Casomorphine-10 (CM-10) Peptide Orchestrates Circadian and Neurodevelopmental Gene Clusters via δ-Opioid Receptor Signaling: Insights from Transcriptome Analysis with δ-Opioid Receptor-Expressing HEK293 Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Experiment
2.1.1. Reagents and Materials
2.1.2. HEK293 and DOR-HEK Cell Culturing
2.1.3. DOR Detection on HEK293 Cells
2.2. Bioinformatic Analysis
2.2.1. mRNA Preparation and Sequencing
2.2.2. mRNA-Seq Data Set Processing
2.2.3. qPCR of the Selected Genes
2.2.4. Statistical Analysis
3. Results
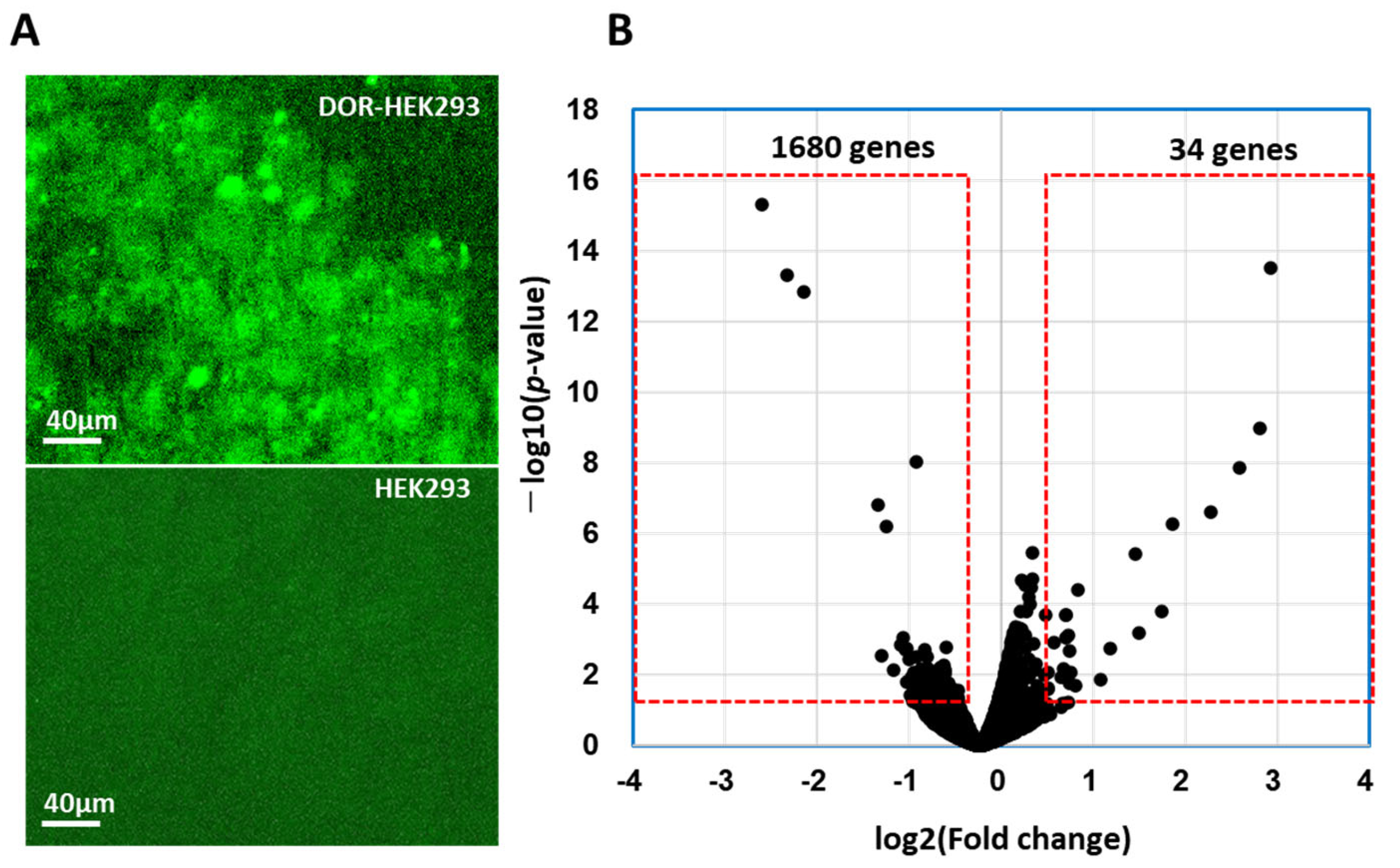
3.1. DOR Expression on DOR-HEK293 Cells
3.2. DEGs in DOR-HEK293 Cells Following CM-10 Treatment
3.3. Ontology Analysis
3.4. Network Analysis of DEGs
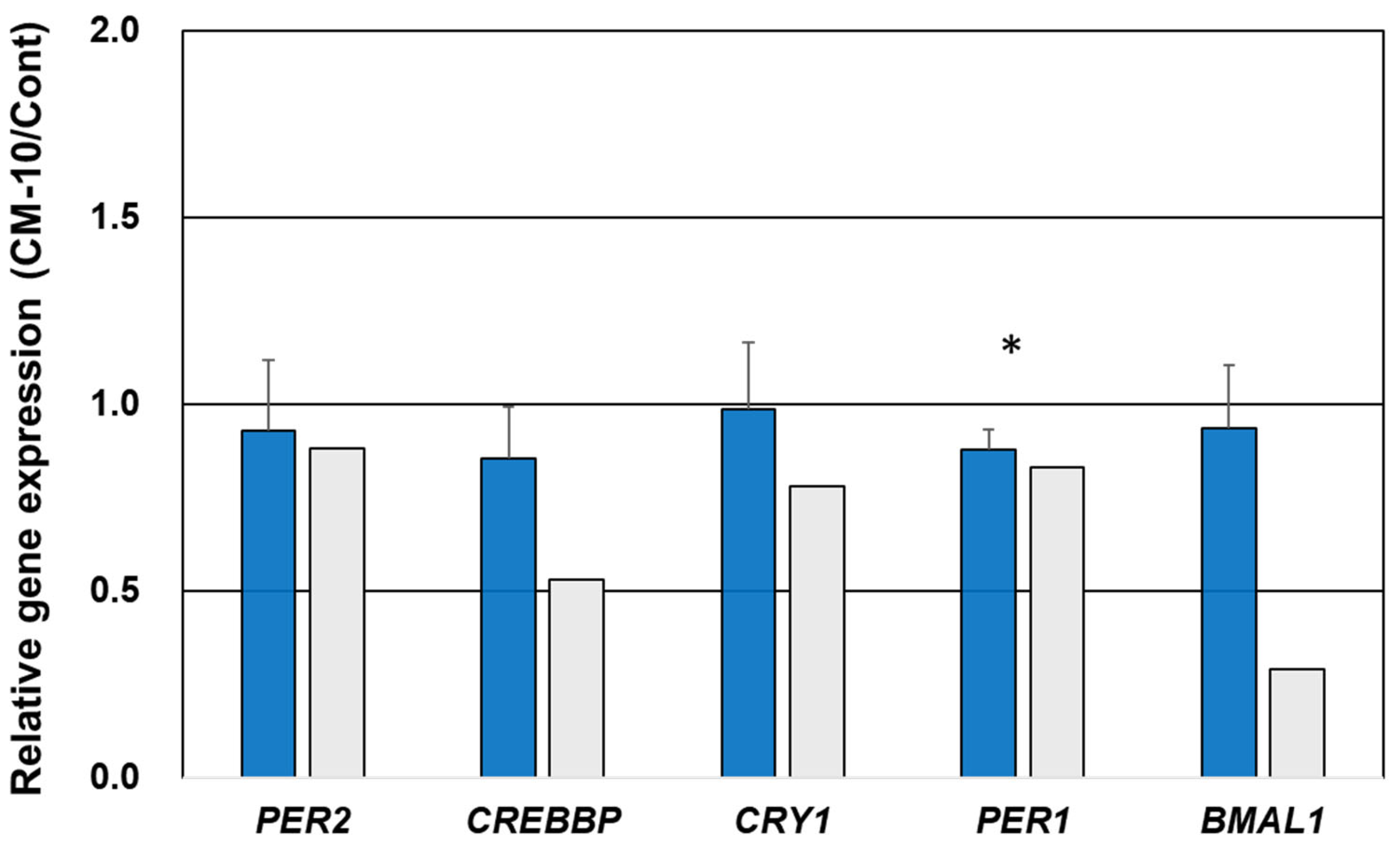
3.5. Gene Expression Validation Using qPCR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martínez-Maqueda, D.; Miralles, B.; Recio, I.; Hernández-Ledesma, B. Antihypertensive peptides from food proteins: A review. Food Funct. 2012, 3, 350. [Google Scholar] [CrossRef]
- Ifeanyi, D.; Nwachukwu, D.; Aluko, R.E. Strucural and functional properties of food protein-derived antioxidant peptides. J. Food Biochem. 2019, 43, e12761. [Google Scholar]
- Santiago-López, L.; Hernández-Mendoza, A.; Vallejo-Cordoba, B.; Mata-Haro, V.; González-Córdova, A.F. Food-derived immunomodulatory peptides. J. Sci. Food Agric. 2016, 96, 3631. [Google Scholar]
- Zhao, Q.; Shi, Y.; Wang, X.; Huang, A. Characterization of a novel antimicrobial peptide from buffalo casein hydrolysate based on live bacteria adsorption. J. Dairy Sci. 2020, 103, 11116. [Google Scholar] [CrossRef] [PubMed]
- Outman, A.; Deracinois, B.; Flahaut, C.; Diab, M.A.; Gressier, B.; Eto, B.; Nedjar, N. Potential of human hemoglobin as a source of bioactive peptides: Comparative study of enzymatic hydrolysis with bovine hemoglobin and the production of active peptide alpha137-141. Int. J. Mol. Sci. 2023, 24, 11921. [Google Scholar]
- Korhonen, H.; Pihlanto, A. Technological options for the production of health-promoting proteins and peptides derived from milk and colostrum. Curr. Pharm. Des. 2007, 13, 829. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, R.; Behare, P.; Rana, R.; Kumar, A.; Kumar, M.; Arora, S.; Morotta, F.; Jain, S.; Yadav, H. Bioactive peptides derived from milk proteins and their health beneficial potentials: An update. Food Funct. 2011, 2, 18. [Google Scholar]
- Nakamoto, K.; Tokuyama, S. Stress-induced changes in the endogenous opioid system cause dysfunction of pain and emotion regulation. Int. J. Mol. Sci. 2023, 24, 11713. [Google Scholar] [CrossRef]
- Higginbotham, J.A.; Markovic, T.; Massaly, N.; Morón, J.A. Endogenous opioid systems alterations in pain and opioid use disorder. Front. Syst. Neurosci. 2022, 16, 1014768. [Google Scholar] [CrossRef]
- Alvarez-Perez, B.; Poras, H.; Maldonado, R. The inhibition of enkephalin catabolism by dual enkephalinase inhibitor: A novel possible therapeutic approach for opioid use disorders. Br. J. Pharmacol. 2023, 180, 879–893. [Google Scholar]
