Anticancer Activity of Demethylincisterol A3 and Related Incisterol-Type Fungal Products
Abstract
1. Introduction
2. Incisterol and Demethylincisterol Derivatives
3. Salimyxins
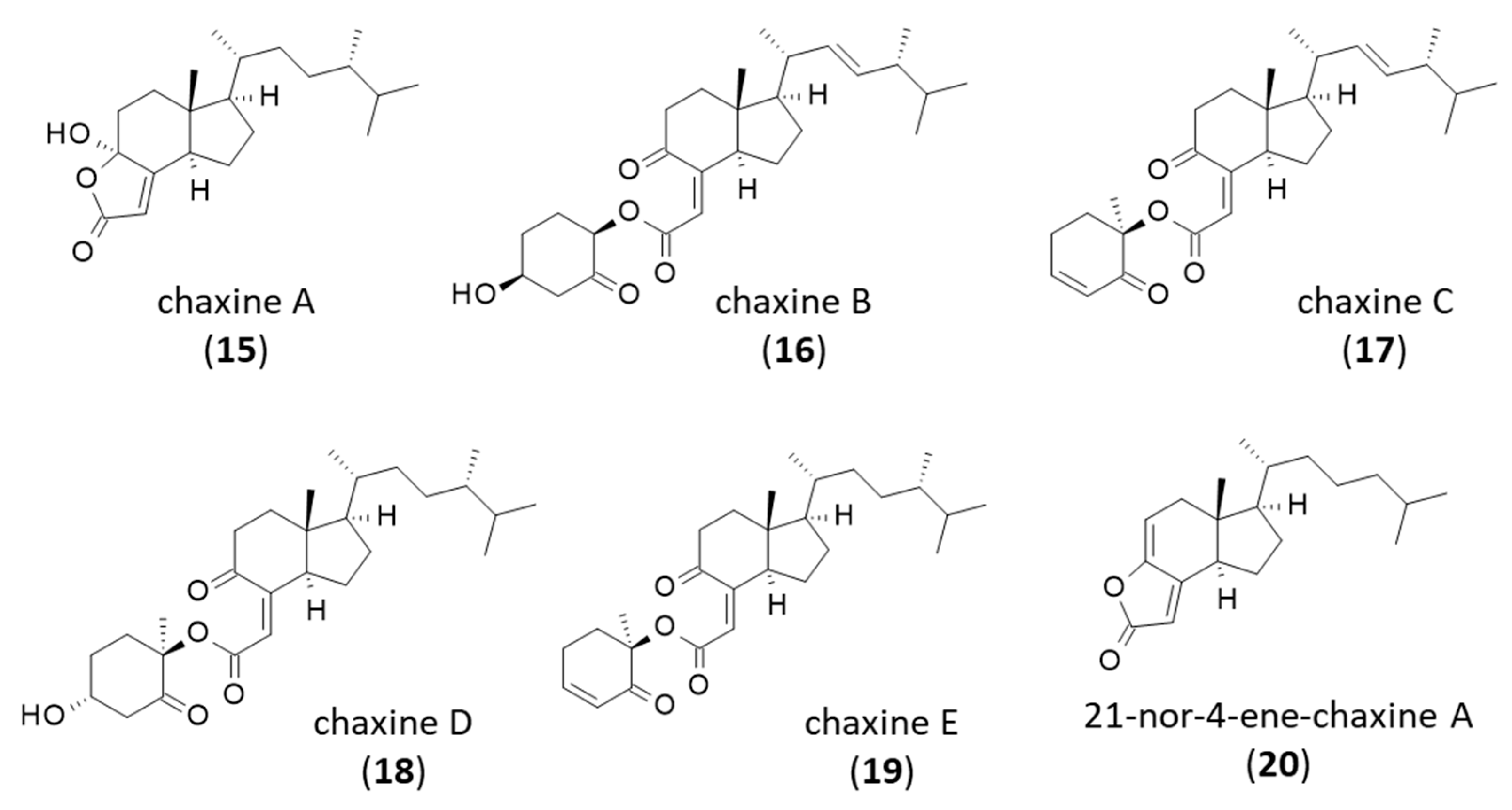
4. Chaxines
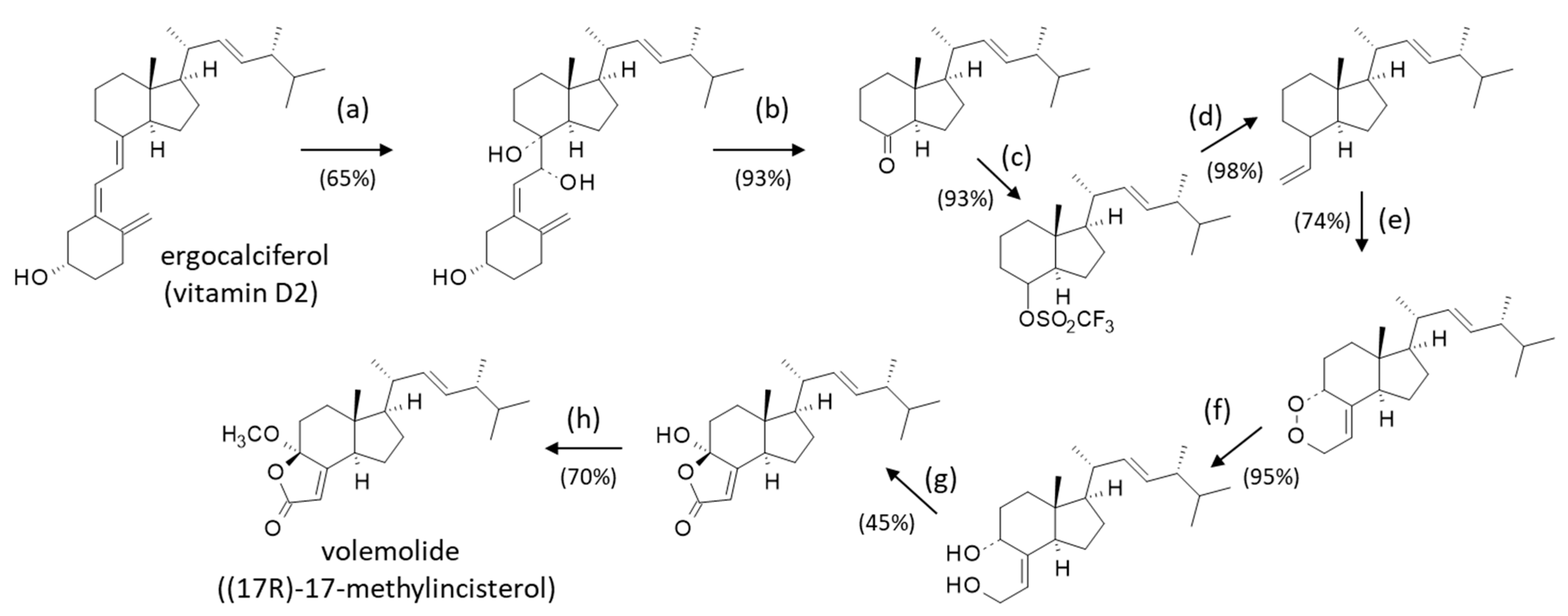
5. Volemolide
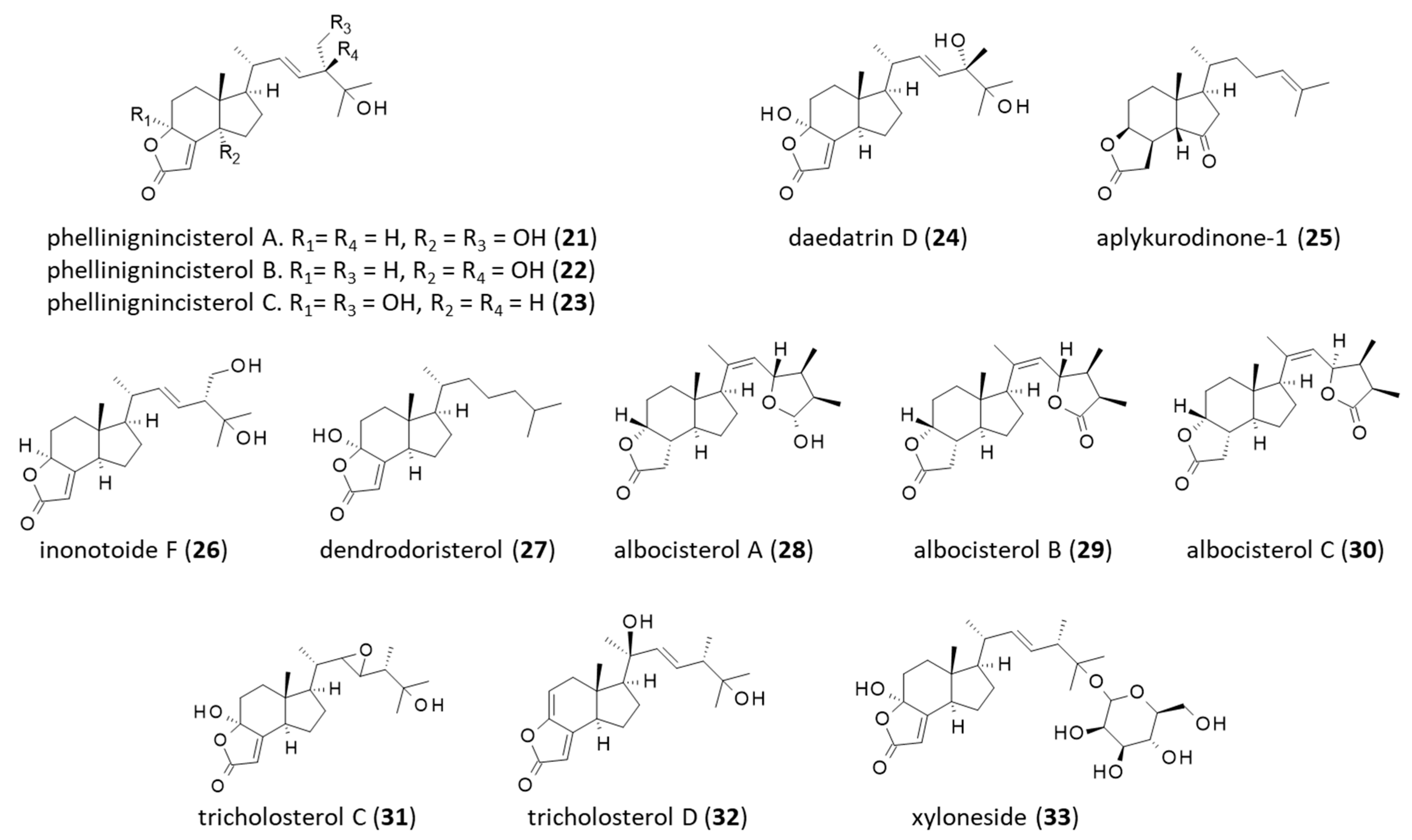
6. Other Derivatives
7. Conclusions
Funding
Conflicts of Interest
Abbreviations
| AchE | Acetylcholinesterase |
| COX-2 | Cyclooxygenase-2 |
| cPGES | Cytosolic prostaglandin E2 synthase |
| DM-A3 | Demethylincisterol A3 |
| IFN-γ | Interferon-γ |
| IL-2 | Interleukin-2 |
| PBMC | Peripheral blood mononuclear cells |
| PXR | Pregnane X receptor |
| TNF-α | Tumor necrosis factor-α |
References
- Poirot, M. Sterol metabolism and cancer. Biochem. Pharmacol. 2022, 196, 114843. [Google Scholar] [CrossRef] [PubMed]
- Evtyugin, D.D.; Evtuguin, D.V.; Casal, S.; Domingues, M.R. Advances and Challenges in Plant Sterol Research: Fundamentals, Analysis, Applications and Production. Molecules 2023, 28, 6526. [Google Scholar] [CrossRef]
- Yalcinkaya, A.; Öztaş, Y.E.; Sabuncuoğlu, S. Sterols in Inflammatory Diseases: Implications and Clinical Utility. Implic. Oxysterols Phytosterols Aging Hum. Dis. 2024, 1440, 261–275. [Google Scholar]
- Wang, Y.; Jiang, Y.; Lin, J. Progress in the Synthesis of Sterols and Related Natural Products by Manipulating the Ergosterol Biosynthetic Pathway in Saccharomyces cerevisiae. ACS Synth. Biol. 2025, 14, 3306–3320. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Gan, Y.J.; Yu, Y.; Zhang, Y. Synthesis and evaluation of new sterol derivatives as potential antitumor agents. RSC Adv. 2018, 8, 26528–26537. [Google Scholar] [CrossRef]
- Sax, J.L.; Hubler, Z.; Allimuthu, D.; Adams, D.J. Screening Reveals Sterol Derivatives with Pro-Differentiation, Pro-Survival, or Potent Cytotoxic Effects on Oligodendrocyte Progenitor Cells. ACS Chem. Biol. 2021, 16, 1288–1297. [Google Scholar] [CrossRef]
- Zhang, H.; Xie, Y.; Cao, F.; Song, X. Gut microbiota-derived fatty acid and sterol metabolites: Biotransformation and immunomodulatory functions. Gut Microbes 2024, 16, 2382336. [Google Scholar] [CrossRef]
- Donova, M.V. Steroid Bioconversions. Methods Mol. Biol. 2017, 1645, 1–13. [Google Scholar]
- Kreit, J. Aerobic catabolism of sterols by microorganisms: Key enzymes that open the 3-ketosteroid nucleus. FEMS Microbiol. Lett. 2019, 366, fnz173. [Google Scholar] [CrossRef]
- Haubrich, B.A. Microbial Sterolomics as a Chemical Biology Tool. Molecules 2018, 23, 2768. [Google Scholar] [CrossRef]
- Ciminiello, P.; Fattorusso, E.; Magno, S.; Mangoni, A.; Pansin, M. Incisterols, a New Class of Highly Degraded Sterols from the Marine Sponge Dictyonella incisa. J. Am. Chem. Soc. 1990, 112, 3505–3509. [Google Scholar] [CrossRef]
- Chianese, G.; Sepe, V.; Limongelli, V.; Renga, B.; D'Amore, C.; Zampella, A.; Taglialatela-Scafati, O.; Fiorucci, S. Incisterols, highly degraded marine sterols, are a new chemotype of PXR agonists. Steroids 2014, 83, 80–85. [Google Scholar] [CrossRef]
- Kawagishi, H.; Akachi, T.; Ogawa, T.; Masuda, K.; Yamaguchi, K.; Yazawa, K.; Takahashi, M. Chaxine A, an osteoclast-forming suppressing substance, from the mushroom Agrocybe chaxingu. Heterocycles 2007, 69, 253–258. [Google Scholar] [CrossRef]
