Applications of Modern Cell Therapies: The Latest Data in Ophthalmology
Abstract
1. Introduction
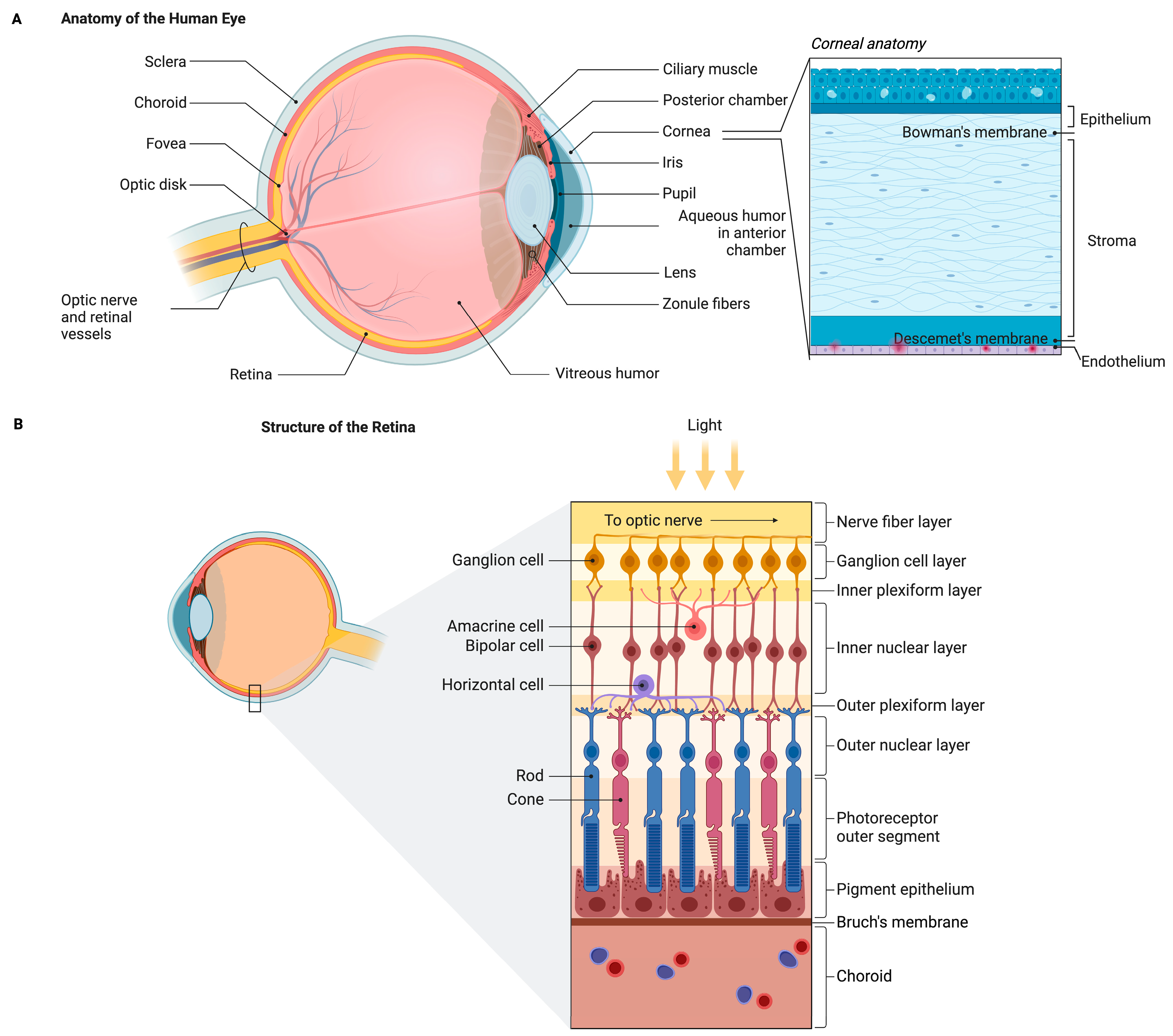
2. Anatomy of the Eye and Its Physiology
2.1. Anatomy of the Eye
2.2. Brief Physiology of the Eye
3. Cellular Therapies in Ophthalmology
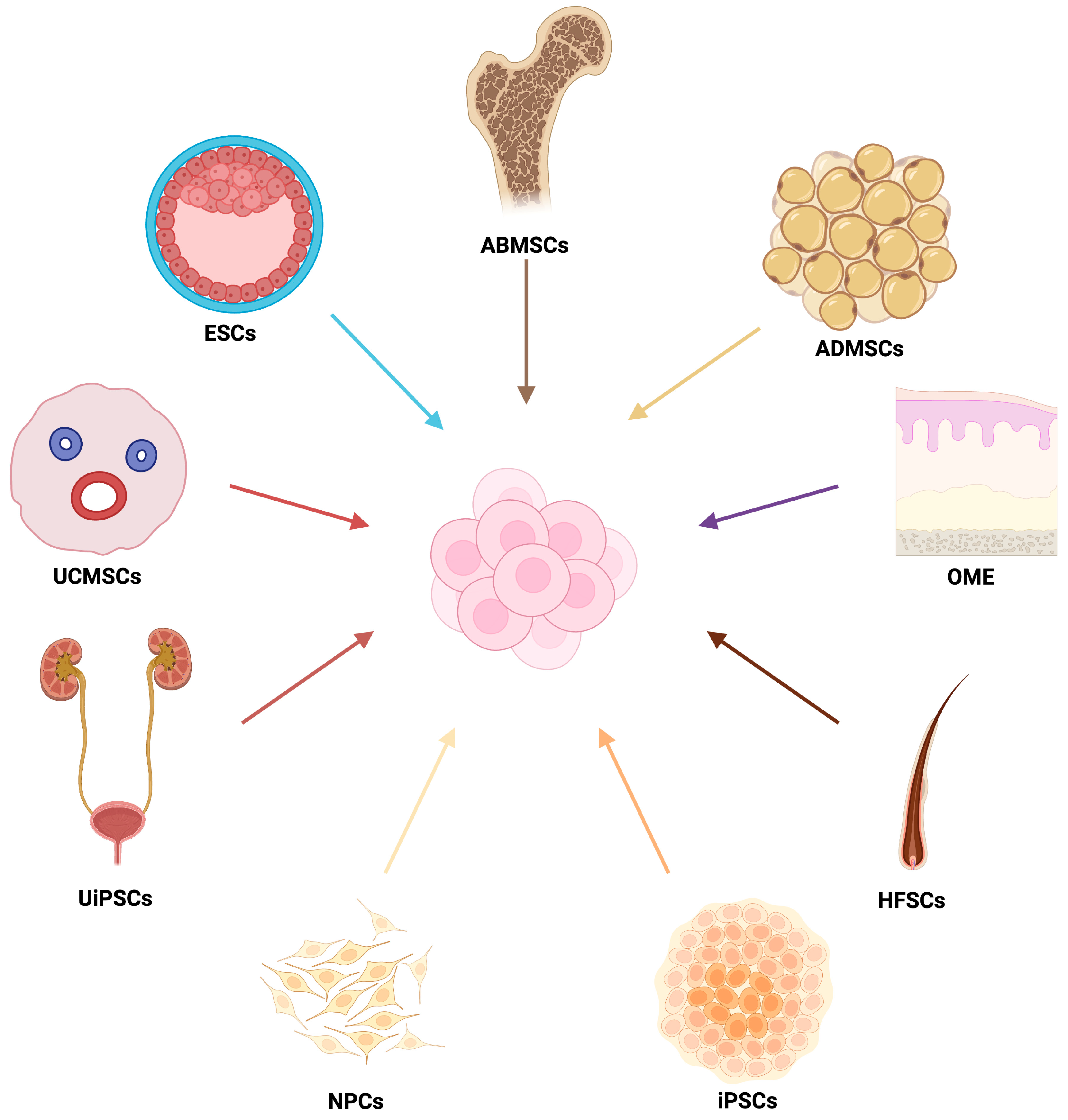
3.1. Types and Strategies of Cell Therapies
3.2. Parthenogenetic Stem Cells
3.3. Mechanisms of Stem Cell Integration in the Eye
3.4. Emerging Immunomodulatory Strategies in Ocular Cell Therapy
3.4.1. Gene-Engineering of Immune-Evasive Grafts
3.4.2. Biomaterial Scaffolds and Engineered Niches for Local Immune Modulation
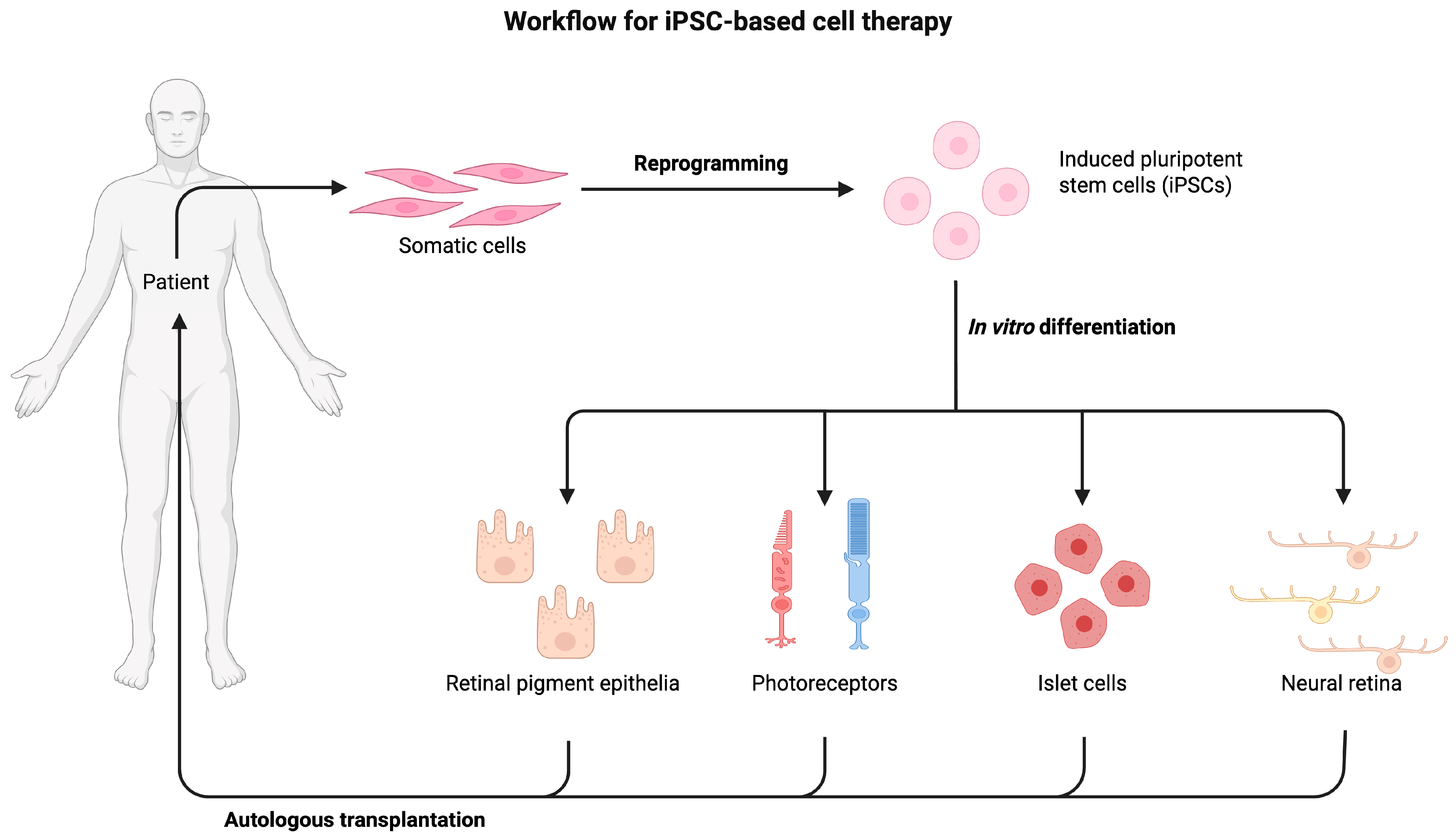
4. Cell Therapy
4.1. Autologous Stem Cell Therapy
4.2. Allogeneic Stem Cell Therapy
4.3. Combine Cellular Encapsulation and Release EVs
4.4. D Bioprinting for Ocular Therapies
5. Imaging Tools for Monitoring Graft Survival and Integration
6. Biomarkers and Functional Endpoints in Retinal Cell Transplantation
6.1. Biomarkers of Graft Integration and Rejection
6.2. Limitations of Best-Corrected Visual Acuity (BCVA)
6.3. Alternative Functional Vision Metrics
7. Applications in Ophthalmologic Diseases
7.1. Corneal Diseases
7.2. Applications for Glaucoma and Optic Neuropathy
7.3. Clinical Applications for Retinal Diseases
7.3.1. Cell Therapy of AMD
7.3.2. Cell Therapy of Retinitis Pigmentosa
7.3.3. Cell Therapy of MacTel
7.3.4. Cell Therapy of Stargardt
7.3.5. Cell Therapy of DR
7.3.6. Stem Cell Therapy for Inherited Retinal Diseases
7.3.7. Stem Cell Therapy for Limbal Stem Cell Deficiency and Corneal Opacification
8. Safety and Adverse Events in Ocular Cell Therapy
| Technique | Target/Delivery Method | Challenges/Risks | Impact on Therapeutic Outcome |
|---|---|---|---|
| Subretinal | Transplantation of hESC-derived RPE cells to the RPE and outer retina. Injection (e.g., 150 μL cell suspension) via cannula into the subretinal space [201] | -Highly invasive; requires pars plana vitrectomy (PPV). -Potential complications: vitreous loss, retinal detachment, retinal hemorrhage, retinal tear, atrophy. -Limited spread of injectate may restrict local efficacy [201] | -Enables localized therapy with potential photoreceptor rescue. -Subretinal space is immune-privileged, favoring graft survival. -Clinical studies reported BCVA improvement in multiple eyes and enhanced vision-related quality of life. -Transplantation in the “transition zone” optimizes integration between atrophic and healthy retina. |
| Intravitreal | Delivery of drugs, cells, or gene therapies directly into the vitreous cavity. Widely used for DME and neovascular AMD [201] | -Requires frequent injections due to the short half-life of agents. -Risk of endophthalmitis, retinal detachment, and vitreous hemorrhage. -MSC delivery may trigger pro-inflammatory effects and proliferative vitreoretinopathy (PVR) [201] | -Provides high bioavailability to retina/vitreous, bypassing some barriers. -Rapid onset of therapeutic action. -MSCs in glaucoma trials showed mixed results: generally, no functional improvement and rare severe complications (e.g., PVR) [148] |
| Suprachoroidal | Targeted delivery of drugs, genes, or cells (e.g., ADMSCs/UCMSCs) into the suprachoroidal space (between sclera and choroid) using microneedles (<1 mm) [202] | -Not immune-privileged; potential local inflammation with AAV vectors or cell therapy. -Technique optimization required (volume, viscosity, injection angle). -Mild to moderate AEs reported (e.g., pain, subconjunctival hemorrhage). | -Enables precise targeting of RPE, retina, and choroid, bypassing ILM and vitreous barriers. - Prolonged duration of action. -MSC/ADMSC implantation demonstrated improved visual acuity and visual field in degenerative diseases (AMD, Stargardt, RP). -Reduced anterior segment exposure lowers the risk of cataract and IOP elevation compared with IVT [201] |
9. Future Directions of Cell Therapy in Ophthalmology
10. Challenges and Limitations of Cell Therapy
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADMSCs | Allogeneic adipose-derived mesenchymal stem cells |
| ADRCs | Adipose-derived regenerative cells |
| AMD | Age-related macular degeneration |
| AI | Artificial intelligence |
| BCVA | Best-corrected visual acuity |
| BMMNCs | Bone marrow mononuclear cells |
| CAD | Computer-aided design |
| cGVHD | Chronic graft-versus-host disease |
| CNTF | Ciliary neurotrophic factor |
| DED | Dry eye disease |
| DR | Diabetic retinopathy |
| ECT | Encapsulated cell technology |
| ESCs | Embryonic stem cells |
| EVs | Extracellular vesicles |
| GA | Geographic atrophy |
| hAECs | Human amniotic epithelial cells |
| hESC-RPE | Human embryonic stem cell-derived retinal pigment epithelium |
| hESCs | Human embryonic stem cells |
| hUTCs | Human umbilical tissue-derived cells |
| IRDs | Inherited retinal dystrophies |
| iPSCs | Induced pluripotent stem cells |
| LSCD | Limbal stem cell deficiency |
| LSCs | Limbal stem cells |
| MD | Macular degeneration |
| MSCs | Mesenchymal stem cells |
| POAG | Primary open-angle glaucoma |
| PSCs | Pluripotent stem cells |
| RGCs | Retinal ganglion cells |
| RP | Retinitis pigmentosa |
| RPE | Retinal pigment epithelium |
| RPCs | Retinal progenitor cells |
| SCT | Stem cell therapy |
References
- Pesudovs, K.; Lansingh, V.C.; Kempen, J.H.; Tapply, I.; Fernandes, A.G.; Cicinelli, M.V.; Arrigo, A.; Leveziel, N.; Resnikoff, S.; Taylor, H.R.; et al. Global Estimates on the Number of People Blind or Visually Impaired by Cataract: A Meta-Analysis from 2000 to 2020. Eye 2024, 38, 2156–2172. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, M.; Hassan, Y.; Vemana, P.P.S.B.; Pattanashetty, M.S.B.; Abdin, Z.U.; Siddiqui, H.F. Age-Related Macular Degeneration: An Exponentially Emerging Imminent Threat of Visual Impairment and Irreversible Blindness. Cureus 2023, 15, e39624. [Google Scholar] [CrossRef]
- Tham, Y.C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.Y. Global Prevalence of Glaucoma and Projections of Glaucoma Burden through 2040: A Systematic Review and Meta-Analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef]
- Gain, P.; Jullienne, R.; He, Z.; Aldossary, M.; Acquart, S.; Cognasse, F.; Thuret, G. Global Survey of Corneal Transplantation and Eye Banking. JAMA Ophthalmol. 2016, 134, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Cross, N.; van Steen, C.; Zegaoui, Y.; Satherley, A.; Angelillo, L. Retinitis Pigmentosa: Burden of Disease and Current Unmet Needs. Clin. Ophthalmol. 2022, 16, 1993. [Google Scholar] [CrossRef]
- Khan, H.; Aziz, A.A.; Sulahria, H.; Khan, H.; Ahmed, A.; Choudhry, N.; Narayanan, R.; Danzig, C.; Khanani, A.M. Emerging Treatment Options for Geographic Atrophy (GA) Secondary to Age-Related Macular Degeneration. Clin. Ophthalmol. 2023, 17, 321. [Google Scholar] [CrossRef] [PubMed]
- Chaibakhsh, S.; Azimi, F.; Shoae-Hassani, A.; Niknam, P.; Ghamari, A.; Dehghan, S.; Nilforushan, N. Evaluating the Impact of Mesenchymal Stem Cell Therapy on Visual Acuity and Retinal Nerve Fiber Layer Thickness in Optic Neuropathy Patients: A Comprehensive Systematic Review and Meta-Analysis. BMC Ophthalmol. 2024, 24, 316. [Google Scholar] [CrossRef]
- Armitage, W.J.; Goodchild, C.; Griffin, M.D.; Gunn, D.J.; Hjortdal, J.; Lohan, P.; Murphy, C.C.; Pleyer, U.; Ritter, T.; Tole, D.M.; et al. High-Risk Corneal Transplantation: Recent Developments and Future Possibilities. Transplantation 2019, 103, 2468. [Google Scholar] [CrossRef]
- Gurnani, B.; Czyz, C.N.; Mahabadi, N.; Havens, S.J. Corneal Graft Rejection. In Mastering Corneal Surgery: Recent Advances and Current Techniques; CRC Press: Boca Raton, FL, USA, 2023; pp. 109–117. [Google Scholar] [CrossRef]
- Radu, M.; Brănișteanu, D.C.; Pirvulescu, R.A.; Dumitrescu, O.M.; Ionescu, M.A.; Zemba, M. Exploring Stem-Cell-Based Therapies for Retinal Regeneration. Life 2024, 14, 668. [Google Scholar] [CrossRef]