- Caputi, F.F.; Rullo, L.; Stamatakos, S.; Candeletti, S.; Romualdi, P. Interplay between the endogenous opioid system and proteasome complex: Beyond signaling. Int. J. Mol. Sci. 2019, 20, 1441. [Google Scholar] [CrossRef] [PubMed]
- Corder, G.; Castro, D.C.; Bruchas, M.R.; Scherrer, G. Endogenous and exogenous opioids in pain. Annu. Rev. Neurosci. 2018, 41, 453. [Google Scholar] [CrossRef] [PubMed]
- Akil, H.; Watson, S.J.; Young, E.; Lewis, M.E.; Khachaturian, H.; Walker, J.M. Endogenous opioids: Biology and function. Annu. Rev. Neurosci. 1984, 7, 223. [Google Scholar] [CrossRef]
- Caputi, F.F.; Rullo, L.; Stamatakos, S.; Candeletti, S.; Romualdi, P. Modulation of the negative affective dimension of pain: Focus on selected neuropeptidergic system contributions. Int. J. Mol. Sci. 2019, 30, 4010. [Google Scholar] [CrossRef]
- Drolet, G.; Dumont, E.C.; Gosselin, I.; Kinkead, R.; Laforest, S.; Trottier, J.F. Role of endogenous opioid system in the regulation of the stress response. Prog. Neuropsychopharmacol. Biol. Psychiatry 2001, 25, 729. [Google Scholar] [CrossRef] [PubMed]
- Le Merrer, J.; Becker, J.A.J.; Befort, K.; Kieffer, B.L. Reward processing by the opioid system in the brain. Physiol. Rev. 2009, 89, 1379. [Google Scholar] [CrossRef]
- Gopalakrishnan, L.; Chatterjee, O.; Ravishankar, N.; Suresh, S.; Raju, R.; Mahadevan, A.; Prasad, T.S.K. ·Opioid receptors signaling network. Cell Commun. Signal. 2022, 16, 475. [Google Scholar] [CrossRef]
- Brantl, V.; Teschemacher, H.; Henschen, A.; Lottspeich, F. Novel opioid peptides derived from casein (b-casomorphins). I. Isolation from bovine casein peptone. Hoppe Seyler’s Z. Physiol. Chem. 1979, 360, 1211. [Google Scholar] [CrossRef]
- Ohinata, K.; Agui, S.; Yoshikawa, M. Soymorphins, novel μ opioid peptides derived from soy β-conglycinin β-subunit, have anxiolytic activities. Biosci. Biotechnol. Biochem. 2007, 71, 2618. [Google Scholar] [CrossRef]
- Fukudome, S.; Yoshikawa, M. Opioid peptides derived from wheat gluten: Their isolation and characterization. FEBS Lett. 1992, 296, 107. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Fukunaga, M.; Iwatani, S.; Miyanaga, K.; Adachi, T.; Yamamoto, N. Release of an anti-anxiety peptide in casein hydrolysate with Aspergillus oryzae protease. Food Funct. 2022, 13, 10449. [Google Scholar] [CrossRef] [PubMed]
- Manabe, S.; Miyano, K.; Fujii, Y.; Ohshima, K.; Yoshida, Y.; Nonaka, M.; Uzu, M.; Matsuoka, Y.; Sato, T.; Uezono, Y.; et al. Possible biased analgesic of hydromorphone through the G protein-over be-ta arrestin-mediated pathway: cAMP, CellKey™, and receptor internalization analyses. J. Pharmacol. Sci. 2019, 140, 171. [Google Scholar] [CrossRef]
- Yamada, D.; Yanagisawa, S.; Yoshizawa, K.; Yanagita, S.; Oka, J.I.; Nagase, H.; Saitoh, A. Selective agonists of the δ-opioid receptor, KNT-127 and SNC80, act differentially on extinction learning of contextual fear memory in mice. Neuropharmacology. 2019, 160, 107792. [Google Scholar] [CrossRef]