- Mansoor, T.A.; Hong, J.; Lee, C.O.; Bae, S.J.; Im, K.S.; Jung, J.H. Cytotoxic sterol derivatives from a marine sponge Homaxinella sp. J. Nat. Prod. 2005, 68, 331–336. [Google Scholar] [CrossRef]
- Yu, S.; Zhu, Y.X.; Peng, C.; Li, J. Two new sterol derivatives isolated from the endophytic fungus Aspergillus tubingensis YP-2. Nat. Prod. Res. 2021, 35, 3277–3284. [Google Scholar] [CrossRef] [PubMed]
- Na, M.W.; Lee, E.; Kang, D.M.; Jeong, S.Y.; Ryoo, R.; Kim, C.Y.; Ahn, M.J.; Kang, K.B.; Kim, K.H. Identification of Antibacterial Sterols from Korean Wild Mushroom Daedaleopsis confragosa via Bioactivity- and LC-MS/MS Profile-Guided Fractionation. Molecules 2022, 27, 1865. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.Y.; Cui, C.Y.; Mao, L.N.; Zhou, Q.; Wang, Z.P. A new steroid from Penicillium brocae G2131. J. Asian Nat. Prod. Res. 2025, 27, 1215–1221. [Google Scholar] [CrossRef] [PubMed]
- Amagata, T.; Tanaka, M.; Yamada, T.; Chen, Y.-P.; Minoura, K.; Numata, A. Additional cytotoxic substances isolated from the sponge-derived Gymnascella dankaliensis. Tetrahedron Lett. 2013, 54, 5960–5962. [Google Scholar] [CrossRef]
- Zhang, F.L.; Yang, H.X.; Wu, X.; Li, J.Y.; Wang, S.Q.; He, J.; Li, Z.H.; Feng, T.; Liu, J.K. Chemical constituents and their cytotoxicities from mushroom Tricholoma imbricatum. Phytochemistry 2020, 177, 112431. [Google Scholar] [CrossRef]
- Ueguchi, Y.; Matsunami, K.; Otsuka, H.; Kondo, K. Constituents of cultivated Agaricus blazei. J. Nat. Med. 2011, 65, 307–312. [Google Scholar] [CrossRef]
- McCloskey, S.; Noppawan, S.; Mongkolthanaruk, W.; Suwannasai, N.; Senawong, T.; Prawat, U. A new cerebroside and the cytotoxic constituents isolated from Xylaria allantoidea SWUF76. Nat. Prod. Res. 2017, 31, 1422–1430. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Zhao, J.L.; Liu, J.M.; Zhang, M.; Chen, R.D.; Xie, K.B.; Chen, D.W.; Dai, J.G. Lanostane triterpenoids and ergostane-type steroids from the cultured mycelia of Ganoderma capense. J. Asian Nat. Prod. Res. 2018, 20, 844–851. [Google Scholar] [CrossRef]
- Tsai, W.J.; Yang, S.C.; Huang, Y.L.; Chen, C.C.; Chuang, K.A.; Kuo, Y.C. 4-Hydroxy-17-methylincisterol from Agaricus blazei Decreased Cytokine Production and Cell Proliferation in Human Peripheral Blood Mononuclear Cells via Inhibition of NF-AT and NF-kappaB Activation. Evid. Based Complement. Altern. Med. 2013, 2013, 435916. [Google Scholar] [CrossRef]
- Yu, R.; Li, X.; Yi, P.; Wen, P.; Wang, S.; Liao, C.; Song, X.; Wu, H.; He, Z.; Li, C. Isolation and Identification of Chemical Compounds from Agaricus blazei Murrill and Their In Vitro Antifungal Activities. Molecules 2023, 28, 7321. [Google Scholar] [CrossRef]
- Zhang, S.S.; Ma, Q.Y.; Zou, X.S.; Dai, H.F.; Huang, S.Z.; Luo, Y.; Yu, Z.F.; Luo, H.R.; Zhao, Y.X. Chemical constituents from the fungus Amauroderma amoiensis and their in vitro acetylcholinesterase inhibitory activities. Planta Medica 2013, 79, 87–91. [Google Scholar] [CrossRef]
- Zeng, Q.; Chen, Y.; Wang, J.; Shi, X.; Che, Y.; Chen, X.; Zhong, W.; Zhang, W.; Wei, X.; Wang, F.; et al. Diverse Secondary Metabolites from the Coral-Derived Fungus Aspergillus hiratsukae SCSIO 5Bn1003. Mar. Drugs 2022, 20, 150. [Google Scholar] [CrossRef] [PubMed]