- Wu, K.Y.; Dhaliwal, J.K.; Sasitharan, A.; Kalevar, A. Cell Therapy for Retinal Degenerative Diseases: Progress and Prospects. Pharmaceutics 2024, 16, 1299. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, G.; Traverso, C.E.; Franzi, A.T.; Zingirian, M.; Cancedda, R.; De Luca, M. Long-Term Restoration of Damaged Corneal Surfaces with Autologous Cultivated Corneal Epithelium. Lancet 1997, 349, 990–993. [Google Scholar] [CrossRef]
- Keivyon, K.R.; Tseng, S.C.G. Limbal Autograft Transplantation for Ocular Surface Disorders. Ophthalmology 1989, 96, 709–723. [Google Scholar] [CrossRef] [PubMed]
- Abbott, A. Behind the Scenes of the World’s First Commercial Stem-Cell Therapy. Nature 2015. [Google Scholar] [CrossRef]
- Ajgaonkar, B.S.; Kumaran, A.; Kumar, S.; Jain, R.D.; Dandekar, P.P. Cell-Based Therapies for Corneal and Retinal Disorders. Stem Cell Rev. Rep. 2023, 19, 2650–2682. [Google Scholar] [CrossRef]
- Ajekiigbe, V.O.; Agbo, C.E.; Ogieuhi, I.J.; Anthony, C.S.; Adewole, O.A.; Ahmed, B.; Akingbola, A.; Nwankwo, C.K.; Kayode, A.T.; Chima, U.E.; et al. Innovative Approaches to Treatment of Eye Diseases: Advances in Stem Cell Therapy Use in Ophthalmology. Int. Ophthalmol. 2025, 45, 113. [Google Scholar] [CrossRef]
- Hussen, B.M.; Taheri, M.; Yashooa, R.K.; Abdullah, G.H.; Abdullah, S.R.; Kheder, R.K.; Mustafa, S.A. Revolutionizing Medicine: Recent Developments and Future Prospects in Stem-Cell Therapy. Int. J. Surg. 2024, 110, 8002. [Google Scholar] [CrossRef]
- Chehelgerdi, M.; Behdarvand Dehkordi, F.; Chehelgerdi, M.; Kabiri, H.; Salehian-Dehkordi, H.; Abdolvand, M.; Salmanizadeh, S.; Rashidi, M.; Niazmand, A.; Ahmadi, S.; et al. Exploring the Promising Potential of Induced Pluripotent Stem Cells in Cancer Research and Therapy. Mol. Cancer 2023, 22, 189. [Google Scholar] [CrossRef]
- Lombardo, M.; Serrao, S.; Lombardo, G. Challenges in Age-Related Macular Degeneration: From Risk Factors to Novel Diagnostics and Prevention Strategies. Front. Med. 2022, 9, 887104. [Google Scholar] [CrossRef]
- Zhang, S.; Ren, J.; Chai, R.; Yuan, S.; Hao, Y. Global Burden of Low Vision and Blindness Due to Age-Related Macular Degeneration from 1990 to 2021 and Projections for 2050. BMC Public Health 2024, 24, 3510. [Google Scholar] [CrossRef]
- Land, M.F. The Human Eye: Structure and Function. Nat. Med. 1999, 5, 1229. [Google Scholar] [CrossRef] [PubMed]
- Pechnikova, N.A.; Poimenidou, M.; Iliadis, I.; Zafeiriou-Chatziefraimidou, M.; Iaremenko, A.V.; Yaremenko, T.V.; Domvri, K.; Yaremenko, A.V. Pre-Clinical and Clinical Advances in Gene Therapy of X-Linked Retinitis Pigmentosa: Hope on the Horizon. J. Clin. Med. 2025, 14, 898. [Google Scholar] [CrossRef]
- Sheardown, L.; Hicks, E.A.; Sheardown, H. Anatomy and Physiology of the Eye. In Ophthalmic Biomaterials; Royal Society of Chemistry: London, UK, 2025; pp. 1–12. [Google Scholar] [CrossRef]
- Nava, A.S.L.d.; Somani, A.N.; Salini, B. Physiology, Vision; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Bartakova, A.; Kunzevitzky, N.J.; Goldberg, J.L. Regenerative Cell Therapy for Corneal Endothelium. Curr. Ophthalmol. Rep. 2014, 2, 81–90. [Google Scholar] [CrossRef] [PubMed]
- West, E.L.; Ribeiro, J.; Ali, R.R. Development of Stem Cell Therapies for Retinal Degeneration. Cold Spring Harb. Perspect. Biol. 2020, 12, a035683. [Google Scholar] [CrossRef]
- Giblin, J.P.; Comes, N.; Strauss, O.; Gasull, X. Ion Channels in the Eye: Involvement in Ocular Pathologies. Adv. Protein Chem. Struct. Biol. 2016, 104, 157–231. [Google Scholar] [CrossRef]
- Haider, K.H. Handbook of Stem Cell Applications; Springer: Singapore, 2023; pp. 65–71. [Google Scholar] [CrossRef]
- Mahla, R.S.; Mukherjee, A.K.; Amin, S.; Jainarayanan, A.; Mouroug-Anand, N.; Nandakumar, A.; Prasad, A.D. Stem Cells Application in Eye Regeneration and Restoration of Vision. In Handbook of Stem Cell Applications; Springer: Singapore, 2023; pp. 1–31. [Google Scholar] [CrossRef]
- Uyama, H.; Mandai, M.; Takahashi, M. Stem-Cell-Based Therapies for Retinal Degenerative Diseases: Current Challenges in the Establishment of New Treatment Strategies. Dev. Growth Differ. 2021, 63, 59–71. [Google Scholar] [CrossRef]
- Chakrabarty, K.; Shetty, R.; Ghosh, A. Corneal Cell Therapy: With IPSCs, It Is No More a Far-Sight. Stem Cell Res. Ther. 2018, 9, 287. [Google Scholar] [CrossRef]
- Niu, Y.; Ji, J.; Yao, K.; Fu, Q. Regenerative Treatment of Ophthalmic Diseases with Stem Cells: Principles, Progress, and Challenges. Adv. Ophthalmol. Pract. Res. 2024, 4, 52–64. [Google Scholar] [CrossRef]
- Zhou, J.; Benito-Martin, A.; Mighty, J.; Chang, L.; Ghoroghi, S.; Wu, H.; Wong, M.; Guariglia, S.; Baranov, P.; Young, M.; et al. Retinal Progenitor Cells Release Extracellular Vesicles Containing Developmental Transcription Factors, MicroRNA and Membrane Proteins. Sci. Rep. 2018, 8, 2823. [Google Scholar] [CrossRef] [PubMed]
- Chopra, P.; Fatima, A.; Mohapatra, S.; Murugaiyan, K.; Vemuganti, G.K.; Rengan, A.K.; Watson, S.L.; Singh, V.; Basu, S.; Singh, S. Extracellular Vesicles in Dry Eye Disease and Sjögren’s Syndrome: A Systematic Review on Their Diagnostic and Therapeutic Role. Surv. Ophthalmol. 2025, 70, 499–515. [Google Scholar] [CrossRef]
- Levi, S.R.; Ryu, J.; Liu, P.K.; Tsang, S.H. Precision Medicine Trials in Retinal Degenerations. Annu. Rev. Vis. Sci. 2021, 7, 851–865. [Google Scholar] [CrossRef] [PubMed]
- Lanza, R.; Russell, D.W.; Nagy, A. Engineering Universal Cells That Evade Immune Detection. Nat. Rev. Immunol. 2019, 19, 723–733. [Google Scholar] [CrossRef]
- Kimbrel, E.A.; Lanza, R. Next-Generation Stem Cells—Ushering in a New Era of Cell-Based Therapies. Nat. Rev. Drug Discov. 2020, 19, 463–479. [Google Scholar] [CrossRef]
- Mannino, G.; Russo, C.; Longo, A.; Anfuso, C.D.; Lupo, G.; Furno, D.L.; Giuffrida, R.; Giurdanella, G. Potential Therapeutic Applications of Mesenchymal Stem Cells for the Treatment of Eye Diseases. World J. Stem Cells 2021, 13, 632–644. [Google Scholar] [CrossRef] [PubMed]
- Puri, M.C.; Nagy, A. Concise Review: Embryonic Stem Cells versus Induced Pluripotent Stem Cells: The Game Is On. Stem Cells 2012, 30, 10–14. [Google Scholar] [CrossRef]
- Cho, C.; Duong, T.T.; Mills, J.A. A Mini Review: Moving IPSC-Derived Retinal Subtypes Forward for Clinical Applications for Retinal Degenerative Diseases. Adv. Exp. Med. Biol. 2019, 1185, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Bose, D.; Maminishkis, A.; Bharti, K. Retinal Pigment Epithelium Replacement Therapy for Age-Related Macular Degeneration—Are We There Yet? Annu. Rev. Pharmacol. Toxicol. 2020, 60, 553. [Google Scholar] [CrossRef]
- Coco-Martin, R.M.; Pastor-Idoate, S.; Pastor, J.C. Cell Replacement Therapy for Retinal and Optic Nerve Diseases: Cell Sources, Clinical Trials and Challenges. Pharmaceutics 2021, 13, 865. [Google Scholar] [CrossRef]
- Puertas-Neyra, K.; Usategui-Martín, R.; Coco, R.M.; Fernandez-Bueno, I. Intravitreal Stem Cell Paracrine Properties as a Potential Neuroprotective Therapy for Retinal Photoreceptor Neurodegenerative Diseases. Neural Regen. Res. 2020, 15, 1631–1638. [Google Scholar] [CrossRef]
- Liu, X.; Chen, F.; Chen, Y.; Lu, H.; Lu, X.; Peng, X.; Kaplan, H.J.; Dean, D.C.; Gao, L.; Liu, Y. Paracrine Effects of Intraocularly Implanted Cells on Degenerating Retinas in Mice. Stem Cell Res. Ther. 2020, 11, 142. [Google Scholar] [CrossRef]
- Froger, N.; Matonti, F.; Roubeix, C.; Forster, V.; Ivkovic, I.; Brunel, N.; Baudouin, C.; Sahel, J.A.; Picaud, S. VEGF Is an Autocrine/Paracrine Neuroprotective Factor for Injured Retinal Ganglion Neurons. Sci. Rep. 2020, 10, 12409. [Google Scholar] [CrossRef] [PubMed]
- Rohowetz, L.J.; Koulen, P. Stem Cell-Derived Retinal Pigment Epithelium Cell Therapy: Past and Future Directions. Front. Cell Dev. Biol. 2023, 11, 1098406. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Huang, S.S.; Lingam, G.; Kai, D.; Su, X.; Liu, Z. Advances in Retinal Pigment Epithelial Cell Transplantation for Retinal Degenerative Diseases. Stem Cell Res. Ther. 2024, 15, 390. [Google Scholar] [CrossRef] [PubMed]
