Acute Sulcal FLAIR Hyperintensity in Severe Tick-Borne Encephalitis: A Potential Prognostic Marker
Abstract
1. Introduction
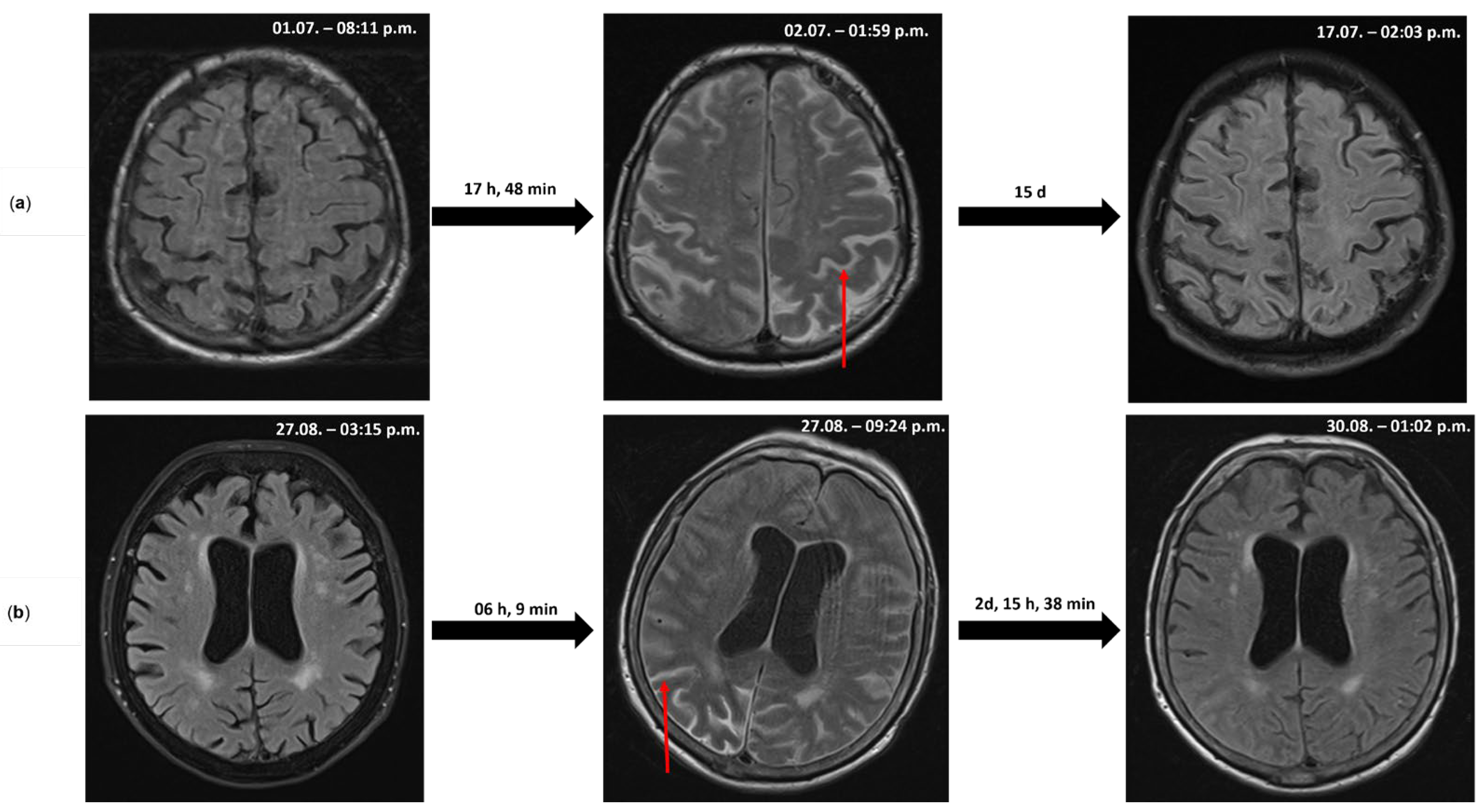
2. Case Reports
2.1. Case 1
2.2. Case 2
3. Discussion
4. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MRI | Magnetic resonance imaging |
| FLAIR | Fluid-attenuated inversion recovery |
| DWI | Diffusion-weighted imaging |
| ADC | Apparent diffusion coefficient |
| TBE | Tick-borne encephalitis |
| CSF | Cerebrospinal fluid |
| EEG | Electroencephalography |
| LPDs | Lateralized periodic discharges |
| NCS | Nerve conduction study |
| PEG | Percutaneous endoscopic gastrostomy |
| mRS | Modified Rankin scale |
| GFR | Glomerular filtration rate |
| CK | Creatine kinase |
| LDH | Lactate dehydrogenase |
| FLCK | Free light chains kappa |
| CXCL13 | C-X-C motif chemokine 13 |
| WNV | West Nile virus |
References
- Worku, D.A. Tick-Borne Encephalitis (TBE): From Tick to Pathology. J. Clin. Med. 2023, 12, 6859. [Google Scholar] [CrossRef] [PubMed]
- Schelling, J.; Einmahl, S.; Torgler, R.; Larsen, C.S. Evidence for a 10-year TBE vaccine booster interval: An evaluation of current data. Expert Rev. Vaccines 2024, 23, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Kohlmaier, B.; Schweintzger, N.A.; Sagmeister, M.G.; Švendová, V.; Kohlfürst, D.S.; Sonnleitner, A.; Leitner, M.; Berghold, A.; Schmiedberger, E.; Fazekas, F.; et al. Clinical characteristics of patients with tick-borne encephalitis: A European multicentre study from 2010 to 2017. Microorganisms 2021, 9, 1420. [Google Scholar] [CrossRef] [PubMed]
- Gudowska-Sawczuk, M.; Mroczko, B. Selected biomarkers of tick-borne encephalitis: A review. Int. J. Mol. Sci. 2021, 22, 10615. [Google Scholar] [CrossRef] [PubMed]
- Zawadzki, R.; Garkowski, A.; Kubas, B.; Zajkowska, J.; Hładuński, M.; Jurgilewicz, D.; Łebkowska, U. Evaluation of imaging methods in tick-borne encephalitis. Pol. J. Radiol. 2017, 82, 742–747. [Google Scholar] [CrossRef] [PubMed]
