Ecology and Population Structure of Two Sympatric Rodents in a Neotropical Forest of Southeastern Brazil
Abstract
1. Introduction
2. Methods
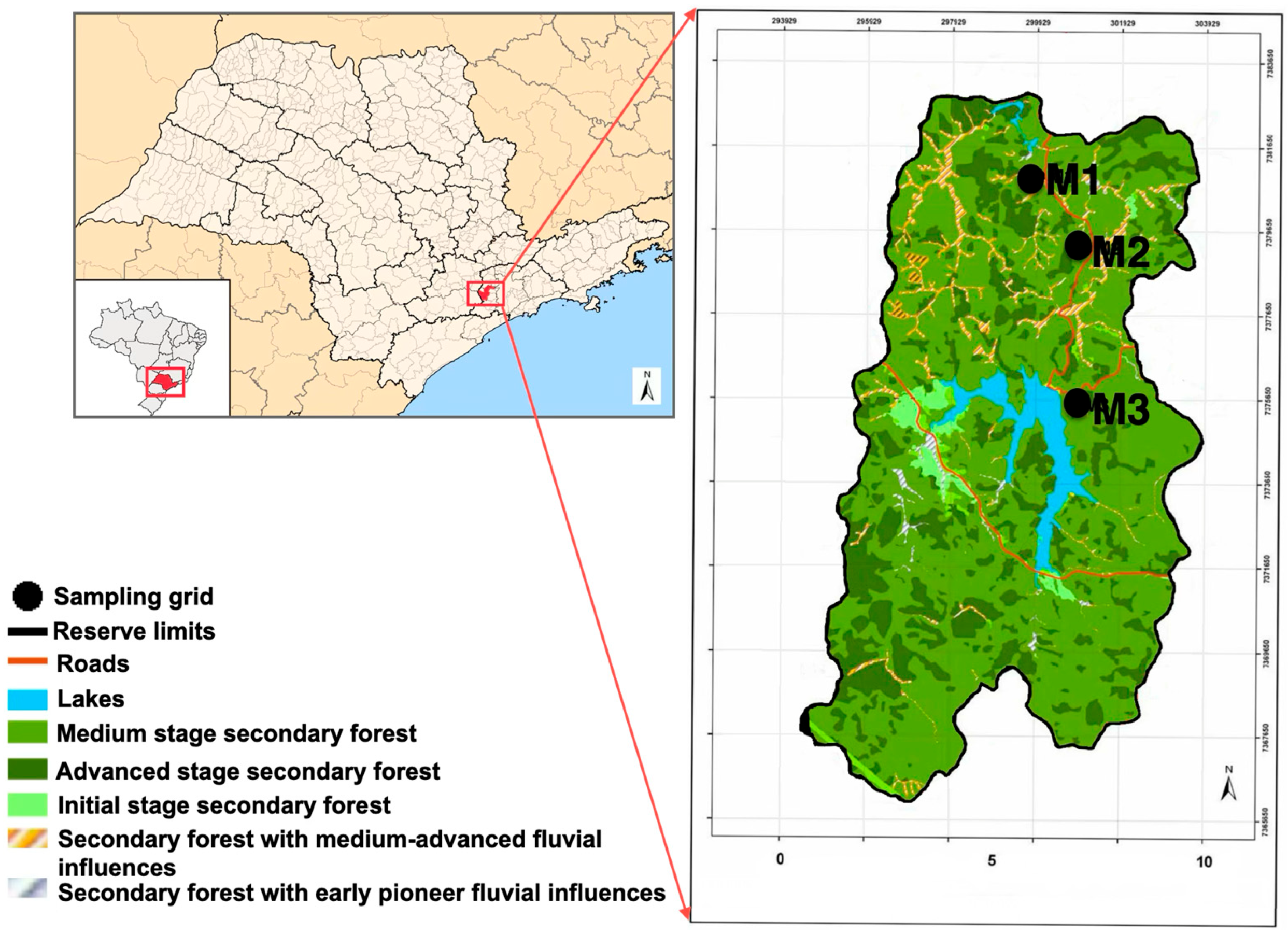
2.1. Study Area
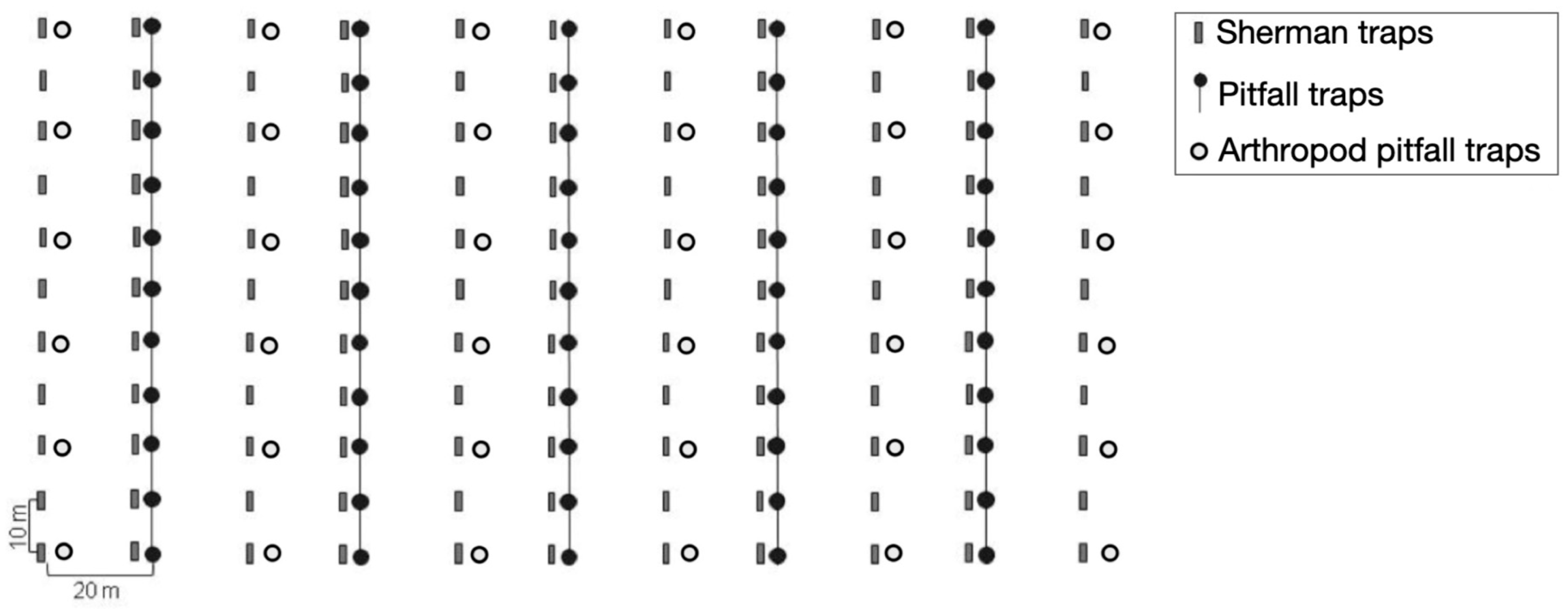
2.2. Sampling Design
2.3. Capture–Recapture
2.4. Biological Data
2.5. Climate Data
2.6. Arthropods