- Dehghan, S.; Mirshahi, R.; Shoae-Hassani, A.; Naseripour, M. Human-Induced Pluripotent Stem Cells-Derived Retinal Pigmented Epithelium, a New Horizon for Cells-Based Therapies for Age-Related Macular Degeneration. Stem Cell Res. Ther. 2022, 13, 217. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.W. Ocular Immune Privilege and Transplantation. Front. Immunol. 2016, 7, 37. [Google Scholar] [CrossRef]
- Perez, V.L.; Mousa, H.M.; Miyagishima, K.J.; Reed, A.A.; Su, A.J.A.; Greenwell, T.N.; Washington, K.M. Retinal Transplant Immunology and Advancements. Stem Cell Rep. 2024, 19, 817–829. [Google Scholar] [CrossRef]
- Karamouzis, M.V.; Konstantinopoulos, P.A.; Papavassiliou, A.G. Roles of CREB-Binding Protein (CBP)/P300 in Respiratory Epithelium Tumorigenesis. Cell Res. 2007, 17, 324–332. [Google Scholar] [CrossRef]
- Hao, J.; Zhu, W.W.; Sheng, C.; Yu, Y.; Zhou, Q. Human Parthenogenetic Embryonic Stem Cells: One Potential Resource for Cell Therapy. Sci. China C Life Sci. 2009, 52, 599–602. [Google Scholar] [CrossRef]
- Mai, Q.; Yu, Y.; Li, T.; Wang, L.; Chen, M.J.; Huang, S.Z.; Zhou, C.; Zhou, Q. Derivation of Human Embryonic Stem Cell Lines from Parthenogenetic Blastocysts. Cell Res. 2007, 17, 1008–1019. [Google Scholar] [CrossRef]
- Steinbrook, R. Egg Donation and Human Embryonic Stem-Cell Research. N. Engl. J. Med. 2006, 354, 324–326. [Google Scholar] [CrossRef]
- Kim, K.; Lerou, P.; Yabuuchi, A.; Lengerke, C.; Ng, K.; West, J.; Kirby, A.; Daly, M.J.; Daley, G.Q. Histocompatible Embryonic Stem Cells by Parthenogenesis. Science 2007, 315, 482–486. [Google Scholar] [CrossRef]
- Tseng, S.C.G.; He, H.; Zhang, S.; Chen, S.Y. Niche Regulation of Limbal Epithelial Stem Cells: Relationship between Inflammation and Regeneration. Ocul. Surf. 2016, 14, 100. [Google Scholar] [CrossRef]
- Soleimani, M.; Cheraqpour, K.; Koganti, R.; Baharnoori, S.M.; Djalilian, A.R. Concise Review: Bioengineering of Limbal Stem Cell Niche. Bioengineering 2023, 10, 111. [Google Scholar] [CrossRef]
- Du, Y.; Xia, Y. Retinal Pigment Epithelium Phagocytosis and Retinal Degenerative Diseases. Aging Dis. 2025, Online ahead of print. [Google Scholar] [CrossRef]
- Kwon, W.; Freeman, S.A. Phagocytosis by the Retinal Pigment Epithelium: Recognition, Resolution, Recycling. Front. Immunol. 2020, 11, 604205. [Google Scholar] [CrossRef]
- Ishida, M.; Sugita, S.; Makabe, K.; Fujii, S.; Futatsugi, Y.; Kamao, H.; Yamasaki, S.; Sakai, N.; Maeda, A.; Mandai, M.; et al. A ROCK Inhibitor Promotes Graft Survival during Transplantation of IPS-Cell-Derived Retinal Cells. Int. J. Mol. Sci. 2021, 22, 3237. [Google Scholar] [CrossRef]
- Ishida, M.; Masuda, T.; Sakai, N.; Nakai-Futatsugi, Y.; Kamao, H.; Shiina, T.; Takahashi, M.; Sugita, S. Graft Survival of Major Histocompatibility Complex Deficient Stem Cell-Derived Retinal Cells. Commun. Med. 2024, 4, 187. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.L.; Pindwarawala, M.; Agosto, M.A. Complex N-Glycosylation of MGluR6 Is Required for Trans-Synaptic Interaction with ELFN Adhesion Proteins. J. Biol. Chem. 2024, 300, 107119. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.Y.; Johnson, T.V. The Internal Limiting Membrane: Roles in Retinal Development and Implications for Emerging Ocular Therapies. Exp. Eye Res. 2021, 206, 108545. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Nguyen, S.; Bhattacharya, S. Optic Nerve Regeneration: Potential Treatment Approaches. Curr. Opin. Pharmacol. 2024, 74, 102428. [Google Scholar] [CrossRef]
- Gowrishankar, S.; Smith, M.E.; Creber, N.; Muzaffar, J.; Borsetto, D. Immunosuppression in Stem Cell Clinical Trials of Neural and Retinal Cell Types: A Systematic Review. PLoS ONE 2024, 19, e0304073. [Google Scholar] [CrossRef]
- Kim, J.; Nam, Y.; Jeon, D.; Choi, Y.; Choi, S.J.; Hong, C.P.; Kim, S.; Jung, H.; Park, N.; Sohn, Y.; et al. Generation of Hypoimmunogenic Universal IPS Cells through HLA-Type Gene Knockout. Exp. Mol. Med. 2025, 57, 686–699. [Google Scholar] [CrossRef]
- Rafiei, M.; Chung, J.T.; Chau, Y. Roles of Biomaterials in Modulating the Innate Immune Response in Ocular Therapy. Front. Drug Deliv. 2023, 3, 1077253. [Google Scholar] [CrossRef]
- Pechnikova, N.A.; Aggeli, A.; Latypova, A.A.; Iaremenko, A.V.; Domvri, K.; Zubarev, I.V.; Liu, C.; Yaremenko, A.V. Implantable Biomaterials for Cancer Immunotherapies. Adv. Funct. Mater. 2024, 35, 2416813. [Google Scholar] [CrossRef]
- Mandai, M.; Watanabe, A.; Kurimoto, Y.; Hirami, Y.; Morinaga, C.; Daimon, T.; Fujihara, M.; Akimaru, H.; Sakai, N.; Shibata, Y.; et al. Autologous Induced Stem-Cell–Derived Retinal Cells for Macular Degeneration. N. Engl. J. Med. 2017, 376, 1038–1046. [Google Scholar] [CrossRef]
- Kuroda, T.; Yasuda, S.; Kusakawa, S.; Hirata, N.; Kanda, Y.; Suzuki, K.; Takahashi, M.; Nishikawa, S.I.; Kawamata, S.; Sato, Y. Highly Sensitive In Vitro Methods for Detection of Residual Undifferentiated Cells in Retinal Pigment Epithelial Cells Derived from Human IPS Cells. PLoS ONE 2012, 7, e37342. [Google Scholar] [CrossRef]
- Soleimani, M.; Masoumi, A.; Momenaei, B.; Cheraqpour, K.; Koganti, R.; Chang, A.Y.; Ghassemi, M.; Djalilian, A.R. Applications of Mesenchymal Stem Cells in Ocular Surface Diseases: Sources and Routes of Delivery. Expert Opin. Biol. Ther. 2023, 23, 509–525. [Google Scholar] [CrossRef] [PubMed]
- Bhujel, B.; Oh, S.-H.; Kim, C.-M.; Yoon, Y.-J.; Kim, Y.-J.; Chung, H.-S.; Ye, E.-A.; Lee, H.; Kim, J.-Y.; Bhujel, B.; et al. Mesenchymal Stem Cells and Exosomes: A Novel Therapeutic Approach for Corneal Diseases. Int. J. Mol. Sci. 2023, 24, 10917. [Google Scholar] [CrossRef]
- Schwartz, S.D.; Regillo, C.D.; Lam, B.L.; Eliott, D.; Rosenfeld, P.J.; Gregori, N.Z.; Hubschman, J.P.; Davis, J.L.; Heilwell, G.; Spirn, M.; et al. Human Embryonic Stem Cell-Derived Retinal Pigment Epithelium in Patients with Age-Related Macular Degeneration and Stargardt’s Macular Dystrophy: Follow-up of Two Open-Label Phase 1/2 Studies. Lancet 2015, 385, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Kim, Y.Y.; Han, J.Y.; Kim, S.W.; Kim, H.; Ku, S.Y. Advancements in Human Embryonic Stem Cell Research: Clinical Applications and Ethical Issues. Tissue Eng. Regen. Med. 2024, 21, 379–394. [Google Scholar] [CrossRef]
- Zhou, W.; Chai, Y.; Lu, S.; Yang, Q.; Tang, L.; Zhou, D. Advances in the Study of Tissue-Engineered Retinal Pigment Epithelial Cell Sheets. Regen. Ther. 2024, 27, 419. [Google Scholar] [CrossRef]
- Klymenko, V.; González Martínez, O.G.; Zarbin, M. Recent Progress in Retinal Pigment Epithelium Cell-Based Therapy for Retinal Disease. Stem Cells Transl. Med. 2024, 13, 317. [Google Scholar] [CrossRef]
- Riedl, J.C.; Wasielica-Poslednik, J.; Giers, B.C.; Buonfiglio, F.; Pfeiffer, N.; Musayeva, A.; Gericke, A. Midterm Results after Allogeneic Simple Limbal Epithelial Transplantation from Deceased-donor Eyes in Patients with Persistent Corneal Epithelial Defects Due to Limbal Stem Cell Deficiency. Acta Ophthalmol. 2024, 103, e125. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Y.; Cortina, M.S.; Greiner, M.A.; Kuo, A.N.; Miller, D.D.; Shtein, R.M.; Veldman, P.B.; Yin, J.; Kim, S.J.; Shen, J.F. Outcomes and Complications of Limbal Stem Cell Allograft Transplantation: A Report by the American Academy of Ophthalmology. Ophthalmology 2024, 131, 1121–1131. [Google Scholar] [CrossRef]
- Humayun, M.S.; Clegg, D.O.; Dayan, M.S.; Kashani, A.H.; Rahhal, F.M.; Avery, R.L.; Salehi-Had, H.; Chen, S.; Chan, C.; Palejwala, N.; et al. Long-Term Follow-up of a Phase 1/2a Clinical Trial of a Stem Cell-Derived Bioengineered Retinal Pigment Epithelium Implant for Geographic Atrophy. Ophthalmology 2024, 131, 682–691. [Google Scholar] [CrossRef] [PubMed]
- OpRegen®—Lineage Cell Therapeutics. Available online: https://lineagecell.com/products-pipeline/opregen/?utm_source=chatgpt.com (accessed on 4 October 2025).
- 36-Month Results from Phase 1/2a Clinical Study of RG6501 Released | Macular Degeneration Association. Available online: https://macularhope.org/36-month-results-from-phase-1-2a-clinical-study-of-rg6501-released/ (accessed on 4 October 2025).
