Effects of Physical Exercise on Cardiorespiratory Fitness and Cardiometabolic Outcomes in Schizophrenia Spectrum Disorders: The FitForLife National Intervention in Sweden
Abstract
1. Introduction
2. Results
2.1. Participant Characteristics at Baseline and Intervention Adherence
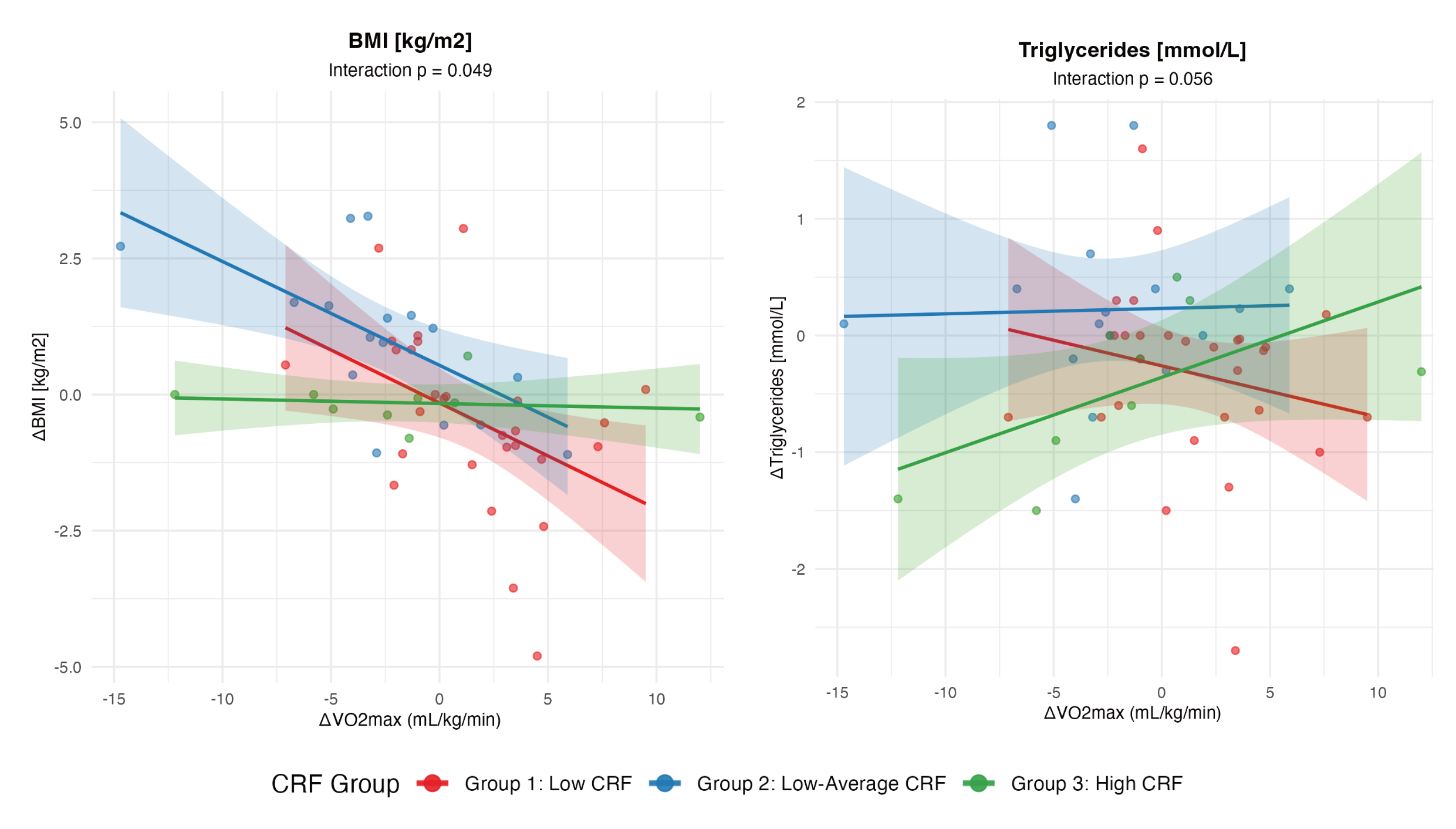
2.2. Pre–Post Change in Cardiometabolic Outcomes
3. Discussion
Strengths and Limitations
4. Materials and Methods
4.1. Study Setting
4.2. Recruitment of Study Participants
4.3. Exercise Regimen
4.4. Examinations and Interviews of Participants
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TLA | Three-letter acronym |
| SSD | Schizophrenia spectrum disorders |
| CRF | Cardiorespiratory fitness |
| CMD | Cardiometabolic diseases |
| VO2max | Volume of oxygen uptake |
| ICD-10 | 10th revision of the International Classification of Diseases |
| BMI | Body mass index |
| WHR | Waist–hip circumference ratio |
| MAP | Mean arterial pressure |
| LDL | Low-density cholesterol |
| HDL | High-density cholesterol |
| GIH | Swedish School of Sport and Medicine |
References
- Laursen, T.M.; Wahlbeck, K.; Hällgren, J.; Westman, J.; Ösby, U.; Alinaghizadeh, H.; Gissler, M.; Nordentoft, M. Life expectancy and death by diseases of the circulatory system in patients with bipolar disorder or schizophrenia in the Nordic countries. PLoS ONE 2013, 8, e67133. [Google Scholar] [CrossRef]
- Fors, B.M.; Isacson, D.; Bingefors, K.; Widerlöv, B. Mortality among persons with schizophrenia in Sweden: An epidemiological study. Nord. J. Psychiatry 2007, 61, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Rossom, R.C.; Hooker, S.A.; O’cOnnor, P.J.; Crain, A.L.; Sperl-Hillen, J.M. Cardiovascular risk for patients with and without schizophrenia, schizoaffective disorder, or bipolar disorder. J. Am. Heart Assoc. 2022, 11, e021444. [Google Scholar] [CrossRef]
- Lindekilde, N.; Scheuer, S.H.; Rutters, F.; Knudsen, L.; Lasgaard, M.; Rubin, K.H.; Henriksen, J.E.; Kivimäki, M.; Andersen, G.S.; Pouwer, F. Prevalence of type 2 diabetes in psychiatric disorders: An umbrella review with meta-analysis of 245 observational studies from 32 systematic reviews. Diabetologia 2022, 65, 440–456. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-J.; Hsieh, H.-M.; Tu, H.-P.; Jiang, H.-J.; Wang, P.-W.; Lin, C.-H. Schizophrenia in type 2 diabetes mellitus: Prevalence and clinical characteristics. Eur. Psychiatry 2018, 54, 102–108. [Google Scholar] [CrossRef]
- Correll, C.U.; Solmi, M.; Veronese, N.; Bortolato, B.; Rosson, S.; Santonastaso, P.; Thapa-Chhetri, N.; Fornaro, M.; Gallicchio, D.; Collantoni, E.; et al. Prevalence, incidence and mortality from cardiovascular disease in patients with pooled and specific severe mental illness: A large-scale meta-analysis of 3,211,768 patients and 113,383,368 controls. World Psychiatry 2017, 16, 163–180. [Google Scholar] [CrossRef]
