Advancing Duodenoscope Reprocessing with Alginate-Coated Calcium Peroxide Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
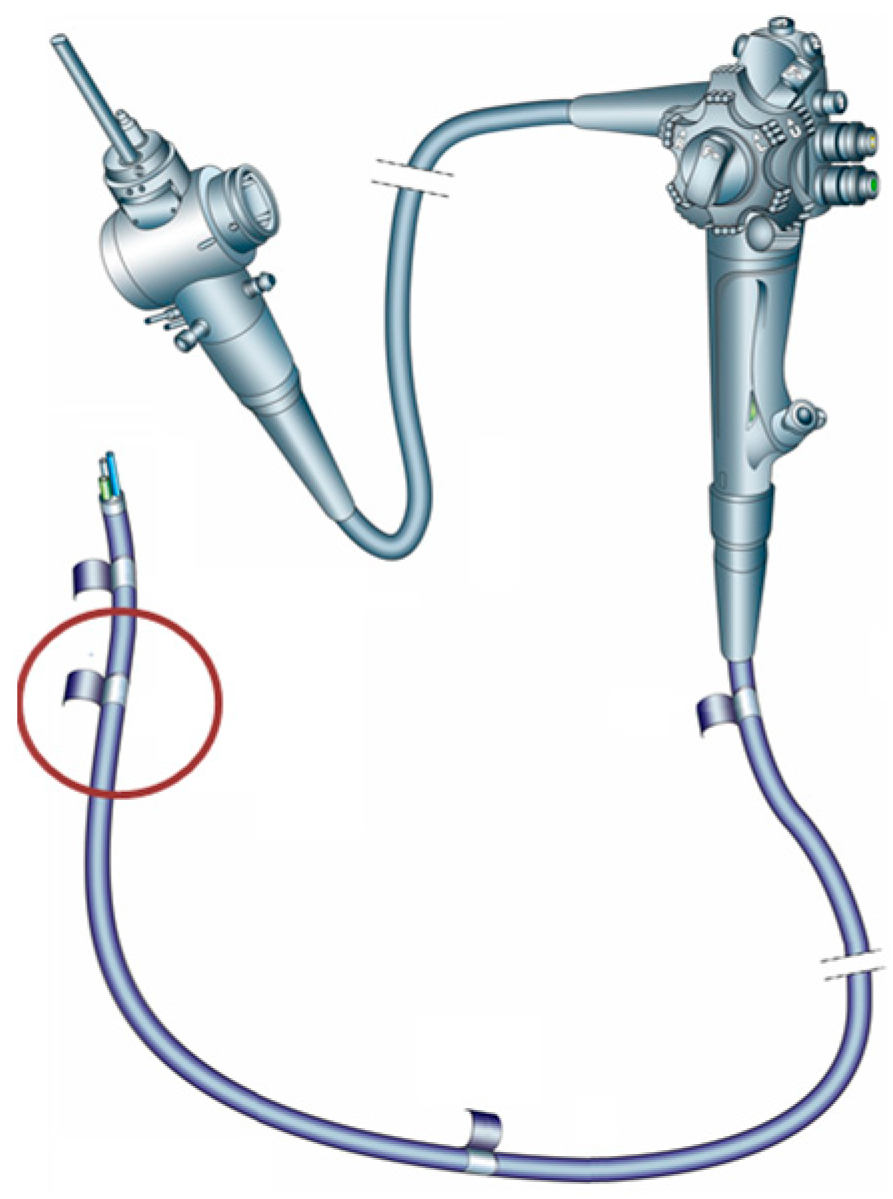
2.1. Duodenoscope Samples
2.2. The CaO2–Alg NPs Synthesis
2.3. Biological Investigation
2.3.1. Evaluation of the Disinfectant Properties of the CaO2–Alg NPs Under Biomimetic Conditions
2.3.2. Evaluation of the Compatibility of the CaO2−Alg NPs with the Polymeric Coating of the Duodenoscope
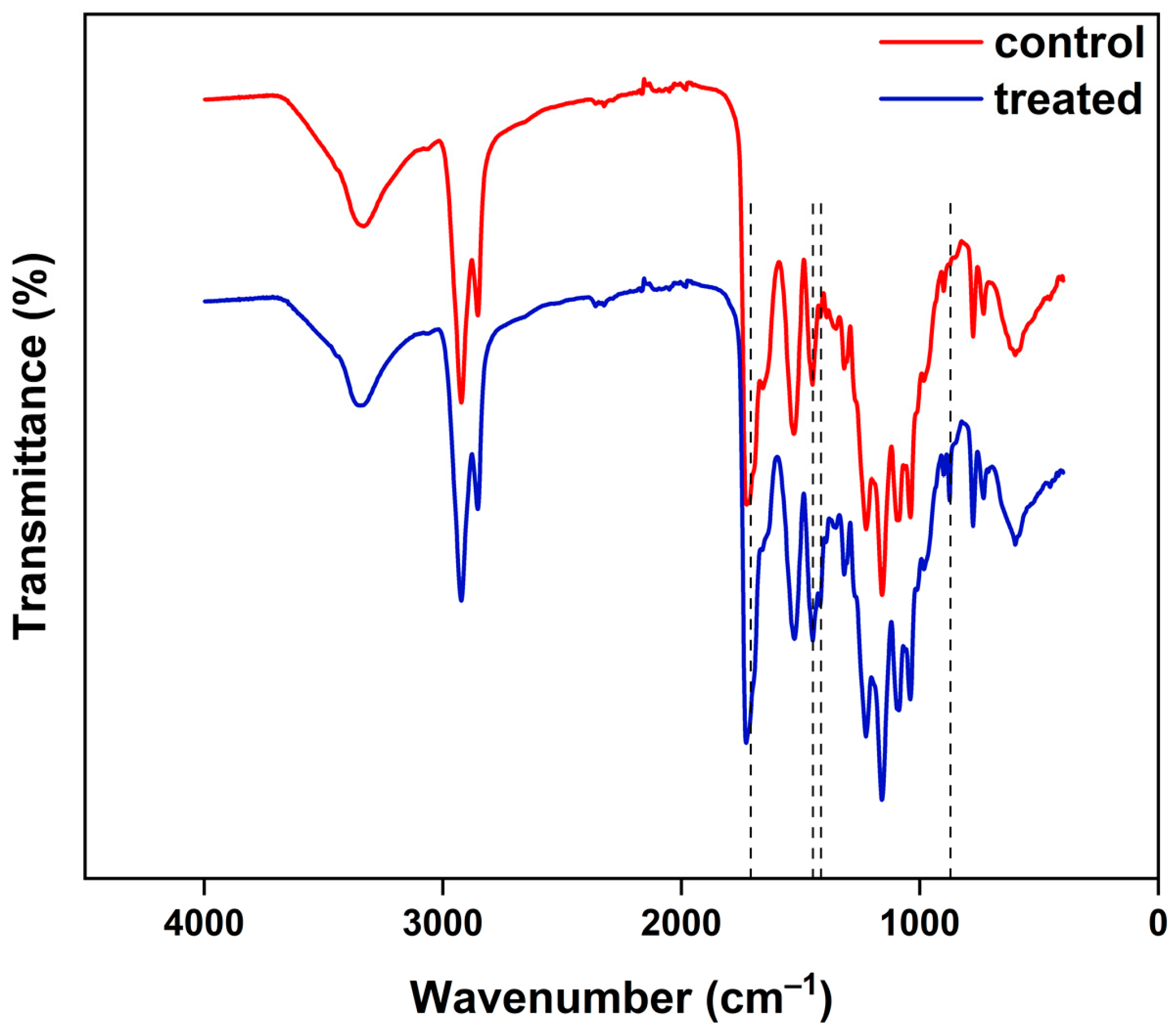
- Fourier Transform Infrared Spectroscopy (FT-IR)
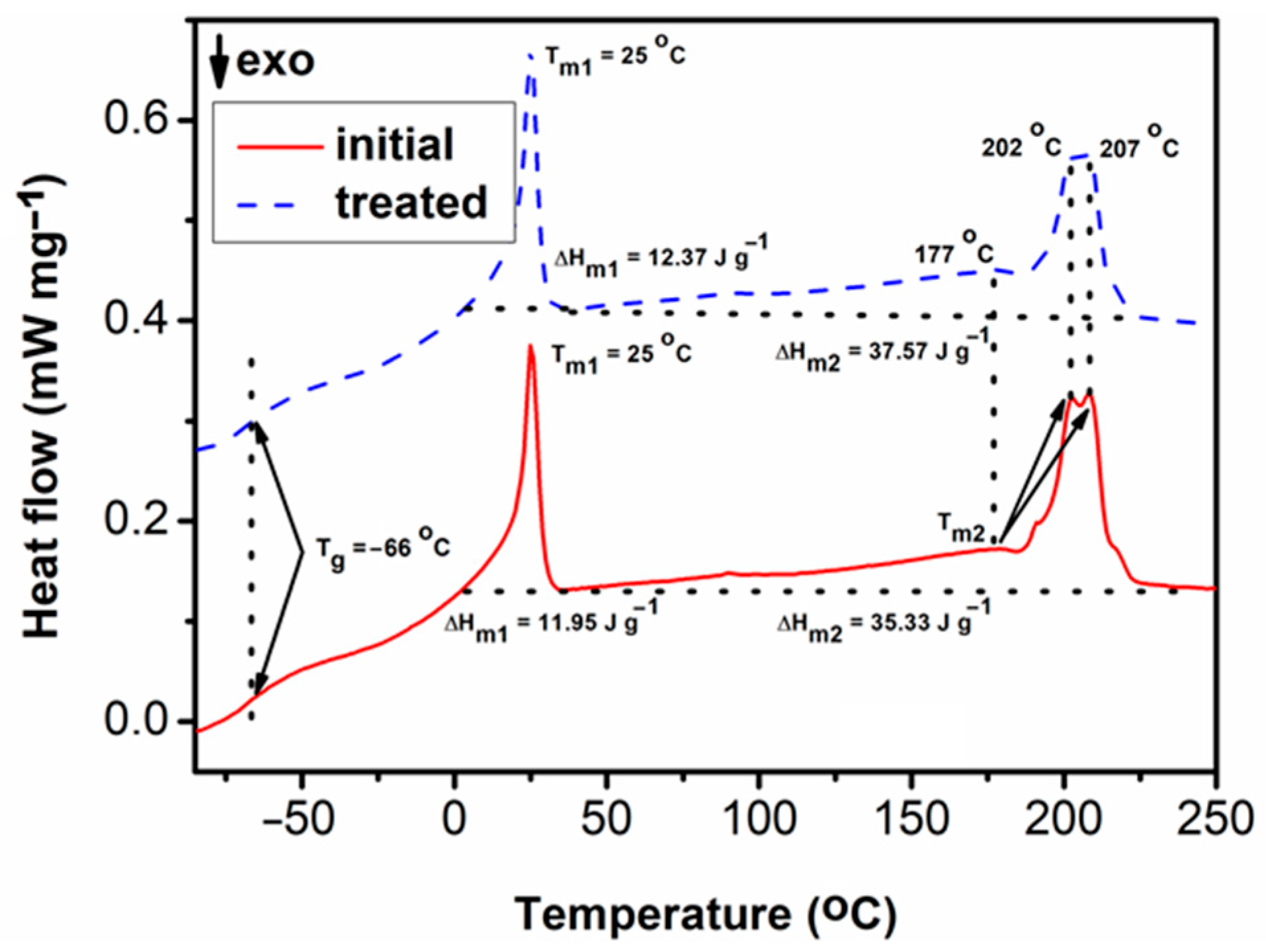
- Differential Scanning Calorimetry (DSC)
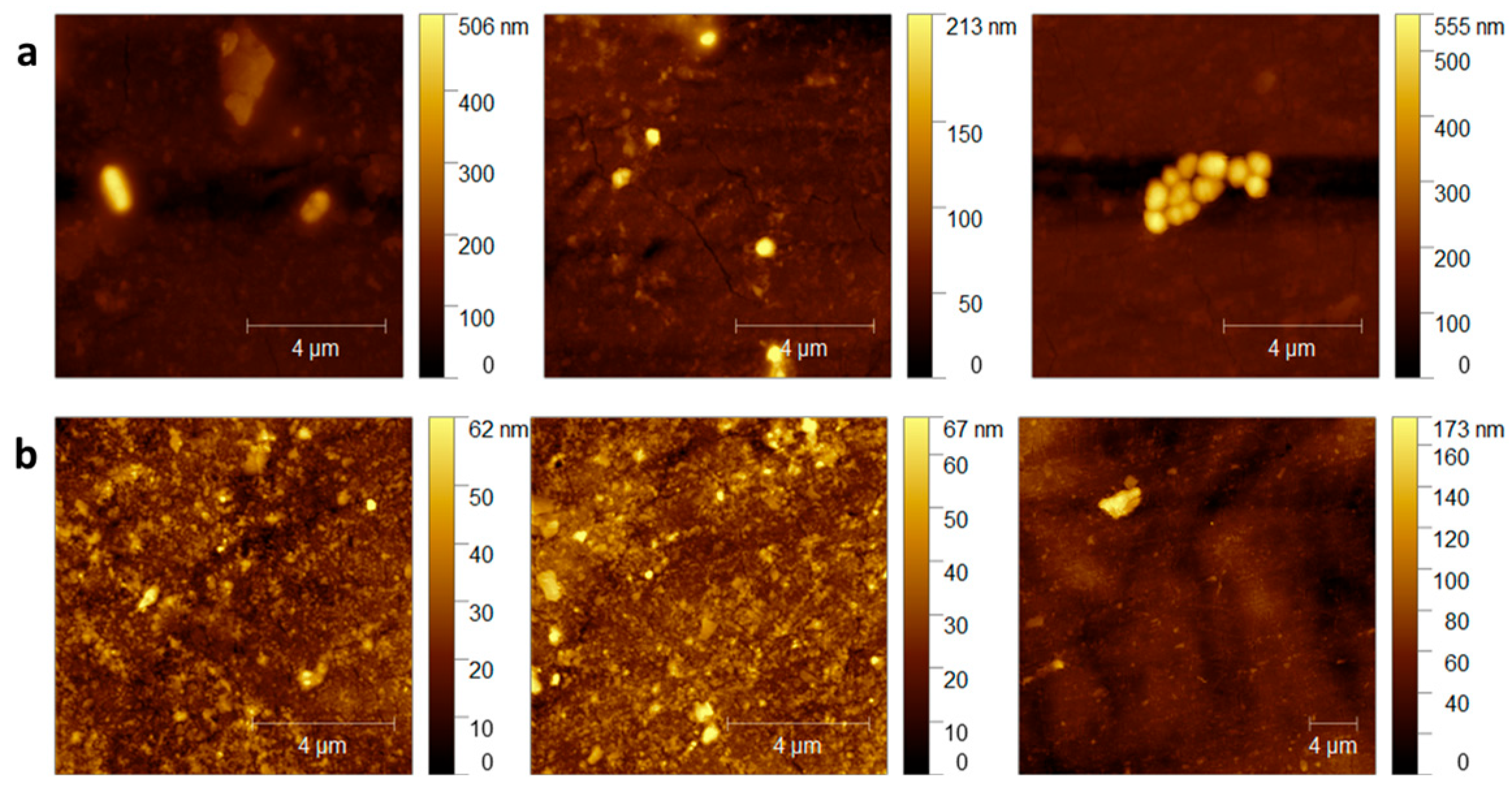
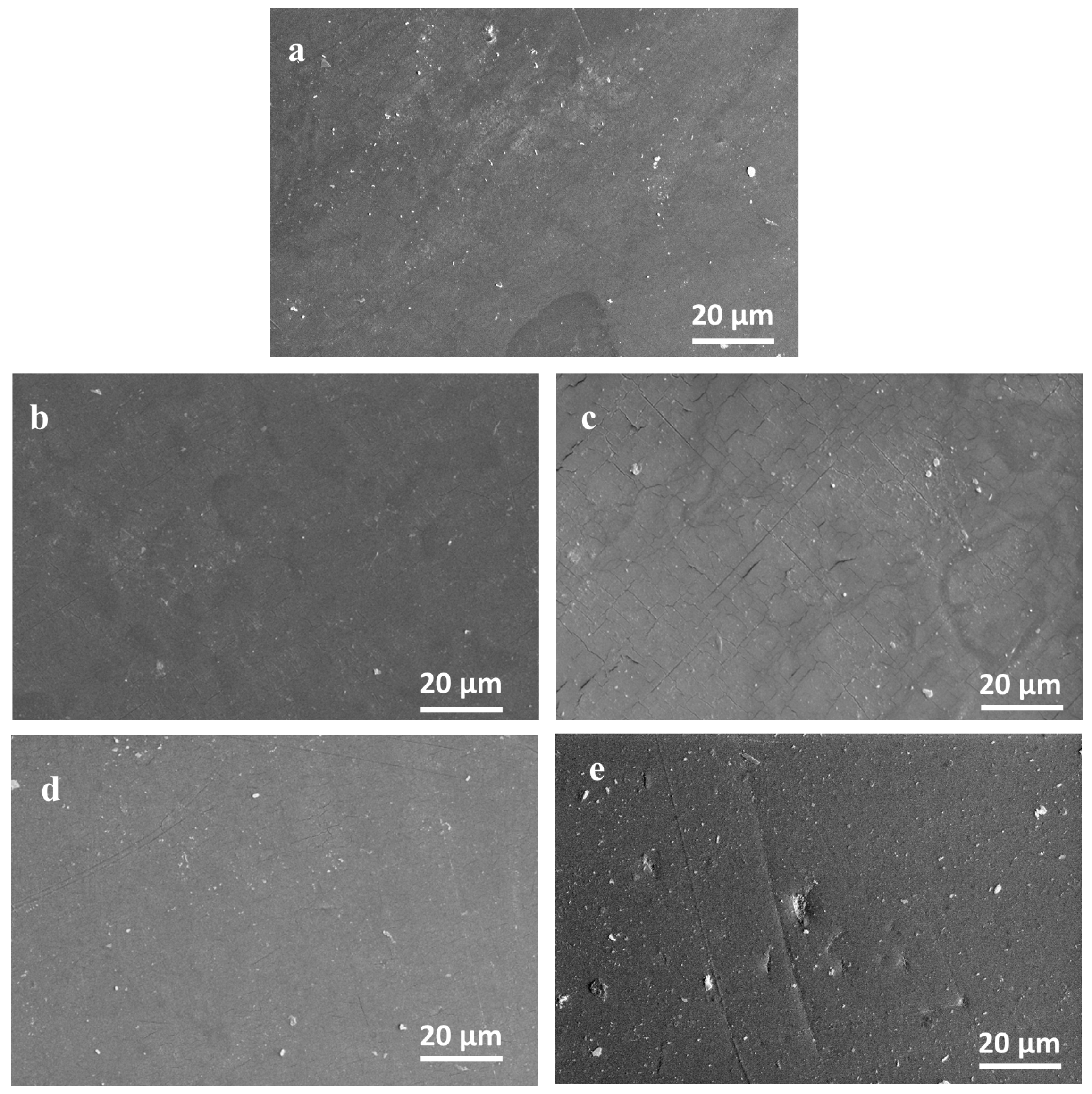
- Atomic Force Microscopy (AFM)
- Scanning Electron Microscopy (SEM)
2.3.3. Evaluation of Microbial Adhesion on the Duodenoscope Surface Following Prolonged Contact with Metal Oxide Nanoparticles
3. Results and Discussion
3.1. Evaluation of the Disinfectant Properties of the CaO2–Alg NPs Under Biomimetic Conditions
3.2. Evaluation of the Compatibility of the CaO2–Alg NPs with the Polymeric Duodenoscope Coating
3.3. Evaluation of Microbial Adhesion on the Duodenoscope Surface Following Prolonged Contact with the CaO2–Alg NPs
3.4. Evaluation of the Economic Burden and Subsequent Challenges of Single-Use Versus Current Multiple-Use Reprocessible Duodenoscopes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Center for Drug Evaluation and Research. Novel Drug Approvals for 2025; FDA: Silver Spring, MD, USA, 2025. [Google Scholar]
- Larsen, S.; Russell, R.V.; Ockert, L.K.; Spanos, S.; Travis, H.S.; Ehlers, L.H.; Mærkedahl, A. Rate and Impact of Duodenoscope Contamination: A Systematic Review and Meta-Analysis. eClinicalMedicine 2020, 25, 100451. [Google Scholar] [CrossRef]