- Sakai, D.; Mandai, M.; Hirami, Y.; Yamamoto, M.; Ito, S.i.; Igarashi, S.; Yokota, S.; Uyama, H.; Fujihara, M.; Maeda, A.; et al. Transplant of Induced Pluripotent Stem Cell–Derived Retinal Pigment Epithelium Strips for Macular Degeneration and Retinitis Pigmentosa. Ophthalmol. Sci. 2025, 5, 100770. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Yamada, T.; Koike, C.; Takahashi, M.; Tachibana, M.; Mandai, M. Transplantation of Genome-Edited Retinal Organoids Restores Some Fundamental Physiological Functions Coordinated with Severely Degenerated Host Retinas. Stem Cell Rep. 2025, 20, 102393. [Google Scholar] [CrossRef]
- Soma, T.; Oie, Y.; Takayanagi, H.; Matsubara, S.; Yamada, T.; Nomura, M.; Yoshinaga, Y.; Maruyama, K.; Watanabe, A.; Takashima, K.; et al. Induced Pluripotent Stem-Cell-Derived Corneal Epithelium for Transplant Surgery: A Single-Arm, Open-Label, First-in-Human Interventional Study in Japan. Lancet 2024, 404, 1929–1939. [Google Scholar] [CrossRef]
- Kashani, A.H.; Lebkowski, J.S.; Hinton, D.R.; Zhu, D.; Faynus, M.A.; Chen, S.; Rahhal, F.M.; Avery, R.L.; Salehi-Had, H.; Chan, C.; et al. Survival of an HLA-Mismatched, Bioengineered RPE Implant in Dry Age-Related Macular Degeneration. Stem Cell Rep. 2022, 17, 448. [Google Scholar] [CrossRef]
- Zhang, K.Y.; Nagalingam, A.; Mary, S.; Aguzzi, E.A.; Li, W.; Chetla, N.; Smith, B.; Paulaitis, M.E.; Edwards, M.M.; Quigley, H.A.; et al. Rare Intercellular Material Transfer as a Confound to Interpreting Inner Retinal Neuronal Transplantation Following Internal Limiting Membrane Disruption. Stem Cell Rep. 2023, 18, 2203–2221. [Google Scholar] [CrossRef]
- Zhang, K.; Hopkins, J.J.; Heier, J.S.; Birch, D.G.; Halperin, L.S.; Albini, T.A.; Brown, D.M.; Jaffe, G.J.; Taoj, W.; Williams, G.A. Ciliary Neurotrophic Factor Delivered by Encapsulated Cell Intraocular Implants for Treatment of Geographic Atrophy in Age-Related Macular Degeneration. Proc. Natl. Acad. Sci. USA 2011, 108, 6241–6245. [Google Scholar] [CrossRef]
- Pellegrini, G.; Ardigò, D.; Milazzo, G.; Iotti, G.; Guatelli, P.; Pelosi, D.; De Luca, M. Navigating Market Authorization: The Path Holoclar Took to Become the First Stem Cell Product Approved in the European Union. Stem Cells Transl. Med. 2018, 7, 146–154. [Google Scholar] [CrossRef]
- Calonge, M.; Pérez, I.; Galindo, S.; Nieto-Miguel, T.; López-Paniagua, M.; Fernández, I.; Alberca, M.; García-Sancho, J.; Sánchez, A.; Herreras, J.M. A Proof-of-Concept Clinical Trial Using Mesenchymal Stem Cells for the Treatment of Corneal Epithelial Stem Cell Deficiency. Transl. Res. 2019, 206, 18–40. [Google Scholar] [CrossRef]
- Toshida, H.; Kasahara, T.; Kiriyama, M.; Iwasaki, Y.; Sugita, J.; Ichikawa, K.; Ohta, T.; Miyahara, K. Early Clinical Outcomes of the First Commercialized Human Autologous Ex Vivo Cultivated Oral Mucosal Epithelial Cell Transplantation for Limbal Stem Cell Deficiency: Two Case Reports and Literature Review. Int. J. Mol. Sci. 2023, 24, 8926. [Google Scholar] [CrossRef]
- Aggarwal, S.; Kumari, M.; Bhatnagar, N. Advancements in Keratoplasty: Exploring Newer Techniques and Imaging Modalities for Enhanced Surgical Outcomes. Saudi J. Ophthalmol. 2024, 10, 4103. [Google Scholar] [CrossRef]
- Singh, R.; Gupta, N.; Vanathi, M.; Tandon, R. Corneal Transplantation in the Modern Era. Indian J. Med. Res. 2019, 150, 7. [Google Scholar] [CrossRef]
- Roberts, H.W.; de Benito-Llopis, L. Comparison of Repeat Penetrating Keratoplasty, DSAEK and DMEK for the Management of Endothelial Failure of Previous PK. Eye 2023, 37, 3596–3601. [Google Scholar] [CrossRef]
- Neokleous, A.; Michail, N.; Herodotou, F.; Athanasiadou, A.; Christodoulou, S.; Kola, D.; Panayidou, K.; Hadjilouka, G.; Palioura, S. Long-Term Monitoring of Corneal Grafts Via Anterior Segment OCT Pachymetry Maps. Ophthalmol. Sci. 2025, 5, 100724. [Google Scholar] [CrossRef]
- Kauper, K.; McGovern, C.; Sherman, S.; Heatherton, P.; Rapoza, R.; Stabila, P.; Dean, B.; Lee, A.; Borges, S.; Bouchard, B.; et al. Two-Year Intraocular Delivery of Ciliary Neurotrophic Factor by Encapsulated Cell Technology Implants in Patients with Chronic Retinal Degenerative Diseases. Investig. Ophthalmol. Vis. Sci. 2012, 53, 7484–7491. [Google Scholar] [CrossRef]
- Hoy, S.M. Revakinagene Taroretcel: First Approval. Mol. Diagn. Ther. 2025, 29, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Guan, Y.; Xie, A.; Yan, Z.; Gao, S.; Li, W.; Rao, L.; Chen, X.; Chen, T. Extracellular Vesicles: A Rising Star for Therapeutics and Drug Delivery. J. Nanobiotechnol. 2023, 21, 231. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.; Kim, J.; Kim, M.J.; Yae, C.G.; Kim, K.H.; Kim, H.K. Development of Human Amniotic Epithelial Cell-Derived Extracellular Vesicles as Cell-Free Therapy for Dry Eye Disease. Ocul. Surf. 2024, 34, 370–380. [Google Scholar] [CrossRef]
- Karttunen, J.; Heiskanen, M.; Navarro-Ferrandis, V.; Das Gupta, S.; Lipponen, A.; Puhakka, N.; Rilla, K.; Koistinen, A.; Pitkänen, A. Precipitation-Based Extracellular Vesicle Isolation from Rat Plasma Co-Precipitate Vesicle-Free MicroRNAs. J. Extracell. Vesicles 2019, 8, 1555410. [Google Scholar] [CrossRef]
- Bai, L.; Wang, Y. Mesenchymal Stem Cells-Derived Exosomes Alleviate Senescence of Retinal Pigment Epithelial Cells by Activating PI3K/AKT-Nrf2 Signaling Pathway in Early Diabetic Retinopathy. Exp. Cell Res. 2024, 441, 114170. [Google Scholar] [CrossRef]
- An, W.; Zhang, W.; Qi, J.; Xu, W.; Long, Y.; Qin, H.; Yao, K. Mesenchymal stem cells and mesenchymal stem cell-derived exosomes: A promising strategy for treating retinal degenerative diseases. Mol. Med. 2025, 31, 75. [Google Scholar] [CrossRef]
- Sun, F.; Sun, Y.; Wang, X.; Zhu, J.; Chen, S.; Yu, Y.; Zhu, M.; Xu, W.; Qian, H. Engineered Mesenchymal Stem Cell-Derived Small Extracellular Vesicles for Diabetic Retinopathy Therapy through HIF-1α/EZH2/PGC-1α Pathway. Bioact. Mater. 2024, 33, 444–459. [Google Scholar] [CrossRef]
- Seyedrazizadeh, S.Z.; Poosti, S.; Nazari, A.; Alikhani, M.; Shekari, F.; Pakdel, F.; Shahpasand, K.; Satarian, L.; Baharvand, H. Extracellular Vesicles Derived from Human ES-MSCs Protect Retinal Ganglion Cells and Preserve Retinal Function in a Rodent Model of Optic Nerve Injury. Stem Cell Res. Ther. 2020, 11, 203. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.; Ioannou, N.; Mathew, E.; Tagalakis, A.D.; Lamprou, D.A.; Yu-Wai-Man, C. 3D Printing in Ophthalmology: From Medical Implants to Personalised Medicine. Int. J. Pharm. 2022, 625, 122094. [Google Scholar] [CrossRef]
- Ruiz-alonso, S.; Villate-beitia, I.; Gallego, I.; Lafuente-merchan, M.; Puras, G.; Saenz-del-burgo, L.; Pedraz, J.L. Current Insights into 3D Bioprinting: An Advanced Approach for Eye Tissue Regeneration. Pharmaceutics 2021, 13, 308. [Google Scholar] [CrossRef] [PubMed]
- Isaacson, A.; Swioklo, S.; Connon, C.J. 3D Bioprinting of a Corneal Stroma Equivalent. Exp. Eye Res. 2018, 173, 188–193. [Google Scholar] [CrossRef]
- Larochelle, R.D.; Mann, S.E.; Ifantides, C. 3D Printing in Eye Care. Ophthalmol. Ther. 2021, 10, 733–752. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.Y.; Tabari, A.; Mazerolle, É.; Tran, S.D. Towards Precision Ophthalmology: The Role of 3D Printing and Bioprinting in Oculoplastic Surgery, Retinal, Corneal, and Glaucoma Treatment. Biomimetics 2024, 9, 145. [Google Scholar] [CrossRef] [PubMed]
- Lorber, B.; Hsiao, W.K.; Martin, K.R. Three-Dimensional Printing of the Retina. Curr. Opin. Ophthalmol. 2016, 27, 262–267. [Google Scholar] [CrossRef]
- Yaremenko, A.V.; Melikov, R.O.; Pechnikova, N.A.; Belyaev, I.B.; Ringaci, A.; Yaremenko, T.V.; Mirkasymov, A.B.; Tamgin, A.A.; Rodionov, V.I.; Dolotova, S.M.; et al. Modification of Contact Lenses via Metal-Organic Frameworks for Glaucoma Treatment. Aggregate 2024, 5, e586. [Google Scholar] [CrossRef]
- Sommer, A.C.; Blumenthal, E.Z. Implementations of 3D Printing in Ophthalmology. Graefe’s Arch. Clin. Exp. Ophthalmol. 2019, 257, 1815–1822. [Google Scholar] [CrossRef]
- Ma, X.; Liu, J.; Zhu, W.; Tang, M.; Lawrence, N.; Yu, C.; Gou, M.; Chen, S. 3D Bioprinting of Functional Tissue Models for Personalized Drug Screening and in Vitro Disease Modeling. Adv. Drug Deliv. Rev. 2018, 132, 235–251. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Bu, Y.; Lau, D.S.A.; Lin, Z.; Sun, T.; Lu, W.W.; Lu, S.; Ruan, C.; Chan, C.H.J. Advances in 3D Bioprinting Technology for Functional Corneal Reconstruction and Regeneration. Front. Bioeng. Biotechnol. 2023, 10, 1065460. [Google Scholar] [CrossRef]
- Poomathi, N.; Singh, S.; Prakash, C.; Patil, R.V.; Perumal, P.T.; Barathi, V.A.; Balasubramanian, K.K.; Ramakrishna, S.; Maheshwari, N.U. Bioprinting in Ophthalmology: Current Advances and Future Pathways. Rapid Prototyp. J. 2019, 25, 496–514. [Google Scholar] [CrossRef]
- Zheng, M.; Paulus, Y.M. Multimodal Imaging in Stem Cell Therapy for Retinal Disease. Photonics 2025, 12, 413. [Google Scholar] [CrossRef]
- Jian, Y.; Zawadzki, R.J.; Sarunic, M.V. Adaptive Optics Optical Coherence Tomography for in vivo Mouse Retinal Imaging. J. Biomed. Opt. 2013, 18, 056007. [Google Scholar] [CrossRef]
- Thomas, B.B.; Lin, B.; Martinez-Camarillo, J.C.; Zhu, D.; McLelland, B.T.; Nistor, G.; Keirstead, H.S.; Humayun, M.S.; Seiler, M.J. Co-Grafts of Human Embryonic Stem Cell Derived Retina Organoids and Retinal Pigment Epithelium for Retinal Reconstruction in Immunodeficient Retinal Degenerate Royal College of Surgeons Rats. Front. Neurosci. 2021, 15, 752958. [Google Scholar] [CrossRef]
- Veckeneer, M.; Augustinus, C.; Feron, E.; Schauwvlieghe, P.P.; Ruys, J.; Cosemans, I.; Van Meurs, J. OCT Angiography Documented Reperfusion of Translocated Autologous Full Thickness RPE-Choroid Graft for Complicated Neovascular Age-Related Macular Degeneration. Eye 2017, 31, 1274–1283. [Google Scholar] [CrossRef]
- Nguyen, V.P.; Karoukis, A.J.; Qian, W.; Chen, L.; Perera, N.D.; Yang, D.; Zhang, Q.; Zhe, J.; Henry, J.; Liu, B.; et al. Multimodal Imaging-Guided Stem Cell Ocular Treatment. ACS Nano 2024, 18, 14893–14906. [Google Scholar] [CrossRef] [PubMed]
- Istomina, M.S.; Pechnikova, N.A.; Korolev, D.V.; Pochkayeva, E.I.; Mazing, D.S.; Galagudza, M.M.; Moshnikov, V.A.; Shlyakhto, E.V. ZaiS-Based Colloidal QDs as Fluorescent Labels for Theranostics: Physical Properties, Biodistribution and Biocompatibility. Bull. Russ. State Med. Univ. 2018, 7, 94–101. [Google Scholar] [CrossRef]
- Pechnikova, N.A.; Domvri, K.; Porpodis, K.; Istomina, M.S.; Iaremenko, A.V.; Yaremenko, A.V. Carbon Quantum Dots in Biomedical Applications: Advances, Challenges, and Future Prospects. Aggregate 2024, 6, e707. [Google Scholar] [CrossRef]
- Liu, Y.V.; Sodhi, S.K.; Xue, G.; Teng, D.; Agakishiev, D.; McNally, M.M.; Harris-Bookman, S.; McBride, C.; Konar, G.J.; Singh, M.S. Quantifiable In Vivo Imaging Biomarkers of Retinal Regeneration by Photoreceptor Cell Transplantation. Transl. Vis. Sci. Technol. 2020, 9, 5. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Singh, R.K.; Seiler, M.J.; Nasonkin, I.O. Survival and Functional Integration of Human Embryonic Stem Cell-Derived Retinal Organoids After Shipping and Transplantation into Retinal Degeneration Rats. Stem Cells Dev. 2024, 33, 201–213. [Google Scholar] [CrossRef]