- Su, J.C.; Pan, Q.; Xu, X.; Wei, X.; Lei, X.; Zhang, P. Structurally diverse steroids from an endophyte of Aspergillus tennesseensis 1022LEF attenuates LPS-induced inflammatory response through the cholinergic anti-inflammatory pathway. Chem. Biol. Interact. 2022, 362, 109998. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wang, K.L.; Liu, M.; She, Z.G.; Wang, C.Y. Bioactive steroid derivatives and butyrolactone derivatives from a gorgonian-derived Aspergillus sp. fungus. Chem. Biodivers. 2015, 12, 1398–1406. [Google Scholar] [CrossRef]
- Shen, Y.; Gu, L.; Zhou, Q.; Zhang, X.; Yu, M.; Li, Q.; Liang, Y.; Chen, C.; Zhang, Y.; Zhu, H. Bipolaristeroid A, a 5,6-seco-9,10-seco-steroid with cytotoxic activity from the fungus Bipolaris maydis. Phytochemistry 2025, 229, 114303. [Google Scholar] [CrossRef]
- Wang, J.H.; Zhang, Z.L.; Wang, Y.Q.; Yang, M.; Wang, C.H.; Li, X.W.; Guo, Y.W. Chemical Constituents from Mycelia and Spores of Fungus Cordyceps cicadae. Chin. Herb. Med. 2017, 9, 188–192. [Google Scholar] [CrossRef]
- Nagarajan, K.; Tong, W.Y.; Leong, C.R.; Tan, W.N. Potential of Endophytic Diaporthe sp. as a New Source of Bioactive Compounds. J. Microbiol. Biotechnol. 2021, 31, 493–500. [Google Scholar] [CrossRef]
- Dai, Z.; Gan, Y.; Zhao, P.; Li, G. Secondary Metabolites from the Endoparasitic Nematophagous Fungus Harposporium anguillulae YMF 1.01751. Microorganisms 2022, 10, 1553. [Google Scholar] [CrossRef] [PubMed]
- Chuluunbaatar, B.; Béni, Z.; Dékány, M.; Kovács, B.; Sárközy, A.; Datki, Z.; Mácsai, L.; Kálmán, J.; Hohmann, J.; Ványolós, A. Triterpenes from the Mushroom Hypholoma lateritium: Isolation, Structure Determination and Investigation in Bdelloid Rotifer Assays. Molecules 2019, 24, 301. [Google Scholar] [CrossRef]
- Yang, X.; Wu, P.; Xue, J.; Li, H.; Wei, X. Seco-pimarane diterpenoids and androstane steroids from an endophytic Nodulisporium fungus derived from Cyclosorus parasiticus. Phytochemistry 2023, 210, 113679. [Google Scholar] [CrossRef]
- Deng, M.; Zhang, E.; Qiu, F.; Fei, Y.; Liu, Y.; Li, L.; Xiong, Y.; Zhou, X. Penicinoid A, an undescribed sesterterpenoid with cytotoxic activity from Penicillium herquei. Nat. Prod. Res. 2025, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.F.; Huang, X.F.; Xiao, H.X.; Hao, Y.J.; Xu, L.; Yan, Q.X.; Zou, Z.B.; Xie, C.L.; Xu, Y.Q.; Yang, X.W. Chemical Constituents of the Marine Fungus Penicillium sp. MCCC 3A00228. Chem. Biodivers. 2021, 18, e2100697. [Google Scholar] [CrossRef]
- Zhou, J.; Li, G.; Deng, Q.; Zheng, D.; Yang, X.; Xu, J. Cytotoxic constituents from the mangrove endophytic Pestalotiopsis sp. induce G0/G1 cell cycle arrest and apoptosis in human cancer cells. Nat. Prod. Res. 2017, 32, 2968–2972. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Liu, Y.; Hu, Y.; Lv, X.; Shi, Z.; Yu, Y.; Jiang, X.; Feng, F.; Xu, J. Neuroprotective Activities of Constituents from Phyllosticta capitalensis, an Endophyte Fungus of Loropetalum chinense var. rubrum. Chem. Biodivers. 2021, 18, e2100314. [Google Scholar] [CrossRef]
- Xiang, S.L.; Xu, K.Z.; Yin, L.J.; Jia, A.Q. An Investigation of Quorum Sensing Inhibitors against Bacillus cereus in The Endophytic Fungus Pithomyces sacchari of the Laurencia sp. Mar. Drugs 2024, 22, 161. [Google Scholar]