- Tek, C.; Kucukgoncu, S.; Guloksuz, S.; Woods, S.W.; Srihari, V.H.; Annamalai, A. Antipsychotic-induced weight gain in first-episode psychosis patients: A meta-analysis of differential effects of antipsychotic medications. Early Interv. Psychiatry 2016, 10, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Vancampfort, D.; Firth, J.; Schuch, F.B.; Rosenbaum, S.; Mugisha, J.; Hallgren, M.; Probst, M.; Ward, P.B.; Gaughran, F.; De Hert, M.; et al. Sedentary behavior and physical activity levels in people with schizophrenia, bipolar disorder and major depressive disorder: A global systematic review and meta-analysis. World Psychiatry 2017, 16, 308–315. [Google Scholar] [CrossRef]
- Stubbs, B.; Firth, J.; Berry, A.; Schuch, F.B.; Rosenbaum, S.; Gaughran, F.; Veronesse, N.; Williams, J.; Craig, T.; Yung, A.R.; et al. How much physical activity do people with schizophrenia engage in? A systematic review, comparative meta-analysis and meta-regression. Schizophr. Res. 2016, 176, 431–440. [Google Scholar] [CrossRef]
- Fornaro, M.; Carvalho, A.F.; De Prisco, M.; Mondin, A.M.; Billeci, M.; Selby, P.; Iasevoli, F.; Berk, M.; Castle, D.J.; de Bartolomeis, A. The prevalence, odds, predictors, and management of tobacco use disorder or nicotine dependence among people with severe mental illness: Systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2022, 132, 289–303. [Google Scholar] [CrossRef]
- Posadzki, P.; Pieper, D.; Bajpai, R.; Makaruk, H.; Könsgen, N.; Neuhaus, A.L.; Semwal, M. Exercise/physical activity and health outcomes: An overview of Cochrane systematic reviews. BMC Public Health 2020, 20, 1724. [Google Scholar] [CrossRef]
- Raghuveer, G.; Hartz, J.; Lubans, D.R.; Takken, T.; Wiltz, J.L.; Mietus-Snyder, M.; Perak, A.M.; Baker-Smith, C.; Pietris, N.; Edwards, N.M. Cardiorespiratory fitness in youth: An important marker of health: A scientific statement from the American Heart Association. Circulation 2020, 142, e101–e118. [Google Scholar] [CrossRef]
- Lin, X.; Zhang, X.; Guo, J.; Roberts, C.K.; McKenzie, S.; Wu, W.-C.; Liu, S.; Song, Y. Effects of exercise training on cardiorespiratory fitness and biomarkers of cardiometabolic health: A systematic review and meta-analysis of randomized controlled trials. J. Am. Heart Assoc. 2015, 4, e002014. [Google Scholar] [CrossRef] [PubMed]
- Kodama, S.; Saito, K.; Tanaka, S.; Maki, M.; Yachi, Y.; Asumi, M.; Sugawara, A.; Totsuka, K.; Shimano, H.; Ohashi, Y.; et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: A meta-analysis. JAMA 2009, 301, 2024–2035. [Google Scholar] [CrossRef] [PubMed]