- Kennelly, K.P.; Holmes, T.M.; Wallace, D.M.; O’Farrelly, C.; Keegan, D.J. Early Subretinal Allograft Rejection Is Characterized by Innate Immune Activity. Cell Transpl. 2017, 26, 983–1000. [Google Scholar] [CrossRef]
- Sugita, S.; Makabe, K.; Fujii, S.; Iwasaki, Y.; Kamao, H.; Shiina, T.; Ogasawara, K.; Takahashi, M. Detection of Retinal Pigment Epithelium-Specific Antibody in IPSC-Derived Retinal Pigment Epithelium Transplantation Models. Stem Cell Rep. 2017, 9, 1501–1515. [Google Scholar] [CrossRef]
- McGill, T.J.; Stoddard, J.; Renner, A.M.; Messaoudi, I.; Bharti, K.; Mitalipov, S.; Lauer, A.; Wilson, D.J.; Neuringer, M. Allogeneic IPSC-Derived RPE Cell Graft Failure Following Transplantation into the Subretinal Space in Nonhuman Primates. Investig. Ophthalmol. Vis. Sci. 2018, 59, 1374–1383. [Google Scholar] [CrossRef]
- Cascalho, M.; Platt, J.L. The Immunological Barrier to Xenotransplantation. Immunity 2001, 14, 437–446. [Google Scholar] [CrossRef]
- Anderson, R.S.; Roark, M.; Gilbert, R.; Sumodhee, D. Expert CONsensus on Visual Evaluation in Retinal Disease ManaGEment: The CONVERGE Study. Br. J. Ophthalmol. 2024, 109, e325310. [Google Scholar] [CrossRef]
- Josan, A.S.; Taylor, L.J.; Xue, K.; Cehajic-Kapetanovic, J.; MacLaren, R.E. Ranked Importance of Visual Function Outcome Measures in Choroideremia Clinical Trials. Investig. Ophthalmol. Vis. Sci. 2024, 65, 58. [Google Scholar] [CrossRef]
- Li, Y.S.; Hu, X.; Zhou, F.Y.; Guo, X.; Yang, X.; Liu, R.; Lin, D.; Dai, M.; Wu, K.; Wu, J.; et al. Reduced Contrast Sensitivity Function and Outer Retina Thickness in Convalescent Vogt-Koyanagi-Harada Disease. Eye 2025, 39, 366–372. [Google Scholar] [CrossRef]
- Khaboushan, A.S.; Ebadpour, N.; Moghadam, M.M.J.; Rezaee, Z.; Kajbafzadeh, A.M.; Zolbin, M.M. Cell Therapy for Retinal Degenerative Disorders: A Systematic Review and Three-Level Meta-Analysis. J. Transl. Med. 2024, 22, 227. [Google Scholar] [CrossRef] [PubMed]
- Ho, A.C.; Banin, E.; Barak, A.; Boyer, D.S.; Ehrlich, R.; Jaouni, T.; MacDonald, H.R.; Riemann, C.D.; Telander, D.; Mones, J.; et al. Safety and Efficacy of a Phase 1/2a Clinical Trial of Transplanted Allogeneic Retinal Pigmented Epithelium (RPE, OpRegen) Cells in Advanced Dry Age-Related Macular Degeneration (AMD). Investig. Ophthalmol. Vis. Sci. 2022, 63, 1862. [Google Scholar]
- Banin, E.; Barak, A.; Boyer, D.S.; Ehrlich, R.; Ho, A.; Jaouni, T.; McDonald, R.; Riemann, C.D.; Telander, D.G.; Zhang, M.; et al. Exploratory Optical Coherence Tomography (OCT) Analysis in Patients with Geographic Atrophy (GA) Treated by OpRegen: Results from the Phase 1/2a Trial. Investig. Ophthalmol. Vis. Sci. 2023, 64, 2826. [Google Scholar]
- Schwartz, S.D.; Hubschman, J.P.; Heilwell, G.; Franco-Cardenas, V.; Pan, C.K.; Ostrick, R.M.; Mickunas, E.; Gay, R.; Klimanskaya, I.; Lanza, R. Embryonic Stem Cell Trials for Macular Degeneration: A Preliminary Report. Lancet 2012, 379, 713–720. [Google Scholar] [CrossRef]
- Plaza Reyes, A.; Petrus-Reurer, S.; Antonsson, L.; Stenfelt, S.; Bartuma, H.; Panula, S.; Mader, T.; Douagi, I.; André, H.; Hovatta, O.; et al. Xeno-Free and Defined Human Embryonic Stem Cell-Derived Retinal Pigment Epithelial Cells Functionally Integrate in a Large-Eyed Preclinical Model. Stem Cell Rep. 2016, 6, 9–17. [Google Scholar] [CrossRef]
- Torado, G.J.; Green, H. Quantitative Studies of the Growth of Mouse Embryo Cells in Culture and Their Development into Established Lines. J. Cell Biol. 1963, 17, 299–313. [Google Scholar] [CrossRef] [PubMed]
- Discher, D.E.; Mooney, D.J.; Zandstra, P.W. Growth Factors, Matrices, and Forces Combine and Control Stem Cells. Science 2009, 324, 1673–1677. [Google Scholar] [CrossRef]
- Rama, P.; Matuska, S.; Paganoni, G.; Spinelli, A.; De Luca, M.; Pellegrini, G. Limbal Stem-Cell Therapy and Long-Term Corneal Regeneration. N. Engl. J. Med. 2010, 363, 147–155. [Google Scholar] [CrossRef]
- Ogasawara, T. Regenerative Medicine Products Autologous Cultured Corneal Epithelium “Nepic®” Regeneration of the Corneal Epithelium Using Corneal Epithelial Stem Cells. Drug Deliv. Syst. 2023, 38, 438–444. [Google Scholar] [CrossRef]
- Jurkunas, U.V.; Kaufman, A.R.; Yin, J.; Ayala, A.; Maguire, M.; Samarakoon, L.; Johns, L.K.; Parekh, M.; Li, S.; Gauthier, A.; et al. Cultivated Autologous Limbal Epithelial Cell (CALEC) Transplantation for Limbal Tem Cell Deficiency: A Phase I/II Clinical Trial of the First Xenobiotic-Free, Serum-Free, Antibiotic-Free Manufacturing Protocol Developed in the US. Nat. Commun. 2025, 16, 1607. [Google Scholar] [CrossRef]
- Cabral, J.V.; Voukali, E.; Smorodinova, N.; Balogh, L.; Kolin, V.; Studeny, P.; Netukova, M.; Jirsova, K. Cultivation and Characterization of Oral Mucosal Epithelial Cells on Fibrin Gel in a Xenobiotic-Free Medium for the Treatment of Limbal Stem Cell Deficiency. Exp. Eye Res. 2025, 253, 110300. [Google Scholar] [CrossRef] [PubMed]
- Cabral, J.V.; Jackson, C.J.; Utheim, T.P.; Jirsova, K. Ex Vivo Cultivated Oral Mucosal Epithelial Cell Transplantation for Limbal Stem Cell Deficiency: A Review. Stem Cell Res. Ther. 2020, 11, 301. [Google Scholar] [CrossRef] [PubMed]
- Gaddipati, S.; Muralidhar, R.; Sangwan, V.S.; Mariappan, I.; Vemuganti, G.K.; Balasubramanian, D. Oral Epithelial Cells Transplanted on to Corneal Surface Tend to Adapt to the Ocular Phenotype. Indian J. Ophthalmol. 2014, 62, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Oliva, J.; Bardag-Gorce, F.; Niihara, Y. Clinical Trials of Limbal Stem Cell Deficiency Treated with Oral Mucosal Epithelial Cells. Int. J. Mol. Sci. 2020, 21, 411. [Google Scholar] [CrossRef]
- El Zarif, M.; Alió, J.L.; Alió del Barrio, J.L.; De Miguel, M.P.; Abdul Jawad, K.; Makdissy, N. Corneal Stromal Regeneration: A Review of Human Clinical Studies in Keratoconus Treatment. Front. Med. 2021, 8, 650724. [Google Scholar] [CrossRef]
- El Zarif, M.; Alió, J.L.; Alió Del Barrio, J.L.; Abdul Jawad, K.; Palazón-Bru, A.; Abdul Jawad, Z.; De Miguel, M.P.; Makdissy, N. Corneal Stromal Regeneration Therapy for Advanced Keratoconus: Long-Term Outcomes at 3 Years. Cornea 2021, 40, 741–754. [Google Scholar] [CrossRef]
- Ong, H.S.; Riau, A.K.; Yam, G.H.F.; Yusoff, N.Z.B.M.; Han, E.J.Y.; Goh, T.W.; Lai, R.C.; Lim, S.K.; Mehta, J.S. Mesenchymal Stem Cell Exosomes as Immunomodulatory Therapy for Corneal Scarring. Int. J. Mol. Sci. 2023, 24, 7456. [Google Scholar] [CrossRef]
- Vilela, C.A.P.; Messias, A.; Calado, R.T.; Siqueira, R.C.; Silva, M.J.L.; Covas, D.T.; Paula, J.S. Retinal Function after Intravitreal Injection of Autologous Bone Marrow-Derived Mesenchymal Stromal Cells in Advanced Glaucoma. Doc. Ophthalmol. 2021, 143, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Tamm, E.R.; Schmetterer, L.; Grehn, F. Status and Perspectives of Neuroprotective Therapies in Glaucoma: The European Glaucoma Society White Paper. Cell Tissue Res. 2013, 353, 347–354. [Google Scholar] [CrossRef]
- Weiss, J.N.; Levy, S.; Benes, S.C. Stem Cell Ophthalmology Treatment Study (SCOTS) for Retinal and Optic Nerve Diseases: A Case Report of Improvement in Relapsing Auto-Immune Optic Neuropathy. Neural Regen. Res. 2015, 10, 1507–1515. [Google Scholar] [CrossRef]
- Chang, E.E.; Goldberg, J.L. Glaucoma 2.0: Neuroprotection, Neuroregeneration, Neuroenhancement. Ophthalmology 2012, 119, 979–986. [Google Scholar] [CrossRef]
- Yousefi, S.; Sakai, H.; Murata, H.; Fujino, Y.; Matsuura, M.; Garway-Heath, D.; Weinreb, R.; Asaoka, R. Rates of Visual Field Loss in Primary Open-Angle Glaucoma and Primary Angle-Closure Glaucoma: Asymmetric Patterns. Investig. Ophthalmol. Vis. Sci. 2018, 59, 5717–5725. [Google Scholar] [CrossRef]
- Goldberg, J.L.; Beykin, G.; Satterfield, K.R.; Nuñez, M.; Lam, B.L.; Albini, T.A. Phase I NT-501 Ciliary Neurotrophic Factor Implant Trial for Primary Open-Angle Glaucoma: Safety, Neuroprotection, and Neuroenhancement. Ophthalmol. Sci. 2023, 3, 100298. [Google Scholar] [CrossRef]
- Aisenbrey, S.; Lafaut, B.A.; Szurman, P.; Hilgers, R.D.; Esser, P.; Walter, P.; Bartz-Schmidt, K.U.; Thumann, G. Iris Pigment Epithelial Translocation in the Treatment of Exudative Macular Degeneration: A 3-Year Follow-Up. Arch. Ophthalmol. 2006, 124, 183–188. [Google Scholar] [CrossRef]
- Song, W.K.; Park, K.M.; Kim, H.J.; Lee, J.H.; Choi, J.; Chong, S.Y.; Shim, S.H.; Del Priore, L.V.; Lanza, R. Treatment of Macular Degeneration Using Embryonic Stem Cell-Derived Retinal Pigment Epithelium: Preliminary Results in Asian Patients. Stem Cell Rep. 2015, 4, 860–872. [Google Scholar] [CrossRef]
- Pierson, R.; Orr, S.C.; Bogert, J.; Ho, A.; Malone, T.; Crosby, R.; Mathias, S.; Chang, T. Health-Related Quality of Life in Patients with Moderate to Advanced Dry Age-Related Macular Degeneration: Results from a Phase 1/2a Clinical Trial of CNTO 2476. Investig. Ophthalmol. Vis. Sci. 2016, 57, 5603. [Google Scholar]
- Ho, A.C.; Chang, T.S.; Samuel, M.; Williamson, P.; Willenbucher, R.F.; Malone, T. Experience with a Subretinal Cell-Based Therapy in Patients with Geographic Atrophy Secondary to Age-Related Macular Degeneration. Am. J. Ophthalmol. 2017, 179, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Sugita, S.; Mandai, M.; Hirami, Y.; Takagi, S.; Maeda, T.; Fujihara, M.; Matsuzaki, M.; Yamamoto, M.; Iseki, K.; Hayashi, N.; et al. HLA-Matched Allogeneic IPS Cells-Derived RPE Transplantation for Macular Degeneration. J. Clin. Med. 2020, 9, 2217. [Google Scholar] [CrossRef] [PubMed]
- Takagi, S.; Mandai, M.; Gocho, K.; Hirami, Y.; Yamamoto, M.; Fujihara, M.; Sugita, S.; Kurimoto, Y.; Takahashi, M. Evaluation of Transplanted Autologous Induced Pluripotent Stem Cell-Derived Retinal Pigment Epithelium in Exudative Age-Related Macular Degeneration. Ophthalmol. Retin. 2019, 3, 850–859. [Google Scholar] [CrossRef]
- Soomro, T.; Georgiadis, O.; Coffey, P.J.; da Cruz, L. Safety, Structure and Function Five Years after HESC-RPE Patch Transplantation in Acute Neovascular AMD with Submacular Haemorrhage. Graefe’s Arch. Clin. Exp. Ophthalmol. 2024, 262, 3057–3060. [Google Scholar] [CrossRef]
- Da Cruz, L.; Fynes, K.; Georgiadis, O.; Kerby, J.; Luo, Y.H.; Ahmado, A.; Vernon, A.; Daniels, J.T.; Nommiste, B.; Hasan, S.M.; et al. Phase 1 Clinical Study of an Embryonic Stem Cell–Derived Retinal Pigment Epithelium Patch in Age-Related Macular Degeneration. Nat. Biotechnol. 2018, 36, 328–337. [Google Scholar] [CrossRef]
- Yin, Z.Q.; Liu, Y.; Li, S.; Xu, H.W.; Wang, Y.; Qian, C.; Zhou, Q. Clincal Trial: Subretinal Transplantation of CTS HESC Derived RPE in the Treatment of Wet Age-Related Macular Degeneration (WAMD). Investig. Ophthalmol. Vis. Sci. 2016, 57, 3742. [Google Scholar]
- Li, S.Y.; Liu, Y.; Wang, L.; Wang, F.; Zhao, T.T.; Li, Q.Y.; Xu, H.W.; Meng, X.H.; Hao, J.; Zhou, Q.; et al. A Phase I Clinical Trial of Human Embryonic Stem Cell-Derived Retinal Pigment Epithelial Cells for Early-Stage Stargardt Macular Degeneration: 5-Years’ Follow-Up. Cell Prolif. 2021, 54, e13100. [Google Scholar] [CrossRef]