- Bomman, S.; Klair, J.S.; Canakis, A.; Muthusamy, A.K.; Nagra, N.; Chandra, S.; Shanmugam, M.; Perisetti, A.; Aggarwal, A.; Gavini, H.K.; et al. Safety and Efficacy of Endoscopic Full Thickness Resection of Upper Gastrointestinal Lesions Using a Full Thickness Resection Device: A Systematic Review and Meta-Analysis. J. Clin. Gastroenterol. 2024, 58, 46–52. [Google Scholar] [CrossRef]
- Rutala, W.A.; Weber, D.J. Best Practices for Disinfection of Noncritical Environmental Surfaces and Equipment in Health Care Facilities: A Bundle Approach. Am. J. Infect. Control 2019, 47, A96–A105. [Google Scholar] [CrossRef]
- Heuvelmans, M.; Wunderink, H.F.; van der Mei, H.C.; Monkelbaan, J.F. A Narrative Review on Current Duodenoscope Reprocessing Techniques and Novel Developments. Antimicrob. Resist. Infect. Control 2021, 10, 171. [Google Scholar] [CrossRef]
- Günșahin, D.; Șandru, V.; Constantinescu, G.; Ilie, M.; Cabel, T.; Popescu, R.Ș.; Ungureanu, B.S.; Miron, V.D.; Balan, G.G.; Cotigă, D.; et al. A Comprehensive Review of Digestive Endoscopy-Associated Infections: Bacterial Pathogens, Host Susceptibility, and the Impact of Viral Hepatitis. Microorganisms 2025, 13, 2128. [Google Scholar] [CrossRef] [PubMed]
- Ofstead, C.L.; Buro, B.L.; Hopkins, K.M.; Eiland, J.E.; Wetzler, H.P.; Lichtenstein, D.R. Duodenoscope-Associated Infection Prevention: A Call for Evidence-Based Decision Making. Endosc. Int. Open 2020, 8, E1769–E1781. [Google Scholar] [CrossRef] [PubMed]
- Verfaillie, C.J.; Bruno, M.J.; Voor in ’t Holt, A.F.; Buijs, J.G.; Poley, J.-W.; Loeve, A.J.; Severin, J.A.; Abel, L.F.; Smit, B.J.; de Goeij, I.; et al. Withdrawal of a Novel-Design Duodenoscope Ends Outbreak of a VIM-2-Producing Pseudomonas aeruginosa. Endoscopy 2015, 47, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Day, L.W.; Muthusamy, V.R.; Collins, J.; Kushnir, V.M.; Sawhney, M.S.; Thosani, N.C.; Wani, S. Multisociety Guideline on Reprocessing Flexible GI Endoscopes and Accessories. Gastrointest. Endosc. 2021, 93, 11–33.e6. [Google Scholar] [CrossRef]
- Speer, T.; Alfa, M.; Jones, D.; Vickery, K.; Griffiths, H.; Sáenz, R.; LeMair, A. WGO Guideline-Endoscope Disinfection Update. J. Clin. Gastroenterol. 2023, 57, 1–9. [Google Scholar] [CrossRef]