- Morales-Mulia, M.; Estrada-Camarena, E.; Amaya, M.I.; Mejía-Mauríes, S.; Sollozo-Dupont, I.; Mengod, G.; de Gortari, P. Anxiolytic effects of ethanol are partially related to a reduced expression of adenylyl cyclase 5 but not to μ-opioid receptor activation in rat nucleus accumbens. Behav. Brain Res. 2012, 235, 189. [Google Scholar] [CrossRef][Green Version]
- Seo, E.J.; Efferth, T.; Panossian, A.S. Curcumin downregulates expression of opioid-related nociceptin receptor gene (OPRL1) in isolated neuroglia cells. Phytomedicine 2018, 50, 285. [Google Scholar] [CrossRef]
- Kim, A.D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357. [Google Scholar] [CrossRef]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290. [Google Scholar] [CrossRef] [PubMed]
- Pertea, M.; Kim, D.; Pertea, G.M.; Leek, J.T.; Salzberg, S.L. Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat. Protoc. 2016, 9, 1650. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2009, 26, 139. [Google Scholar] [CrossRef]
- Satoh, M.; Minami, M. Molecular pharmacology of the opioid receptors. Pharmacol. Ther. 1995, 68, 343. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Décaillot, F.M.; Devi, L.A. Targeting opioid receptor heterodimers: Strategies for screening and drug development. AAPS J. 2006, 8, E153. [Google Scholar] [CrossRef]
- Levitt, E.S.; Purington, L.C.; Traynor, J.R. Gi/o-coupled receptors compete for signaling to adenylyl cyclase in SH-SY5Y cells and reduce opioid-mediated cAMP overshoot. Mol. Pharmacol. 2011, 79, 461. [Google Scholar] [CrossRef] [PubMed]
- Clark, M.J.; Linderman, J.J.; Traynor, J.R. Endogenous regulators of G protein signaling differentially modulate full and partial mu-opioid agonists at adenylyl cyclase as predicted by a collision coupling model. Mol. Pharmacol. 2008, 73, 1538. [Google Scholar] [CrossRef]
- Reiss, D.; Maurin, H.; Audouard, E.; Martínez-Navarro, M.; Xue, Y.; Herault, Y.; Maldonado, R.; Cabañero, D.; Gaveriaux-Ruff, C. Delta opioid receptor in astrocytes contributes to neuropathic cold pain and analgesic tolerance in female mice. Front. Cell Neurosci. 2021, 15, 745178. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wang, X.; Zhao, F.; Zhao, Q.; Kong, S.; Ma, P.; Wu, G.; Wang, W.; Zhang, X. Centrosomal protein CEP135 regulates the migration and angiogenesis of endothelial cells in a microtubule-dependent manner. Front. Biosci. 2023, 28, 303. [Google Scholar] [CrossRef]
- Galindo, A.; Planelles-Herrero, V.J.; Degliesposti, G.; Munro, S. Cryo-EM structure of metazoan TRAPPIII, the multi-subunit complex that activates the GTPase Rab1. EMBO J. 2021, 40, e107608. [Google Scholar] [CrossRef]
- Walsh, T.G.; Li, Y.; Williams, C.M.; Aitken, E.W.; Andrews, R.K.; Poole, A.W. Loss of the exocyst complex component, EXOC3, promotes hemostasis and accelerates arterial thrombosis. Blood Adv. 2021, 5, 674. [Google Scholar] [CrossRef]
