Beyond the Pain: A Critical Examination of the Psychopathological and Neuropsychological Dimensions of Primary Headaches in Pediatric Populations
Abstract
1. Introduction
1.1. Pediatric Perspective
1.2. Comorbidity, Psychosocial Factors, and Neurocognitive Profile
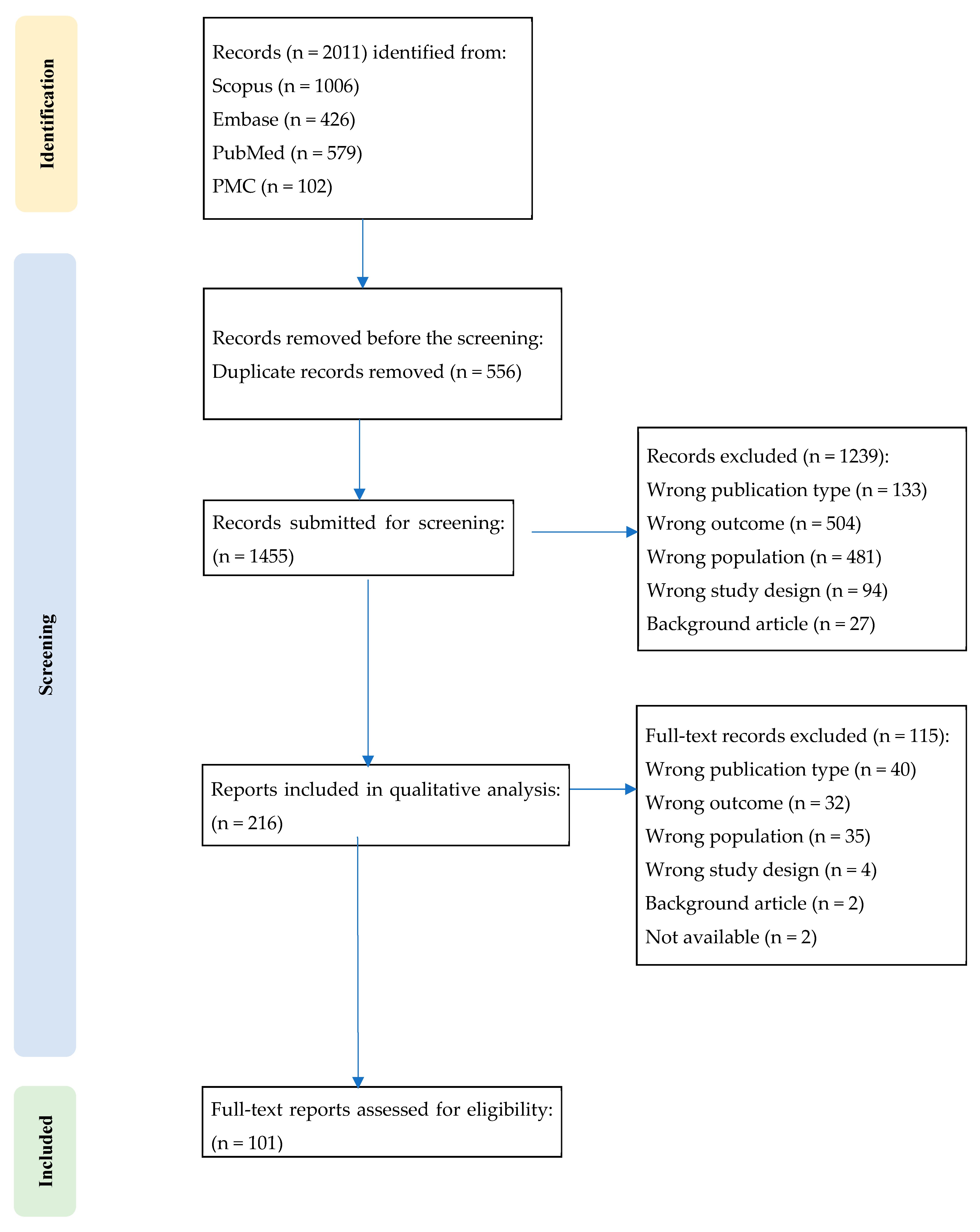
2. Methods
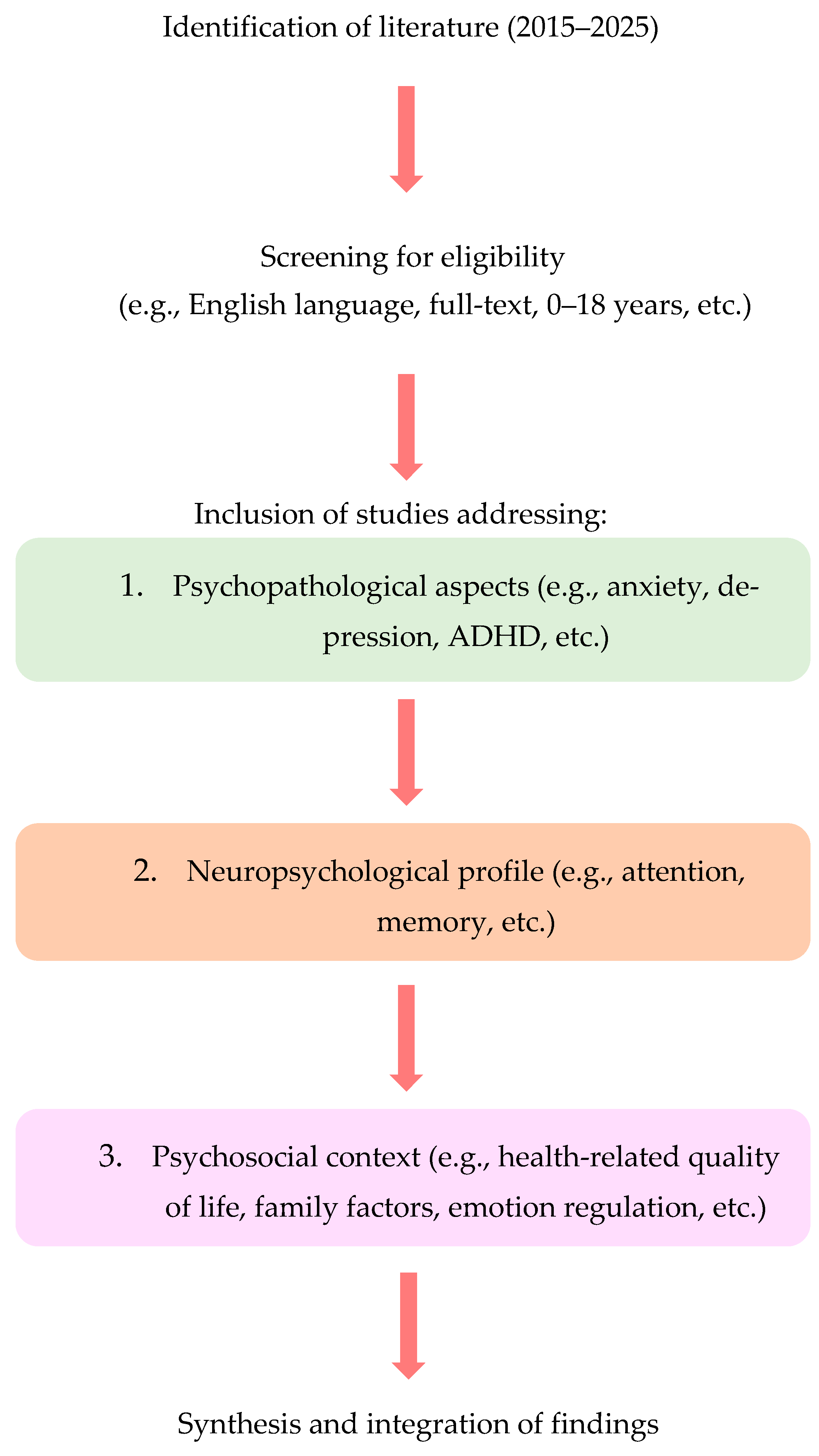
2.1. Design and Scope
2.2. Eligibility Criteria
- Population: Children and adolescents aged 0–18 years. Mixed samples (e.g., pediatric+adult) were eligible only when pediatric-specific data or analyses were reported.