- Rutala, W.A.; Weber, D.J. Disinfection and Sterilization: An Overview. Am. J. Infect. Control 2013, 41, S2–S5. [Google Scholar] [CrossRef]
- Beilenhoff, U.; Biering, H.; Blum, R.; Brljak, J.; Cimbro, M.; Dumonceau, J.-M.; Hassan, C.; Jung, M.; Kampf, B.; Neumann, C.; et al. Reprocessing of Flexible Endoscopes and Endoscopic Accessories Used in Gastrointestinal Endoscopy: Position Statement of the European Society of Gastrointestinal Endoscopy (ESGE) and European Society of Gastroenterology Nurses and Associates (ESGENA)—Update 2018. Endoscopy 2018, 50, 1205–1234. [Google Scholar] [CrossRef] [PubMed]
- Epstein, L.; Hunter, J.C.; Arwady, M.A.; Tsai, V.; Stein, L.; Gribogiannis, M.; Frias, M.; Guh, A.Y.; Laufer, A.S.; Black, S.; et al. New Delhi Metallo-β-Lactamase–Producing Carbapenem-Resistant Escherichia coli Associated with Exposure to Duodenoscopes. JAMA 2014, 312, 1447–1455. [Google Scholar] [CrossRef] [PubMed]
- Gromski, M.A.; Sherman, S. Technological Review: Developments in Innovative Duodenoscopes. Gastrointest. Endosc. 2022, 95, 42–50. [Google Scholar] [CrossRef] [PubMed]
- van der Ploeg, K.; Haanappel, C.P.; Voor In ’t Holt, A.F.; de Groot, W.; Bulkmans, A.J.C.; Erler, N.S.; Mason-Slingerland, B.C.G.C.; Severin, J.A.; Vos, M.C.; Bruno, M.J. Unveiling 8 Years of Duodenoscope Contamination: Insights from a Retrospective Analysis in a Large Tertiary Care Hospital. Gut 2024, 73, 613–621. [Google Scholar] [CrossRef]
- Balan, G.G.; Rosca, I.; Ursu, E.-L.; Fifere, A.; Varganici, C.-D.; Doroftei, F.; Turin-Moleavin, I.-A.; Sandru, V.; Constantinescu, G.; Timofte, D.; et al. Duodenoscope-Associated Infections beyond the Elevator Channel: Alternative Causes for Difficult Reprocessing. Molecules 2019, 24, 2343. [Google Scholar] [CrossRef]