- Liu, X.H.; Song, Y.P.; Wang, B.G.; Ji, N.Y. Sesquiterpenes and lipids from the algicolous fungus Trichoderma atroviride RR-dl-3-9. Phytochem. Lett. 2021, 45, 6–12. [Google Scholar] [CrossRef]
- Wang, Y.; Jia, J.; Wang, Q.; Wei, Y.; Yuan, H. Secondary Metabolites from the Cultures of Medicinal Mushroom Vanderbylia robiniophila and Their Tyrosinase Inhibitory Activities. J. Fungi 2023, 9, 702. [Google Scholar] [CrossRef]
- Yang, Z.; Wu, K.; Ji, W.; Yin, Y.; Wang, X.; Shao, L.; Ge, M.; Xu, Y. A novel biologically active xylaphenoside from the endophytic fungus Xylaria CGMCC No.5410. J. Antibiot. 2023, 76, 239–243. [Google Scholar] [CrossRef]
- Yin, G.P.; Li, Y.J.; Li, B.; Liu, X.M.; Zhu, J.J.; Wang, Z.M.; Hu, C.H. Secondary metabolites of endophyte fungi Xylaria sp. from Coptis chinensis. J. Chin. Mater. Medica 2022, 47, 2165–2169. [Google Scholar]
- Jin, Y.P.; Yang, Z.J.; Luo, M.Y. Metabolites of Endophytic Fungus Xylaria sp. with Biological Activities. Chin. Pharm. J. 2015, 50, 1853–1856. [Google Scholar]
- Zhang, M.; Zhao, L.; Tang, F.; Gao, J.M.; Qi, J. Chemical Structures, Biological Activities, and Biosynthetic Analysis of Secondary Metabolites from Agaricus Mushrooms: A Review. J. Agric. Food Chem. 2024, 72, 12387–12397. [Google Scholar] [CrossRef]
- Huang, K.; El-Seedi, H.R.; Xu, B. Critical review on chemical compositions and health-promoting effects of mushroom Agaricus blazei Murill. Curr. Res. Food Sci. 2022, 5, 2190–2203. [Google Scholar] [CrossRef]
- Zhabinskii, V.N.; Drasar, P.; Khripach, V.A. Structure and Biological Activity of Ergostane-Type Steroids from Fungi. Molecules 2022, 27, 2103. [Google Scholar] [CrossRef]
- Sun, M.; Zhou, D.; Wu, J.; Zhou, J.; Xu, J. Sdy-1 Executes Antitumor Activity in HepG2 and HeLa Cancer Cells by Inhibiting the Wnt/β-Catenin Signaling Pathway. Mar. Drugs 2022, 20, 125. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fu, H.; Zuo, L. Synergistic Cytotoxicity Effect of 5-Fluorouracil and SHP2 Inhibitor Demethylincisterol A3 on Cervical Cancer Cell. Anticancer Agents Med. Chem. 2022, 22, 1313–1319. [Google Scholar] [CrossRef]
- Chen, C.; Liang, F.; Chen, B.; Sun, Z.; Xue, T.; Yang, R.; Luo, D. Identification of demethylincisterol A(3) as a selective inhibitor of protein tyrosine phosphatase Shp2. Eur. J. Pharmacol. 2017, 795, 124–133. [Google Scholar] [CrossRef]
- Pérez-Baena, M.J.; Cordero-Pérez, F.J.; Pérez-Losada, J.; Holgado-Madruga, M. The Role of GAB1 in Cancer. Cancers 2023, 15, 4179. [Google Scholar] [CrossRef]
- Liu, P.; Chen, J. Targeting SHP2: Dual breakthroughs in colorectal cancer therapy-from signaling pathway modulation to immune microenvironment remodeling. World J. Gastrointest. Oncol. 2025, 17, 107380. [Google Scholar] [CrossRef]
- Guo, Z.; Duan, Y.; Sun, K.; Zheng, T.; Liu, J.; Xu, S.; Xu, J. Advances in SHP2 tunnel allosteric inhibitors and bifunctional molecules. Eur. J. Med. Chem. 2024, 275, 116579. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.; Cen, C.; Tian, Y.; Cao, X.; Hao, L.; Tao, X.; Cao, Z. Targeting Shp2 as a therapeutic strategy for neurodegenerative diseases. Transl. Psychiatry 2025, 15, 6. [Google Scholar] [CrossRef] [PubMed]