- Li, P.; Chen, J.; Zou, J.; Zhu, W.; Zang, Y.; Li, H. Circular RNA coiled-coil domain containing 66 regulates malignant development of papillary thyroid carcinoma by upregulating La ribonucleoprotein 1 via the sponge effect on miR-129-5p. Bioengineered 2022, 13, 7181. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Wang, T.; Li, Y.F.; Deng, Y.N.; He, X.L.; Wang, L.J. N-acetylcysteine improves autism-like behavior by recovering autophagic deficiency and decreasing Notch-1/Hes-1 pathway activity. Exp. Biol. Med. 2023, 248, 966. [Google Scholar] [CrossRef]
- Polge, A.; Durand, N.; Miret, A.; Lumbroso, S.; Francannet, C.; Mouzat, K. Discovering the ANK2-related autism phenotype. Clin. Genet. 2023, 104, 384. [Google Scholar]
- Kim, S.-H.; Serezani, C.H.; Okunishi, K.; Zaslona, Z.; Peters-Golden, D.M.A.M. Distinct protein kinase A anchoring proteins direct prostaglandin E2 modulation of Toll-like receptor signaling in Alveolar Macrophages. J. Biol. Chem. 2011, 286, 8875. [Google Scholar] [CrossRef]
- Holmstrom, S.R.; Wijayatunge, R.; McCrum, K.; Mgbemena, V.E.; Ross, T.S. Functional interaction of BRCA1and CREBBP in murine hematopoiesis. iScience 2019, 19, 809. [Google Scholar] [CrossRef]
- Ghosh, S.; Lu, Y.; Hu, Y. A role of CREB in BRCA1 constitutive promoter activity and aromatase basal expression. Int. J. Biomed. Sci. 2008, 4, 260. [Google Scholar] [CrossRef]
- Lee, P.T.; Lin, G.; Lin, W.W. A kinase-dependent feedforward loop affects CREBB stability and long term memory formation. eLife 2018, 7, e33007. [Google Scholar] [CrossRef]
- Sánchez-Rivera, L.; Santos, P.F.; Sevilla, M.A.; Montero, M.J.; Recio, I.; Miralles, B. Implication of opioid receptors in the antihypertensive effect of a bovine casein hydrolysate and alpha(s1)-casein-derived peptides. J. Agric. Food Chem. 2020, 78, 1877. [Google Scholar] [CrossRef] [PubMed]
- Bounouala, F.Z.; Roudj, S.; Karam, N.-E.; Recio, I.; Miralles, B. Casein hydrolysates by Lactobacillus brevis and Lactococcus lactis proteases: Peptide profile discriminates strain-dependent enzyme specificity. J. Agric. Food Chem. 2017, 65, 9324. [Google Scholar] [PubMed]
- Kapusta, D.R.; Jones, S.Y.; Kopp, U.C.; Recio, I.; Miralles, B. Role of renal nerves in excretory responses to exogenous and endogenous opioid peptides. J. Pharmacol. Exp. Ther. 1989, 248, 1039. [Google Scholar] [CrossRef]
- Dremencov, E.; Grinchii, D.; Romanova, Z.; Chomanic, P.; Lacinova, L.; Jezova, D. Effects of chronic delta-opioid receptor agonist on the excitability of hippocampal glutamate and brainstem monoamine neurons, anxiety, locomotion, and habituation in rats. Pharmacol. Rep. 2023, 75, 585. [Google Scholar] [CrossRef]
- Angelova, H.; Krumova, E.; Dzhambazova, E.; Pechlivanova, D. Effects of the antinociceptive dipeptide L-tyrosine-L-arginine (kyotorphin) on the motivation, anxiety, and memory in rats. Folia Med. 2021, 63, 189. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.; Nurgali, K.; Mishra, V.K. Food proteins as source of opioid peptides-a review. Curr. Med. Chem. 2016, 23, 893. [Google Scholar]
- Rubin, G.M.; Yandell, M.D.; Wortman, J.R.; Miklos, G.L.G.; Nelson, C.R.; Hariharan, I.K.; Fortini, M.E.; Li, P.W.; Apweiler, R.; Fleischmann, W.; et al. Comparative genomics of the eukaryotes. Science 2000, 287, 2204. [Google Scholar] [CrossRef]