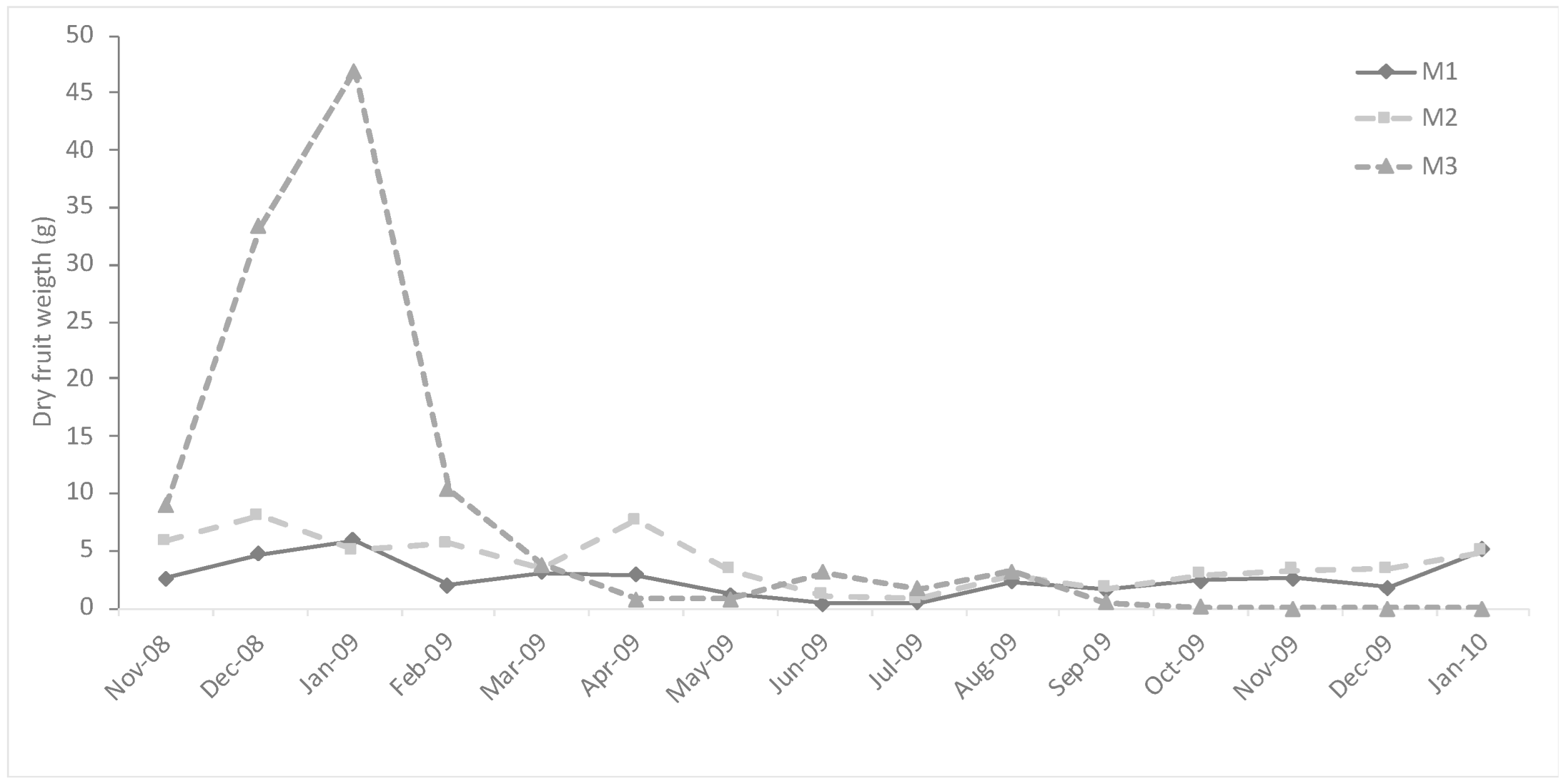
2.7. Fruits
2.8. Data Analysis
3. Results
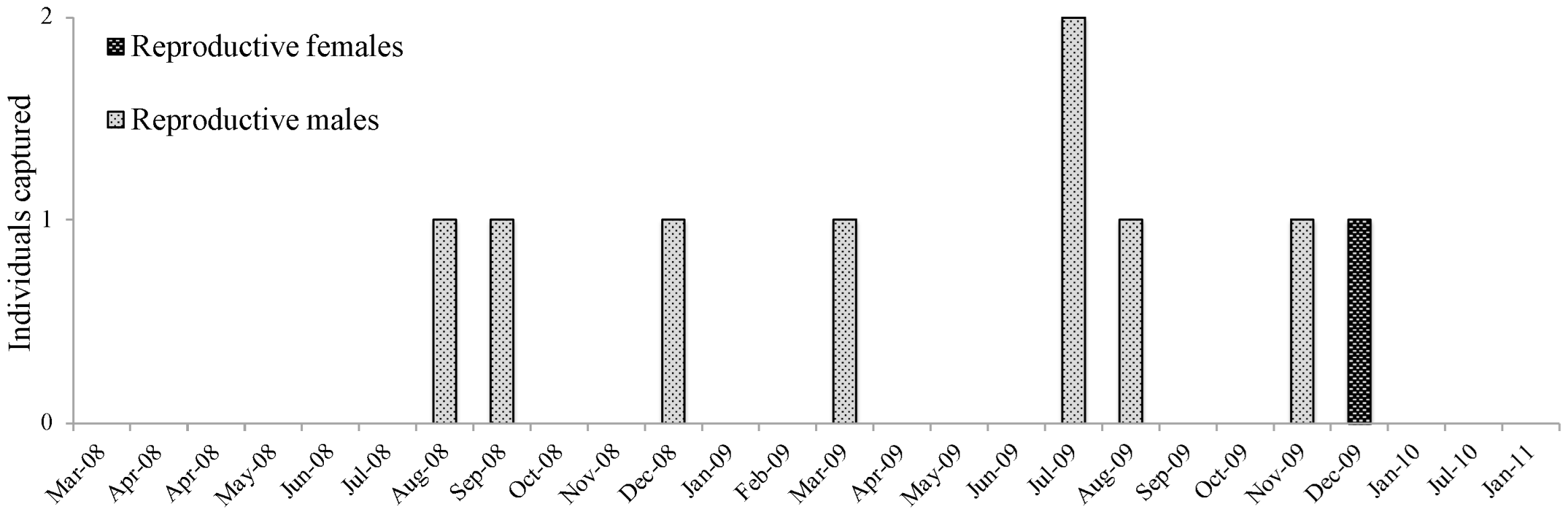
3.1. Biological Data
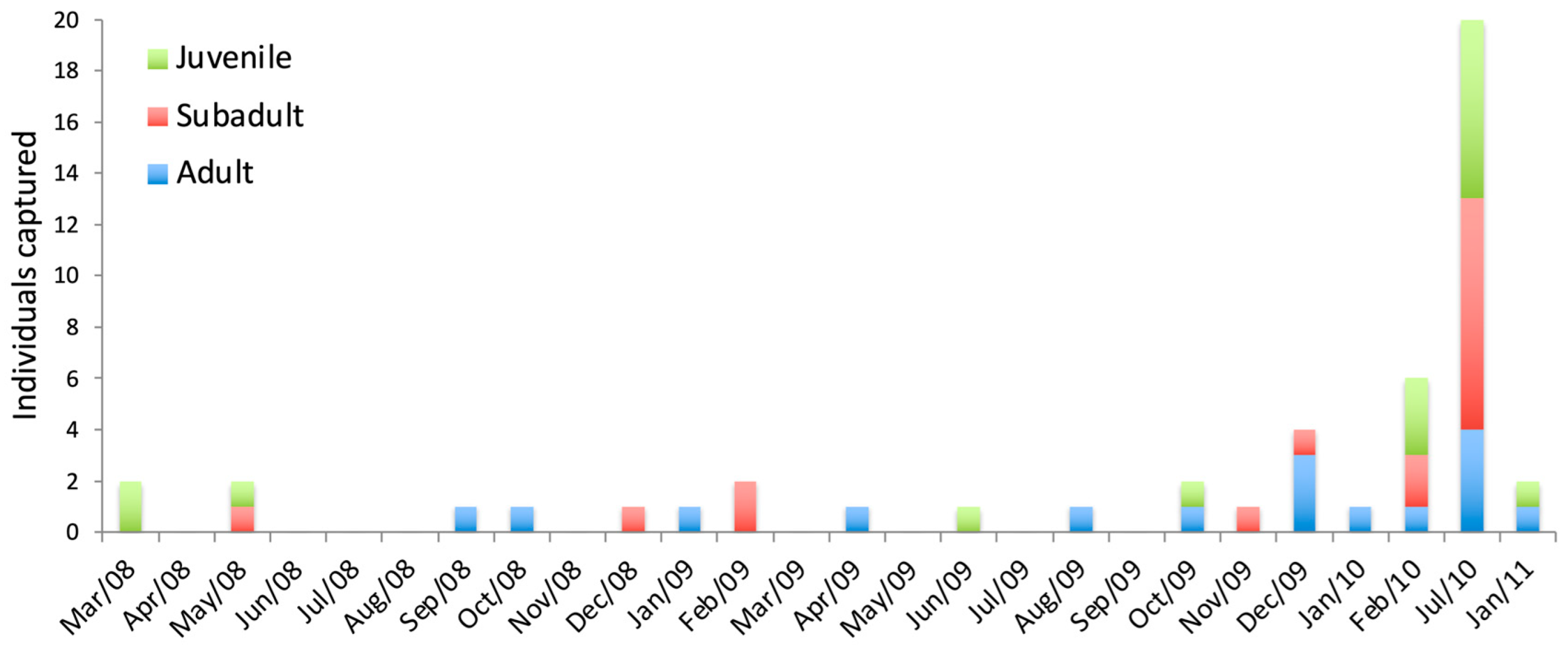
3.1.1. Sooretamys angouya
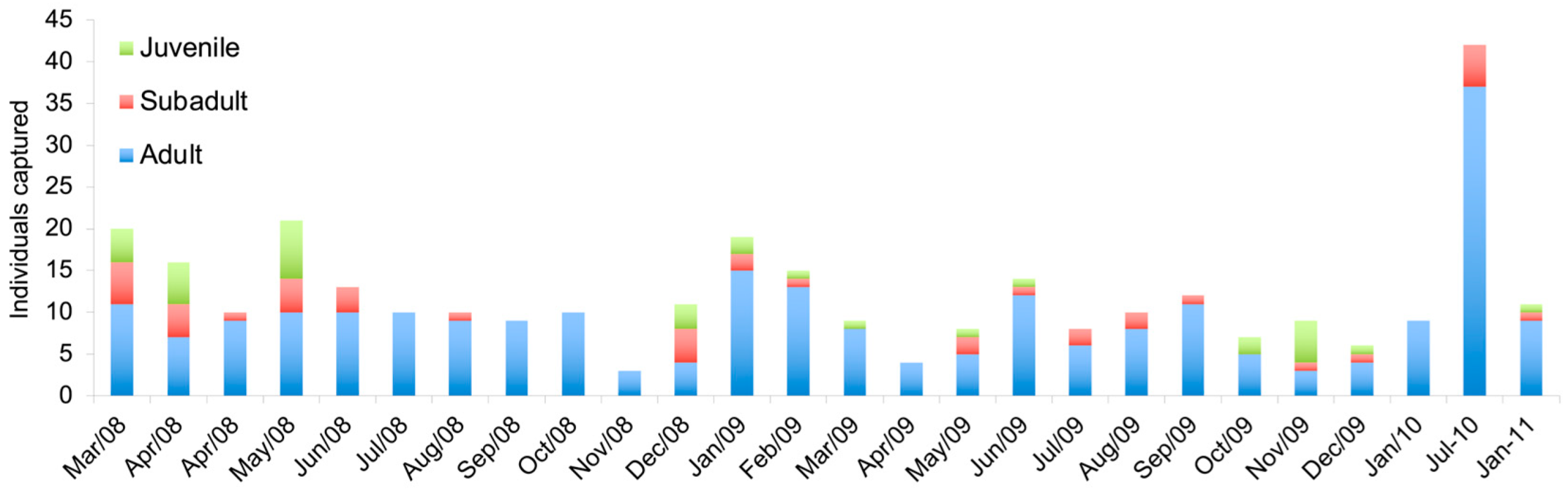
3.1.2. Euryoryzomys russatus
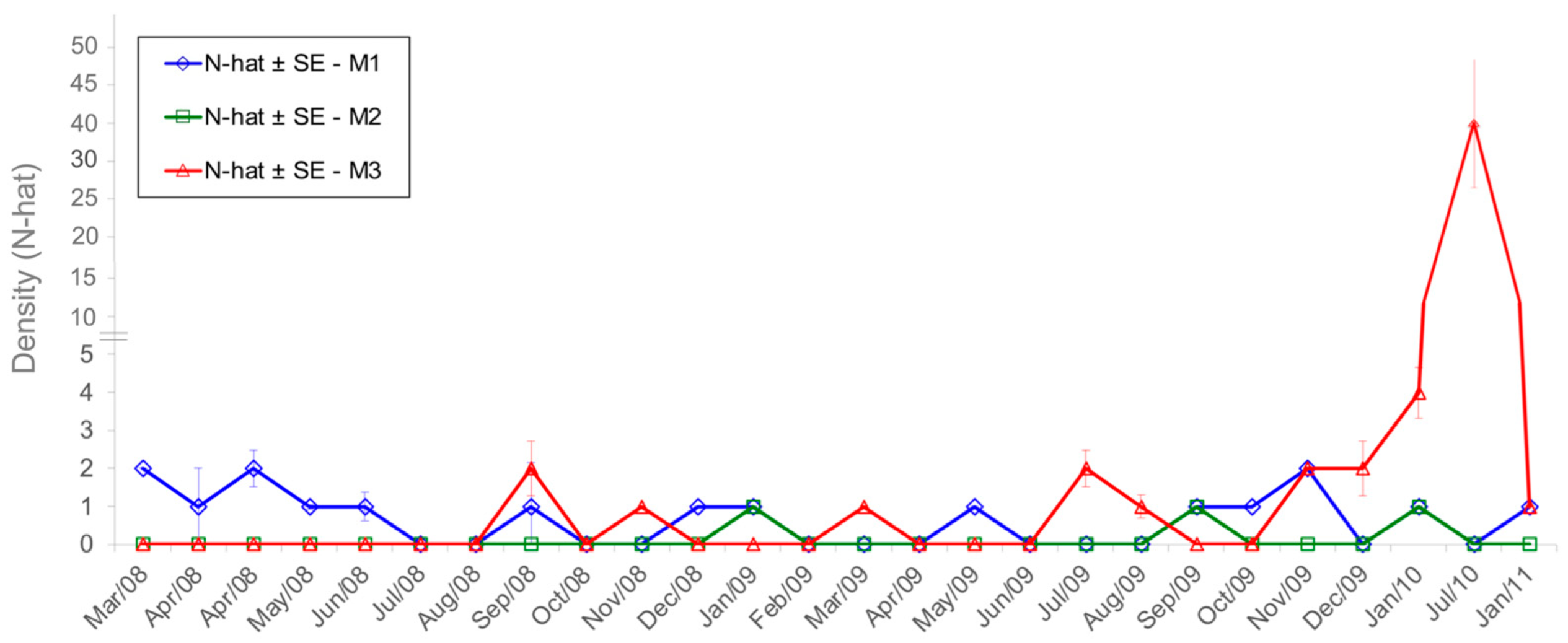
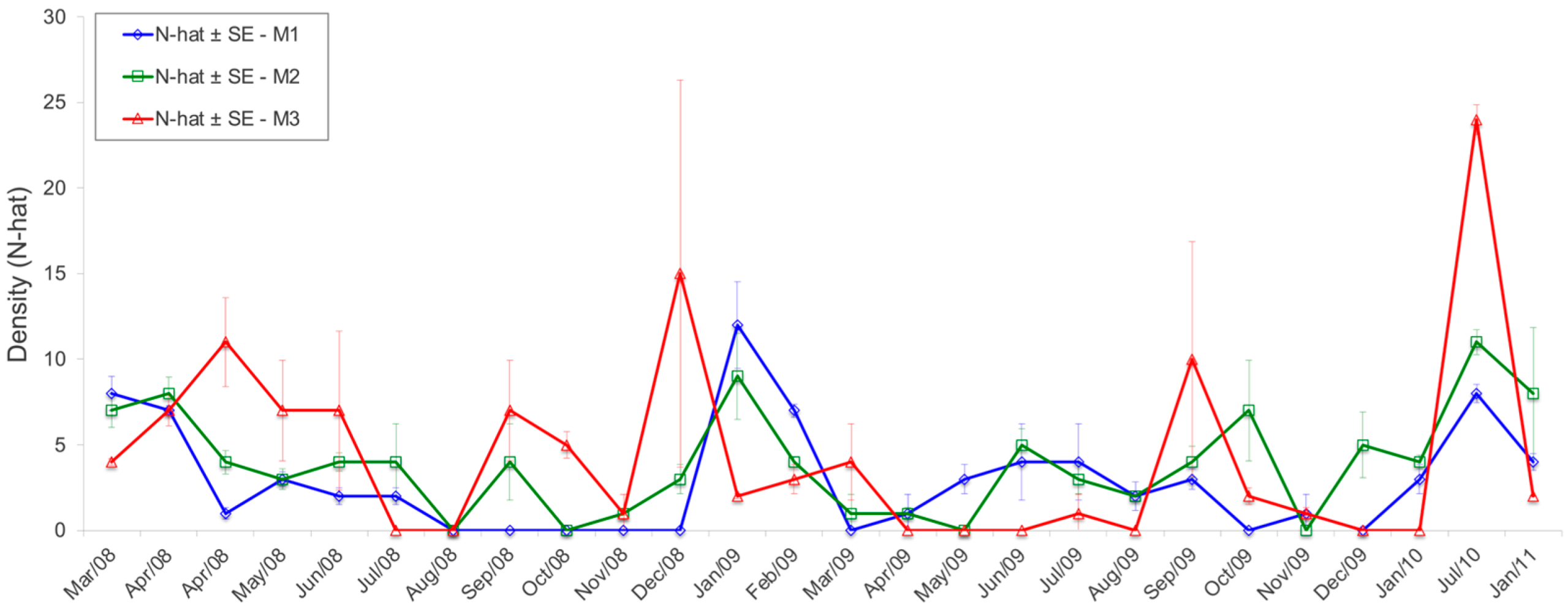
3.2. Capture–Recapture
