and vascular aging in the japanese population. PLoS ONE 2025, 20, e0323778. [Google Scholar] [CrossRef]
- Kullmann, S.; Schweizer, F.; Veit, R.; Fritsche, A.; Preissl, H. Compromised white matter integrity in obesity. Obes. Rev. 2015, 16, 273–281. [Google Scholar] [CrossRef]
- Daoust, J.; Schaffer, J.; Zeighami, Y.; Dagher, A.; García-García, I.; Michaud, A. White matter integrity differences in obesity: A meta-analysis of diffusion tensor imaging studies. Neurosci. Biobehav. Rev. 2021, 129, 133–141. [Google Scholar] [CrossRef]
- Corstjens, H.; Dicanio, D.; Muizzuddin, N.; Neven, A.; Sparacio, R.; Declercq, L.; Maes, D. Glycation associated skin autofluorescence and skin elasticity are related to chronological age and body mass index of healthy subjects. Exp. Gerontol. 2008, 43, 663–667. [Google Scholar] [CrossRef] [PubMed]
- Addor, F. Beyond photoaging: Additional factors involved in the process of skin aging. Clin. Cosmet. Investig. Dermatol. 2018, 11, 437–443. [Google Scholar] [CrossRef]
- Goodman, G.D.; Kaufman, J.; Day, D.; Weiss, R.; Kawata, A.K.; Garcia, J.K.; Santangelo, S.; Gallagher, C.J. Impact of smoking and alcohol use on facial aging in women: Results of a large multinational, multiracial, cross-sectional survey. J. Clin. Aesthetic Dermatol. 2019, 12, 28. [Google Scholar]
- Oyetakin-White, P.; Suggs, A.; Koo, B.; Matsui, M.S.; Yarosh, D.; Cooper, K.D.; Baron, E.D. Does poor sleep quality affect skin ageing? Clin. Exp. Dermatol. 2015, 40, 17–22. [Google Scholar] [CrossRef]
- Sundelin, T.; Lekander, M.; Kecklund, G.; Van Someren, E.J.W.; Olsson, A.; Axelsson, J. Cues of fatigue: Effects of sleep deprivation on facial appearance. Sleep 2013, 36, 1355–1360. [Google Scholar] [CrossRef]
- Spencer, R.L.; Hutchison, K.E. Alcohol, aging, and the stress response. Alcohol Res. Health 1999, 23, 272. [Google Scholar]
- Szatkowska, J.; Teofilak, M.; Śpiołek, O.; Siwiec, J.; Smyl, N.; Kędziora, F.; Wąsowicz, A.; Słowikowska, A.; Sztyler-Krąkowska, M.; Fabian, D. The role of physical activity in enhancing and preserving skin health. J. Educ. Health Sport 2024, 76, 56455. [Google Scholar] [CrossRef]
- Rittié, L.; Fisher, G.J. UV-light-induced signal cascades and skin aging. Ageing Res. Rev. 2002, 1, 705–720. [Google Scholar] [CrossRef]
- Gonzaga, E.R. Role of UV light in photodamage, skin aging, and skin cancer: Importance of photoprotection. Am. J. Clin. Dermatol. 2009, 10, 19–24. [Google Scholar] [CrossRef]
- Farage, M.A.; Miller, K.W.; Zouboulis, C.C.; Piérard, G.E.; Maibach, H.I. Gender differences in skin aging and the changing profile of the sex hormones with age. J. Steroids Horm. Sci. 2012, 3, 109. [Google Scholar] [CrossRef]
- Watanabe, K.; Kakeda, S.; Nemoto, K.; Onoda, K.; Yamaguchi, S.; Kobayashi, S.; Yamakawa, Y. Grey-matter brain healthcare quotient and cognitive function: A large cohort study of an MRI brain screening system in Japan. Cortex 2021, 145, 97–104. [Google Scholar] [CrossRef]
- Nemoto, K.; Kokubun, K.; Ogata, Y.; Koike, Y.; Arai, T.; Yamakawa, Y. Dark chocolate intake may reduce fatigue and mediate cognitive function and gray matter volume in healthy middle-aged adults. Behav. Neurol. 2022, 2022, 1–8. [Google Scholar] [CrossRef]
- Fujino, M.; Watanabe, K.; Yamakawa, Y. The personal trait of spiritual growth is correlated with the white matter integrity of the brain. Front. Hum. Neurosci. 2022, 16, 890160. [Google Scholar] [CrossRef] [PubMed]
- Kokubun, K.; Nemoto, K.; Yamakawa, Y. Brain conditions mediate the association between aging and happiness. Sci. Rep. 2022, 12, 4290. [Google Scholar] [CrossRef] [PubMed]
- Kokubun, K.; Ogata, Y.; Koike, Y.; Yamakawa, Y. Brain condition may mediate the association between training and work engagement. Sci. Rep. 2020, 10, 6848. [Google Scholar] [CrossRef] [PubMed]
- Pineda, J.C.D.; Kokubun, K.; Ikaga, T.; Yamakawa, Y. Housing quality and behavior affect brain health and anxiety in healthy japanese adults. Sci. Rep. 2021, 11, 11999. [Google Scholar] [CrossRef]
- Ni, Y.; Kokubun, K.; Nemoto, K.; Yamakawa, Y. Examination of the impact of curiosity and fatigue on brain condition. Sci. Rep. 2025, 15, 13005. [Google Scholar] [CrossRef]
- ITU-T H.861.1; Requirements on Establishing Brain Healthcare Quotients. Series H: Audiovisual and Multimedia Systems, E-Health Multimedia Services and Applications—Multimedia e-Health Data Exchange Services. International Telecommunication Union Telecommunication Standardization Bureau: Geneva, Switzerland, 2018. Available online: https://www.itu.int/rec/T-REC-H.861.1 (accessed on 30 September 2025).