- Bălan, G.G.; Roșca, I.; Ursu, E.-L.; Doroftei, F.; Bostănaru, A.-C.; Hnatiuc, E.; Năstasă, V.; Șandru, V.; Ștefănescu, G.; Trifan, A.; et al. Plasma-Activated Water: A New and Effective Alternative for Duodenoscope Reprocessing. Infect. Drug Resist. 2018, 11, 727–733. [Google Scholar] [CrossRef]
- Ailincai, D.; Turin Moleavin, I.-A.; Sarghi, A.; Fifere, A.; Dumbrava, O.; Pinteala, M.; Balan, G.G.; Rosca, I. New Hydrogels Nanocomposites Based on Chitosan, 2-Formylphenylboronic Acid, and ZnO Nanoparticles as Promising Disinfectants for Duodenoscopes Reprocessing. Polymers 2023, 15, 2669. [Google Scholar] [CrossRef]
- Li, A.; Qian, C.; Wen, J.; Wang, T. Synchronous Removal of Organic Pollutants and Phosphorus from Emergency Wastewater in Chemical Industry Park by Plasma Catalysis System Based on Calcium Peroxide. Catalysts 2025, 15, 486. [Google Scholar] [CrossRef]
- Xu, Q.; Huang, Q.-S.; Wei, W.; Sun, J.; Dai, X.; Ni, B.-J. Improving the Treatment of Waste Activated Sludge Using Calcium Peroxide. Water Res. 2020, 187, 116440. [Google Scholar] [CrossRef]
- Shasha, Z.; Chuanchuan, H.; Yawen, Z. The Progress and Prospect of Calcium Peroxide Nanoparticles in Antibacterial Activity. Colloid Interface Sci. Commun. 2024, 61, 100793. [Google Scholar] [CrossRef]
- Mollajavadi, M.Y.; Saadatmand, M.; Ghobadi, F. Effect of Calcium Peroxide Particles as Oxygen-Releasing Materials on Cell Growth and Mechanical Properties of Scaffolds for Tissue Engineering. Iran. Polym. J. 2023, 32, 599–608. [Google Scholar] [CrossRef]
- Daneshmandi, L.; Laurencin, C.T. Regenerative Engineered Vascularized Bone Mediated by Calcium Peroxide. J. Biomed. Mater. Res. Part A 2020, 108, 1045–1057. [Google Scholar] [CrossRef] [PubMed]
- Turin Moleavin, I.A.; Sarghi, A.; Ursu, E.L.; Sandu, A.I.; Balan, G.G.; Rosca, I.; Fifere, A.; Pinteala, M. Alginate-Coated Calcium Peroxide Nanoparticles as a Disinfectant for Duodenoscope Reprocessing. ACS Appl. Nano Mater. 2023, 6, 23103–23113. [Google Scholar] [CrossRef]
- JIS Z 2801; Antibacterial Products—Test for Antibacterial Activity and Efficacy. Japan Standards Association (JSA): Tokyo, Japan, 2010.
