Abstract
Dirofilariosis, a parasitic disease caused by nematodes of the genus Dirofilaria, primarily affects dogs but can also infect other carnivores and, more rarely, humans. In Europe, the most commonly involved species are D. immitis and D. repens, transmitted through the bites of mosquito vectors. This study, conducted in Tulcea County between April and October 2024, aimed to determine the prevalence of D. immitis and D. repens in mosquitoes. A total of 1507 mosquitoes were collected and grouped into 76 pools, and subsequently molecular analysis was carried out using qPCR. The estimated infection rate (EIR) was calculated using statistical methods available in the ‘binGroup’ package in R, which allow the determination of the point estimate and confidence interval (CI) for a single binomial proportion in group testing. The study revealed a high infection rate with D. immitis (48%), while D. repens was identified in only two pools. The species with the highest vector potential was Anopheles maculipennis (PTP = 75%, EIR = 0.1168 with both Dirofilaria species), followed by Aedes vexans. Notably, Aedes albopictus was identified for the first time in Tulcea, and all individuals were positive for D. immitis. Simulations of local thermal conditions using the proposed model show that the favorable time window for mosquitoes will increase until 2100. Our results indicate an established and active transmission cycle of D. immitis in the region, a situation projected to intensify with climate change requiring urgent monitoring.
1. Introduction
Dirofilariosis is a parasitic disease caused by nematodes of the genus Dirofilaria, primarily D. immitis and D. repens, which are prevalent in Europe [1,2]. Both circulate in an enzootic cycle between mosquitoes and domestic dogs, although other carnivores such as red foxes or gray wolves can serve as reservoirs [3,4]. These parasites are transmitted through the bites of infected mosquitoes and affect carnivorous mammals worldwide, primarily dogs. They can also infect cats and, more rarely, humans [3,5,6,7,8,9,10]. In Europe, the disease has become more and more common. It now affects the health of companion animals and, in some cases, also impacts human health [11,12,13,14,15]. There are about 70 known species of Dirofilaria. Human infections are most often caused by D. immitis, D. repens, and D. tenuis, while other species such as D. striata, D. ursi, D. spectans, and D. magnilarvata are reported only occasionally. The main natural hosts for these species are dogs and wild canids (D. immitis and D. repens) and raccoons (D. tenuis) [16,17,18,19,20].
Countries previously considered free of Dirofilaria are now regarded as endemic [21]. Climate warming is believed to be the main factor allowing nematodes to develop successfully in mosquitoes [22], along with increased dog movement facilitated by European travel regulations for pets [20]. Recently, more cases have been reported in Southeastern Europe, Sri Lanka, and southern India. This highlights the need for molecular testing and large-scale epidemiological studies.
Initially, dirofilariosis was mostly reported in southern European countries like Italy, Spain, Greece, and France [23,24]. However, in the last few decades, it has spread into northern regions such as Germany, Austria, and even southern Sweden [25]. This spread is attributed to climate changes that favor mosquito proliferation, especially Aedes and Culex species [15,17]. Pet travel and animal migrations have also contributed to spreading the disease in non-endemic areas [26].
In Romania, Dirofilaria spp. was first identified about two decades ago [27]. Studies on Dirofilaria spp. prevalence in dogs have shown infection rates from 3% up to over 60% [28,29,30,31]. D. immitis and D. repens have also been found in wild carnivores such as red foxes, golden jackals, weasels, wild cats, and gray wolves. These animals probably act as important reservoirs in natural areas where the disease occurs [7,32]. However, human cases in Romania remain low, possibly due to limited physician awareness concerning this parasite.
To date, research on the circulation of Dirofilaria species in Romania has primarily focused on monitoring canine populations, with evidence of parasite circulation documented as early as 2007 [27], whereas vector-related studies have been limited. More recently, Tomazatos et al. (2018) [33] conducted an investigation in the Danube Delta, reporting a D. immitis prevalence of 4.53% in mosquito samples (with pools containing up to 250 specimens) and 19.44% in dog blood samples.
D. immitis remains largely restricted to southern European countries with Mediterranean climates (Cs—hot & dry summers and cool & wet winters according to Köppen climate classification system) but has gradually expanded north [22]. Most European countries have now become endemic, with significant distribution changes in the last 20 years [34,35]. Predictions suggest dirofilariosis will spread rapidly, urging implementation of monitoring and control programs at the European level [36]. The increase in global trade, travel, and environmental changes in recent decades has greatly contributed to the fast spread of vector-borne diseases [37].