- Al-Hasani, R.; Bruchas, M.R. Molecular mechanisms of opioid receptordependent signaling and behavior. Anesthesiology 2011, 115, 1363. [Google Scholar] [CrossRef]
- Aline, A.-C.; Laurent, B.; Dario, D. Regulation of G protein-coupled receptor signaling by a-kinase anchoring proteins. J. Recept. 2006, 26, 631. [Google Scholar]
- Alberini, C.M. Transcription factors in long-term memory and synaptic plasticity. Physiol. Rev. 2009, 89, 121. [Google Scholar] [CrossRef] [PubMed]
- Dyson, H.J.; Wright, P.E. Role of intrinsic protein disorder in the function and interactions of the transcriptional coactivators CREB-binding protein (CBP) and p300. J. Biol. Chem. 2016, 291, 6714. [Google Scholar] [CrossRef]
- Wang, H.; Xu, J.; Lazarovici, P.; Zheng, W. cAMP response element-binding protein (CREB): A possible signaling molecule link in the pathophysiology of schizophrenia. Front. Mol. Neurosci. 2018, 11, 255. [Google Scholar] [CrossRef]
- Ren, X.; Rizavi, H.S.; Khan, M.A.; Bhaumik, R.; Dwivedi, Y.; Pandey, G.N. Alteration of cyclic-AMP response element binding protein in the postmortem brain of subjects with bipolar disorder and schizophrenia. J. Affect. Disord. 2014, 15, 326. [Google Scholar] [CrossRef]
- McGirr, A.; Lipina, T.V.; Mun, H.S.; Georgiou, J.; Al-Amri, A.H.; Ng, E.; Zhai, D.; Elliott, C.; Cameron, R.T.; Mullins, J.G.; et al. Specific inhibition of phosphodiesterase-4B results in anxiolysis and facilitates memory acquisition. Neuropsychopharmacology 2016, 14, 1080. [Google Scholar] [CrossRef]
- Lyu, J.W.; Yuan, B.; Cheng, T.L.; Qiu, Z.L.; Zhou, W.H. Reciprocal regulation of autism-related genes MeCP2 and PTEN via microRNAs. Sci. Rep. 2016, 6, 20392. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.-J.; Xu, N.; Kong, L.; Sun, S.-C.; Xu, X.-F.; Jia, M.-Z.; Wang, Y.; Chen, Z.-Y. The antidepressant roles of Wnt2 and Wnt3 in stress-induced depression-like behaviors. Transl. Psychiatry 2016, 6, e892. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Ying, Z.; Li, Z.; Sun, S.C.; Xu, X.F.; Jia, M.Z.; Wang, Y.; Chen, Z.Y. Danzhi Xiaoyao powder promotes neuronal regeneration by downregulating Notch signaling pathway in the treatment of generalized anxiety disorder. Front. Pharmacol. 2021, 12, 772576. [Google Scholar] [CrossRef]
- Steine, I.M.; Zayats, T.; Stansberg, C.; Pallesen, S.; Mrdalj, J.; Håvik, B.; Soulé, J.; Haavik, J.; Milde, A.M.; Skrede, S.; et al. Implication of NOTCH1 gene in susceptibility to anxiety and depression among sexual abuse victims. Transl. Psychiatry 2016, 6, e977. [Google Scholar] [CrossRef]
- Teunissen, M.W.A.; Lewerissa, E.; van Hugte, E.J.H.; Wang, S.; Ockeloen, C.W.; Koolen, D.A.; Pfundt, R.; Marcelis, C.L.M.; Brilstra, E.; Howe, J.L.; et al. ANK2 loss-of-function variants are associated with epilepsy, and lead to impaired axon initial segment plasticity and hyperactive network activity in hiPSC-derived neuronal networks. Hum. Mol. Genet. 2023, 32, 2373. [Google Scholar] [CrossRef]