- Khodaveisi, J.; Banejad, H.; Afkhami, A.; Olyaie, E.; Lashgari, S.; Dashti, R. Synthesis of Calcium Peroxide Nanoparticles as an Innovative Reagent for in Situ Chemical Oxidation. J. Hazard. Mater. 2011, 192, 1437–1440. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Zhang, X.; Xue, Y. Application of Calcium Peroxide in Water and Soil Treatment: A Review. J. Hazard. Mater. 2017, 337, 163–177. [Google Scholar] [CrossRef]
- Wu, J.; Shen, P.; Qin, X.; Yang, Y.; Lin, C.; Li, X.; Geng, W.; Gao, P.; Chen, L.; Miao, L.; et al. Self-Supply of H2O2 and O2 by a Composite Nanogenerator for Chemodynamic Therapy/Hypoxia Improvement and Rapid Therapy of Biofilm-Infected Wounds. Chem. Eng. J. 2023, 459, 141507. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, Y.; Li, T.; Chen, Z.; Wang, Y.; Qin, C. Properties of Calcium Peroxide for Release of Hydrogen Peroxide and Oxygen: A Kinetics Study. Chem. Eng. J. 2016, 303, 450–457. [Google Scholar] [CrossRef]
- Zhang, M.; Song, R.; Liu, Y.; Yi, Z.; Meng, X.; Zhang, J.; Tang, Z.; Yao, Z.; Liu, Y.; Liu, X.; et al. Calcium-Overload-Mediated Tumor Therapy by Calcium Peroxide Nanoparticles. Chem 2019, 5, 2171–2182. [Google Scholar] [CrossRef]
- Wu, D.; Zhu, Z.-Q.; Tang, H.-X.; Shi, Z.-E.; Kang, J.; Liu, Q.; Qi, J. Efficacy-Shaping Nanomedicine by Loading Calcium Peroxide into Tumor Microenvironment-Responsive Nanoparticles for the Antitumor Therapy of Prostate Cancer. Theranostics 2020, 10, 9808–9829. [Google Scholar] [CrossRef]
- Wang, Y.; He, X.; Zhou, C.; Bai, Y.; Li, T.; Liu, J.; Ju, S.; Wang, C.; Xiang, G.; Xiong, B. Nanoscale CaO2 Materials for Synergistic Transarterial Chemoembolization in a VX2 Orthotopic Rabbit Liver Cancer Model. Acta Biomater. 2022, 154, 536–548. [Google Scholar] [CrossRef]
- Ruan, S.; Yin, W.; Chang, J.; Yang, Y.; Sun, J.; Ma, X.; Liu, Y.; Zang, J.; Liu, Y.; Li, Y.; et al. Acidic and Hypoxic Tumor Microenvironment Regulation by CaO2-Loaded Polydopamine Nanoparticles. J. Nanobiotechnol. 2022, 20, 544. [Google Scholar] [CrossRef] [PubMed]