- Czupryna, P.; Mroczko, B.; Pancewicz, S.; Muszynski, P.; Grygorczuk, S.; Dunaj, J.; Borawski, K.; Róg-Makal, M.; Świerzbińska, R.; Zajkowska, J.; et al. Assessment of the tau protein concentration in patients with tick-borne encephalitis. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Safriel, Y.; Sohi, J.; Llave, A.; Weathers, S. West Nile virus infection: MR imaging findings in the nervous system. AJNR Am. J. Neuroradiol. 2005, 26, 289–297. [Google Scholar] [PubMed]
- Perillo, T.; Capasso, R.; Pinto, A. Neuroimaging of the most common meningitis and encephalitis of adults: A narrative review. Diagnostics 2024, 14, 1064. [Google Scholar] [CrossRef] [PubMed]
- Duong, M.T.; Rudie, J.D.; Mohan, S. Neuroimaging patterns of intracranial infections: Meningitis, cerebritis, and their complications. Neuroimaging Clin. N. Am. 2023, 33, 11–41. [Google Scholar] [CrossRef] [PubMed]
- Kuwahara, S.; Kawada, M.; Uga, S. Cryptococcal meningoencephalitis presenting with an unusual magnetic resonance imaging appearance: Case report. Neurol. Med. Chir. 2001, 41, 517–521. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Panteleienko, L.; Banerjee, G.; Mallon, D.H.; Harvey, V.; Oliver, R.; Hotton, G.; Knight, W.; Datta, S.; Zandi, M.S.; Jäger, H.R.; et al. Sulcal hyperintensity as an early imaging finding in cerebral amyloid angiopathy-related inflammation. Neurology 2024, 103, e210084. [Google Scholar] [CrossRef] [PubMed]
- Maeda, M.; Yagishita, A.; Yamamoto, T.; Sakuma, H.; Takeda, K. Abnormal hyperintensity within the subarachnoid space evaluated by fluid-attenuated inversion-recovery MR imaging: A spectrum of central nervous system diseases. Eur. Radiol. 2003, 13 (Suppl. 6), L192–L201. [Google Scholar] [CrossRef] [PubMed]
- Bozzao, A.; Floris, R.; Fasoli, F.; Fantozzi, L.; Colonnese, C.; Simonetti, G. Cerebrospinal fluid changes after intravenous injection of gadolinium chelate: Assessment by FLAIR MR imaging. Eur. Radiol. 2003, 13, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Frigon, C.; Shaw, D.W.W.; Heckbert, S.R.; Weinberger, E.; Jardine, D.S. Supplemental oxygen causes increased signal intensity in subarachnoid cerebrospinal fluid on brain FLAIR MR images obtained in children during general anesthesia. Radiology 2004, 233, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Braga, F.T.; da Rocha, A.J.; Filho, G.H.; Arikawa, R.K.; Ribeiro, I.M.; Fonseca, R.B. Relationship between the concentration of supplemental oxygen and signal intensity of CSF depicted by fluid-attenuated inversion recovery imaging. AJNR Am. J. Neuroradiol. 2003, 24, 1863–1868. [Google Scholar] [PubMed]