- Iossifov, M.; Ronemus, D.; Levy, Z.; Wang, Z.; Hakker, I.; Rosenbaum, J.; Yamrom, B.; Lee, Y.H.; Narzisi, G.; Leotta, A.; et al. De novo gene disruptions in children on the autistic spectrum. Neuron 2012, 74, 285. [Google Scholar] [CrossRef] [PubMed]
- Kawano, S.; Baba, M.; Fukushima, H.; Miura, D.; Hashimoto, H.; Nakazawa, T. Autism-associated ANK2 regulates embryonic neurodevelopment. Biochem. Biophys. Res. Commun. 2022, 605, 45. [Google Scholar] [CrossRef] [PubMed]
- Łysiak, K.; Łysiak, A.; Maziarz, B.; Przywara, P.; Bogumiłło, N.; Ignacak, J.; Ściurka, K.; Borek, M.; Grzelak, O. Insomnia in the elderly and contemporary treatment methods. Prospects 2024, 22, 74–80. [Google Scholar] [CrossRef]
- Mahfoud, Y.; Talih, F.; Streem, D.; Budur, K. Sleep disorders in substance abusers: How common are they? Psychiatry 2009, 6, 38. [Google Scholar]
- Huhn, A.S.; Ellis, J.D.; Dunn, K.E.; Sholler, D.L.; Tabaschek, P.; Burns, R.; Strain, E.C. Patient-reported sleep outcomes in randomize-controlled trials in persons with substance use disorders: A systematic review. Drug Alcohol Depend. 2022, 237, 109508. [Google Scholar] [CrossRef]
- Huhn, S.; Finan, P.H.; Gamaldo, C.E.; Hammond, A.H.; Umbricht, A.; Bergeria, C.L.; Strain, E.C.; Dunn, K.E. Suvorexant ameliorated sleep disturbance, opioid withdrawal, and craving during a buprenorphine taper. Sci. Transl. Med. 2022, 14, eabn8238. [Google Scholar] [CrossRef]
- Eacret, D.; Veasey, S.C.; Blendy, J.A. Bidirectional relationship between opioids and disrupted sleep: Putative mechanisms. Mol. Pharmacol. 2020, 98, 445–453. [Google Scholar] [CrossRef]
- Eacret, D.; Manduchi, E.; Noreck, J.; Tyner, E.; Fenik, P.; Dunn, A.D.; Schug, J.; Veasey, S.C.; Blendy, J.A. Mu-opioid receptor-expressing neurons in the paraventricular thalamus modulate chronic morphine-induced wake alterations. Transl. Psychiatry 2023, 13, 78. [Google Scholar] [CrossRef] [PubMed]
- Oyefeso, A.; Sedgwick, P.; Ghodse, H. Subjective sleep-wake parameters in treatment-seeking opiate addicts. Drug Alcohol Depend. 1997, 48, 9. [Google Scholar] [CrossRef]
- Stinus, L.; Robert, C.; Karasinski, P.; Limoge, A. Continuous quantitative monitoring of spontaneous opiate withdrawal: Locomotor activity and sleep disorders. Pharmacol. Biochem. Behav. 1998, 59, 83. [Google Scholar] [CrossRef]
- Bering, T.; Carstensen, M.B.; Wortwein, G.; Weikop, P.; Rath, M.F. The circadian oscillator of the cerebral cortex: Molecular, biochemical and behavioral effects of deleting the Arntl clock gene in cortical neurons. Cereb. Cortex 2017, 28, 644. [Google Scholar] [CrossRef]
- Zhang, P.; Moye, L.S.; Southey, B.R.; Dripps, I.; Sweedler, J.V.; Pradhan, A.; Rodriguez-Zas, S.L. Opioid-induced hyperalgesia is associated with dysregulation of circadian rhythm and adaptive immune pathways in the mouse trigeminal ganglia and Nucleus Accumbens. Mol. Neurobiol. 2019, 56, 7929. [Google Scholar] [CrossRef]
- Meijer, J.H.; Ruijs, A.C.; Albus, H.; van de Geest, B.; Duindam, H.; Zwinderman, A.H.; Dahan, A. Fentanyl, a upsilon-opioid receptor agonist, phase shifts the hamster circadian pacemaker. Brain Res. 2000, 868, 135. [Google Scholar] [CrossRef]