- Vancampfort, D.; Rosenbaum, S.; Probst, M.; Soundy, A.; Mitchell, A.J.; De Hert, M.; Stubbs, B. Promotion of cardiorespiratory fitness in schizophrenia: A clinical overview and meta-analysis. Acta Psychiatr. Scand. 2015, 132, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Vancampfort, D.; Rosenbaum, S.; Ward, P.B.; Stubbs, B. Exercise improves cardiorespiratory fitness in people with schizophrenia: A systematic review and meta-analysis. Schizophr. Res. 2015, 169, 453–457. [Google Scholar] [CrossRef]
- Marston, N.A.; Giugliano, R.P.; Im, K.; Silverman, M.G.; O’donoghue, M.L.; Wiviott, S.D.; Ference, B.A.; Sabatine, M.S. Association between triglyceride lowering and reduction of cardiovascular risk across multiple lipid-lowering therapeutic classes: A systematic review and meta-regression analysis of randomized controlled trials. Circulation 2019, 140, 1308–1317. [Google Scholar] [CrossRef]
- Korman, N.; Stanton, R.; Vecchio, A.; Chapman, J.; Parker, S.; Martland, R.; Siskind, D.; Firth, J. The effect of exercise on global, social, daily living and occupational functioning in people living with schizophrenia: A systematic review and meta-analysis. Schizophr. Res. 2023, 256, 98–111. [Google Scholar] [CrossRef]
- Noone, J.; Mucinski, J.M.; DeLany, J.P.; Sparks, L.M.; Goodpaster, B.H. Understanding the variation in exercise responses to guide personalized physical activity prescriptions. Cell Metab. 2024, 36, 702–724. [Google Scholar] [CrossRef]
- Fernández-Abascal, B.; Suárez-Pinilla, M.; Cobo-Corrales, C.; Crespo-Facorro, B.; Suárez-Pinilla, P. Lifestyle intervention based on exercise and behavioural counselling and its effect on physical and psychological health in outpatients with schizophrenia spectrum disorders: An exploratory, pragmatic randomized clinical trial. Schizophr. Res. 2023, 261, 256–268. [Google Scholar] [CrossRef]
- Gorini, S.; Camajani, E.; Feraco, A.; Armani, A.; Karav, S.; Filardi, T.; Aulisa, G.; Cava, E.; Strollo, R.; Padua, E.; et al. Exploring gender differences in the effects of diet and physical activity on metabolic parameters. Nutrients 2025, 17, 354. [Google Scholar] [CrossRef] [PubMed]
- Waters, L.A.; Galichet, B.; Owen, N.; Eakin, E. Who participates in physical activity intervention trials? J. Phys. Act. Health 2011, 8, 85–103. [Google Scholar] [CrossRef]
- Statistics Sweden. Population in the Country, Counties and Municipalities on 31 December 2023 and Population Change in 2024. 2024. Available online: https://www.scb.se/en/finding-statistics/statistics-by-subject-area/population/population-composition/population-statistics/pong/tables-and-graphs/population-statistics---year/population-in-the-country-counties-and-municipalities-on-31-december-2023-and-population-change-in-2023/ (accessed on 10 April 2025).