- Thi, P.L.; Lee, Y.; Tran, D.L.; Thi, T.T.H.; Park, K.M.; Park, K.D. Calcium Peroxide-Mediated in Situ Formation of Multifunctional Hydrogels with Enhanced Mesenchymal Stem Cell Behaviors and Antibacterial Properties. J. Mater. Chem. B 2020, 8, 11033–11043. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.; Nguyen, T.T. Strategy for Treatment of Infected Diabetic Foot Ulcers. Acc. Chem. Res. 2021, 54, 1080–1093. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Pradhan, D.; Halder, J.; Biswasroy, P.; Rai, V.K.; Dubey, D.; Kar, B.; Ghosh, G.; Rath, G. Metal Nanoparticles against Multi-Drug-Resistance Bacteria. J. Inorg. Biochem. 2022, 237, 111938. [Google Scholar] [CrossRef]
- Seixas, A.F.; Quendera, A.P.; Sousa, J.P.; Silva, A.F.Q.; Arraiano, C.M.; Andrade, J.M. Bacterial Response to Oxidative Stress and RNA Oxidation. Front. Genet. 2022, 12, 821535. [Google Scholar] [CrossRef]
- Chaudhary, P.; Janmeda, P.; Docea, A.O.; Yeskaliyeva, B.; Abdull Razis, A.F.; Modu, B.; Calina, D.; Sharifi-Rad, J. Oxidative Stress, Free Radicals and Antioxidants: Potential Crosstalk in the Pathophysiology of Human Diseases. Front. Chem. 2023, 11, 1158198. [Google Scholar] [CrossRef]
- Asad, N.R.; Asad, L.M.B.O.; de Almeida, C.E.B.; Felzenszwalb, I.; Cabral-Neto, J.B.; Leitão, A.C. Several Pathways of Hydrogen Peroxide Action That Damage the E. coli Genome. Genet. Mol. Biol. 2004, 27, 291–303. [Google Scholar] [CrossRef]
- Gu, P.; Wang, Y.; Wu, H.; Chen, L.; Zhang, Z.; Yang, K.; Zhang, Z.; Ren, X.; Miao, H.; Zheng, Z. Efficient Control of Cyanobacterial Blooms with Calcium Peroxide: Threshold and Mechanism. Sci. Total Environ. 2023, 882, 163591. [Google Scholar] [CrossRef]
- Liu, L.; Pan, Y.; Zhi, X.; Chen, L.; Zhu, H. Bacterial Antioxidant Mechanism in Calcium Peroxide Aided Sludge Anaerobic Fermentation. Bioresour. Technol. 2023, 384, 129327. [Google Scholar] [CrossRef]