3.2.1. Sooretamys angouya
3.2.2. Euryoryzomys russatus
4. Discussion
4.1. Capture Efficiency and Trap Effectiveness
4.2. Population Structure and Dynamics
4.3. Capture Success and Habitat Influence
4.4. Grid Permanence and Sex Ratios
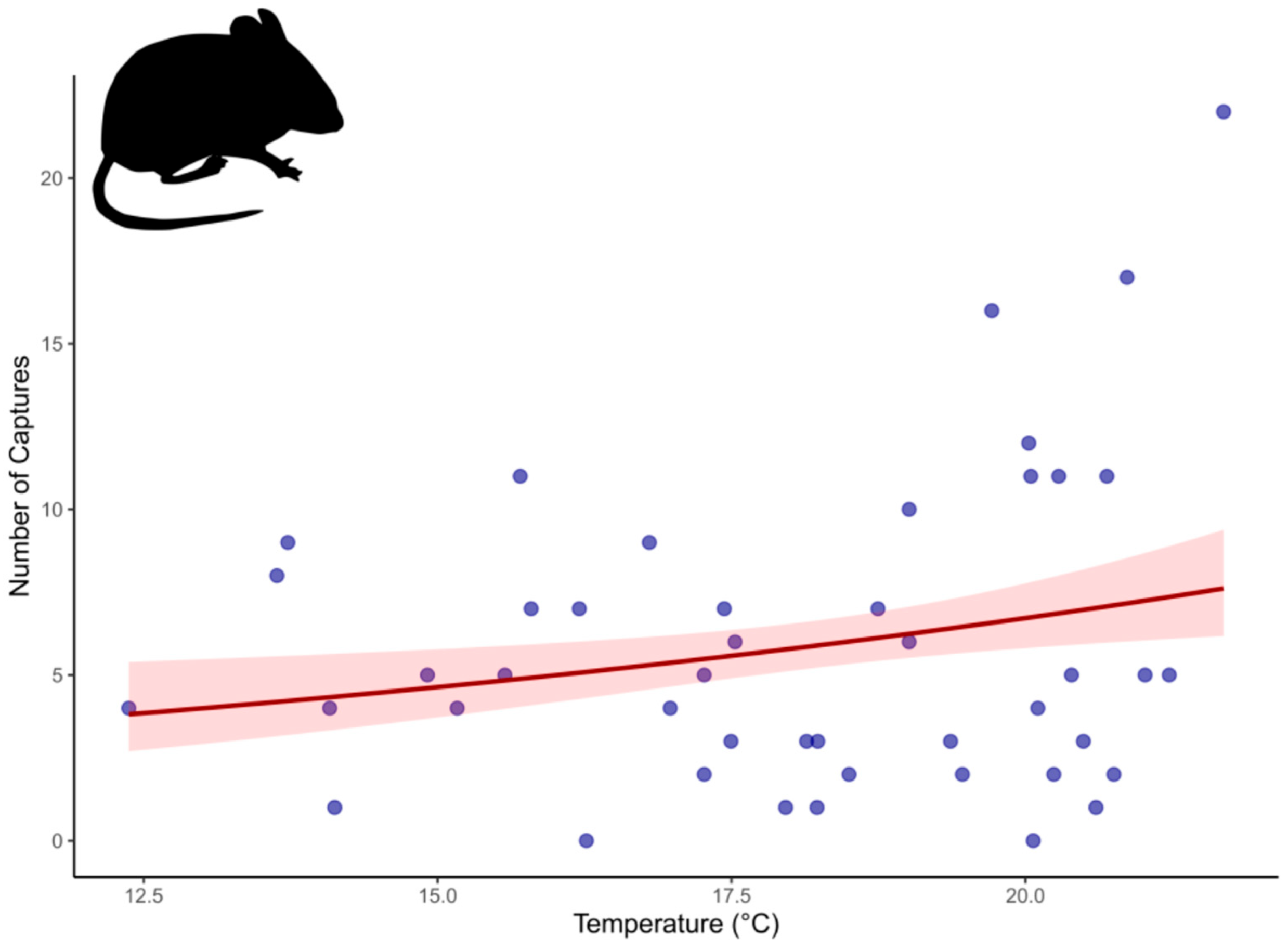
4.5. Body Size, Reproduction, and Resource Availability
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A



References
- Emmons, L.H. Neotropical Rainforest Mammals: A Field Guide, 2nd ed.; Chicago University Press: Chicago, IL, USA, 1997; p. 307. [Google Scholar]
- Wilson, D.E.; Reeder, D.M. Mammal Species of the World. A Taxonomic and Geographic Reference; Johns Hopkins University Press: Baltimore, MD, USA, 2005; Volume 2, p. 2000. [Google Scholar]
- Emmons, L.H. Mammals of rain forest canopies. In Forest Canopies; Lowman, M.D., Nadkarni, N.M., Eds.; Academic Press: London, UK, 1995; Volume 1, pp. 199–223. [Google Scholar]
- IUCN. The IUCN Red List of Threatened Species. Version 2024-2. 2024. Available online: https://www.iucnredlist.org (accessed on 5 December 2024).