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; LaMonte, M.J.; Lee, I.-M.; Nieman, D.C.; Swain, D.P. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Med. Sci. Sports Exerc. 2011, 43, 1334–1359. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Waist Circumference and Waist-Hip Ratio: Report of a WHO Expert Consultation; World Health Organization: Geneva, Switzerland, 2011. Available online: https://www.who.int/publications/i/item/9789241501491 (accessed on 10 April 2025).
- World Health Organization. The SuRF Report 2: Surveillance of Risk Factors Related to Noncommunicable Diseases: Current Status of Global Data; World Health Organization: Geneva, Switzerland, 2005.
- DeMers, D.; Wachs, D. Physiology, mean arterial pressure. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK538226/ (accessed on 10 April 2025).
- Nicolaisen, S.K.; Pedersen, L.; Witte, D.R.; Sørensen, H.T.; Thomsen, R.W. HbA1c-defined prediabetes and progression to type 2 diabetes in Denmark: A population-based study based on routine clinical care laboratory data. Diabetes Res. Clin. Pract. 2023, 203, 110829. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Chen, M.; Shen, H.; Yin, P.; Fan, L.; Chen, X.; Wu, J.; Xu, Z.; Zhang, J. Predictive value of LDL/HDL ratio in coronary atherosclerotic heart disease. BMC Cardiovasc. Disord. 2022, 22, 273. [Google Scholar] [CrossRef]
- Mayo Clinic. Triglycerides: Why do They Matter? Available online: https://www.mayoclinic.org/diseases-conditions/high-blood-cholesterol/in-depth/triglycerides/art-20048186 (accessed on 12 January 2023).
- Kierkegaard, S. The Ekblom-Bak Test. The Åstrand Laboratory, Swedish School of Sport and Health Sciences. n.d. Available online: https://www.gih.se/english/research/laboratories/the-astrand-laboratory/the-ekblom-bak-test (accessed on 10 April 2025).
- Björkman, F.; Ekblom, Ö.; Ekblom-Bak, E.; Bohman, T. The ability of a submaximal cycle ergometer test to detect longitudinal changes in VO2max. BMC Sports Sci. Med. Rehabil. 2021, 13, 154. [Google Scholar] [CrossRef]
- Williams, N. The Borg Rating of Perceived Exertion (RPE) scale. Occup. Med. 2017, 67, 404–405. [Google Scholar] [CrossRef]
- Kierkegaard, S. The Ekblom-Bak Test: Test Protocol EKBLOM-BAK Test Excel. The Åstrand Laboratory, Swedish School of Sport and Health Sciences. 2022. Available online: https://www.gih.se/download/18.119eb01e1840e0d095c7e22b/1668767217912/TestProtocol_EBtest_eng.xlsx (accessed on 10 April 2025).