- Vansteensel, M.J.; Magnone, M.C.; van Oosterhout, F.; Baeriswyl, S.; Albrecht, U.; Albus, H.; Dahan, A.; Meijer, J.H. The opioid fentanyl affects light input, electrical activity and Per gene expression in the hamster suprachiasmatic nuclei. Eur. J. Neurosci. 2005, 21, 2958. [Google Scholar] [CrossRef]
- Kakar, N.; Goebel, I.; Daud, S.; Nürnberg, G.; Agha, N.; Ahmad, A.; Nürnberg, P.; Kubisch, C.; Ahmad, J.; Borck, G. A homozygous splice site mutation in TRAPPC9 causes intellectual disability and microcephaly. Eur. J. Med. Genet. 2012, 55, 727. [Google Scholar] [CrossRef] [PubMed]
- Tierno, A.; Fiore, P.; Gannon, R.L. Delta opioid inhibition of light-induced phase advances in hamster circadian activity rhythms. Brain Res. 2002, 937, 66. [Google Scholar] [CrossRef] [PubMed]


| Gene | Gene Name | Forward Primer Sequence | Reverse Primer Sequence |
|---|---|---|---|
| PER1 | period circadian clock 1 | 5’-GCT CCT ACC AGC AGA TCA AC-3’ | 5’-GAG GCA CAT TTA CGC TTA GTG-3’ |
| PER2 | period circadian clock 2 | 5’-CAC AAA GCA AAA ATG AAC ACT G-3’ | 5’-CTC TCT GTC CTC CTT CAA AAC-3’ |
| CRY1 | cryptochrome circadian regulator 1 | 5’-CTC CGA TTT GGT TGT TTG TC-3’ | 5’-GAA AAA TTC ACG CCA TAA CAG-3’ |
| BMAL1 | basic helix-loop-helix ARNT like 1 | 5’-GAC AAC GAA CCA GAC AAT GAG-3’ | 5’-GTG CCG AGA AAC ATA TTC CAT AG-3’ |
| CREBBP | CREB binding protein | 5’-GAG ATG ATG GAG GAG GAT TTG-3’ | 5’-CGT TAC TGC TAC TCT CTT CTT C-3’ |
| ACTIN | actin beta (ACTB), mRNA | 5’-TGG CAC CCA GCA CAA TGA A-3’ | 5’-CTA AGT CAT AGT CCG CCT AGA AGC A-3’ |
| Cluster | Genes | Gene Names in the Cluster | Function |
|---|---|---|---|
| Cluster 1 | 32 | AKAP11, ATAD2, C2CD3, CCDC14, CDK5R1, CDK5RAP2, CENPE, CEP135, CEP152, CEP295, CHML, CNTROB, DNAJC5, FGD6, IREB2, KIAA0753, KNL1, MINK1, NCKAP5L, NIN, ODF2, OFD1, PLK2, PLK3, RAB3A, SLC11A2, SLC38A1, SLC7A5, STX3, STXBP5, TFRC, ZRANB3 | Cell cycle regulation |
| Cluster 2 | 42 | ALG10, ALG10B, BMS1, CARMIL1, CCSER2, CD2AP, EDEM3, FAM160A2, FAM160B1, FTSJ3, HOOK1, INF2, ITPR3, NDEL1, NECTIN3, NLE1, NOL10, OBSCN, OGDH, PACS2, PAFAH1B2, PDCD11, PDCD6IP, PDK4, PPAN, PVR, RBM19, RRP1, RRP12, RRP1B, SHROOM3, STT3B, SUN1, SUN2, TBL3, TMEM201, TPM1, TPM4, TRMT2A, ULBP3, UTP15, UTP20 | Nuclear migration and matric anchoring, endonucleolytic cleavage |
| Cluster 3 | 30 | ASB7, CERS5, CERS6, COL4A1, CUL5, EXOC1, EXOC4, EXOC6, EXOC6B, GCC2, GOLGA4, ITGAV, ITGB8, MACF1, MAP11, PAQR3, RAB43, RHOBTB3, SGPL1, SMPD4, TENT4B, TMF1, TRAPP, TRAPPC10, TRAPPC11, TRAPPC2L, TRAPPC6B, TRAPPC8, TRAPPC9, TUT4, ZCCHC14 | Autophagy, Mental retardation |
| Cluster 4 | 20 | AQR, CCDC18, CCDC66, CCDC97, CTTNBP2NL, INTS1, INTS7, KIN, PCNX3, PPIL2, PPME1, PPP2R1B, PPP2R5E, PRPF3, PRPF4B, RBM41, SF3B1, SLMAP, SRRM2, STRN | mRNA splicing, RNA processing |