- Campbell, M.; Doyle, S.L. Current Perspectives on Established and Novel Therapies for Pathological Neovascularization in Retinal Disease. Biochem. Pharmacol. 2019, 164, 321–325. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, Z.; Gu, P. Stem/Progenitor Cell-Based Transplantation for Retinal Degeneration: A Review of Clinical Trials. Cell Death Dis. 2020, 11, 793. [Google Scholar] [CrossRef]
- Tang, Z.; Liu, Y.; Xiang, H.; Dai, X.; Huang, X.; Ju, Y.; Ni, N.; Huang, R.; Gao, H.; Zhang, J.; et al. Bifunctional MXene-Augmented Retinal Progenitor Cell Transplantation for Retinal Degeneration. Adv. Sci. 2023, 10, 2302747. [Google Scholar] [CrossRef]
- Monville, C.; Bertin, S.; Devisme, C.; Brazhnikova, E.; Jaillard, C.; Walter, H.; Plancheron, A.; Jarraya, M.; Bejanariu, A.; Abbas, S.; et al. Phase I/II Open-Label Study of Implantation into One Eye of HESC-Derived RPE in Patients with Retinitis Pigmentosa Due to Monogenic Mutation: First Safety Results. Investig. Ophthalmol. Vis. Sci. 2023, 64, 3829. [Google Scholar]
- Yamasaki, S.; Sugita, S.; Horiuchi, M.; Masuda, T.; Fujii, S.; Makabe, K.; Kawasaki, A.; Hayashi, T.; Kuwahara, A.; Kishino, A.; et al. Low Immunogenicity and Immunosuppressive Properties of Human ESC- and IPSC-Derived Retinas. Stem Cell Rep. 2021, 16, 851–867. [Google Scholar] [CrossRef]
- Aharony, I.; Michowiz, S.; Goldenberg-Cohen, N. The Promise of Stem Cell-Based Therapeutics in Ophthalmology. Neural Regen. Res. 2017, 12, 173–180. [Google Scholar] [CrossRef]
- Hirami, Y.; Mandai, M.; Sugita, S.; Maeda, A.; Maeda, T.; Yamamoto, M.; Uyama, H.; Yokota, S.; Fujihara, M.; Igeta, M.; et al. Safety and Stable Survival of Stem-Cell-Derived Retinal Organoid for 2 Years in Patients with Retinitis Pigmentosa. Cell Stem Cell 2023, 30, 1585–1596.e6. [Google Scholar] [CrossRef]
- Siqueira, R.C. Stem Cell Therapy in Retinal Diseases? Rev. Bras. Hematol. Hemoter. 2012, 34, 222–226. [Google Scholar] [CrossRef]
- Siqueira, R.C.; Messias, A.; Messias, K.; Arcieri, R.S.; Ruiz, M.A.; Souza, N.F.; Martins, L.C.; Jorge, R. Quality of Life in Patients with Retinitis Pigmentosa Submitted to Intravitreal Use of Bone Marrow-Derived Stem Cells (Reticell -Clinical Trial). Stem Cell Res. Ther. 2015, 6, 29. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, R.C.; Messias, A.; Voltarelli, J.C.; Scott, I.U.; Jorge, R. Intravitreal Injection of Autologous Bone Marrow-Derived Mononuclear Cells for Hereditary Retinal Dystrophy: A Phase i Trial. Retina 2011, 31, 1207–1214. [Google Scholar] [CrossRef]
- Sen, S.; de Guimaraes, T.A.C.; Filho, A.G.; Fabozzi, L.; Pearson, R.A.; Michaelides, M. Stem Cell-Based Therapies for Retinal Diseases: Focus on Clinical Trials and Future Prospects. Ophthalmic Genet. 2024, 46, 324–337. [Google Scholar] [CrossRef]
- Satarian, L.; Nourinia, R.; Safi, S.; Kanavi, M.R.; Jarughi, N.; Daftarian, N.; Arab, L.; Aghdami, N.; Ahmadieh, H.; Baharvand, H. Intravitreal Injection of Bone Marrow Mesenchymal Stem Cells in Patients with Advanced Retinitis Pigmentosa; A Safety Study. J. Ophthalmic Vis. Res. 2017, 12, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.T.; Kawai, K.; Abdo, D.; Comanita, L.; Ortin-Martinez, A.; Ueno, Y.; Tsao, E.; Rastgar-Moghadam, A.; Xue, C.; Cui, H.; et al. Transplanted Human Photoreceptors Transfer Cytoplasmic Material but not to the Recipient Mouse Retina. Stem Cell Res. Ther. 2024, 15, 79. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.T.; Ortin-Martinez, A.; Yan, N.E.; Comanita, L.; Gurdita, A.; Pham Truong, V.; Cui, H.; Wallace, V.A.; Shoichet, M.S. Hydrogel Assisted Photoreceptor Delivery Inhibits Material Transfer. Biomaterials 2023, 298, 122140. [Google Scholar] [CrossRef]
- Thanos, C.G.; Bell, W.J.; O’Rourke, P.; Kauper, K.; Sherman, S.; Stabila, P.; Tao, W. Sustained Secretion of Ciliary Neurotrophic Factor to the Vitreous, Using the Encapsulated Cell Therapy-Based NT-501 Intraocular Device. Tissue Eng. 2004, 10, 1617–1622. [Google Scholar] [CrossRef] [PubMed]
- Kauper, K.; Orecchio, L.; Nystuen, A.; Medeiros, K.; Lee, A.; Cavallaro, V.; Duncan, J.L.; Aaberg, T. Continuous Intraocular Drug Delivery Lasting Over a Decade: Ciliary Neurotrophic Factor (CNTF) Secreted from Neurotech’s NT-501 Implanted in Subjects with Retinal Degenerative Disorders. Investig. Ophthalmol. Vis. Sci. 2023, 64, 3680. [Google Scholar]
- Tuekprakhon, A.; Sangkitporn, S.; Trinavarat, A.; Pawestri, A.R.; Vamvanij, V.; Ruangchainikom, M.; Luksanapruksa, P.; Pongpaksupasin, P.; Khorchai, A.; Dambua, A.; et al. ongsri. Intravitreal Autologous Mesenchymal Stem Cell Transplantation: A Non-Randomized Phase I Clinical Trial in Patients with Retinitis Pigmentosa. Stem Cell Res. Ther. 2021, 12, 52. [Google Scholar] [CrossRef]
- Song, D.J.; Bao, X.L.; Fan, B.; Li, G.Y. Mechanism of Cone Degeneration in Retinitis Pigmentosa. Cell Mol. Neurobiol. 2023, 43, 1037–1048. [Google Scholar] [CrossRef]
- Schwartz, S.D.; Tan, G.; Hosseini, H.; Nagiel, A. Subretinal Transplantation of Embryonic Stem Cell–Derived Retinal Pigment Epithelium for the Treatment of Macular Degeneration: An Assessment at 4 Years. Investig. Ophthalmol. Vis. Sci. 2016, 57, ORSFc1–ORSFc9. [Google Scholar] [CrossRef]
- Gu, X.; Yu, X.; Zhao, C.; Duan, P.; Zhao, T.; Liu, Y.; Li, S.; Yang, Z.; Li, Y.; Qian, C.; et al. Efficacy and Safety of Autologous Bone Marrow Mesenchymal Stem Cell Transplantation in Patients with Diabetic Retinopathy. Cell. Physiol. Biochem. 2018, 49, 40–52. [Google Scholar] [CrossRef]
- Santos, G.S.P.; Prazeres, P.H.D.M.; Mintz, A.; Birbrair, A. Role of Pericytes in the Retina. Eye 2018, 32, 483–486. [Google Scholar] [CrossRef]
- Labrador-Velandia, S.; Alonso-Alonso, M.L.; Alvarez-Sanchez, S.; González-Zamora, J.; Carretero-Barrio, I.; Pastor, J.C.; Fernandez-Bueno, I.; Srivastava, G.K. Mesenchymal Stem Cell Therapy in Retinal and Optic Nerve Diseases: An Update of Clinical Trials. World J. Stem Cells 2016, 8, 376. [Google Scholar] [CrossRef]
- Buckland, K.F.; Bobby Gaspar, H. Gene and Cell Therapy for Children—New Medicines, New Challenges? Adv. Drug Deliv. Rev. 2014, 73, 162–169. [Google Scholar] [CrossRef]
- Chen, H.Y.; Lehmann, O.J.; Swaroop, A. Genetics and Therapy for Pediatric Eye Diseases. eBioMedicine 2021, 67, 103360. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xu, N.; Li, J.; Zhao, M.; Huang, L. Stem Cell Therapy for Inherited Retinal Diseases: A Systematic Review and Meta-Analysis. Stem Cell Res. Ther. 2023, 14, 286. [Google Scholar] [CrossRef]
- Jauregui, R.; Cho, G.Y.; Takahashi, V.K.L.; Takiuti, J.T.; Bassuk, A.G.; Mahajan, V.B.; Tsang, S.H. Caring for Hereditary Childhood Retinal Blindness. Asia-Pac. J. Ophthalmol. 2018, 7, 183–191. [Google Scholar] [CrossRef]
- Li, S.; Sun, H.; Chen, L.; Fu, Y. Targeting Limbal Epithelial Stem Cells: Master Conductors of Corneal Epithelial Regeneration from the Bench to Multilevel Theranostics. J. Transl. Med. 2024, 22, 794. [Google Scholar] [CrossRef]
- Thokala, P.; Singh, A.; Kumar Singh, V.; Rathi, V.M.; Basu, S.; Singh, V.; MacNeil, S.; Singh Sangwan, V. Clinical Science Economic, Clinical and Social Impact of Simple Limbal Epithelial Transplantation for Limbal Stem Cell Deficiency. Br. J. Ophthalmol. 2022, 106, 923–928. [Google Scholar] [CrossRef]
- Garg, A.; Goel, K.; Gour, A.; Sapra, M.; Sangwan, V.S.; Tripathi, R.; Tiwari, A. Unveiling the Molecular Mechanisms Underlying the Success of Simple Limbal Epithelial Transplantation (SLET). Cells 2025, 14, 200. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.J.; Myklebust Ernø, I.T.; Ringstad, H.; Tønseth, K.A.; Dartt, D.A.; Utheim, T.P. Simple Limbal Epithelial Transplantation: Current Status and Future Perspectives. Stem Cells Transl. Med. 2020, 9, 316–327. [Google Scholar] [CrossRef]
- Li, Y.; Xu, X.; Wang, L.; Liu, G.; Li, Y.; Wu, X.; Jing, Y.; Li, H.; Wang, G. Senescent Mesenchymal Stem Cells Promote Colorectal Cancer Cells Growth via Galectin-3 Expression. Cell Biosci. 2015, 5, 21. [Google Scholar] [CrossRef]
- Neri, S. Genetic Stability of Mesenchymal Stromal Cells for Regenerative Medicine Applications: A Fundamental Biosafety Aspect. Int. J. Mol. Sci. 2019, 20, 2406. [Google Scholar] [CrossRef]
- Liu, M.; Henick, B.; Cheng, K. Translational Inhalable Extracellular Vesicle-Based MRNA Therapy for the Treatment of Lung Cancer. Clin. Transl. Med. 2025, 15, e70186. [Google Scholar] [CrossRef] [PubMed]
- Xian, B.; Huang, B. The Immune Response of Stem Cells in Subretinal Transplantation. Stem Cell Res. Ther. 2015, 6, 161. [Google Scholar] [CrossRef] [PubMed]
- Intonti, S.; Kokona, D.; Zinkernagel, M.S.; Enzmann, V.; Stein, J.V.; Conedera, F.M. Glia Modulates Immune Responses in the Retina Through Distinct MHC Pathways. Glia 2025, 73, 822–839. [Google Scholar] [CrossRef]
- Honda, N.; Watanabe, Y.; Tokuoka, Y.; Hanajima, R. Roles of Microglia/Macrophage and Antibody in Cell Sheet Transplantation in the Central Nervous System. Stem Cell Res. Ther. 2022, 13, 470. [Google Scholar] [CrossRef]
- Ren, Q.; Lu, F.; Hao, R.; Chen, Y.; Liang, C. Subretinal Microglia Support Donor Photoreceptor Survival in Rd1 Mice. Stem Cell Res. Ther. 2024, 15, 436. [Google Scholar] [CrossRef]
- Wu, K.Y.; Fujioka, J.K.; Gholamian, T.; Zaharia, M.; Tran, S.D.; Almeida, H.; Puglia, C.; Santonocito, D.; Wu, K.Y.; Fujioka, J.K.; et al. Suprachoroidal Injection: A Novel Approach for Targeted Drug Delivery. Pharmaceuticals 2023, 16, 1241. [Google Scholar] [CrossRef]
- Hartman, R.R.; Kompella, U.B. Intravitreal, Subretinal, and Suprachoroidal Injections: Evolution of Microneedles for Drug Delivery. J. Ocul. Pharmacol. Ther. 2018, 34, 141–153. [Google Scholar] [CrossRef]
- Iwama, Y.; Sugase-Miyamoto, Y.; Onoue, K.; Uyama, H.; Matsuda, K.; Hayashi, K.; Akiba, R.; Masuda, T.; Yokota, S.; Yonemura, S.; et al. Transplantation of Human Pluripotent Stem Cell-Derived Retinal Sheet in a Primate Model of Macular Hole. Stem Cell Rep. 2024, 19, 1524–1533. [Google Scholar] [CrossRef]
- Luo, L.J.; Nguyen, D.D.; Lai, J.Y. Benzoic Acid Derivative-Modified Chitosan-g-Poly(N-Isopropylacrylamide): Methoxylation Effects and Pharmacological Treatments of Glaucoma-Related Neurodegeneration. J. Control. Release 2020, 317, 246–258. [Google Scholar] [CrossRef]
- Hu, B.Y.; Xin, M.; Chen, M.; Yu, P.; Zeng, L.Z. Mesenchymal Stem Cells for Repairing Glaucomatous Optic Nerve. Int. J. Ophthalmol. 2024, 17, 748. [Google Scholar] [CrossRef] [PubMed]
- Sugita, S.; Iwasaki, Y.; Makabe, K.; Kimura, T.; Futagami, T.; Suegami, S.; Takahashi, M. Lack of T Cell Response to IPSC-Derived Retinal Pigment Epithelial Cells from HLA Homozygous Donors. Stem Cell Rep. 2016, 7, 619–634. [Google Scholar] [CrossRef]
- Tu, H.Y.; Watanabe, T.; Shirai, H.; Yamasaki, S.; Kinoshita, M.; Matsushita, K.; Hashiguchi, T.; Onoe, H.; Matsuyama, T.; Kuwahara, A.; et al. Medium- to Long-Term Survival and Functional Examination of Human IPSC-Derived Retinas in Rat and Primate Models of Retinal Degeneration. eBioMedicine 2019, 39, 562–574. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Tang, J.; Liu, L.; Liu, Z.; Xue, J.; Ge, J.; Zhuo, Y.; Li, Y. Emerging Therapeutic Strategies for Optic Nerve Regeneration. Trends Pharmacol. Sci. 2025, 46, 45–61. [Google Scholar] [CrossRef] [PubMed]