- Togashi, H.; Mizushina, Y.; Takemura, M.; Sugawara, F.; Koshino, H.; Esumi, Y.; Uzawa, J.; Kumagai, H.; Matsukage, A.; Yoshida, S.; et al. 4-Hydroxy-17-methylincisterol, an inhibitor of DNA polymerase-alpha activity and the growth of human cancer cells in vitro. Biochem. Pharmacol. 1998, 56, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Liu, L.; Ye, S.; Li, J.; Wu, L.; Li, J.; Jia, H.; Long, Y. New steroids from mangrove-associated fungus Trichoderma asperellum SCNU-F0048. Steroids 2024, 208, 109449. [Google Scholar] [CrossRef]
- Jiao, Y.; Li, G.; Wang, H.Y.; Liu, J.; Li, X.B.; Zhang, L.L.; Zhao, Z.T.; Lou, H.X. New metabolites from endolichenic fungus Pleosporales sp. Chem. Biodivers. 2015, 12, 1095–1104. [Google Scholar]
- Kongkaew, N.; Hengphasatporn, K.; Shigeta, Y.; Rungrotmongkol, T.; Harada, R. Preferential Door for Ligand Binding and Unbinding Pathways in Inhibited Human Acetylcholinesterase. J. Phys. Chem. Lett. 2024, 15, 5696–5704. [Google Scholar] [CrossRef]
- Rizvi, S.M.; Shaikh, S.; Naaz, D.; Shakil, S.; Ahmad, A.; Haneef, M.; Abuzenadah, A.M. Kinetics and Molecular Docking Study of an Anti-diabetic Drug Glimepiride as Acetylcholinesterase Inhibitor: Implication for Alzheimer's Disease-Diabetes Dual Therapy. Neurochem. Res. 2016, 41, 1475–1482. [Google Scholar] [CrossRef]
- Wu, Y.J.; Wang, L.; Ji, C.F.; Gu, S.F.; Yin, Q.; Zuo, J. The Role of α7nAChR-Mediated Cholinergic Anti-inflammatory Pathway in Immune Cells. Inflammation 2021, 44, 821–834. [Google Scholar] [CrossRef]
- Ványolós, A.; Muszyńska, B.; Chuluunbaatar, B.; Gdula-Argasińska, J.; Kała, K.; Hohmann, J. Extracts and Steroids from the Edible Mushroom Hypholoma lateritium Exhibit Anti-Inflammatory Properties by Inhibition of COX-2 and Activation of Nrf2. Chem. Biodivers. 2020, 17, e2000391. [Google Scholar] [CrossRef]
- Felder, S.; Kehraus, S.; Neu, E.; Bierbaum, G.; Schäberle, T.F.; König, G.M. Salimyxins and enhygrolides: Antibiotic, sponge-related metabolites from the obligate marine myxobacterium Enhygromyxa salina. Chembiochem 2013, 14, 1363–1371. [Google Scholar] [CrossRef]
- Qiao, Y.; Tu, K.; Feng, W.; Liu, J.; Xu, Q.; Tao, L.; Zhu, H.; Chen, C.; Wang, J.; Xue, Y.; et al. Polyketide and Prenylxanthone Derivatives from the Endophytic Fungus Aspergillus sp. TJ23. Chem. Biodivers. 2018, 15, e1800395. [Google Scholar] [CrossRef]
- Choi, J.H.; Ogawa, A.; Abe, N.; Masuda, K.; Koyama, T.; Yazawa, K.; Kawagishi, H. Chaxines B, C, D, and E from the edible mushroom Agrocybe chaxingu. Tetrahedron 2009, 65, 9850–9853. [Google Scholar] [CrossRef]
- Hirata, Y.; Nakazaki, A.; Kawagishi, H.; Nishikawa, T. Biomimetic synthesis and structural revision of chaxine B and its analogues. Org. Lett. 2017, 19, 560–563. [Google Scholar] [CrossRef] [PubMed]
- Niki, M.; Hirata, Y.; Nakazaki, A.; Wu, J.; Kawagishi, H.; Nishikawa, T. Biomimetic Synthesis of Chaxine and its Related Compounds. J. Org. Chem. 2020, 85, 4848–4860. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, Z.Y.; Fu, Y.; Gu, Y.C.; Li, S.W.; Zhang, H.Y.; Guo, Y.W. Anti-inflammatory Steroids from the South China Sea Sponge Spongia officinalis. Chem. Biodivers. 2024, 21, e202400519. [Google Scholar] [CrossRef] [PubMed]
- Ge, F.H.; Duan, M.H.; Li, J.; Shi, Q.L. Ganoderin A, a novel 9,11-secosterol from Ganoderma lucidum spores oil. J. Asian Nat. Prod. Res. 2017, 19, 1252–1257. [Google Scholar] [CrossRef] [PubMed]