- Yaffe, K.; Ferriero, D.; Barkovich, A.J.; Rowley, H. Reversible MRI abnormalities following seizures. Neurology 1995, 45, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.M.; Kim, J.H.; Kim, E.; Choi, B.S.; Bae, Y.J.; Bae, H.J. Early Stage of Hyperintense Acute Reperfusion Marker on Contrast-Enhanced FLAIR Images in Patients With Acute Stroke. AJR Am. J. Roentgenol. 2016, 206, 1272–1275. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-A.; Chung, J.I.; Yoon, P.H.; Kim, D.I.; Chung, T.-S.; Kim, E.-J.; Jeong, E.-K. Transient MR signal changes in patients with generalized tonicoclonic seizure or status epilepticus: Periictal diffusion-weighted imaging. AJNR Am. J. Neuroradiol. 2001, 22, 1149–1160. [Google Scholar] [PubMed]
- Williams, J.A.; Bede, P.; Doherty, C.P. An exploration of the spectrum of peri-ictal MRI change; a comprehensive literature review. Seizure 2017, 50, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Mariajoseph, F.P.; Sagar, P.; Muthusamy, S.; Amukotuwa, S.; Seneviratne, U. Seizure-induced reversible MRI abnormalities in status epilepticus: A systematic review. Seizure 2021, 92, 166–173. [Google Scholar] [CrossRef] [PubMed]
| Consecutive Serology Testing | |||||||
|---|---|---|---|---|---|---|---|
| Case 1 | Case 2 | ||||||
| Days after onset of neurological symptoms | 1 | 3 | 17 | 4 | 6 | 19 | |
| Days after presentation | 1 | 3 | 17 | 2 | 4 | 17 | |
| Leukocytes (G/L) [4.4–9.4] | 13.1 | 10.5 | |||||
| Thrombocytes (G/L) [186–400] | 235 | 157 | |||||
| Hemoglobin (g/dL) [12.3–15.3] | 14.1 | 12.9 | |||||
| CRP (mg/dL) [0–0.5] | 1.3 | 0.7 | |||||
| Sodium (mmol/L) [136–145] | 126 | 137 | |||||
| Potassium (mmol/L) [3.5–4.5] | 3.9 | 3.5 | |||||
| GFR (mL/min/1.7) [70–130] | 87 | 87 | |||||
| CK (U/L) [0–170] | 70 | 97 | |||||
| LDH (U/L) [135–250] | 140 | 259 | |||||
| TBE IgM (Ratio) [0.80] | 3.76 | 5.16 | 2.82 | 6.15 | 6.07 | 6.21 | |
| TBE IgG (RU/mL) [0–16] | 16.4 | 18.53 | 165.51 | 48.08 | 132.79 | >200 | |
| Consecutive CSF Testing | ||||||||