- Condition: Primary headache disorders—migraine (and subtypes), tension-type headache, and cluster headache—classified according to ICHD-3 and national guideline terminology (SISC).
- Outcomes: At least one psychopathological outcome (e.g., anxiety, depression, ADHD, conduct problems) and/or neuropsychological functioning (e.g., attention, memory, executive functions), including emotional/behavioral profiles.
- Publication characteristics: Articles in English, published 2015–2025, with full text available.
- No geographical restrictions were applied; studies from any country were eligible if all other inclusion criteria were met.
- Studies not clearly distinguishing primary from secondary headaches, or where headache was only a marginal manifestation of broader genetic/systemic conditions.
- Publications not reporting data relevant to either psychopathology or neuropsychology.
- Editorials, opinion pieces, unstructured single-case reports, and records without retrievable full text.
- Adult-only samples or mixed samples without extractable pediatric data.
2.3. Information Sources and Search Strategy
- Scopus: (Title/Abstract/Keywords): (“primary headache” OR migraine OR “tension-type headache” OR “cluster headache”) AND (child* OR adolescen* OR pediatric OR paediatric OR youth) AND (psychopathology* OR neuropsychology* OR “cognitive function*” OR “executive function*” OR attention OR memory OR “behavioral problem*” OR “emotional problem*” OR “mental health”).
- Embase: (ti,ab,kw): analogous terms with year (2015–2025) and English-language limits applied.
- PubMed: MeSH and title/abstract terms for primary headache disorders, pediatric populations, and psychopathology/neuropsychology constructs; English and 2015–2025 limits were applied.
2.4. Study Selection
2.5. Data Charting and Synthesis
2.6. Flow of Analysis and Synthesis
3. Results
3.1. Results from Primary Studies (Experimental/Observational Evidence)
3.1.1. Emotional–Behavioral Difficulties and Psychiatric Comorbidity
3.1.2. Neurodevelopmental Conditions and Learning
3.1.3. Sleep Alterations and Clinical Impact
3.1.4. Neurocognitive Profile and Brain Function
3.1.5. Pain-Related Cognitions, Affective Traits, and Personality
3.1.6. Quality of Life, School Participation, and Health-Related Behaviors (Including Eating-Related Symptoms)
3.1.7. Measures Used Across Studies
3.2. Results from Reviews and Meta-Analyses (Secondary Evidence)
3.2.1. Psychopathology (Anxiety/Depression) and Headache
3.2.2. Sleep and Headache
3.2.3. ADHD and Other Neurodevelopmental Conditions
3.2.4. Neurocognition
3.2.5. School/Academics, Learning, and QoL
3.2.6. Obesity and Migraine
3.2.7. Integrated/Biobehavioral Models
3.2.8. Methodological Limitations and Research Agenda
4. Discussion
4.1. Psychopathology
4.2. Sleep
4.3. Family and Process-Level Factors
4.4. Neurocognition
4.5. Clinical and Educational Implications
4.6. Methodological Considerations and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ICHD-3 | International Classification of Headache Disorders, 3rd edition |
| TTH | Tension-Type Headache |
| YLDs | Years Lived with Disability |
| ASD | Autism Spectrum Disorder |
| ADHD | Attention Deficit Hyperactivity Disorder |
| QoL | Quality of Life |
| TAC | Trigeminal Autonomic Cephalalgia |
| LD | Learning Disabilities |
| RLS | Restless Legs Syndrome |
| PSG | Polysomnography |
| ACE | Adverse Childhood Experience |
| OSAS | Obstructive Sleep Apnea Syndrome |
References
- Mammen, V.E. WHO-Facts Sheet. Kuwait Med. J. 2024, 56, 86–98. [Google Scholar]
- Ashina, M.; Katsarava, Z.; Do, T.P.; Buse, D.C.; Pozo-Rosich, P.; Özge, A.; Krymchantowski, A.V.; Lebedeva, E.R.; Ravishankar, K.; Yu, S.; et al. Migraine: Epidemiology and Systems of Care. Lancet 2021, 397, 1485–1495. [Google Scholar] [CrossRef] [PubMed]
- Lipton, R.B.; Bigal, M.E.; Steiner, T.J.; Silberstein, S.D.; Olesen, J. Classification of Primary Headaches. Neurology 2004, 63, 427–435. [Google Scholar] [CrossRef]
- Mier, R.W.; Dhadwal, S. Primary Headaches. Dent. Clin. N. Am. 2018, 62, 611–628. [Google Scholar] [CrossRef]
- González-Quintanilla, V.; Pascual, J. Other Primary Headaches: An Update. Neurol. Clin. 2019, 37, 871–891. [Google Scholar] [CrossRef]
- Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd Edition. Cephalalgia 2018, 38, 1–211. [Google Scholar] [CrossRef] [PubMed]
- Raggi, A.; Leonardi, M.; Arruda, M.; Caponnetto, V.; Castaldo, M.; Coppola, G.; Della Pietra, A.; Fan, X.; Garcia-Azorin, D.; Gazerani, P.; et al. Hallmarks of Primary Headache: Part 1—Migraine. J. Headache Pain 2024, 25, 189. [Google Scholar] [CrossRef]
- Onan, D.; Younis, S.; Wellsgatnik, W.D.; Farham, F.; Andruškevičius, S.; Abashidze, A.; Jusupova, A.; Romanenko, Y.; Grosu, O.; Moldokulova, M.Z.; et al. Debate: Differences and Similarities between Tension-Type Headache and Migraine. J. Headache Pain 2023, 24, 92. [Google Scholar] [CrossRef]
- Pan, L.-L.H.; Ling, Y.-H.; Wang, S.-J.; Al-Hassany, L.; Chen, W.-T.; Chiang, C.-C.; Cho, S.-J.; Chu, M.K.; Coppola, G.; Pietra, A.D.; et al. Hallmarks of Primary Headache: Part 2—Tension-Type Headache. J. Headache Pain 2025, 26, 164. [Google Scholar] [CrossRef] [PubMed]