- Väisänen, D.; Ekblom, B.; Wallin, P.; Andersson, G.; Ekblom-Bak, E. Reference values for estimated VO2max by two submaximal cycle tests: The Åstrand-test and the Ekblom-Bak test. Eur. J. Appl. Physiol. 2024, 124, 1747–1756. [Google Scholar] [CrossRef]
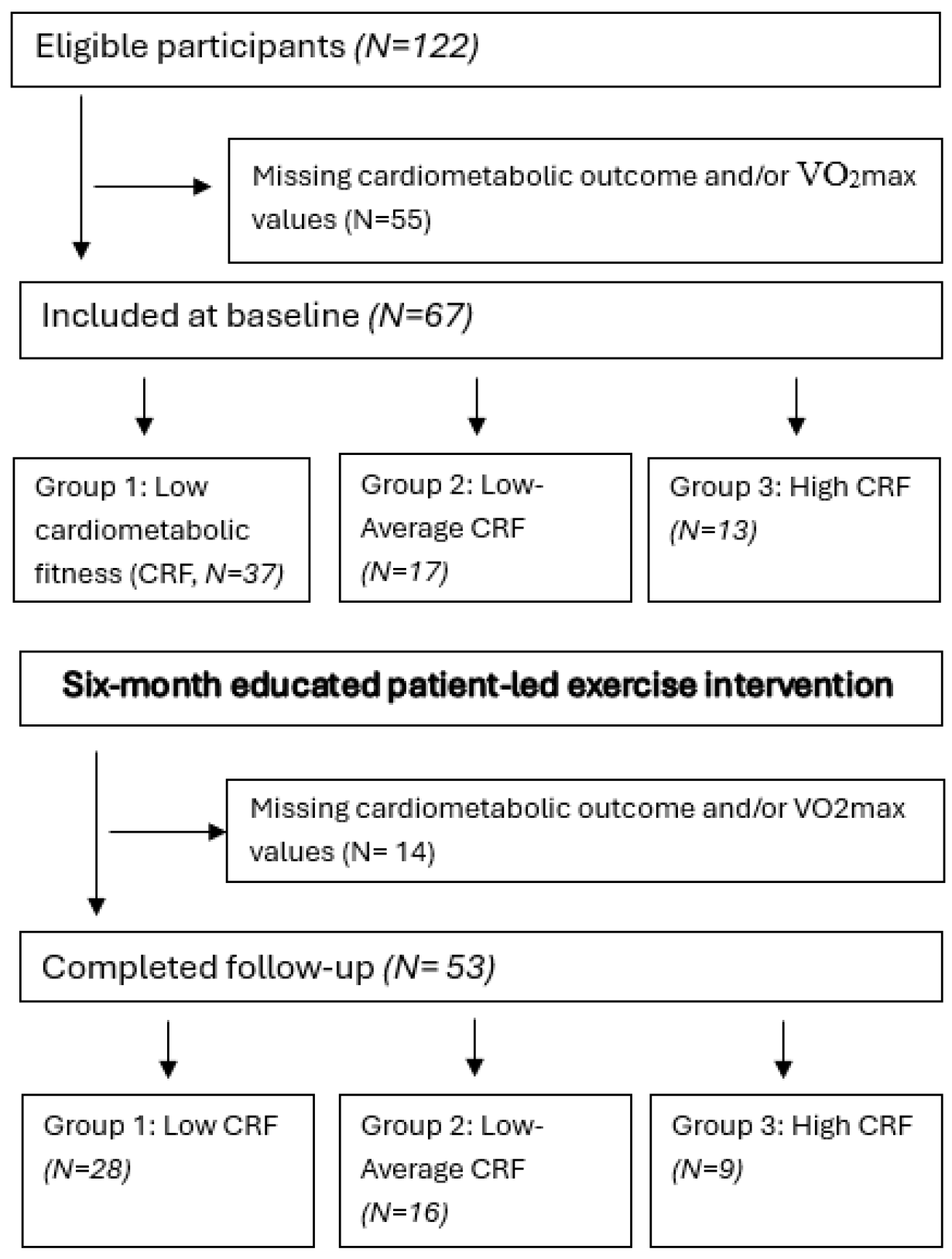

| Characteristic | Group 1 Low CRF (N = 37) | Group 2 Low–Average CRF (N = 17) | Group 3 High CRF (N = 13) | Total (N = 67) |
|---|---|---|---|---|
| Male sex; % | 46.0 | 58.8 | 46.2 | 49.3 |
| Age; Mean (S.D.) | 44.6 (10.2) | 47.5 (11.2) | 50.5 (9.1) | 46.5 (10.4) |
| Employed or student; % * | 21.6 | 23.5 | 15.4 | 20.9 |
| Number of exercise sessions in clinic; Mean (S.D.) | 18.0 (10.9) | 24.7 (10.3) | 28.6 (10.1) | 21.7 (11.3) |
| Psychiatric service utilization | ||||
| SSD as first ICD-10 F-diagnosis; % ** | 81.1 | 82.4 | 100.0 | 85.1 |
| Number of times inpatient; Mean (S.D.) | 3.0 (2.5) | 5.3 (3.3) | 3.9 (3.0) | 3.7 (2.9) |
| Current antipsychotic prescription; % | 89.2 | 94.1 | 84.6 | 89.6 |
| Cardiometabolic risk factors | ||||
| WHR; Mean (S.D.) | 0.93 (0.09) | 0.95 (0.09) | 0.93 (0.11) | 0.94 (0.09) |
| Normal (♂ ≤ 0.89, ♀ ≤ 0.84); % | 24.32 | 11.76 | 30.77 | 22.39 |
| Elevated (♂ ≥ 0.90, ♀ ≥ 0.85); % | 75.68 | 88.24 | 69.23 | 77.61 |
| BMI [kg/m2]; Mean (S.D.) | 32.39 (5.53) | 28.03 (3.56) | 25.43 (3.50) | 29.93 (5.51) |
| Normal (≤24.9); % | 8.1 | 17.7 | 46.2 | 17.9 |
| Overweight (25.0–29.9); % | 21.6 | 52.9 | 46.2 | 34.3 |
| Obese (30–39.9); % | 59.5 | 29.4 | 7.7 | 41.8 |
| Severely obese (≥40.0) % | 10.8 | 0.0 | 0.0 | 6.0 |
| MAP blood pressure [mmHg]; Mean (S.D.) | 98.0 (11.9) | 100.1 (8.9) | 93.9 (11.5) | 97.8 (11.2) |
| Normal MAP (60.9–99.9); % | 56.8 | 47.1 | 53.9 | 53.7 |
| High MAP (≥100.00); % | 43.2 | 52.9 | 46.2 | 46.3 |