- Smith, S.M.; Vale, W.W. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin. Neurosci. 2006, 8, 383–395. [Google Scholar] [CrossRef]
- Elewa, R.M.; Abdallah, M.; Youssef, N.; Zouboulis, C.C. Aging-related changes in cutaneous corticotropin-releasing hormone system reflect a defective neuroendocrine-stress response in aging. Rejuvenation Res. 2012, 15, 366–373. [Google Scholar] [CrossRef]
- Picó-Pérez, M.; Radua, J.; Steward, T.; Menchón, J.M.; Soriano-Mas, C. Emotion regulation in mood and anxiety disorders: A meta-analysis of fMRI cognitive reappraisal studies. Prog. Neuropsychopharmacol. Biol. Psychiatry 2017, 79, 96–104. [Google Scholar] [CrossRef]
- Howell, B.R.; McCormack, K.M.; Grand, A.P.; Sawyer, N.T.; Zhang, X.; Maestripieri, D.; Hu, X.; Sanchez, M.M. Brain white matter microstructure alterations in adolescent rhesus monkeys exposed to early life stress: Associations with high cortisol during infancy. Biol. Mood Anxiety Disord. 2013, 3, 21. [Google Scholar] [CrossRef]
- Rinne-Albers, M.A.W.; van der Werff, S.J.A.; van Hoof, M.-J.; van Lang, N.D.; Lamers-Winkelman, F.; Rombouts, S.A.; Vermeiren, R.R.J.M.; van der Wee, N.J.A. Abnormalities of white matter integrity in the corpus callosum of adolescents with PTSD after childhood sexual abuse: A DTI study. Eur. Child Adolesc. Psychiatry 2016, 25, 869–878. [Google Scholar] [CrossRef]
- Paul, R.H. The relationship between early life stress and microstructural integrity of the corpus callosum in a non-clinical population. Neuropsychiatr. Dis. Treat. 2008, 4, 193–201. [Google Scholar] [CrossRef][Green Version]
- Jackowski, A.P.; Douglas-Palumberi, H.; Jackowski, M.; Win, L.; Schultz, R.T.; Staib, L.W.; Krystal, J.H.; Kaufman, J. Corpus callosum in maltreated children with posttraumatic stress disorder: A diffusion tensor imaging study. Psychiatry Res. Neuroimaging 2008, 162, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Teicher, M.H.; Andersen, S.L.; Polcari, A.; Anderson, C.M.; Navalta, C.P.; Kim, D.M. The neurobiological consequences of early stress and childhood maltreatment. Neurosci. Biobehav. Rev. 2003, 27, 33–44. [Google Scholar] [CrossRef]
- Villarreal, G.; Hamilton, D.A.; Graham, D.P.; Driscoll, I.; Qualls, C.; Petropoulos, H.; Brooks, W.M. Reduced area of the corpus callosum in posttraumatic stress disorder. Psychiatry Res. Neuroimaging 2004, 131, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, K.; Yasuno, F.; Kishimoto, T.; Yamamoto, A.; Kiuchi, K.; Kosaka, J.; Nagatsuka, K.; Iida, H.; Kudo, T. Microstructural differences in the corpus callosum in patients with bipolar disorder and major depressive disorder. J. Clin. Psychiatry 2016, 77, 1915. [Google Scholar] [CrossRef]
- Rotarska-Jagiela, A.; Schönmeyer, R.; Oertel, V.; Haenschel, C.; Vogeley, K.; Linden, D.E. The corpus callosum in schizophrenia-volume and connectivity changes affect specific regions. Neuroimage 2008, 39, 1522–1532. [Google Scholar] [CrossRef]
- Galinowski, A.; Miranda, R.; Lemaitre, H.; Martinot, M.-L.P.; Artiges, E.; Vulser, H.; Goodman, R.; Penttilä, J.; Struve, M.; Barbot, A.; et al. Resilience and corpus callosum microstructure in adolescence. Psychol. Med. 2015, 45, 2285–2294. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).