- Baglioni, V.; Orecchio, S.; Esposito, D.; Faedda, N.; Natalucci, G.; Guidetti, V. Tension-Type Headache in Children and Adolescents. Life 2023, 13, 825. [Google Scholar] [CrossRef]
- Genizi, J.; Bugdnoskya, V.; Aboud, A.; Segal, I.; Assaf, N.; Srugo, I.; Kerem, N.C. Migraine and Tension-Type Headache Among Children and Adolescents: Application of International Headache Society Criteria in a Clinical Setting. J. Child Neurol. 2021, 36, 618–624. [Google Scholar] [CrossRef]
- Leonardi, M.; Grazzi, L.; D’Amico, D.; Martelletti, P.; Guastafierro, E.; Toppo, C.; Raggi, A. Global Burden of Headache Disorders in Children and Adolescents 2007–2017. Int. J. Environ. Res. Public Health 2021, 18, 250. [Google Scholar] [CrossRef] [PubMed]
- Caponnetto, V.; Deodato, M.; Robotti, M.; Koutsokera, M.; Pozzilli, V.; Galati, C.; Nocera, G.; De Matteis, E.; De Vanna, G.; Fellini, E.; et al. Comorbidities of Primary Headache Disorders: A Literature Review with Meta-Analysis. J. Headache Pain 2021, 22, 71. [Google Scholar] [CrossRef]
- Abu-Arafeh, I. Headache and Psychological Comorbidities: An Appraisal of the Evidence. J. Clin. Med. 2023, 12, 2683. [Google Scholar] [CrossRef]
- Abu Bakar, N.; Tanprawate, S.; Lambru, G.; Torkamani, M.; Jahanshahi, M.; Matharu, M. Quality of Life in Primary Headache Disorders: A Review. Cephalalgia 2016, 36, 67–91. [Google Scholar] [CrossRef]
- Bottiroli, S.; Renzi, A.; Ballante, E.; De Icco, R.; Sances, G.; Tanzilli, A.; Vecchi, T.; Tassorelli, C.; Galli, F. Personality in Chronic Headache: A Systematic Review with Meta-Analysis. Pain Res. Manag. 2023, 2023, 6685372. [Google Scholar] [CrossRef]
- McCracken, H.T.; Lee, A.A.; Smitherman, T.A. Headache and Psychological Variables as Predictors of Disability in Individuals with Primary Headache Disorders. Headache 2023, 63, 1259–1270. [Google Scholar] [CrossRef]
- Miscioscia, M.; Di Riso, D.; Spaggiari, S.; Poli, M.; Gaiga, G.; Randazzo, G.; Pelizza, M.F.; Galdiolo, L.; Raffagnato, A.; Sartori, S.; et al. Emotional Experience and Regulation in Juvenile Primary Headaches: A Cross-Sectional Pilot Study. Children 2022, 9, 1630. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, S.; De Ranieri, C.; Dionisi, C.; Gagliardi, V.; Capuano, A.; Vigevano, F.; Gentile, S.; Valeriani, M. Migraine Equivalents and Related Symptoms, Psychological Profile and Headache Features: Which Relationship? J. Headache Pain 2015, 16, 536. [Google Scholar] [CrossRef]
- Tarantino, S.; De Ranieri, C.; Dionisi, C.; Gagliardi, V.; Paniccia, M.F.; Capuano, A.; Frusciante, R.; Balestri, M.; Vigevano, F.; Gentile, S.; et al. Role of the Attachment Style in Determining the Association Between Headache Features and Psychological Symptoms in Migraine Children and Adolescents. An Analytical Observational Case–Control Study. Headache 2017, 57, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, H. Emotional Problems in Pediatric Headache Patients. Curr. Pain Headache Rep. 2022, 26, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Begasse de Dhaem, O.; Robbins, M.S. Cognitive Impairment in Primary and Secondary Headache Disorders. Curr. Pain Headache Rep. 2022, 26, 391–404. [Google Scholar] [CrossRef]
- Pizer, J.H.; Hernandez, K.A.; Aita, S.L.; Ikonomou, V.C.; Myers, M.A.; Hawley, N.A.; Brasil, K.M.; Borgogna, N.C.; Spiegel, J.A.; Smitherman, T.A.; et al. Neuropsychological Functioning in Pediatric Primary Headache Disorders: A Meta-Analysis. Pediatrics 2025, 155, e2024067838. [Google Scholar] [CrossRef]
- Richardson, S.; Diaz-Orueta, U. In Search of a Neuropsychological Profile for Migraine: A Scoping Review. Eur. J. Pain 2024, 28, 1033–1068. [Google Scholar] [CrossRef]
- Tarantino, S.; Proietti Checchi, M.; Papetti, L.; Ursitti, F.; Sforza, G.; Ferilli, M.A.N.; Moavero, R.; Monte, G.; Capitello, T.G.; Vigevano, F.; et al. Interictal Cognitive Performance in Children and Adolescents with Primary Headache: A Narrative Review. Front. Neurol. 2022, 13, 898626. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Grant, M.J.; Booth, A. A Typology of Reviews: An Analysis of 14 Review Types and Associated Methodologies. Health Inf. Libr. J. 2009, 26, 91–108. [Google Scholar] [CrossRef] [PubMed]
- Arruda, M.A.; Arruda, R.; Guidetti, V.; Bigal, M.E. Psychosocial Adjustment of Children with Migraine and Tension-Type Headache—A Nationwide Study. Headache 2015, 55 (Suppl. S1), 39–50. [Google Scholar] [CrossRef]
- Orr, S.L.; Potter, B.K.; Ma, J.; Colman, I. Migraine and Mental Health in a Population-Based Sample of Adolescents. Can. J. Neurol. Sci. 2017, 44, 44–50. [Google Scholar] [CrossRef]
- Donnelly, T.J.; Bott, A.; Bui, M.; Goh, S.; Jaaniste, T.; Chapman, C.; Crawford, M.; Hopper, J.L.; Champion, D. Common Pediatric Pain Disorders and Their Clinical Associations. Clin. J. Pain 2017, 33, 1131–1140. [Google Scholar] [CrossRef]
- Wagner, J.L.; Wilson, D.A.; Smith, G.; Malek, A.; Selassie, A.W. Neurodevelopmental and Mental Health Comorbidities in Children and Adolescents with Epilepsy and Migraine: A Response to Identified Research Gaps. Dev. Med. Child Neurol. 2015, 57, 45–52. [Google Scholar] [CrossRef]