- SMA (Secretaria do Meio Ambiente do Estado de São Paulo). Espécies da Fauna Silvestre do Estado de São Paulo Regionalmente Extintas, as Ameaçadas de Extinção, as Quase Ameaçadas e as Com Dados Insuficientes Para Avaliação de Seu Grau de Conservação. DECRETO Nº 63.853, DE 27 DE NOVEMBRO DE 2018. 2018. Available online: https://www.al.sp.gov.br/repositorio/legislacao/decreto/2018/decreto-63853-27.11.2018.html (accessed on 5 December 2024).
- Vieira, E.M.; Monteiro Filho, E.L.A. Vertical stratfication of small mammals in the Atlantic rain forest of south-eastern Brazil. J. Trop. Ecol. 2003, 19, 501–507. [Google Scholar] [CrossRef]
- Cerboncini, R.A.S.; Rubio, M.B.G.; Bernardi, I.P.; Braga, T.V.; Roper, J.J.; Passos, F.C. Small mammal community structure and vertical space use preferences in nonfragmented Atlantic Forest. Mammalia 2014, 78, 429–436. [Google Scholar] [CrossRef]
- Bubadué, J.; Cáceres, N.; Melo, G.; Sponchiado, J.; Battistella, T.; Newton, J.; Meloro, C. Niche partitioning in small mammals: Interspecific and biome-level analyses using stable isotopes. J. Mammal. 2021, 102, 1235–1248. [Google Scholar] [CrossRef]
- Cunha, A.A.; Vieira, M.V. Support diameter, incline, and vertical movements of four didelphid marsupials in the Atlantic forest of Brazil. J. Zool. 2002, 258, 419–426. [Google Scholar] [CrossRef]
- Camargo, N.F.d.; Sano, N.Y.; Vieira, E.M. Forest vertical complexity affects alpha and beta diversity of small mammals. J. Mammal. 2018, 99, 1444–1454. [Google Scholar] [CrossRef]
- Metzger, J.P.; Alves, L.F.; Goulart, W.; Teixeira, A.M.d.G.; Simões, S.J.C.; Catharino, E.L.M. Uma área de relevante interesse biológico, porém pouco conhecida: A Reserva Florestal do Morro Grande. Biota Neotrop. 2006, 6, 1–33. [Google Scholar] [CrossRef]
- Püttker, T.; Barros, C.S.; Pinotti, B.T.; Bueno, A.A.; Pardini, R. Co-occurrence patterns of rodents at multiple spatial scales: Competitive release of generalists following habitat loss? J. Mammal. 2019, 100, 1229–1242. [Google Scholar] [CrossRef]
- Prado, J.R.; Percequillo, A.R. Geographic distribution of the genera of the tribe Oryzomyini (Rodentia: Cricetidae: Sigmodontinae) in South America: Patterns of distribution and diversity. Arq. De Zool. 2013, 44, 1–120. [Google Scholar] [CrossRef]
- Pardini, R.; de Souza, S.M.; Braga-Neto, R.; Metzger, J.P. The role of forest structure, fragment size and corridors in maintaining small mammal abundance and diversity in an Atlantic forest landscape. Biol. Conserv. 2005, 124, 253–266. [Google Scholar] [CrossRef]
- Pardini, R.; Umetsu, F. Pequenos mamíferos não-voadores da Reserva Florestal do Morro Grande: Distribuição das espécies e da diversidade em uma área de Mata Atlântica. Biota Neotrop. 2006, 6, 1–22. [Google Scholar] [CrossRef]
- Umetsu, F.; Naxara, L.; Pardini, R. Evaluating the efficiency of pitfall traps for sampling small mammals in the neotropics. J. Mammal. 2006, 87, 757–765. [Google Scholar] [CrossRef]
- Rocha, M.F.; Passamani, M.; Louzada, J. A small mammal community in a forest fragment, vegetation corridor and coffee matrix system in the Brazilian Atlantic forest. PLoS ONE 2011, 6, e23312. [Google Scholar] [CrossRef]
- Koeppen, W. Climatologia: Con un estudio de los climas de la tierra. Das geographische System der Klimate. In Handbuch der Klimatologie, Band I, Teil C.; Köppen, W., Geiger, R., Eds.; Gebrüder Borntraeger: Berlin, Germany, 1948. [Google Scholar]
- Metzger, J.P.; Alves, L.F.; Pardini, R.; Dixo, M.; Nogueira, A.d.A.; Negrão, M.d.F.F.; Martensen, A.C.; Catharino, E.L.M. Características ecológicas e implicações para a conservação da Reserva Florestal do Morro Grande. Biota Neotrop. 2006, 6, 1–13. [Google Scholar] [CrossRef]
- Catharino, E.L.M.; Bernacci, L.C.; Franco, G.A.D.C.; Durigan, G.; Metzger, J.P. Aspectos da composição e diversidade do componente arbóreo das florestas da Reserva Florestal do Morro Grande, Cotia, SP. Biota Neotrop. 2006, 6. [Google Scholar] [CrossRef]
- Barros, C.S.; Martins, T.K.; Puettker, T.; Pardini, R. Long distance and short time movement of a small neotropical marsupial. Oecologia Aust. 2016, 20, 75–79. [Google Scholar] [CrossRef]
- Paglia, A.P.; Da Fonseca, G.A.; Rylands, A.B.; Herrmann, G.; Aguiar, L.M.; Chiarello, A.G.; Leite, Y.; Costa, L.P.; Siciliano, S.; Kierulff, M.C.; et al. Lista Anotada dos Mamíferos do Brasil 2ª Edição/annotated checklist of Brazilian mammals. Occas. Pap. Conserv. Biol. 2012, 6. [Google Scholar]
- Patton, J.L.; Pardiñas, U.F.; D’Elía, G. Mammals of South America, Volume 2: Rodents; University of Chicago Press: Chicago, IL, USA, 2020; Volume 2. [Google Scholar]
- Sikes, R.S.; Animal Care and Use Committee of the American Society of Mammalogists. 2016 Guidelines of the American Society of Mammalogists for the use of wild mammals in research and education. J. Mammal. 2016, 97, 663–688. [Google Scholar] [CrossRef]
- Almeida, L.M.; Ribeiro-Costa, C.S.; Marinoni, L. Manual de Coleta, Conservação, Montagem e Identificação de Insetos; Holos: Ribeirão Preto, Brazil, 1998; p. 78. [Google Scholar]
- Efford, M.G. secr: Spatially Explicit Capture–Recapture Models, R Package Version 5.2. 2024. Available online: https://CRAN.R-project.org/package=secr (accessed on 24 June 2025).