- Zanganeh, M.; Jiang, Y.; Waugh, N.; Brown, A.; Chen, Y.-F.; Arasaradnam, R.P.; Andronis, L.; “SUMU Endo” Project Group. Single-Use versus Reusable Endoscopes in Gastroenterology: Systematic Review of Full and Partial Economic Evaluations. Endosc. Int. Open 2025, 13, a26451463. [Google Scholar] [CrossRef]
- Rodríguez de Santiago, E.; Dinis-Ribeiro, M.; Pohl, H.; Agrawal, D.; Arvanitakis, M.; Baddeley, R.; Bak, E.; Bhandari, P.; Bretthauer, M.; Burga, P.; et al. Reducing the Environmental Footprint of Gastrointestinal Endoscopy: European Society of Gastrointestinal Endoscopy (ESGE) and European Society of Gastroenterology and Endoscopy Nurses and Associates (ESGENA) Position Statement. Endoscopy 2022, 54, 797–826. [Google Scholar] [CrossRef]
- Hassan, C.; Ponchon, T.; Bisschops, R.; van Hooft, J.E.; Messmann, H.; Gralnek, I.M.; Dinis-Ribeiro, M. European Society of Gastrointestinal Endoscopy (ESGE) Publications Policy—Update 2020. Endoscopy 2020, 52, 123–126. [Google Scholar] [CrossRef]
- Lenzen, M.; Malik, A.; Li, M.; Fry, J.; Weisz, H.; Pichler, P.-P.; Chaves, L.S.M.; Capon, A.; Pencheon, D. The Environmental Footprint of Health Care: A Global Assessment. Lancet Planet. Health 2020, 4, e271–e279. [Google Scholar] [CrossRef]
- Ma, G.K.; Pegues, D.A.; Kochman, M.L.; Alby, K.; Fishman, N.O.; Saunders, M.; Grous, C.; Dempsey, D.T.; Ginsberg, G.G. Implementation of a Systematic Culturing Program to Monitor the Efficacy of Endoscope Reprocessing: Outcomes and Costs. Gastrointest. Endosc. 2018, 87, 104–109.e3. [Google Scholar] [CrossRef]
- Barakat, M.; Ghosh, S.; Banerjee, S. 775 Cost Utility Analysis Comparing Duodenoscope Reprocessing/Sterilization, Novel Duodenoscopes with Disposable Endcaps and Fully Disposable Duodenoscopes. Gastrointest. Endosc. 2020, 91, AB67–AB68. [Google Scholar] [CrossRef]
- Kwakman, J.A.; Poley, M.J.; Vos, M.C.; Bruno, M.J. Single-Use Duodenoscopes Compared with Reusable Duodenoscopes in Patients Carrying Multidrug-Resistant Microorganisms: A Break-Even Cost Analysis. Endosc. Int. Open 2023, 11, E571–E580. [Google Scholar] [CrossRef]