- Hommer, R.; Lateef, T.; He, J.-P.; Merikangas, K. Headache and Mental Disorders in a Nationally Representative Sample of American Youth. Eur. Child Adolesc. Psychiatry 2022, 31, 39–49. [Google Scholar] [CrossRef]
- Finning, K.; Neochoriti Varvarrigou, I.; Ford, T.; Panagi, L.; Ukoumunne, O.C. Mental Health and School Absenteeism in Children with Long-Term Physical Conditions: A Secondary Analysis of the British Child and Adolescent Mental Health Surveys 2004 and 2007. Child Care Health Dev. 2022, 48, 110–119. [Google Scholar] [CrossRef]
- Yavuz, A.; Ersöz Alan, B.; Çak Esen, T. Assessment of Headache in Children with Psychiatric Symptoms. Neurol. Asia 2023, 28, 991–998. [Google Scholar] [CrossRef]
- Fielding, J.; Young, S.; Martin, P.R.; Waters, A.M. Headache Symptoms Consistent with Migraine and Tension-Type Headaches in Children with Anxiety Disorders. J. Anxiety Disord. 2016, 40, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Güler, G.; Kütük, M.Ö.; Toros, F.; Özge, A.; Taşdelen, B. The High Level of Psychiatric Disorders Associated with Migraine or Tension-Type Headache in Adolescents. J. Neurol. Sci. 2017, 34, 312–321. [Google Scholar] [CrossRef]
- Uyar Cankay, T.; Besenek, M. Negative Effects of Accompanying Psychiatric Disturbances on Functionality among Adolescents with Chronic Migraine. BMC Neurol. 2021, 21, 97. [Google Scholar] [CrossRef]
- Romano, C.; Cho, S.Y.; Marino, S.; Raucci, U.; Fiumara, A.; Falsaperla, R.; Massimino, C.R.; Taibi, R.; Greco, F.; Venti, V.; et al. Primary Headache in Childhood Associated with Psychiatric Disturbances: An Update. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 6893–6898. [Google Scholar] [CrossRef]
- Amouroux, R.; Rousseau-Salvador, C.; Pillant, M.; Antonietti, J.-P.; Tourniaire, B.; Annequin, D. Longitudinal Study Shows That Depression in Childhood Is Associated with a Worse Evolution of Headaches in Adolescence. Acta Paediatr. Int. J. Paediatr. 2017, 106, 1961–1965. [Google Scholar] [CrossRef] [PubMed]
- Kemper, K.J.; Heyer, G.; Pakalnis, A.; Binkley, P.F. What Factors Contribute to Headache-Related Disability in Teens? Pediatr. Neurol. 2016, 56, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Blaauw, B.A.; Dyb, G.; Hagen, K.; Holmen, T.L.; Linde, M.; Wentzel-Larsen, T.; Zwart, J.-A. The Relationship of Anxiety, Depression and Behavioral Problems with Recurrent Headache in Late Adolescence—A Young-HUNT Follow-up Study. J. Headache Pain 2015, 16, 10. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Yoon, J.-R.; Yi, Y.Y.; Eom, S.; Lee, J.S.; Kim, H.D.; Cheon, K.-A.; Kang, H.-C. Screening for Depression and Anxiety Disorder in Children with Headache. Korean J. Pediatr. 2015, 58, 64–68. [Google Scholar] [CrossRef]
- von Gontard, A.; Overs, C.; Moritz, A.-M.; Thomé-Granz, S.; Hussong, J. Incontinence and Headache in Preschool Children. Neurourol. Urodyn. 2019, 38, 2280–2287. [Google Scholar] [CrossRef] [PubMed]
- Jafari, E.; Kazemizadeh, H.; Togha, M.; Haghighi, S.; Salami, Z.; Shahamati, D.; Martami, F.; Baigi, V.; Etesam, F. The Influence of Anxiety and Depression on Headache in Adolescent Migraineurs: A Case-Control Study. Expert Rev. Neurother. 2023, 22, 1019–1023. [Google Scholar] [CrossRef] [PubMed]
- Açlkel, S.B.; Bilgiç, A.; Derin, H.; Eroǧlu, A.; Akça, O.F.; Çaksen, H. Erratum: Comparison of Children with Migraine and Those with Tension-Type Headache for Psychiatric Symptoms and Quality of Life (Journal of Pediatric Neurology (2019) DOI: 10.1055/s-0039-1692138). J. Pediatr. Neurol. 2021; e1. [Google Scholar] [CrossRef]
- Gerstl, L.; Tadych, N.; Heinen, F.; Kainz, C.; Bonfert, M.V.; Hannibal, I.; Huss, K.; Ruscheweyh, R.; Straube, A.; Obermeier, V.; et al. Migraine and the Development of Additional Psychiatric and Pain Disorders in the Transition from Adolescence to Adulthood. Cephalalgia 2021, 41, 1342–1347. [Google Scholar] [CrossRef]
- Galli, F.; Caputi, M.; Gallucci, M.; Termine, C.; Chiappedi, M.; Balottin, U. Headache and Psychological Disorders in Children and Adolescents: A Cross-Generational Study. Minerva Pediatr. 2017, 69, 231–238. [Google Scholar] [CrossRef]
- Şentürk, A.; Abanoz, Y.; Abanoz, Y.G.; Şentürk, Ö.; Saip, S. The Prevalence and Impact of Primary Headaches in Orphaned Children: A Cross-Sectional, Observational Study in a Boarding School. Vulnerable Child. Youth Stud. 2023, 18, 30–45. [Google Scholar] [CrossRef]
- Arruda, M.A.; Arruda, R.; Guidetti, V.; Bigal, M.E. ADHD Is Comorbid to Migraine in Childhood: A Population-Based Study. J. Atten. Disord. 2017, 24, 990–1001. [Google Scholar] [CrossRef]
- Hsu, T.-W.; Chen, M.-H.; Chu, C.-S.; Tsai, S.-J.; Bai, Y.-M.; Su, T.-P.; Chen, T.-J.; Liang, C.-S. Attention Deficit Hyperactivity Disorder and Risk of Migraine: A Nationwide Longitudinal Study. Headache 2022, 62, 634–641. [Google Scholar] [CrossRef]
- Genizi, J.; Khourieh Matar, A.; Schertz, M.; Zelnik, N.; Srugo, I. Pediatric Mixed Headache -The Relationship between Migraine, Tension-Type Headache and Learning Disabilities—In a Clinic-Based Sample. J. Headache Pain 2016, 17, 42. [Google Scholar] [CrossRef]