- Efford, M.G.; Borchers, D.L.; Byrom, A.E. Density estimation by spatially explicit capture–recapture: Likelihood-based methods. In Modeling Demographic Processes in Marked Populations; Springer: Berlin/Heidelberg, Germany, 2009; pp. 255–269. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing Version 4.3.1; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 24 June 2025).
- Bovendorp, R.S.; Heming, N.M.; Percequillo, A.R. Bottom-up effect: A rodent outbreak following the bamboo blooming in a Neotropical rainforest. Mammal Res. 2020, 65, 535–543. [Google Scholar] [CrossRef]
- O’Connell, M.A. Population dynamics of neotropical small mammals in seasonal habitats. J. Mammal. 1989, 70, 532–548. [Google Scholar] [CrossRef]
- Barros, C.S.; Püttker, T.; Pinotti, B.T.; Pardini, R. Determinants of capture-recapture success: An evaluation of trapping methods to estimate population and community parameters for Atlantic forest small mammals. Zoologia 2015, 32, 334–344. [Google Scholar] [CrossRef]
- Bovendorp, R.S.; Mccleery, R.A.; Galetti, M. Optimising sampling methods for small mammal communities in Neotropical rainforests. Mammal Rev. 2017, 47, 148–158. [Google Scholar] [CrossRef]
- Graipel, M.c.E.; Cherem, J.J.; Monteiro-Filho, E.L.A.; Glock, L. Dinâmica populacional de marsupiais e roedores no Parque Municipal da Lagoa do Peri, Ilha de Santa Catarina, sul do Brasil. Mastozoología Neotrop. 2006, 13, 31–49. [Google Scholar]
- Graipel, M.E.; Miller, P.R.M.; Glock, L. Padrão de atividade de Akodon montensis e Oryzomys russatus na reserva Volta Velha, Santa Catarina, sul do Brasil. Mastozoología Neotrop. 2003, 10, 255–260. [Google Scholar]
- Bergallo, H.G. Ecology of a Small Mammal Community in an Atlantic Forest Area in Southeastern Brazil. Stud. Neotrop. Fauna Environ. 1994, 29, 197–217. [Google Scholar] [CrossRef]
- Pardini, R.; Bueno Ade, A.; Gardner, T.A.; Prado, P.I.; Metzger, J.P. Beyond the fragmentation threshold hypothesis: Regime shifts in biodiversity across fragmented landscapes. PLoS ONE 2010, 5, e13666. [Google Scholar] [CrossRef]
- Passamani, M.; Fernandez, F.A.S. Movements of small mammals among Atlantic Forest fragments in Espírito Santo, Southeastern Brazil. Mammalia 2011, 75, 83–86. [Google Scholar] [CrossRef]
- Bonvicino, C.R.; Lindbergh, S.M.; Maroja, L.S. Small non-flying mammals from conserved and altered areas of Atlantic forest and Cerrado: Comments on their potencial use for monitoring environment. Braz. J. Biol. 2002, 62, 765–774. [Google Scholar] [CrossRef]
- Moreira, J.C.; Manduca, E.G.; Gonçalves, P.R.; Morais Júnior, M.M.d.; Pereira, R.F.; Lessa, G.; Dergam, J.A. Small mammals from Serra do Brigadeiro State Park, Minas Gerais, southeastern Brazil: Species composition and elevational distribution. Arq. Do Mus. Nac. 2009, 67, 103–118. [Google Scholar]
- Lima, D.O.d.; Azambuja, B.O.; Camilotti, V.L.; Cáceres, N.C. Small mammal community structure and microhabitat use in the austral boundary of the Atlantic Forest, Brazil. Zoologia 2010, 27, 99–105. [Google Scholar] [CrossRef][Green Version]
- Cherem, J.J.; Perez, D.M. Mamíferos terrestres de floresta de araucária no município de Três Barras, Santa Catarina, Brasil. Biotemas 1996, 9, 29–46. [Google Scholar][Green Version]
- Cáceres, N.C.; Monteiro Filho, E.L.A. Population dynamics of the Common Oppossum, Didelphis marsupialis (Mammalia, Marsupialia), in southern Brazil. Z. Saugetierkd. 1998, 63, 169–172. [Google Scholar][Green Version]
- Bergallo, H.G.; Magnusson, W.E. Factors affecting the use of space by two rodent species in Brazilian Atlantic forest. Mammalia 2004, 68, 121–132. [Google Scholar] [CrossRef]
- Uchôa, T. Comunidades dos Pequenos Mamíferos em dois Estágios Sucessionais de Floresta ATLÂNTICA e suas Implicações à Ecologia e Conservação. Master’s Thesis, Universidade Federal do Paraná, Curitiba, Brazil, 2006. Available online: https://acervodigital.ufpr.br/xmlui/bitstream/handle/1884/19817/Tatiane%20Uchoa.pdf?sequence=1&isAllowed=y (accessed on 24 June 2025).