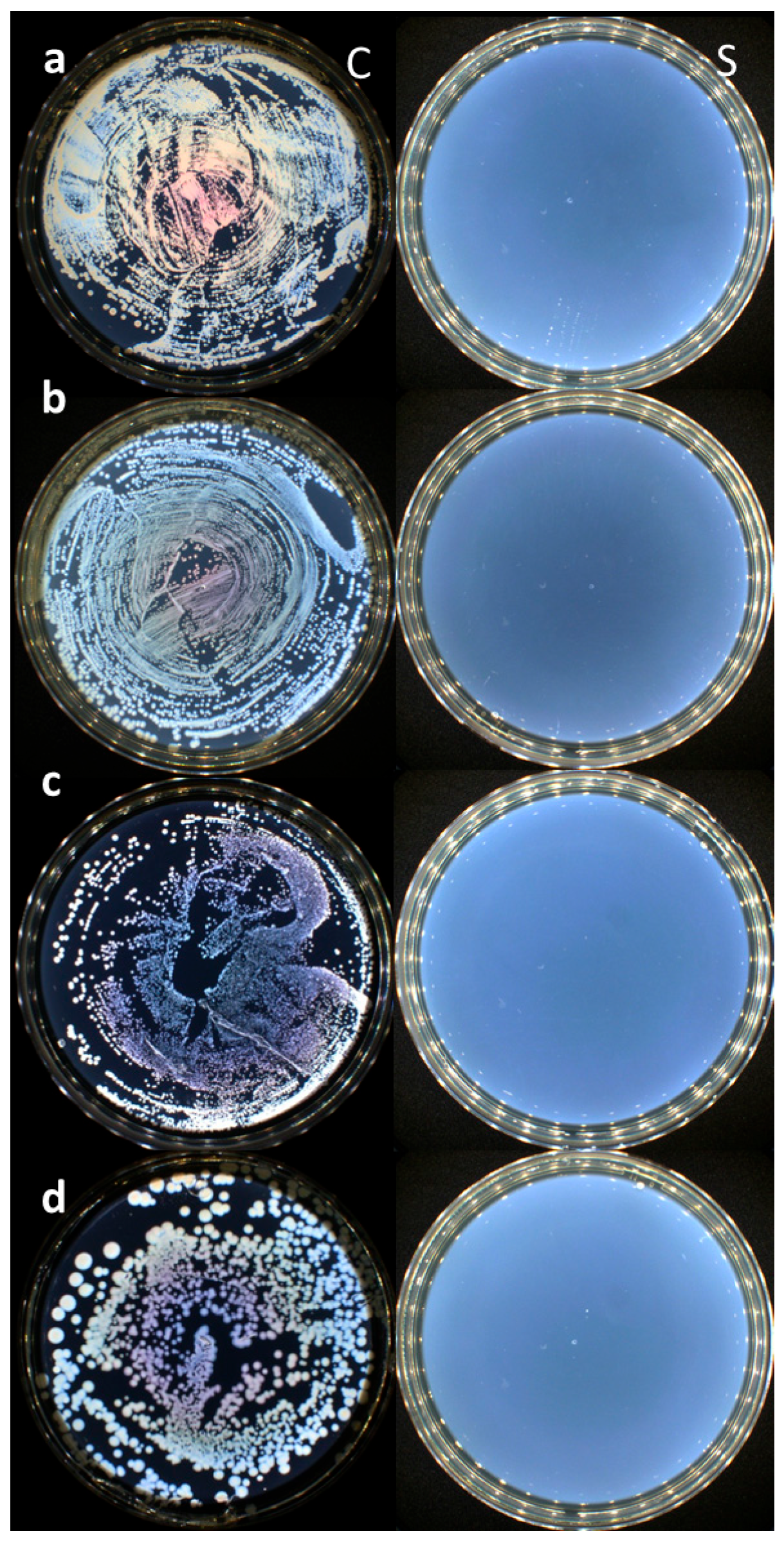
| Tested Strain | 5 min | 10 min | 15 min | 20 min | 30 min |
|---|---|---|---|---|---|
| E. coli | + | + | + | − | − |
| K. pneumoniae | + | + | + | − | − |
| E. faecalis | + | + | + | − | − |
| S. aureus | + | + | + | − | − |
| Element | Control | Treated Sample | ||
|---|---|---|---|---|
| Weight % | Atomic % | Weight % | Atomic % | |
| C | 59.14 | 66.59 | 59.65 | 68.72 |
| N | 11.98 | 11.56 | 5.40 | 5.33 |
| O | 22.07 | 18.65 | 25.09 | 21.70 |
| Na | 1.47 | 0.86 | 0.57 | 0.34 |
| Mg | 0.50 | 0.28 | 0.40 | 0.23 |
| P | 2.68 | 1.17 | 4.29 | 1.92 |
| S | 1.71 | 0.72 | 1.28 | 0.55 |
| K | 0.47 | 0.16 | 0.63 | 0.22 |
| Ca | - | - | 2.29 | 0.79 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fifere, A.; Varganici, C.-D.; Ursu, E.-L.; Pinteala, T.; Sandru, V.; Turin-Moleavin, I.-A.; Rosca, I.; Balan, G.G. Advancing Duodenoscope Reprocessing with Alginate-Coated Calcium Peroxide Nanoparticles. Life 2025, 15, 1643. https://doi.org/10.3390/life15111643
Fifere A, Varganici C-D, Ursu E-L, Pinteala T, Sandru V, Turin-Moleavin I-A, Rosca I, Balan GG. Advancing Duodenoscope Reprocessing with Alginate-Coated Calcium Peroxide Nanoparticles. Life. 2025; 15(11):1643. https://doi.org/10.3390/life15111643
Chicago/Turabian StyleFifere, Adrian, Cristian-Dragos Varganici, Elena-Laura Ursu, Tudor Pinteala, Vasile Sandru, Ioana-Andreea Turin-Moleavin, Irina Rosca, and Gheorghe G. Balan. 2025. "Advancing Duodenoscope Reprocessing with Alginate-Coated Calcium Peroxide Nanoparticles" Life 15, no. 11: 1643. https://doi.org/10.3390/life15111643
APA StyleFifere, A., Varganici, C.-D., Ursu, E.-L., Pinteala, T., Sandru, V., Turin-Moleavin, I.-A., Rosca, I., & Balan, G. G. (2025). Advancing Duodenoscope Reprocessing with Alginate-Coated Calcium Peroxide Nanoparticles. Life, 15(11), 1643. https://doi.org/10.3390/life15111643