- Soundararajan, L.; Surendran, H.; Patlolla, N.; Battu, R.; Stoddard, J.; Arrizabalaga, S.; Liu, Z.; Lingam, G.; Su, X.; Ryals, R.C.; et al. Allogeneic RPE Cell Suspension Manufactured at Scale Demonstrating Preclinical Safety and Efficacy Led to IND Approval. NPJ Regen. Med. 2025, 10, 19. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.Y.; Chan, H.C.; Chan, C.M. Can Stem Cell Therapy Revolutionize Ocular Disease Treatment? A Critical Review of Preclinical and Clinical Advances. Stem Cell Rev. Rep. 2025, 21, 1160–1185. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.S.; Park, S.S.; Albini, T.A.; Canto-Soler, M.V.; Klassen, H.; MacLaren, R.E.; Takahashi, M.; Nagiel, A.; Schwartz, S.D.; Bharti, K. Retinal Stem Cell Transplantation: Balancing Safety and Potential. Prog. Retin. Eye Res. 2020, 75, 100779. [Google Scholar] [CrossRef]
- Daley, G.Q. The Promise and Perils of Stem Cell Therapeutics. Cell Stem Cell 2012, 10, 740–749. [Google Scholar] [CrossRef]
- Chiang, M.C.; Chern, E. Current Development, Obstacle and Futural Direction of Induced Pluripotent Stem Cell and Mesenchymal Stem Cell Treatment in Degenerative Retinal Disease. Int. J. Mol. Sci. 2022, 23, 2529. [Google Scholar] [CrossRef]
- Wei, L.; Yan, W.; Shah, W.; Zhang, Z.; Wang, M.; Liu, B.; Xue, Z.; Cao, Y.; Hou, X.; Zhang, K.; et al. Advancements and Challenges in Stem Cell Transplantation for Regenerative Medicine. Heliyon 2024, 10, e35836. [Google Scholar] [CrossRef]
- Yu, J.; Vodyanik, M.A.; Smuga-Otto, K.; Antosiewicz-Bourget, J.; Frane, J.L.; Tian, S.; Nie, J.; Jonsdottir, G.A.; Ruotti, V.; Stewart, R.; et al. Induced Pluripotent Stem Cell Lines Derived from Human Somatic Cells. Science 2007, 318, 1917–1920. [Google Scholar] [CrossRef]
- Merkl, C.; Saalfrank, A.; Riesen, N.; Kühn, R.; Pertek, A.; Eser, S.; Hardt, M.S.; Kind, A.; Saur, D.; Wurst, W.; et al. Efficient Generation of Rat Induced Pluripotent Stem Cells Using a Non-Viral Inducible Vector. PLoS ONE 2013, 8, e55170. [Google Scholar] [CrossRef]
- Lee, S.; Huh, J.Y.; Turner, D.M.; Lee, S.; Robinson, J.; Stein, J.E.; Shim, S.H.; Hong, C.P.; Kang, M.S.; Nakagawa, M.; et al. Repurposing the Cord Blood Bank for Haplobanking of HLA-Homozygous IPSCs and Their Usefulness to Multiple Populations. Stem Cells 2018, 36, 1552–1566. [Google Scholar] [CrossRef]
- Rodríguez-Fuentes, D.E.; Fernández-Garza, L.E.; Samia-Meza, J.A.; Barrera-Barrera, S.A.; Caplan, A.I.; Barrera-Saldaña, H.A. Mesenchymal Stem Cells Current Clinical Applications: A Systematic Review. Arch. Med. Res. 2021, 52, 93–101. [Google Scholar] [CrossRef]
- Yamanaka, S. Pluripotent Stem Cell-Based Cell Therapy—Promise and Challenges. Cell Stem Cell 2020, 27, 523–531. [Google Scholar] [CrossRef]
- Schlereth, S.L.; Hos, D.; Matthaei, M.; Hamrah, P.; Schmetterer, L.; O’leary, O.; Ullmer, C.; Horstmann, J.; Bock, F.; Wacker, K.; et al. New Technologies in Clinical Trials in Corneal Diseases and Limbal Stem Cell Deficiency: Review from the European Vision Institute Special Interest Focus Group Meeting. Ophthalmic Res. 2021, 64, 145–167. [Google Scholar] [CrossRef]
- Viganò, M.; Budelli, S.; Lavazza, C.; Montemurro, T.; Montelatici, E.; De Cesare, S.; Lazzari, L.; Orlandi, A.R.; Lunghi, G.; Giordano, R. Tips and Tricks for Validation of Quality Control Analytical Methods in Good Manufacturing Practice Mesenchymal Stromal Cell Production. Stem Cells Int. 2018, 2018, 3038565. [Google Scholar] [CrossRef] [PubMed]
- Coulon, S.J.; Schuman, J.S.; Du, Y.; Bahrani Fard, M.R.; Ethier, C.R.; Stamer, W.D. A Novel Glaucoma Approach: Stem Cell Regeneration of the Trabecular Meshwork. Prog. Retin. Eye Res. 2022, 90, 101063. [Google Scholar] [CrossRef]
- Lechanteur, C.; Briquet, A.; Bettonville, V.; Baudoux, E.; Beguin, Y. Msc Manufacturing for Academic Clinical Trials: From a Clinical-Grade to a Full Gmp-Compliant Process. Cells 2021, 10, 1320. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, C.-J.; Jiang, J.-Y.; Jin, Z.-B. The Next-Generation Therapies in Ophthalmology for Blindness Worldwide. Eye ENT Res. 2024, 1, 20–38. [Google Scholar] [CrossRef]
- Kakroodi, F.A.; Khodadoust, E.; Alizadeh, M.; Hayaei Tehrani, R.S.; Sarabi, P.A.; Rahmanian, M.; Vosough, M. Current Challenges and Future Directions of ATMPs in Regenerative Medicine. Regen. Ther. 2025, 30, 358–370. [Google Scholar] [CrossRef]
- Atewologun, F.A.; Okesanya, O.J.; Okon, I.I.; Kayode, H.H.; Ukoaka, B.M.; Olaleke, N.O.; Ogaya, J.B.; Okikiola, L.A.; Manirambona, E.; Lucero-Prisno, D.E. Examining the Potentials of Stem Cell Therapy in Reducing the Burden of Selected Non-Communicable Diseases in Africa. Stem Cell Res. Ther. 2024, 15, 253. [Google Scholar] [CrossRef] [PubMed]
- Francis, N.; Aho, J.; Ben-Nun, I.F.; Bharti, K.; Dianat, N.; Makovoz, B.; Nouri, P.; Rothberg, J.; Song, H.; Zamilpa, R.; et al. Scaling up Pluripotent Stem Cell-Based Therapies-Considerations, Current Challenges and Emerging Technologies: Perspectives from the ISCT Emerging Regenerative Medicine Working Group. Cytotherapy 2025, 27, 1031–1042. [Google Scholar] [CrossRef] [PubMed]
- Neofytou, E.; O’Brien, C.G.; Couture, L.A.; Wu, J.C. Hurdles to Clinical Translation of Human Induced Pluripotent Stem Cells. J. Clin. Investig. 2015, 125, 2551–2557. [Google Scholar] [CrossRef]
- Guidelines—International Society for Stem Cell Research. Available online: https://www.isscr.org/guidelines (accessed on 4 October 2025).
- Regulation-1394/2007-EN-EUR-Lex. Available online: https://eur-lex.europa.eu/eli/reg/2007/1394/oj (accessed on 4 October 2025).
- Testa, F.; Bacci, G.; Falsini, B.; Iarossi, G.; Melillo, P.; Mucciolo, D.P.; Murro, V.; Salvetti, A.P.; Sodi, A.; Staurenghi, G.; et al. Voretigene Neparvovec for Inherited Retinal Dystrophy Due to RPE65 Mutations: A Scoping Review of Eligibility and Treatment Challenges from Clinical Trials to Real Practice. Eye 2024, 38, 2504–2515. [Google Scholar] [CrossRef]
- Fischer, M.D.; Simonelli, F.; Sahni, J.; Holz, F.G.; Maier, R.; Fasser, C.; Suhner, A.; Stiehl, D.P.; Chen, B.; Audo, I.; et al. Real-World Safety and Effectiveness of Voretigene Neparvovec: Results up to 2 Years from the Prospective, Registry-Based PERCEIVE Study. Biomolecules 2024, 14, 122. [Google Scholar] [CrossRef] [PubMed]
- Drag, S.; Dotiwala, F.; Upadhyay, A.K. Gene Therapy for Retinal Degenerative Diseases: Progress, Challenges, and Future Directions. Investig. Ophthalmol. Vis. Sci. 2023, 64, 39. [Google Scholar] [CrossRef] [PubMed]
- Brar, A.S.; Parameswarappa, D.C.; Takkar, B.; Narayanan, R.; Jalali, S.; Mandal, S.; Fujinami, K.; Padhy, S.K. Gene Therapy for Inherited Retinal Diseases: From Laboratory Bench to Patient Bedside and Beyond. Ophthalmol. Ther. 2024, 13, 21–50. [Google Scholar] [CrossRef] [PubMed]
| Cell Type | Source | Differentiation Potential | Applications in Ophthalmology | Advantages | Limitations/Challenges | Development Stage |
|---|---|---|---|---|---|---|
| hESCs | Early-stage human embryos | High—can differentiate into RPE, neural, and corneal cells | AMD, Stargardt disease, RPE replacement | Pluripotent, well-studied protocols | Ethical concerns, immunogenicity | Clinical trials (I–II phase) |
| iPSCs | Reprogrammed somatic cells | High—similar to hESCs | RPE transplantation, genetic correction (e.g., Leber’s disease) | Autologous use is possible, avoiding ethical issues | Risk of mutations, complex reprogramming | Preclinical study |
| MSCs | Bone marrow, adipose tissue, and umbilical cord | Limited—mainly support or stromal roles | Neuroprotection, anti-inflammation, DR | Immunomodulatory, easy to isolate | Poor differentiation to RPE, short survival | Clinical trials (I phase) |
| LSCs | Corneal-conjunctival junction | Differentiate into the corneal epithelium | Corneal regeneration, LSCD | Autologous use, clinically established | Limited to LSCD, surgical collection | Approved (Holoclar®—EMA) |
| Genetically modified cells (GMCs) | Derived from iPSCs or other engineered cells | Varies depending on the source | Monogenic inherited retinal diseases | Personalized therapy potential | Safety, regulatory, and long-term expression control | Preclinical study |
| Comparative clinical trials: design, endpoints, and outcomes for 2023–2025 | ||||||
| Condition | Trial/Identifier | Cell type and delivery | Study design/N/Follow-up | Pre-specified endpoints | Key clinical outcomes (timepoint) | Notes |
| AMD (GA) | Stem cell-derived bioengineered RPE implant (CPCB-RPE1)—long-term follow-up) [79] | hESC-RPE on a synthetic scaffold; subretinal | Phase 1/2a; single-arm, open-label; N = 16 (15 implanted); median 3 y follow-up | Primary: Safety; Secondary: BCVA, multimodal imaging (OCT, fundus), IOP; systemic humoral immune monitoring | Safety met; implanted eyes more likely to gain >5 ETDRS letters and less likely to lose >5 vs. fellow eyes at median 3 years; implant stable in position; anticipated hemorrhage mitigated with surgical refinement (cohort 2) (years 3) | First peer-reviewed multi-year outcomes for scaffolded RPE in GA; efficacy signals vs. fellow eye; randomized data still pending [79] |
| AMD (GA) | OpRegen (RG6501)—sponsor/meeting 36-mo readout; Phase 2a ongoing (NCT05626114) | hESC-RPE suspension; subretinal | Phase 1/2a; single-arm, open-label; cohorts by GA severity; 36 months follow-up reported | Safety; BCVA; OCT (outer retinal structure, lesion coverage) | Mean +6.2 ETDRS letters at 36 months in less advanced cohort; greater lesion coverage associated with larger BCVA and structural gains; durability signals to 36 months (non-randomized) | Peer-reviewed primary paper pending for 36-months efficacy; Phase 2a GAlette enrolling (NCT05626114) [80,81] |
| AMD/RP (RPE loss) | Allogeneic iPSC-RPE strips (HLA-mismatched) [82] | iPSC-RPE strips (pre-formed); subretinal | First-in-human, Phase 1-type; single-arm, open-label; N = 3 (1 dry AMD, 2 MERTK-RP); ≥6–12 months follow-up | Primary: Safety/feasibility; Secondary: BCVA, OCT (strip position/continuity), AEs; systemic IS for 24 weeks | Acceptable safety with HLA-mismatch under short IS; anatomic graft survival on OCT; functional measures stabilized/improved in some eyes (early follow-up) [82] | Extends prior RPE scaffolds/suspensions with strip format; very small N; randomized data lacking |
| RP (advanced) | Genome-edited retinal organoid sheets—case series [83] | Patient-matched genome-edited retinal sheets; subretinal | Clinical case series; 2 eyes/2 patients; 24 months | Safety, anatomical survival; exploratory function | Stable survival of sheets and safety to 24 months; exploratory signals of local structural integration; functional endpoints limited in end-stage disease (24 months) [83] | Pioneering but very small; controlled functional efficacy not established |
| LSCD (cornea) | iPSC-derived corneal epithelial sheets [84] | iPSC-corneal epithelium sheets; ocular surface | Single-arm, open-label, first-in-human; N = 4; 52 weeks | Primary: Safety; Secondary: corneal epithelialization, BCVA, AS-OCT, neovascularization, QOL | Epithelialization restored and surface stabilized in most eyes; BCVA improved or stabilized by 52 weeks; no serious graft-related AEs; effect greater in less severe cases (52 weeks) | First iPSC-corneal sheet FIH study; small N; no comparator; durability >1 year needs further tracking |