- Ostfeld, R.S. The ecology of territoriality in small mammals. Trends Ecol. Evol. 1990, 5, 411–415. [Google Scholar] [CrossRef]
- Wolff, J.O. Why Are Female Small Mammals Territorial? Oikos 1993, 68, 364–370. [Google Scholar] [CrossRef]
- Hendrie, C.A.; Weiss, S.M.; Eilam, D. Behavioural response of wild rodents to the calls of an owl: A comparative study. J. Zool. 1998, 245, 439–446. [Google Scholar] [CrossRef]
- Bergallo, H.G.; Magnusson, W.E. Effects of climate and food availability on four rodent species in southeastern Brazil. J. Mammal. 1999, 80, 472–486. [Google Scholar] [CrossRef]
- Moscarella, R.A.; Benado, M.; Aguilera, M. A Comparative Assessment of Growth Curves as Estimators of Male and Female Ontogeny in Oryzomys albigularis. J. Mammal. 2001, 82, 520–526. [Google Scholar] [CrossRef]
- Austin, C.R.; Austin, C.R.; Short, R.V. Reproduction in Mammals: Volume 4, Reproductive Fitness; Cambridge University Press: Cambridge, UK, 1985. [Google Scholar]
- Bergallo, H.G.; Magnusson, W.E. Effects of weather and food availability on the condition and growth of two species of rodents in Southeastern Brazil. Mammalia 2002, 66, 17–31. [Google Scholar] [CrossRef]
- Pinotti, B.T.; Naxara, L.; Pardini, R. Diet and food selection by small mammals in an old-growth Atlantic forest of south-eastern Brazil. Stud. Neotrop. Fauna Environ. 2011, 46, 1–9. [Google Scholar] [CrossRef]
- Fonseca, G.; Kierulff, M. Biology and natural history of Brazilian Atlantic forest small mammals. Bull. Fla. State Mus. Biol. Sci. 1989, 34, 99–152. [Google Scholar] [CrossRef]
- Smith, M.H.; Blessing, R.W. Trap Response and Food Availability. J. Mammal. 1969, 50, 368–369. [Google Scholar] [CrossRef]
- Vieira, E.M.; Pizo, M.A.; Izar, P. Fruit and seed exploitation by small rodents of the Brazilian Atlantic forest. Mammalia 2003, 67, 533–539. [Google Scholar] [CrossRef]
- Briani, D.C.; Vieira, E.M.; Vieira, M.V. Nests and nesting sites of Brazilian forest rodents (Nectomys squamipes and Oryzomys intermedius) as revealed by a spool-and-line device. Acta Theriol. 2001, 46, 331–334. [Google Scholar] [CrossRef]
- Skupien, F.L.; Rodrigues, D.P.; Sausen, J.D.O.; Gonçalves, G.L.; Lima, D.O.D. Small mammals and microhabitat selection in forest fragments in the transition zone between Atlantic Forest and Pampa biome. Papéis Avulsos Zool. 2022, 62, e202262039. [Google Scholar] [CrossRef]
| Species | Stage | Sex | Avg. Body Mass (g) (Range) | n | Avg. Body Length (mm) (Range) | Sex Ratio (F:M) |
|---|---|---|---|---|---|---|
| S. angouya | Juvenile | Female | 27 (15–36) | 12 | 98 (80–133) | 6:1 |
| Male | 23 (22–24) | 2 | 97.5 (90–105) | |||
| Subadult | Female | 54.2 (33.5–66) | 9 | 122 (112–130) | 1.1:1 | |
| Male | 51 (36–60) | 8 | 120 (106–130) | |||
| Adult | Female | 99.1 (56.1–158) | 10 | 148 (120–169) | 1.2:1 | |
| Male | 85.1 (70.1–116) | 8 | 146 (132–157) | |||
| E. russatus | Juvenile | Female | 18.9 (10–31) | 15 | 89 (76–101) | 1:1.1 |
| Male | 17.2 (12.5–28) | 17 | 83 (71–95) | |||
| Subadult | Female | 38.9 (19–63) | 23 | 110 (85–130) | 1.9:1 | |
| Male | 35.6 (26–56) | 12 | 108 (89–127) | |||
| Adult | Female | 62.7 (42–104) | 45 | 132 (94–150) | 1.2:1 | |
| Male | 69.8 (38–128) | 95 | 133 (89–161) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bovendorp, R.; Moreno, G.; Feitosa, M.; Percequillo, A. Ecology and Population Structure of Two Sympatric Rodents in a Neotropical Forest of Southeastern Brazil. Life 2025, 15, 1642. https://doi.org/10.3390/life15111642
Bovendorp R, Moreno G, Feitosa M, Percequillo A. Ecology and Population Structure of Two Sympatric Rodents in a Neotropical Forest of Southeastern Brazil. Life. 2025; 15(11):1642. https://doi.org/10.3390/life15111642
Chicago/Turabian StyleBovendorp, Ricardo, Gabriela Moreno, Matheus Feitosa, and Alexandre Percequillo. 2025. "Ecology and Population Structure of Two Sympatric Rodents in a Neotropical Forest of Southeastern Brazil" Life 15, no. 11: 1642. https://doi.org/10.3390/life15111642
APA StyleBovendorp, R., Moreno, G., Feitosa, M., & Percequillo, A. (2025). Ecology and Population Structure of Two Sympatric Rodents in a Neotropical Forest of Southeastern Brazil. Life, 15(11), 1642. https://doi.org/10.3390/life15111642