- Goenka, A.; Fonseca, L.D. Rate and Predictors of Intractable Status Migrainosus among Patients Aged 13–18 Years. Neurohospitalist 2023, 13, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Attygalle, U.R.; Hewawitharana, G.; Wijesinghe, C.J. Migraine, Attention Deficit Hyperactivity Disorder and Screen Time in Children Attending a Sri Lankan Tertiary Care Facility: Are They Associated? BMC Neurol. 2020, 20, 275. [Google Scholar] [CrossRef] [PubMed]
- Onofri, A.; Olivieri, L.; Silva, P.; Bernassola, M.; Tozzi, E. Correlation between Primary Headaches and Learning Disabilities in Children and Adolescents. Minerva Pediatr. 2022, 74, 1–6. [Google Scholar] [CrossRef]
- Maltese, A.; Salerno, M.; Tripi, G.; Romano, P.; Ricciardi, A.; di Folco, A.; di Filippo, T.; Parisi, L. Internalizing Problems Are Related to Sleep Patterns Disordered in Children Affected by Primary Headache. Acta Med. Mediterr. 2017, 33, 729–735. [Google Scholar] [CrossRef]
- Armoni Domany, K.; Nahman-Averbuch, H.; King, C.D.; Dye, T.; Xu, Y.; Hossain, M.; Hershey, A.D.; Simakajornboon, N. Clinical Presentation, Diagnosis and Polysomnographic Findings in Children with Migraine Referred to Sleep Clinics. Sleep Med. 2019, 63, 57–63. [Google Scholar] [CrossRef]
- Nita, S.A.; Teleanu, R.I.; Bajenaru, O.A. The Role of Polysomnography in Identifying Sleep Disorders in Children with Migraine. J. Med. Life 2020, 13, 64–67. [Google Scholar] [CrossRef]
- Clementi, M.A.; Kienzler, C.; Yonker, M.; Harmon, M.; Simon, S.L. Preliminary Exploration of a Multidimensional Sleep Health Composite in Adolescent Females with Frequent Migraine. Headache 2023, 63, 1437–1447. [Google Scholar] [CrossRef] [PubMed]
- Lateef, T.; Witonsky, K.; He, J.; Ries Merikangas, K. Headaches and Sleep Problems in US Adolescents: Findings from the National Comorbidity Survey—Adolescent Supplement (NCS-A). Cephalalgia 2019, 39, 1226–1235. [Google Scholar] [CrossRef]
- Nahman-Averbuch, H.; Schneider, V.J., 2nd; Lee, G.R.; Peugh, J.L.; Hershey, A.D.; Powers, S.W.; de Zambotti, M.; Coghill, R.C.; King, C.D. New Insight into the Neural Mechanisms of Migraine in Adolescents: Relationships with Sleep. Headache 2022, 62, 668–680. [Google Scholar] [CrossRef]
- Rabner, J.; Kaczynski, K.J.; Simons, L.E.; LeBel, A. Pediatric Headache and Sleep Disturbance: A Comparison of Diagnostic Groups. Headache 2018, 58, 217–228. [Google Scholar] [CrossRef]
- Erdoğan, A.; Tuncel, D.; Işikay, S.; Okyay, R.A. Restless Legs Syndrome and Headache Cause Sleepiness and Consequent Poor School Performance: A Community-Based Study from Turkey. Minerva Pediatr. 2025, 77, 314–319. [Google Scholar] [CrossRef]
- Esposito, M.; Antinolfi, L.; Carotenuto, M. Neuropsychological Profile in Pediatric Migraine without Aura: A Pilot Study. Brain Sci. 2021, 11, 1582. [Google Scholar] [CrossRef]
- Costa-Silva, M.A.; de Almeida Prado, A.C.; de Souza, L.C.; Gomez, R.S.; Teixeira, A.L. Cognitive Functioning in Adolescents with Migraine. Dement. Neuropsychol. 2016, 10, 47–51. [Google Scholar] [CrossRef]
- Villa, T.R.; Agessi, L.M.; Moutran, A.R.C.; Gabbai, A.A.; Carvalho, D. de S. Visual Attention in Children with Migraine: The Importance of Prophylaxis. J. Child Neurol. 2016, 31, 569–572. [Google Scholar] [CrossRef]
- Giricz, Z.; Pertich, Á.; Őze, A.; Puszta, A.; Fehér, Á.; Eördegh, G.; Kóbor, J.; Bihari, K.; Pálinkás, É.; Braunitzer, G.; et al. Visually Guided Associative Learning in Pediatric and Adult Migraine without Aura. Cephalalgia 2021, 41, 176–184. [Google Scholar] [CrossRef]
- Agessi, L.M.; Villa, T.R.; de Souza Carvalho, D.; Pereira, L.D. Auditory Processing in Children with Migraine: A Controlled Study. Neuropediatrics 2017, 48, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Colon, E.; Ludwick, A.; Wilcox, S.L.; Youssef, A.M.; Danehy, A.; Fair, D.A.; Lebel, A.A.; Burstein, R.; Becerra, L.; Borsook, D. Migraine in the Young Brain: Adolescents vs. Young Adults. Front. Hum. Neurosci. 2019, 13, 87. [Google Scholar] [CrossRef] [PubMed]
- Genizi, J.; Samet, H.; Zaitoon, H.; Elimelech, U.; Kerem, N.C.; Kessel, A.; Shalata, A.; Nathan, K.; Engel-Yeger, B. Executive Functions, Anxiety, Social Participation and Quality of Life in Children with Migraine During COVID-19. Life 2025, 15, 528. [Google Scholar] [CrossRef]
- Operto, F.F.; Scuoppo, C.; Padovano, C.; Vivenzio, V.; Belfiore, G.; de Simone, V.; Pistola, I.; Rinaldi, R.; Diaspro, G.; Mazza, R.; et al. Migraine and Epilepsy: Social Cognition Skills in Pediatric Population. Eur. J. Paediatr. Neurol. 2022, 37, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Albanês Oliveira Bernardo, A.; Lys Medeiros, F.; Sampaio Rocha-Filho, P.A. Osmophobia and Odor-Triggered Headaches in Children and Adolescents: Prevalence, Associated Factors, and Importance in the Diagnosis of Migraine. Headache 2020, 60, 954–966. [Google Scholar] [CrossRef]
- Sciruicchio, V.; D’Agnano, D.; Clemente, L.; Rutigliano, A.; Laporta, A.; de Tommaso, M. Clinical Correlates of Osmophobia in Primary Headaches: An Observational Study in Child Cohorts. J. Clin. Med. 2023, 12, 2939. [Google Scholar] [CrossRef]