- Yajima, A.; Kagohara, Y.; Shikai, K.; Katsuta, R.; Nukada, T. Synthesis of two osteoclast-forming suppressors, demethylincisterol A3 and chaxine A. Tetrahedron 2012, 68, 1729–1735. [Google Scholar] [CrossRef]
- Kobata, K.; Wada, T.; Hayashi, Y.; Shibata, H. Volemolide, a novel norsterol from the fungus Lactarius volemus. Biosci. Biotechnol. Biochem. 1994, 58, 1542–1544. [Google Scholar] [CrossRef]
- Liu, X.H.; Miao, F.P.; Li, X.D.; Yin, X.L.; Ji, N.Y. A new sesquiterpene from an endophytic Aspergillus versicolor strain. Nat. Prod. Commun. 2012, 7, 819–820. [Google Scholar] [CrossRef]
- Yoneyama, T.; Takahashi, H.; Grudniewska, A.; Ban, S.; Umeyama, A.; Noji, M. Ergostane-Type Sterols From Several Cordyceps Strains. Nat. Prod. Commun. 2022, 17, 1934578X221105363. [Google Scholar] [CrossRef]
- De Riccardis, F.; Spinella, A.; Izzo, I.; Giordano, A.; Sodano, G. Synthesis of (17R)-17-methylincisterol, a highly degraded marine steroid. Tetrahedron Lett. 1995, 36, 4303–4306. [Google Scholar] [CrossRef]
- Wu, X.; Lin, S.; Zhu, C.; Yue, Z.; Yu, Y.; Zhao, F.; Liu, B.; Dai, J.; Shi, J. Homo- and heptanor-sterols and tremulane sesquiterpenes from cultures of Phellinus igniarius. J. Nat. Prod. 2010, 73, 1294–1300. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.Y.; Feng, T.; Li, Z.H.; Dong, Z.J.; Zhang, H.B.; Liu, J.K. Sesquiterpenoids and an ergosterol from cultures of the fungus Daedaleopsis tricolor. Nat. Prod. Bioprospecting 2013, 3, 271–276. [Google Scholar] [CrossRef]
- Gavagnin, M.; Carbone, M.; Nappo, M.; Mollo, E.; Roussis, R.; Cimino, G. First chemical study of anaspidean Syphonota geographica: Structure of degraded sterols aplykurodinone-1 and -2. Tetrahedron 2005, 61, 617–621. [Google Scholar] [CrossRef]
- Zou, C.X.; Hou, Z.L.; Bai, M.; Guo, R.; Lin, B.; Wang, X.B.; Huang, X.X.; Song, S.J. Highly modified steroids from Inonotus obliquus. Org. Biomol. Chem. 2020, 18, 3908–3916. [Google Scholar] [CrossRef] [PubMed]
- Huong, P.T.M.; Phong, N.V.; Thao, N.P.; Binh, P.T.; Thao, D.T.; Thanh, N.V.; Cuong, N.X.; Nam, N.H.; Thung, D.C.; Minh, C.V. Dendrodoristerol, a cytotoxic C20 steroid from the Vietnamese nudibranch mollusk Dendrodoris fumata. J. Asian Nat. Prod. Res. 2020, 22, 193–200. [Google Scholar] [CrossRef]
- Chen, Z.M.; Yang, X.Y.; Fan, Q.Y.; Li, Z.H.; Wei, K.; Chen, H.P.; Feng, T.; Liu, J.K. Three novel degraded steroids from cultures of the Basidiomycete Antrodiella albocinnamomea. Steroids 2014, 87, 21–25. [Google Scholar] [CrossRef]
- Jin, Y.X.; Chi, M.J.; Wei, W.K.; Zhao, Y.Q.; Wang, G.K.; Feng, T. Tricholosterols A-D, four new ergosterol derivatives from the mushroom Tricholoma terreum. Steroids 2023, 191, 109157. [Google Scholar] [CrossRef]
- Lee, S.R.; Kreuzenbeck, N.B.; Jang, M.; Oh, T.; Ko, S.K.; Ahn, J.S.; Beemelmanns, C.; Kim, K.H. Xyloneside A: A New Glycosylated Incisterol Derivative from Xylaria sp. FB. Chembiochem 2020, 21, 2253–2258. [Google Scholar] [CrossRef]
- Liu, J.; Wu, X.; Yang, M.; Gu, Y.C.; Yao, L.G.; Huan, X.J.; Miao, Z.H.; Luo, H.; Guo, Y.W. Erectsterates A and B, a pair of novel highly degraded steroid derivatives from the South China Sea soft coral Sinularia erecta. Steroids 2020, 161, 108681. [Google Scholar] [CrossRef]
- Ratnaweera, P.B.; Williams, D.E.; Patrick, B.O.; de Silva, E.D.; Andersen, R.J. Solanioic Acid, an Antibacterial Degraded Steroid Produced in Culture by the Fungus Rhizoctonia solani Isolated from Tubers of the Medicinal Plant Cyperus rotundus. Org. Lett. 2015, 17, 2074–2077. [Google Scholar] [CrossRef]