|---|---|---|---|---|---|---|---|---|
| Case 1 | Case 2 | |||||||
| Days after onset of neurological symptoms | 1 | 3 | 17 | 36 | 4 | 6 | 19 | |
| Day after presentation | 1 | 3 | 17 | 36 | 2 | 4 | 17 | |
| Leukocytes (/uL) [0–4] | 880 | 120 | 15 | 11 | 41 | 74 | 44 | |
| Glucose-Ratio [0.5–0.7] | 0.51 | 0.5 | 0.5 | 0.6 | 0.52 | 0.42 | 0.5 | |
| Lactate (mmol/L) [1–2.1] | 3.7 | 4.15 | 2.61 | 2.14 | 3.1 | 3.2 | 3.17 | |
| Protein levels (mg/dL) [15–45] | 121 | 147 | 136 | 137 | 151 | 123 | 98.8 | |
| CSF to serum albumin ratio (/1000) [0.50–10] | 23.9 | 28.8 | 40.4 | 30.4 | 19.7 | |||
| Tau Protein (pg/mL) [146–404] | 480 | 801 | 876 | |||||
| TBE IgM (L/S-Index) [0–1.3] | 2.35 | 4.71 | 6.27 | 3 | 2 | 18.91 | ||
| TBE IgG (L/S-Index) [0–1.3] | negative | 2.7 | 0.72 | 1.89 | 4.22 | 1.84 | ||
| FLCK (L/S) × 1000 [0–16.8] | 128.14 | 297.49 | 366.22 | 696.43 | ||||
| CXCL13 (pg/mL) [0–20] | <4 | 101.6 | 95.2 | 189.8 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Böhm, V.; Ianosi, B.-A.; Kulyk, C.; Gruber, F.; Lorenz, M.; Mitterling, T.; Hauser, A.; Eger, S.; Köhl, U.; Weis, S.; et al. Acute Sulcal FLAIR Hyperintensity in Severe Tick-Borne Encephalitis: A Potential Prognostic Marker. Life 2025, 15, 1655. https://doi.org/10.3390/life15111655
Böhm V, Ianosi B-A, Kulyk C, Gruber F, Lorenz M, Mitterling T, Hauser A, Eger S, Köhl U, Weis S, et al. Acute Sulcal FLAIR Hyperintensity in Severe Tick-Borne Encephalitis: A Potential Prognostic Marker. Life. 2025; 15(11):1655. https://doi.org/10.3390/life15111655
Chicago/Turabian StyleBöhm, Vincent, Bogdan-Andrei Ianosi, Caterina Kulyk, Franz Gruber, Maria Lorenz, Thomas Mitterling, Amadeus Hauser, Stephan Eger, Ulrike Köhl, Serge Weis, and et al. 2025. "Acute Sulcal FLAIR Hyperintensity in Severe Tick-Borne Encephalitis: A Potential Prognostic Marker" Life 15, no. 11: 1655. https://doi.org/10.3390/life15111655
APA StyleBöhm, V., Ianosi, B.-A., Kulyk, C., Gruber, F., Lorenz, M., Mitterling, T., Hauser, A., Eger, S., Köhl, U., Weis, S., Wimmer, S., Sonnberger, M., & Helbok, R. (2025). Acute Sulcal FLAIR Hyperintensity in Severe Tick-Borne Encephalitis: A Potential Prognostic Marker. Life, 15(11), 1655. https://doi.org/10.3390/life15111655