- Sciruicchio, V.; Simeone, M.; Barbaro, M.G.F.; Tanzi, R.C.; Delussi, M.D.; Libro, G.; D’Agnano, D.; Basiliana, R.; De Tommaso, M. Pain Catastrophizing in Childhood Migraine: An Observational Study in a Tertiary Headache Center. Front. Neurol. 2019, 10, 114. [Google Scholar] [CrossRef]
- Caruso, A.; Grolnick, W.; Mueller, C.; Kaczynski, K.; Chang, C.Y.-H.; Lebel, A. Health Mindsets in Pediatric Chronic Headache. J. Pediatr. Psychol. 2022, 47, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Güler Aksu, G.; Kayar, O.; Tufan, A.E.; Kütük, M.Ö.; Sucu, D.H.; Taşdelen, B.; Toros, F.; Özge, A. Early Maladaptive Schemas Differing According to Sex May Contribute to Migraine among the Youth. Brain Dev. 2022, 44, 427–437. [Google Scholar] [CrossRef]
- Güler Aksu, G.; Kayar, O.; Tufan, A.E.; Kütük, M.Ö.; Özdağ Acarli, A.N.; Sucu, D.H.; Taşdelen, B.; Toros, F.; Özge, A. Early Maladaptive Schemas in Episodic and Chronic Migraine in Adolescents. Front. Neurol. 2023, 14, 1128953. [Google Scholar] [CrossRef] [PubMed]
- Balottin, L.; Mannarini, S.; Candeloro, D.; Mita, A.; Chiappedi, M.; Balottin, U. Rorschach Evaluation of Personality and Emotional Characteristics in Adolescents with Migraine Versus Epilepsy and Controls. Front. Neurol. 2018, 9, 160. [Google Scholar] [CrossRef]
- Cerutti, R.; Valastro, C.; Tarantino, S.; Valeriani, M.; Faedda, N.; Spensieri, V.; Guidetti, V. Alexithymia and Psychopathological Symptoms in Adolescent Outpatients and Mothers Suffering from Migraines: A Case Control Study. J. Headache Pain 2016, 17, 39. [Google Scholar] [CrossRef][Green Version]
- Gatta, M.; Spitaleri, C.; Balottin, U.; Spoto, A.; Balottin, L.; Mangano, S.; Battistella, P.A. Alexithymic Characteristics in Pediatric Patients with Primary Headache: A Comparison between Migraine and Tension-Type Headache. J. Headache Pain 2015, 16, 98. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Natalucci, G.; Faedda, N.; Quinzi, A.; Alunni Fegatelli, D.; Vestri, A.; Turturo, G.; Verdecchia, P.; Bellini, B.; Pirisi, C.; Calderoni, D.; et al. Alexithymia, Metacognition, and Theory of Mind in Children and Preadolescents with Migraine Without Aura (MWoA): A Case-Control Study. Front. Neurol. 2019, 10, 774. [Google Scholar] [CrossRef]
- Natalucci, G.; Faedda, N.; Quinzi, A.; Fegatelli, D.A.; Fazi, M.; Verdecchia, P.; Sabatello, U.; Catino, E.; Cerutti, R.; Guidetti, V. Metacognition and Theory of Mind in Children with Migraine and Children with Internalizing Disorders. Neurol. Sci. 2019, 40, 187–189. [Google Scholar] [CrossRef]
- Basile, C.; Terenzi, F.; Abbracciavento, G. Personality Traits through Psychological Dimensions in Adolescents with Headache: An Investigation on How Social Isolation during the Pandemic Influenced Mental Health—A Pilot Study. Neuropsychiatr. Neuropsychol. 2023, 18, 148–151. [Google Scholar] [CrossRef]
- Hartberg, S.; Clench-Aas, J.; Raanaas, R.K.; Lundqvist, C. Coping Strategies among Adolescents with Chronic Headache and Mental Health Problems: A Cross-Sectional Population-Based Study. Springerplus 2015, 4, 801. [Google Scholar] [CrossRef]
- Genizi, J.; Halevy, A.; Schertz, M.; Osman, K.; Assaf, N.; Segal, I.; Srugo, I.; Kessel, A.; Engel-Yeger, B. Sensory Processing Patterns Affect Headache Severity among Adolescents with Migraine. J. Headache Pain 2020, 21, 48. [Google Scholar] [CrossRef] [PubMed]
- Operto, F.F.; Craig, F.; Peschechera, A.; Mazza, R.; Lecce, P.A.; Margari, L. Parenting Stress and Emotional/Behavioral Problems in Adolescents with Primary Headache. Front. Neurol. 2018, 8, 749. [Google Scholar] [CrossRef] [PubMed]
- Philipp, J.; Zeiler, M.; Wöber, C.; Wagner, G.; Karwautz, A.F.K.; Steiner, T.J.; Wöber-Bingöl, Ç. Prevalence and Burden of Headache in Children and Adolescents in Austria—A Nationwide Study in a Representative Sample of Pupils Aged 10–18 Years. J. Headache Pain 2019, 20, 101. [Google Scholar] [CrossRef]
- Togha, M.; Jafari, E.; Salami, Z.; Kamali, K.; Mirzaee Godarzee, H.; Mirzaee Godarzee, M.; Bavarnegin, S. The Prevalence and Impact of Tension-Type Headache in School-Aged Children in Iran. Front. Neurol. 2023, 14, 1259624. [Google Scholar] [CrossRef]
- Torres-Ferrus, M.; Vila-Sala, C.; Quintana, M.; Ajanovic, S.; Gallardo, V.J.; Gomez, J.B.; Alvarez-Sabin, J.; Macaya, A.; Pozo-Rosich, P. Headache, Comorbidities and Lifestyle in an Adolescent Population (The TEENs Study). Cephalalgia 2019, 39, 91–99. [Google Scholar] [CrossRef]
- Öztop, D.B.; Taşdelen, B.İ.; PoyrazoğLu, H.G.; Ozsoy, S.; Yilmaz, R.; Şahın, N.; Per, H.; Bozkurt, S. Assessment of Psychopathology and Quality of Life in Children and Adolescents with Migraine. J. Child Neurol. 2016, 31, 837–842. [Google Scholar] [CrossRef]
- Canfora, M.; Pallotto, I.K.; Davis, J.K.; Farley, S.; Khayata, M.J.; Hornik, C.P.; Reeve, B.B.; Rikhi, A.; Gelfand, A.A.; Szperka, C.L.; et al. More Than a Headache: Lived Experience of Migraine in Youth. Pediatr. Neurol. 2023, 146, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Magnerou, A.M.; Doumbe, J.N.; Paule Rose, D.T.; Massi-Gams, D.; Chimi-Mbonda, P.C.; Bila-Gueumekane, E.L.; Eyoum, C.; Sini, V.; Iyawa, H.; Mapoure, Y.N.; et al. Prevalence and Impact of Primary Headaches among Students Aged 8–12 Years in Sub-Saharan Africa: Cameroon Experience. Cephalalgia 2024, 44, 03331024241288523. [Google Scholar] [CrossRef]