| Cluster 5 | 42 | AMER1, ANK2, ARIH2, AXIN1, CANX, CELSR1, CHUK, CUL9, DLST, DVL1, FZD1, FZD5, GCDH, HIPK2, IKBKB, IL6ST, ITPR2, KITLG, MATK, NCSTN, NOTCH1, NOTCH2, PELI1, PLXNA1, PLXNB1, PLXNB2, PLXND1, PORCN, RFXAP, SEMA3F, SEMA4D, SEMA4G, SEMA6A, SFRP1, SPTB, SPTBN1, STIM1, TAB1, TAB3, TMOD2, WNT11, ZMYND11 | Semaphorin receptor complex, cellular response to tumor |
| Cluster 6 | 32 | AGO1, AGO2, AHR, ARNT2, ARNTL, ASXL3, CHD3, CHD6, CIPC, CLOCK, CNOT1, CNOT2, DAB2IP, DDX6, DGCR8, EPG5, EZH2, JARID2, KDM6A, KDM6B, MAU2, NR1D1, NR1D2, PDE12, PDS5A, PER1, RAI1, SIM2, SMARCC2, TET1, TXLNA, ZNF512B | CLOC-BMAL regulation, Histone methylation |
| Cluster 7 | 41 | ARL4A, ATG9A, BRD1, BRPF3, BTAF1, CAPRIN1, CCPG1, EIF3B, EIF3C, EIF4G3, EPOR, INO80, KAT6A, LIFR, LSM12, MAPKAP1, MAX, MBTD1, MGA, MLST8, MXD4, PCF11, RB1CC1, RPRD1A, RPRD2, RPTOR, SEPTIN10, SEPTIN12, SLC30A7, SLC39A10, SLC39A6, SRCAP, STAM, STAM2, TAF2, TAF4B, TELO2, TRRAP, TTF2, TYK2, YEATS2 | Histone acetylation, TOR complex |
| Cluster 8 | 49 | ADGRB2, ARID1B, ARID2, BCL9, BCL9L, BICRA, BRD9, CDC73, DCAF16, DCAF4, DCAF8, EHD1, GAN, GATAD1, ICE1, KBTBD8, KLC3, KLC4, KLHL21, LZTR1, MAPK8IP3, NEMP1, NUP155, NUP210, NUP58, PHKA2, PHKG2, POM121, PYGB, PYGO1, PYGO2, RAB11FIP1, RAB11FIP2, RANGAP1, SENP5, SENP6, SIN3B, SMG5, SMG6, SMG7, SNAP47, TGFBRAP1, TLE4, TNRC18, USPL1, VPS18, VPS33A, VPS39, WDTC1 | RNA export from nucleus |
| Cluster 9 | 22 | AKAP10, CABYR, CLUAP1, DOP1A, GLI3, IFT81, MON2, MPP6, NCR3LG1, POLA1, PRIM2, PRKAR1A, PRKAR2A, TCTN2, TEN1, TMEM216, TMEM67, TRAF3IP1, TTC21B, TTC30A, TULP3, VPS26B | DNA replication, cAMP—dependent kinase repression |
| Cluster 10 | 30 | BARD1, BRCA1, BRCC3, BRIP1, CDC27, CREBBP, ERBB2, ESPL1, FBH1, GEN1, GINS1, IFITM | DNA repair and recombination |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fukunaga, M.; Watanabe, S.; Orihara, K.; Yamamoto, N. Casomorphine-10 (CM-10) Peptide Orchestrates Circadian and Neurodevelopmental Gene Clusters via δ-Opioid Receptor Signaling: Insights from Transcriptome Analysis with δ-Opioid Receptor-Expressing HEK293 Cells. Life 2025, 15, 1636. https://doi.org/10.3390/life15101636
Fukunaga M, Watanabe S, Orihara K, Yamamoto N. Casomorphine-10 (CM-10) Peptide Orchestrates Circadian and Neurodevelopmental Gene Clusters via δ-Opioid Receptor Signaling: Insights from Transcriptome Analysis with δ-Opioid Receptor-Expressing HEK293 Cells. Life. 2025; 15(10):1636. https://doi.org/10.3390/life15101636
Chicago/Turabian StyleFukunaga, Moe, Shin Watanabe, Kanami Orihara, and Naoyuki Yamamoto. 2025. "Casomorphine-10 (CM-10) Peptide Orchestrates Circadian and Neurodevelopmental Gene Clusters via δ-Opioid Receptor Signaling: Insights from Transcriptome Analysis with δ-Opioid Receptor-Expressing HEK293 Cells" Life 15, no. 10: 1636. https://doi.org/10.3390/life15101636
APA StyleFukunaga, M., Watanabe, S., Orihara, K., & Yamamoto, N. (2025). Casomorphine-10 (CM-10) Peptide Orchestrates Circadian and Neurodevelopmental Gene Clusters via δ-Opioid Receptor Signaling: Insights from Transcriptome Analysis with δ-Opioid Receptor-Expressing HEK293 Cells. Life, 15(10), 1636. https://doi.org/10.3390/life15101636