| Triglycerides [mmol/L]; Mean (S.D.) | 1.75 (1.03) | 1.56 (0.69) | 1.52 (0.9) | 1.66 (0.93) |
| Normal (≤1.6); % | 59.5 | 52.9 | 69.2 | 59.7 |
| Elevated (≥1.7); % | 40.5 | 47.1 | 30.8 | 40.3 |
| LDL/HDL ratio; Mean (S.D) | 2.72 (0.91) | 2.63 (0.77) | 2.24 (0.96) | 2.61 (0.9) |
| Normal (<2); % | 21.6 | 23.5 | 46.2 | 26.9 |
| Elevated (≥2.0); % | 78.4 | 76.5 | 53.9 | 73.1 |
| HbA1c [mmol/mol]; Mean (S.D.) | 36.4 (4.8) | 35.4 (3.7) | 36.2 (5.3) | 36.1 (4.6) |
| Normal (≤41); % | 86.5 | 94.1 | 92.3 | 89.6 |
| Pre-diabetes and diabetes (≥42); % | 13.5 | 5.9 | 7.7 | 10.5 |
| Outcome Variable (N = 53) | Baseline: Mean (S.D.) | Follow-Up: Mean (S.D.) | Pre–Post Change: Mean (95%CI) |
|---|---|---|---|
| VO2max [mL/(kg ∗ min)] | 37.2 (9.2) | 37.0 (8.5) | −0.2 (−1.5, 1.1) |
| WHR | 0.94 (0.09) | 0.96 (0.09) | 0.02 (0.00, 0.04) |
| BMI [kg/m2] | 30.34 (5.37) | 30.38 (5.23) | 0.04 (−0.39, 0.47) |
| MAP [mmHg] | 98.8 (10.9) | 96.3 (9.8) | −2.5 (−5.8, 0.9) |
| Triglycerides [mmol/L] | 1.72 (0.91) | 1.53 (0.77) | −0.18 (−0.40, 0.04) |
| Ldl/Hdl ratio | 2.63 (0.92) | 2.62 (1.11) | 0.00 (−0.19, 0.18) |
| HbA1c [mmol/mol] | 36.5 (4.8) | 36.3 (5.8) | −0.21 (−1.11, 0.65) |
| Males (N = 25) | |||
| VO2max [mL/(kg ∗ min)] | 42.9 (6.7) | 41.7 (7.5) | −1.6 (−3.4, 1.1) |
| WHR | 0.99 (0.08) | 1.00 (0.07) | 0.01 (0.00, 0.03) |
| BMI [kg/m2] | 29.66 (4.77) | 30.21 (4.94) | 0.55 (0.03, 1.07) |
| MAP [mmHg] | 98.9 (11.0) | 98.1 (7.7) | −0.8 (−5.6, 3.9) |
| Triglycerides [mmol/L] | 1.63 (0.78) | 1.71 (0.95) | 0.08 (−0.24, 0.41) |
| Ldl/Hdl ratio | 2.91 (0.68) | 3.01 (1.19) | 0.10 (−0.25, 0.44) |
| HbA1c [mmol/mol] | 35.5 (3.5) | 35.6 (4.1) | 0.12 (−0.85, 1.09) |
| Females (N = 28) | |||
| VO2max [mL/(kg ∗ min)] | 32.1 (8.2) | 32.8 (7.1) | 0.6 (−1.0, 2.2) |