| AMD (GA, earlier cohort) | Bioengineered RPE implant—early HLA-mismatch findings (safety/engraftment) [85] | hESC-RPE on scaffold; subretinal | Phase 1/2a subset; single-arm; HLA-mismatch | Safety/immune monitoring; imaging | No clinical signs of intraocular inflammation or serologic response despite HLA mismatch; RPE survival and functionality on imaging/histology (early) | Included here as mechanistic/immune context supporting the 2024 follow-up |
| Modality | Primary Indication(s) | Graft/Format | Mechanism of Benefit | Integration Requirement | Durability Considerations | Immunogenicity/IS | Surgical Complexity | Scalability |
|---|---|---|---|---|---|---|---|---|
| RPE sheet/patch (PSC-derived) [47,76] | AMD-GA/RPE loss | Polarized monolayer on a scaffold | Structural and metabolic support; OS phagocytosis; barrier/transport | High: adherent, continuous, polarized RPE on Bruch’s | Tied to monolayer integrity and immune milieu | Allogeneic risk; HLA-aware strategies emerging | Subretinal surgery/device handling | Batch manufacture; QA for polarity/purity |
| RPE suspension (PSC-derived) [47] | AMD | Single cells, subretinal | Trophic support ± local repopulation | Moderate: In vivo re-sheeting is inconsistent | Often early peaks; variable if the sheet does not reform | Similar to above | Subretinal injection | Easier to produce/freeze |
| Photoreceptor precursors/retinal organoid sheets [76,86] | RP/outer retinal degeneration | Suspension or laminated sheets | Neuronal replacement ± trophic aid | High for true vision restoration (synapses with bipolar cells) | Gains are short-lived if synaptogenesis is limited; sheets may be superior | Allogeneic risk | Subretinal surgery | Complex differentiation |
| MSCs (neuroprotection) [76] | RP, DR, glaucoma; ocular surface | Intravitreal/subretinal/subconjunctival | Paracrine immunomodulation and trophic effects | Low (no structural replacement) | Transient if cells do not persist; repeat dosing | Generally low; route-dependent safety | Injection-based | Readily scalable |
| LSC/epithelial constructs (Holoclar®, Nepic®, CALEC) [87,88,89,90] | LSCD | Autologous epithelial sheet | Structural resurfacing of the cornea | High: stable, avascular epithelium; limbal niche | Durable with niche/vascular control | Autologous minimal; allogeneic rejection risk | Ocular surface surgery | Autologous bespoke |
| KLAL/lr-CLAL (allogeneic limbal) [78] | Bilateral LSCD | Donor limbal tissue | Restores the stem cell pool | High | Variable; immune-mediated failure is common without IS | High; systemic IS typical | Ocular surface surgery | Donor dependent |
| Traditional corneal transplantation (PKP, DSAEK/DMEK) [91,92,93,94] | Corneal opacity/endothelial failure | Donor tissue | Tissue replacement | N/A | Good mid-term; endothelial attrition over the years | Rejection risk (esp. endothelium) | Microsurgery | Donor-limited |
| Disease or setting | Retinal degenerations (mixed; includes AMD/RP/SMD across modalities) | Inherited retinal diseases (IRDs) | Optic neuropathies (context for neuro-retinal VA effects) | AMD (dry; selected early-phase cohorts) |
| Cell modality (studies pooled) | Mixed cell therapies (hPSC-RPE, MSCs, RPCs) [131] | Mixed stem-cell interventions [131] | MSCs (autologous/allogeneic) [7] | hESC-/iPSC-RPE (injection or patch) [7,132,133,134,135] |
| Pooled/reported BCVA finding | Overall modest BCVA improvement with substantial heterogeneity across designs and indications | Directionally favorable BCVA change, but wide CIs and non-uniform durability | Statistically significant BCVA gains in pooled analysis; clinical magnitude modest | Reported BCVA gains ranging from single-digit to ~+20 letters in small cohorts (varies by program); attenuation on longer follow-up in some series; no AMD-only meta-estimate in these sources |
| Typical follow-up | Mostly 3–12 months | Up to ~12 months | 3–12 months | 6–12+ months |
| Notes/heterogeneity | Authors emphasize BCVA limitations and recommend multi-metric outcomes; durability beyond 12 months is uncertain. | Recommends adding microperimetry/ERG; calls for standardized designs and longer follow-up. | Study quality and heterogeneity limit durability inference; included as supportive evidence in related neuro-retinal settings. | Outcomes depend on establishing a durable, polarized RPE monolayer; variability by format (suspension vs. patch) and baseline severity. |
| NCT Number | Study Name | Phases, Study Status | Conditions | Date of Start |
|---|---|---|---|---|
| NCT05279157 | Autologous adipose-derived adult stem cell implantation for corneal diseases | II, Completed | Corneal diseases | 19-04-2022 |
| NCT04932629 | To evaluate the clinical safety and efficacy of LSCs for the treatment of superficial corneal pathologies | I, Unknown | Corneal scars and opacities | 07-2021 |
| NCT04626583 | Safety of locally delivered allogeneic AMSCs | I, Completed | Corneal defect | 05-03-2021 |
| NCT04615455 | MSCs therapy of DED in patients with Sjögren’s syndrome | II, Completed | Keratoconjunctivitis Sicca, Sjögren’s Syndrome | 03-11-2020 |
| NCT04484402 | Treatment of patients with inflammatory-dystrophic diseases of the cornea using autologous stem cells | I–II, Completed | Corneal ulcer, corneal disease, corneal dystrophy | 03-10-2016 |
| NCT03878628 | Treatment with allogeneic adipose-derived MSCs in patients with aqueous-deficient DED | I, Completed | Dry eye, keratoconjunctivitis sicca, aqueous tear deficiency | 16-10-2019 |
| NCT03302273 | Corneal epithelial stem cells and DED | NA, Completed | Dry eye syndromes, dry eye, ocular inflammation, ocular surface disease, ocular discomfort, blepharitis | 01-02-2019 |
| NCT02592330 | LSCD treatment with cultivated stem cell (CALEC) graft | I–II, Completed | LSCD | 01-08-2016 |
| NCT02577861 | Efficacy and safety of autologous cultivated LSCs transplantation (ACLSCT) for restoration of corneal epithelium in patients with LSCD | IV, Completed | LSCD | 10-2015 |
| NCT01562002 | Safety study of stem cell transplant to treat limbus insufficiency syndrome | I–II, Completed | Limbus cornea insufficiency syndrome | 03-2012 |
| NCT Number | Study Name | Phases, Study Status | Results | Conditions | Date of Start |
|---|---|---|---|---|---|
| NCT06557460 | A phase IIb clinical trial to assess the safety and efficacy of subretinal implantation of the CPCB-RPE1 implant in subjects with advanced dry AMD | II, not yet recruiting | no | Dry AMD, GA | 10-2024 |
| NCT06394232 | Safety and efficacy of EYECYTE-RPE™ in patients with GA secondary to dry AMD | I–II, recruiting | no | RD, AMD, GA | 04-06-2024 |
| NCT05626114 | A study to optimize subretinal surgical delivery and to evaluate the safety and activity of Opregen in participants with GA | II, recruiting | no | GA | 23-03-2023 |
| NCT05445063 | Safety and efficacy of autologous transplantation of iPSC-RPE in the treatment of MD | I, recruiting | no | MD | 08-2022 |
| NCT04339764 | Autologous transplantation of iPSCs-derived RPE for GA associated with AMD | I–II, recruiting | no | Dry AMD, GA | 23-09-2020 |
| NCT03944239 | Safety and efficacy of subretinal transplantation of clinical hESCs derived RPE in the treatment of RP | I, unknown | no | RP | 05-2020 |
| NCT03305029 | The safety and tolerability of sub-retinal transplantation of SCNT-hES-RPE cells in patients with advanced dry AMD | I, unknown | no | Dry AMD | 05-2016 |
| NCT03178149 | A study of the safety and tolerability of ASP7317 in senior adults who are losing their clear, sharp central vision due to GA secondary to dry AMD | I, recruiting | no | GA, AMD | 13-07-2018 |
| NCT03046407 | Treatment of dry AMD with RPE derived from hESCs | I–II, unknown | no | Dry AMD | 06-09-2017 |
| NCT02903576 | Stem cell therapy for outer retinal degenerations | I–II, completed | no | AMD, Stargardt’s disease | 08-2015 |
| NCT02755428 | Subretinal Transplantation of RPE in the treatment of AMD | I–II, unknown | no | Dry AMD MD | 01-2018 |
| NCT02749734 | Clinical study of subretinal transplantation of hESCs derived RPE in the treatment of MD diseases | I–II, unknown | no | MD, Stargardt’s macular dystrophy | 05-2015 |
| NCT02590692 | Study of subretinal implantation of hESC RPE cells in advanced dry AMD | I–II, unknown | no | Dry MD, GA | 16-02-2016 |
| NCT02286089 | Safety and efficacy study of OpRegen for treatment of advanced dry AMD | I–II, active | yes | Dry AMD | 01-04-2015 |
| NCT01691261 | A study of the implantation of RPE in subjects with Acute wet AMD | I, completed | no | Wet AMD | 14-10-2021 |
| NCT01674829 | A study to determine the safety and tolerability of MA09-hRPE cells in patients with advanced dry AMD | I–II, terminated | no | Dry AMD | 09-2012 |
| NCT01625559 | Safety and tolerability of MA09-hRPE cells in patients with Stargardt’s macular dystrophy | III, completed | no | Stargardt’s macular dystrophy | 09-2012 |
| NCT01469832 | Safety and tolerability of sub-retinal transplantation of hESC-RPE cells in patients with Stargardt’s macular dystrophy | I–II, completed | no | Stargardt’s macular dystrophy | 13-12-2011 |
| NCT01345006 | Sub-retinal transplantation of hESC-derived RPE (MA09-hRPE) cells in patients with Stargardt’s macular dystrophy | I–II, completed | no | Stargardt’s macular dystrophy | 16-06-2011 |
| NCT01344993 | Safety and tolerability of sub-retinal transplantation of hESC-derived RPE (MA09-hRPE) cells in patients with advanced dry AMD | I–II, completed | no | Dry AMD | 09-06-2011 |
| UMIN000011929 | A study of transplantation of autologous iPSC-derived RPE cell sheet in subjects with exudative AMD | I–II, completed | yes | Wet AMD | 2013 |
| UMIN000026003 | A Study of transplantation of allogenic iPSC-derived RPE cell suspension in subjects with neovascular AMD. | I, completed | - | Wet AMD | 2017 |
| jRCTa050210178 | Clinical research of allogeneic iPSC-RPE cell strip transplantation for RPE impaired disease | I–II, active | - | RPE impaired disease | 2022 |
| jRCTa050200027 | Safety Study of allogenic hiPSC-retinas in RP | I, completed | - | RP | 2020 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iliadis, I.; Pechnikova, N.A.; Poimenidou, M.; Almaliotis, D.D.; Tsinopoulos, I.; Yaremenko, T.V.; Yaremenko, A.V. Applications of Modern Cell Therapies: The Latest Data in Ophthalmology. Life 2025, 15, 1610. https://doi.org/10.3390/life15101610
Iliadis I, Pechnikova NA, Poimenidou M, Almaliotis DD, Tsinopoulos I, Yaremenko TV, Yaremenko AV. Applications of Modern Cell Therapies: The Latest Data in Ophthalmology. Life. 2025; 15(10):1610. https://doi.org/10.3390/life15101610
Chicago/Turabian StyleIliadis, Ioannis, Nadezhda A. Pechnikova, Malamati Poimenidou, Diamantis D. Almaliotis, Ioannis Tsinopoulos, Tamara V. Yaremenko, and Alexey V. Yaremenko. 2025. "Applications of Modern Cell Therapies: The Latest Data in Ophthalmology" Life 15, no. 10: 1610. https://doi.org/10.3390/life15101610
APA StyleIliadis, I., Pechnikova, N. A., Poimenidou, M., Almaliotis, D. D., Tsinopoulos, I., Yaremenko, T. V., & Yaremenko, A. V. (2025). Applications of Modern Cell Therapies: The Latest Data in Ophthalmology. Life, 15(10), 1610. https://doi.org/10.3390/life15101610