- de Oliveira-Souza, A.I.S.; da Silva Freitas, D.; Ximenes, R.C.C.; Raposo, M.C.F.; de Oliveira, D.A. The Presence of Migraine Symptoms Was Associated with a Higher Likelihood to Present Eating Disorders Symptoms among Teenage Students. Eat. Weight Disord. 2022, 27, 1661–1667. [Google Scholar] [CrossRef]
- Gibler, R.C.; Marzouk, M.A.; Peugh, J.; Reidy, B.L.; Ernst, M.M.; Daffin, M.L.; Powers, S.W.; Kabbouche Samaha, M.; Kacperski, J.; Hershey, A.D.; et al. Clinic-Based Characterization of Adolescents and Young Adults with Migraine: Psychological Functioning, Headache Days, and Disability. Neurol. Clin. Pract. 2024, 14, e200294. [Google Scholar] [CrossRef]
- Uçar, H.N.; Tekin, U.; Tekin, E. Irritability and Its Relationships with Psychological Symptoms in Adolescents with Migraine: A Case-Control Study. Neurol. Sci. 2020, 41, 2461–2470. [Google Scholar] [CrossRef]
- Law, E.F.; Powers, S.W.; Blume, H.; Palermo, T.M. Screening Family and Psychosocial Risk in Pediatric Migraine and Tension-Type Headache: Validation of the Psychosocial Assessment Tool (PAT). Headache 2019, 59, 1516–1529. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.M.; Koh, M.S.; Lim, H.-C.; Ryu, S.; Kim, Y.J.; Moon, J.-H. Assessment of Parenting Attitudes by Children and Adolescents with Migraine. Ann. Child Neurol. 2022, 30, 180–188. [Google Scholar] [CrossRef]
- Margari, L.; Palumbi, R.; Lecce, P.A.; Craig, F.; Simone, M.; Margari, M.; Seccia, S.M.C.; Buttiglione, M. Non-Verbal Cognitive Abilities in Children and Adolescents Affected by Migraine and Tension-Type Headache: An Observational Study Using the Leiter-3. Front. Neurol. 2018, 9, 78. [Google Scholar] [CrossRef]
- Falla, K.; Kuziek, J.; Mahnaz, S.R.; Noel, M.; Ronksley, P.E.; Orr, S.L. Anxiety and Depressive Symptoms and Disorders in Children and Adolescents with Migraine: A Systematic Review and Meta-Analysis. JAMA Pediatr. 2022, 176, 1176–1187. [Google Scholar] [CrossRef]
- Dyb, G.; Stensland, S.; Zwart, J.-A. Psychiatric Comorbidity in Childhood and Adolescence Headache. Curr. Pain Headache Rep. 2015, 19, 5. [Google Scholar] [CrossRef]
- O’Brien, H.L.; Slater, S.K. Comorbid Psychological Conditions in Pediatric Headache. Semin. Pediatr. Neurol. 2016, 23, 68–70. [Google Scholar] [CrossRef]
- Gelfand, A.A. Psychiatric Comorbidity and Paediatric Migraine: Examining the Evidence. Curr. Opin. Neurol 2015, 28, 261–264. [Google Scholar] [CrossRef] [PubMed]
- Polese, D.; Belli, A.; Esposito, D.; Evangelisti, M.; Luchetti, A.; Di Nardo, G.; Parisi, P.; Bruni, O. Psychological Disorders, Adverse Childhood Experiences and Parental Psychiatric Disorders in Children Affected by Headache: A Systematic Review. Neurosci. Biobehav. Rev. 2022, 140, 104798. [Google Scholar] [CrossRef]
- Orr, S.L. Headache in Children and Adolescents. Contin. Lifelong Learn. Neurol. 2024, 30, 438–472. [Google Scholar] [CrossRef]
- Basile, V.; Tittarelli, S.; Stella, N.; Mazzone, L.; Moavero, R.; Papetti, L.; Valeriani, M. Primary Headaches and Sleep Disorders: Review of Literature about Comorbidity in Children and Adolescents. Neurol. Sci. Neurophysiol. 2024, 41, 1–6. [Google Scholar] [CrossRef]
- Dosi, C.; Figura, M.; Ferri, R.; Bruni, O. Sleep and Headache. Semin. Pediatr. Neurol. 2015, 22, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Salem, H.; Vivas, D.; Cao, F.; Kazimi, I.F.; Teixeira, A.L.; Zeni, C.P. ADHD Is Associated with Migraine: A Systematic Review and Meta-Analysis. Eur. Child Adolesc. Psychiatry 2018, 27, 267–277. [Google Scholar] [CrossRef]
- Paolino, M.C.; Ferretti, A.; Villa, M.P.; Parisi, P. Headache and ADHD in Pediatric Age: Possible Physiopathological Links. Curr. Pain Headache Rep. 2015, 19, 25. [Google Scholar] [CrossRef]
- Yum, J.; Chu, M.K. Unraveling the Connections between Migraine and Psychiatric Comorbidities: A Narrative Review. Brain Dev. 2025, 47, 104392. [Google Scholar] [CrossRef] [PubMed]
- Genizi, J.; Guidetti, V.; Arruda, M.A. Primary Headaches and School Performance-Is There a Connection? Curr. Pain Headache Rep. 2017, 21, 31. [Google Scholar] [CrossRef]
- Langdon, R.; DiSabella, M.; Strelzik, J.; Fletcher, A. Pediatric Migraine and Academics. Curr. Pain Headache Rep. 2020, 24, 40. [Google Scholar] [CrossRef]
- Ayta, S.; Uludüz, D.; Poyraz Findik, O.T.; Özge, A. Quality of Life in Children and Adolescents with Primary Headache Disorders. J. Neurol. Sci. 2016, 33, 185–193. [Google Scholar]
- Eidlitz Markus, T.; Toldo, I. Obesity and Migraine in Childhood. Curr. Pain Headache Rep. 2018, 22, 42. [Google Scholar] [CrossRef]
- Guidetti, V.; Cerutti, R.; Faedda, N.; Natalucci, G. Migraine in Childhood: An Organic, Biobehavioral, or Psychosomatic Disorder? Neurol. Sci. 2019, 40, 93–98. [Google Scholar] [CrossRef]
- Chiappedi, M.; Mensi, M.; Antonaci, E.; Zavani, E.; Tronconi, L.; Termine, C.; Balottin, U. Intellectual Profile of Adolescents with Headache: A Case-Control Study Using the WISC-IV. Front. Neurol. 2018, 9, 128. [Google Scholar] [CrossRef]