| WHR | 0.9 (0.08) | 0.92 (0.09) | 0.03 (−0.02, 0.07) |
| BMI [kg/m2] | 30.95 (5.87) | 30.54 (5.57) | −0.41 (−1.06, 0.23) |
| MAP [mmHg] | 98.7 (10.9) | 94.8 (11.3) | −3.9 (−8.9, 1.1) |
| Triglycerides [mmol/L] | 1.79 (1.01) | 1.37 (0.53) | −0.42 (−0.71, −0.13) |
| Ldl/Hdl ratio | 2.38 (1.03) | 2.28 (0.93) | −0.10 (−0.29, 0.10) |
| Hba1c [mmol/mol] | 37.4 (5.6) | 36.9 (7.0) | −0.50 (−1.93, 0.93) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Forsell, Y.; Skott, M.; Yel Bektash, B.; Syvertsen, A.; Ekblom, Ö.; Lavebratt, C. Effects of Physical Exercise on Cardiorespiratory Fitness and Cardiometabolic Outcomes in Schizophrenia Spectrum Disorders: The FitForLife National Intervention in Sweden. Life 2025, 15, 1637. https://doi.org/10.3390/life15101637
Forsell Y, Skott M, Yel Bektash B, Syvertsen A, Ekblom Ö, Lavebratt C. Effects of Physical Exercise on Cardiorespiratory Fitness and Cardiometabolic Outcomes in Schizophrenia Spectrum Disorders: The FitForLife National Intervention in Sweden. Life. 2025; 15(10):1637. https://doi.org/10.3390/life15101637
Chicago/Turabian StyleForsell, Yvonne, Maria Skott, Buse Yel Bektash, Astrid Syvertsen, Örjan Ekblom, and Catharina Lavebratt. 2025. "Effects of Physical Exercise on Cardiorespiratory Fitness and Cardiometabolic Outcomes in Schizophrenia Spectrum Disorders: The FitForLife National Intervention in Sweden" Life 15, no. 10: 1637. https://doi.org/10.3390/life15101637
APA StyleForsell, Y., Skott, M., Yel Bektash, B., Syvertsen, A., Ekblom, Ö., & Lavebratt, C. (2025). Effects of Physical Exercise on Cardiorespiratory Fitness and Cardiometabolic Outcomes in Schizophrenia Spectrum Disorders: The FitForLife National Intervention in Sweden. Life, 15(10), 1637. https://doi.org/10.3390/life15101637