Monitoring dog infections is difficult, as the disease is often asymptomatic or presents mild symptoms, but severe forms can cause congestive heart failure, chronic cough, weakness, and death [38,39]. In humans, D. repens infections are more common and may cause subcutaneous nodules, sometimes painful or inflamed. Also it can cause pulmonary inflammation triggered by dead adult worms, visible as coin lesions on X-rays [40,41,42]. However, most human cases are asymptomatic. When symptoms appear, they include cough (sometimes with blood), chest pain, fever, and pleural effusion [35,43]. Rarely, D. immitis worms have been found outside the lungs (e.g., brain, eyes, testicles) [11,44]. D. repens and D. tenuis typically cause subcutaneous nodules or, occasionally, are found in the conjunctiva [45,46,47,48,49,50]. Some reports describe viable D. repens microfilariae in human blood [51,52]. Diagnosis involves serological testing, PCR, and blood microscopy. Ultrasound may also detect adult worms in the heart or other organs [53].
Disease spread is favored by mosquito presence, freshwater, high humidity, and warm temperatures. Higher ambient temperatures shorten larval development time in the vector [54]. The number of mosquito species implicated in Dirofilaria transmission increases yearly, including both endemic and invasive species spreading due to climate change [9,54]. Currently, at least 77 mosquito species (Diptera: Culicidae) from the genera Culex, Aedes, Anopheles, Mansonia, Coquillettidia, Psorophora, and Culiseta are considered to act as vectors [55]. Among these, Aedes vexans is highly efficient due to its aggressive hematophagy and wide distribution. Culex pipiens, adapted to urban and suburban settings, plays a major role in temperate regions [55,56]. Anopheles mosquitoes, especially A. maculipennis, maintain the transmission cycle in rural areas of Europe and Asia [57,58]. Ochlerotatus species, like O. caspius, are also competent vectors, particularly in Mediterranean and subtropical regions [59].
2. Materials and Methods
- (a)
- Biological material
The objectives of the study aimed to establish the prevalence of D. repens and D. immitis in mosquitoes from the Tulcea area, as well as to predict the evolution of this prevalence under global warming conditions. Between April and October 2024, mosquito traps were installed to assess the transmission risk of D. immitis and D. repens in Tulcea County. The collection points selected were: Somova commune, human dwelling in Mineri village (longitude 28°43′27.8″ E, latitude 45°10′10.5″ N), Nufăru commune, human dwelling in Victoria village (longitude 28°57′55.0″ E, latitude 45°10′58.9″ N), veterinary clinic with animal housing in Murighiol commune (longitude 29°09′58.3″ E, latitude 45°02′15.1″ N), and Tulcea city public animal shelter (longitude 28°45′17.1″ E, latitude 45°10′58.9″ N).
CDC Light traps, model 1012 (John W. Hock Company, Gainesville, FL, USA), were used on the first and last weekend of each month during the study period (a total of four traps were employed, all being simultaneously positioned at the predetermined sites). The traps were deployed overnight from 19:00 to 08:00. Mosquito species were identified based on morphological features, using the identification keys by Norbert Becker [59] and the interactive keys from the MosKeyTool v2.1 software (Pasteur Institute, Paris, France). After morphological identification, mosquitoes were stored at −80 °C and grouped into species-specific pools, each pool containing 20 mosquitoes. The pools were divided by species for each location. Since the pool positivity was very similar across all locations, the prevalence of D. immitis and D. repens was calculated for the entire study area. Pools are made in order to reduce the overall costs; however, care should be taken regarding the size of pooled samples not to exceed the detection limit. Therefore, the smaller the pool, the better chance of identifying positive samples.