- Bergstrand, L.H.; Cardenas, E.; Holert, J.; Van Hamme, J.D.; Mohn, W.W. Delineation of Steroid-Degrading Microorganisms through Comparative Genomic Analysis. MBio 2016, 7, e00166, Erratum in MBio 2016, 7, e00865–e16. [Google Scholar] [CrossRef]
- Golmei, P.; Kasna, S.; Roy, K.P.; Kumar, S. A review on pharmacological advancement of ellagic acid. J. Pharmacol. Pharmacother. 2024, 15, 93–104. [Google Scholar] [CrossRef]
- Qian, Y.K.; Chan, A.W.; Madhavan, R.; Peng, H.B. The function of Shp2 tyrosine phosphatase in the dispersal of acetylcholine receptor clusters. BMC Neurosci. 2008, 9, 70. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Peng, H.B. Roles of tyrosine kinases and phosphatases in the formation and dispersal of acetylcholine receptor clusters. Neurosci. Lett. 2020, 733, 135054. [Google Scholar] [CrossRef] [PubMed]


| Species | Observations | References |
|---|---|---|
| Agaricus blazei Murill | Isolation of a few metabolites including DM-A3 and volemolide from this terrestrial basidiomycete. | [20,23,24] |
| Amauroderma amoiensis (= A. rugosum) | Identification of 14 metabolites including DM-A3 and (17R)-17-methylincisterol. Modest inhibition of AchE (46.3% at 10 µM with cpd 5) | [25] |
| Aspergillus hiratsukae SCSIO 5Bn1003 | Isolation of DM-A3 and 12 other metabolites from this fungus. Its cytotoxic activities against four tumor cell lines (34 < IC50 < 50 µM). Inhibition of α-glucosidase (IC50 = 35.73 µM) and Bacillus subtilis (MIC = 10.26 µg/mL) | [26] |
| Aspergillus tennesseensis 1022LEF | Identification of DM-A3 and characterization of its capacity to inhibit acetylcholinesterase (AchE), NO production, and cytokine expression in activated macrophages. | [27] |
| Aspergillus tubingensis YP-2 | Discovery of demethylincisterol A5 from this endophytic fungus and characterization of its cytotoxic properties. | [15] |
| Aspergillus sp. | Isolation of chaxine and (4S,17R)-4-hydroxy-17-methylincisterol (DM-A3) from a gorgonian-derived fungus, and their antifouling activity. | [28] |
| Bipolaris maydis | Identification of DM-A3 together with 11 new steroids (bipolaristeroids A-K) from this corn pathogenic fungus. Cytotoxic effects of DM-A3 toward different cancer cells in vitro. | [29] |
| Cordyceps cicadae | Identification of 4-hydroxy-17R-methylincisterol (DM-A3) and other metabolites from C. cicadae mycelia and spores. | [30] |
| Daedaleopsis confragosa | Identification of DM-A3 and a few other sterols from this fungus. A mild anti-H. pylori activity at 100 µM. | [16] |
| Diaporthe sp. LG23 | Identification of DM-A3 and volemolide from this fungus on the host plant Mahonia fortune. | [31] |
| Ganoderma capense (Lloyd) Teng | Isolation of DM-A3 and its derivative11α-hydroxy-21-hydroxy-DM-A3 from the cultured mycelia of this medicinal fungus. No associated bioactivities. | [22] |
| Gymnascella dankaliensis | Identification of DM-A3 and dankastatin C from this fungus. | [18] |
| Harposporium anguillulae YMF 1.01751 | Isolation of 7 metabolites including (17R)-17-methylincisterol (volemolide) from this endoparasitic nematophagous fungus. | [32] |