- Shimomura, H.; Tokunaga, S.; Taniguchi, N.; Inoue, K.; Okuda, M.; Kato, T.; Takeshima, Y. Emotional and Behavioral Problems in Pediatric Patients with Migraine and Tension-Type Headache. Brain Dev. 2021, 43, 826–832. [Google Scholar] [CrossRef] [PubMed]
- Öksüz, N.; Aksu, G.G.; Özdemir, A.A.; Özge, A. Internalizing Disorders Rather than ADHD Are Risk Factors for Chronicity in Pediatric Migraine Patients. Turk. J. Med. Sci. 2024, 54, 930–937. [Google Scholar] [CrossRef] [PubMed]
- Strong, E.; Pierce, E.L.; Langdon, R.; Strelzik, J.; McClintock, W.; Cameron, M.; Furda, M.; DiSabella, M. New Daily Persistent Headache in a Pediatric Population. J. Child Neurol. 2021, 36, 888–893. [Google Scholar] [CrossRef]
- Walter, S.M.; Dai, Z.; Wang, K. Obesity, Migraine, and Overlapping Comorbidities in a Rural Pediatric Population. J. Neurosci. Rural Pract. 2021, 12, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, B.A.; Kuziek, J.; Cho, L.Y.; Ronksley, P.E.; Noel, M.N.; Orr, S.L. Anxiety and Depressive Symptoms and Migraine-Related Outcomes in Children and Adolescents. Headache 2024, 64, 342–351. [Google Scholar] [CrossRef]
- Syed, S.E.; Mullick, M.S.I. Psychiatric Co-Morbidities Among Children and Adolescents with Headache: Findings from a Cross-Sectional Study in Bangladesh. Brain Behav. 2025, 15, e70599. [Google Scholar] [CrossRef]
- Bonuccelli, A.; Depietri, G.; Baldaccini, T.; Ricciutelli, I.; Peroni, D.; Spalice, A.; Massimetti, G.; Morganti, R.; Orsini, A.; Striano, P. Essential Headaches in Developmental Age: What Is Changed before, during and after the Lockdown for COVID-19 Pandemic. Clinical Study. Front. Pediatr. 2023, 11, 1166984. [Google Scholar] [CrossRef]

| Database | Search Query | Filters |
|---|---|---|
| Scopus | (“primary headache” OR migraine OR “tension-type headache” OR “cluster headache”) AND (child* OR adolescen* OR pediatric OR paediatric OR youth) AND (psychopathology* OR neuropsychology* OR “cognitive function*” OR “executive function*” OR attention OR memory OR “behavioral problem*” OR “emotional problem*” OR “mental health”) | Language: English Title/abstract/keywords Last 10 years |
| Embase | (‘primary headache’:ti,ab,kw OR migraine:ti,ab,kw OR ‘tension-type headache’:ti,ab,kw OR ‘cluster headache’:ti,ab,kw) AND (child*:ti,ab,kw OR adolescen*:ti,ab,kw OR pediatric:ti,ab,kw OR paediatric:ti,ab,kw OR youth:ti,ab,kw) AND (psychopathology:ti,ab,kw OR neuropsychology*:ti,ab,kw OR ‘cognitive function*’:ti,ab,kw OR ‘executive function*’:ti,ab,kw OR attention:ti,ab,kw OR memory:ti,ab,kw OR ‘behavioral problem*’:ti,ab,kw OR ‘emotional problem*’:ti,ab,kw OR ‘mental health’:ti,ab,kw) AND [2015–2025]/py AND [english]/lim | Language: English Title/abstract/keywords Last 10 years |
| PubMed | ((“Primary Headache Disorders”[MeSH Terms] OR “Migraine Disorders”[MeSH Terms] OR “Tension-Type Headache”[MeSH Terms] OR “Cluster Headache”[MeSH Terms] OR “primary headache”[Title/Abstract] OR migraine[Title/Abstract] OR “tension-type headache”[Title/Abstract] OR “cluster headache”[Title/Abstract]) AND (“Child”[MeSH Terms] OR “Adolescent”[MeSH Terms] OR child*[Title/Abstract] OR adolescen*[Title/Abstract] OR pediatric[Title/Abstract] OR paediatric[Title/Abstract] OR youth[Title/Abstract]) AND (“Psychopathology”[MeSH Terms] OR “Neuropsychology”[MeSH Terms] OR “Cognition”[MeSH Terms] OR “Mental Disorders”[MeSH Terms] OR “Executive Function”[MeSH Terms] OR psychopathology[Title/Abstract] OR neuropsychology*[Title/Abstract] OR “cognitive function*”[Title/Abstract] OR “executive function*”[Title/Abstract] OR attention[Title/Abstract] OR memory[Title/Abstract] OR “behavioral problem*”[Title/Abstract] OR “emotional problem*”[Title/Abstract] OR “mental health”[Title/Abstract])) | Language: English Title/abstract/keywords Last 10 years |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Accogli, G.; Nicolardi, V.; Ferrante, C.; Carlucci, G.; Scoditti, S.; Trabacca, A. Beyond the Pain: A Critical Examination of the Psychopathological and Neuropsychological Dimensions of Primary Headaches in Pediatric Populations. Life 2025, 15, 1641. https://doi.org/10.3390/life15101641
Accogli G, Nicolardi V, Ferrante C, Carlucci G, Scoditti S, Trabacca A. Beyond the Pain: A Critical Examination of the Psychopathological and Neuropsychological Dimensions of Primary Headaches in Pediatric Populations. Life. 2025; 15(10):1641. https://doi.org/10.3390/life15101641
Chicago/Turabian StyleAccogli, Giuseppe, Valentina Nicolardi, Camilla Ferrante, Giorgia Carlucci, Sara Scoditti, and Antonio Trabacca. 2025. "Beyond the Pain: A Critical Examination of the Psychopathological and Neuropsychological Dimensions of Primary Headaches in Pediatric Populations" Life 15, no. 10: 1641. https://doi.org/10.3390/life15101641
APA StyleAccogli, G., Nicolardi, V., Ferrante, C., Carlucci, G., Scoditti, S., & Trabacca, A. (2025). Beyond the Pain: A Critical Examination of the Psychopathological and Neuropsychological Dimensions of Primary Headaches in Pediatric Populations. Life, 15(10), 1641. https://doi.org/10.3390/life15101641