- (b)
- Molecular screening
The pools created at the moment of the morphological identification each contain 20 mosquito specimens, as was previously described. We have classified the samples as tissues; therefore, we added an extra step: preincubation of the samples, using 20 μL of proteinase K and 180 μL PureLink™ Genomic Digestion Buffer in each tube, according to the extraction protocol. We set for overnight digestion while incubating at 55 °C with periodic vortexing. Molecular screening began with DNA extraction from pools using the PureLink Genomic DNA Mini Kit (Thermo Fisher Scientific, Waltham, MA, USA). DNA was extracted from 200 µL of digested pool, following the manufacturer’s protocol, and each pool eluate (100 μL) was stored at −20 °C until molecular detection was performed. For pathogen detection, a multiplex qPCR protocol was used, combining TaqMan (D. immitis CAT CCT GAG GTT TAT GTT ATT ATT TT, CWG TAT ACA TAT GAT GRC CYC A, and 6FAM-CGG TGT TTG GGA TTG TTA GTG-MGB) and SYBR Green (D. repens with TM 70: GTG TGC TGC GCT ACA TCG ATG TT, ATA AAC CGC TCT GTC TCA CGA CG) principles. QuantiNova Multiplex PCR Master Mix 4× (Qiagen, Venlo, The Netherlands) was used in real-time tests performed in a C1000™ thermocycler (Bio-Rad Laboratories Inc., Hercules, CA, USA), using the CFX96™ Real-Time Detection System. Amplification reactions for all qPCRs included 5 μL of QuantiNova Multiplex PCR Master Mix (Qiagen, Venlo, The Netherlands), 5 μL of DNA template, primers (0.4 μM), probes (0.25 μM), and molecular-grade water up to a final volume of 20 μL. The result validation comprised both an endogenous control (Cy5) that targeted a housekeeping gene to evaluate extraction and amplification efficiency, as well as an exogenous internal control (for possible amplification inhibitions)—HEX [60], and also a positive (Ct = 22.3) and negative control.
The thermocycling program consisted of an initial denaturation step at 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s and 62 °C for 1 min. Melting temperature (Tm) measurements were taken between 65 and 88 °C at 0.5 s intervals [61,62]. Fluorescent signals collected from FAM and HEX channels were analyzed using CFX Manager Software Version 3.1. Primers (Bio-Rad Laboratories Inc., Hercules, CA, USA) recommended by Pękacz [63] were used for D. repens detection, and primers recommended by Negron [64] were used for D. immitis detection (Table 1).

Table 1.
Primers used for RT-PCR detection of D. repens and D. immitis.
- (c)
- Estimation infection rate method
In the laboratory, we used the qPCR technique to identify the presence of D. repens and D. immitis in mosquito samples. In epidemiology, mosquito populations are classified into pools to determine whether they are infected or not [33,57]. Mathematically, we estimated the infection rate in the pool using a statistical method for group testing to determine the probability of positive material in the pool [65]. This method is based on binomial group testing, a complex algorithm typically involving probability models and statistical estimation techniques.
Estimated infection rate and confidence interval (CI) were calculated using the ‘bgtCI’ function from the ‘binGroup’ package in R, which accounts for pooled testing [66]. The proportion of positive pools was calculated according to a 95% confidence level [66].
The proportion of tested pools (PTP) that are positive was calculated using:
The prevalence of Dirofilaria immitis and Dirofilaria repens infection in the collected mosquitoes was also estimated using the Minimum Infection Rate (MIR), i.e., the minimum proportion of infected mosquitoes expressed as a percentage: MIR = (p/N) × 100%, where p represents the number of positive pools and N is the total number of mosquitoes tested. This method assumes that in each positive pool there is at most one infected mosquito.
- (d)
- Climatic model
In the present study, we analyzed the thermal characteristics of the air near the surface (2 m) during the period 1961–2024, making estimates of long-term temperature changes in order to understand the influence of these changes on development of mosquito populations.
We used a simplified and adapted climatic model based on the global climate modeling project CMIP6 (Coupled Model Intercomparison Project Phase 6), taking into consideration the local climatic conditions in Tulcea County (elevation ranging from sea level up to 467 m). The CMIP6 estimates the air temperature near the surface (at 2 m) based on the heat balance determined as radiative forcing (RF) of the air using the three possible scenarios: SSP126 (RFSSP126 = 2.6 W/m2), SSP245 (RFSSP245 = 4.5 W/m2) and SSP585 (RFSSP585 = 5.8 W/m2). In this approach, the temperature projections are made by considering different hypothetical scenarios given by the air carbon dioxide concentrations. The NetCDF output files from CMIP6 archives estimate surface air temperatures (‘tas’ variable) on long term until 2100 by using the IPSL–CM6A–LR climatic model [67]. We identified the fitting parameters of tas variables that range from IPSL–CM6A–LR to obtain the fingerprint of global warming curve. The fitting parameters were used for Tulcea temperature range to simulate the values for long-term 2070–2100 (Figure 1). In proposed model, the input data used was the range of average daily temperatures measured during the period 1961–2024 at Tulcea weather station from Romania (STAID: 967 from the European Climate Assessment & Dataset—ECA&D).