| Hypholoma lateritium (Schae.) P. Kumm. | Isolation of DM-A2 and other metabolites from this brick cap mushroom. | [33] |
| Nodulisporium sp. SC-J597 | Isolation of DM-A3 and other metabolites from this endophytic fungus. Cytotoxic effects of DM-A3 toward several cancer cell lines in vitro. | [34] |
| Penicillium brocae G2131 | Identification of DM-A3 and a closely related compound from this fungus. | [17] |
| Penicillium herquei | Identification of DM-A3 and volemolide from this endophytic fungus to the roots of the Chinese plant Aconitum carmichaelii. | [35] |
| Penicillium sp. MCCC 3A00228 | Isolation of DM-A3 together with 14 other metabolites from this marine fungus. | [36] |
| Pestalotiopsis sp. | Identification of DM-A3 and ten other metabolites from this fungus endophyte to the Chinese mangrove Rhizophora mucronata. Highly potent cytotoxic activity of DM-A3 to HeLa cells (IC50 = 0.17 nM). | [37] |
| Phyllosticta capitalensis | An endophyte fungus of the shrub Loropetalum chinense var. rubrum. Identification of 13 metabolites including demethylincisterol A | [38] |
| Plakortis cfr. lita | Isolation of incisterol A2, A5, and A6 from this marine sponge and characterization of the PXR agonist activity of A5 and A6. Binding to the ligand binding domain (LBD) of pregnane X receptor (PXR). | [12] |
| Pithomyces sacchari | Isolation of DM-A3 and characterization of antibacterial effects, notably on QS, biofilm formation, and virulence factors in B. cereus. | [39] |
| Trichoderma atroviride RR-dl-3-9 | Identification of 4-(p-hydroxyphenethoxy)demethylincisterol A3 from this algicolous fungus. The crystal structure of the compound was determined by X-ray diffraction. Modest anti-phytoplankton and antibacterial effects. | [40] |
| Tricholoma imbricatum | Identification of DM-A3 and volemolide from this fungus. Cytotoxicity against different cancer cell lines. | [19] |
| Vanderbylia robiniophila | Identification of 4-hydroxy-17R-methylincisterol (DM-A3) and other metabolites from this medicinal mushroom (Tametes robiniophila). No effect on tyrosinase. | [41] |
| Xylaria allantoidea SWUF76 | Identification of DM-A3 and chaxine C and characterization of their cytotoxic properties. | [21] |
| Xylaria CGMCC No.5410 | An endophytic fungus from the leaves of spikemoss Selaginella moellendorffii Hieron. Isolation of DM-A3 and coumarin derivatives | [42] |
| Xylaria sp. | Identification of DM-A3 and other metabolites from this endophytic fungus isolated from traditional Chinese medicinal herb Coptis chinensis Franch. | [43,44] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bailly, C. Anticancer Activity of Demethylincisterol A3 and Related Incisterol-Type Fungal Products. Life 2025, 15, 1638. https://doi.org/10.3390/life15101638
Bailly C. Anticancer Activity of Demethylincisterol A3 and Related Incisterol-Type Fungal Products. Life. 2025; 15(10):1638. https://doi.org/10.3390/life15101638
Chicago/Turabian StyleBailly, Christian. 2025. "Anticancer Activity of Demethylincisterol A3 and Related Incisterol-Type Fungal Products" Life 15, no. 10: 1638. https://doi.org/10.3390/life15101638
APA StyleBailly, C. (2025). Anticancer Activity of Demethylincisterol A3 and Related Incisterol-Type Fungal Products. Life, 15(10), 1638. https://doi.org/10.3390/life15101638