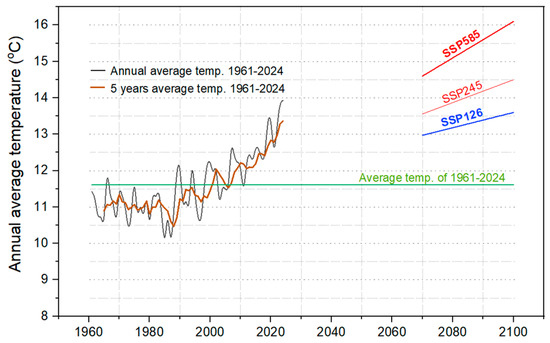
Figure 1.
Temperature evolution over the past six decades and projected trends for the last decades of 21st century. The annual (black line) and 5-year (brown line) averages were calculated for diurnal temperatures recorded at a height of 2 m in the Tulcea weather station (1961–2024). Long-term projections were generated using an adapted local version of the CMIP6 climate scenarios model. The three temperature scenarios for 2070–2100 were simulated: (1) SSP126 (blue line)—representing low greenhouse gas emissions (GHGE); (2) SSP245 (thin red line)—representing an intermediate-emission scenario and (3) SSP584 (thick red line)—a high-emission scenario.
- (e)
- Heartworm Development Unit
The heartworm development unit (HDU) is a bioclimatic index that establishes the existing incubation periods of Dirofilaria spp. based on accumulated temperature. Studies show that the minimum biological temperature threshold for the development of Dirofilaria spp. in mosquitoes is an average diurnal temperature (Td) of 14 °C. From thermal point of view, the population of mosquitoes passes from microfilaria (L1) to the infective (L3) stages, it is necessary to accumulate 130 HDUs in less than 30 days [68]:
3. Results
A total of 1507 mosquitoes, identified to species or complex level based on morphological criteria, were tested for the presence of Dirofilaria spp. Pools were created with the species Aedes caspius, Aedes vexans, Aedes albopictus, Culex pipiens s.s./Cx. torrentium, Anopheles hyrcanus, Anopheles maculipennis s.l., and Uranotaenia unguiculata, with 20 specimens included in each pool. In total, 76 pools were tested (Table 2).

Table 2.
Results of pool-based identification of D. immitis and D. repens.
The pools were classified according to the seven mosquito species sampled from the studied area (pooling by species was performed across all collection sites, as the samples were processed fresh and in order to ensure a consistent number of specimens per pool): Uranotaenia unguiculata, Aedes albopictus, Aedes vexans, Anopheles hyrcanus, Anopheles maculipennis s.l., Culex pipiens s.s./Cx. torrentium and Aedes caspius. The Culex pipiens s.s./Cx. torrentium was the most numerous (580 mosquitoes), while Aedes albopictus was the least numerous (7 mosquitoes) (Table 2). The small number of Aedes albopictus identified in the field means the data are not statistically significant, allowing us to only indicate the presence of Dirofilaria immitis in this potential competent vector. We specify that this is the first report of the species Aedes albopictus in Tulcea County, with the nearest place where it has been previously reported being the Great Brăila Island in 2023 [69]. The 48% PTP value for all examined species raises an alarm that climate change is having a significant impact, and, in the absence of monitoring and vector control measures, the risk of Dirofilaria immitis infection has reached a very high level.
Within the 76 mosquito pools tested in the laboratory using the qPCR technique to identify Dirofilaria species DNA sequences, a high rate of positive infections (39 pools) was identified. The proportion of positive D. immitis pools was very high for all tested samples (PTP > 29%) but low for D. repens infection (PTP = 16.67%) in all the mosquito populations tested.
The average infection probability for each pool of Dirofilaria is EIR = 0.03406, with a confidence interval (CI) distribution of [0.02489, 0.04682]. The mosquito species with the highest probability of being a vector for both Dirofilaria species is Anopheles maculipennis, with an estimated infection probability of EIR = 0.1168 per pool and a CI = [0.05061, 0.1898] at a 95% confidence level. In contrast, Anopheles hyrcanus is the least vulnerable species to becoming a vector for Dirofilaria spp. (EIR = 0.01033; CI = [0.00340, 0.02772]).
We can state that Anopheles maculipennis shows the highest importance as a vector for Dirofilaria immitis and Dirofilaria repens, with a PTP value of 75%, followed by Aedes vexans with a PTP of 50%. For Aedes albopictus, we can only indicate the presence of Dirofilaria immitis, since only one pool consisting of seven specimens was analyzed, but we emphasize the vectorial potential and the rapid spread of this invasive species in Romania.
All mosquito species identified in the field had individuals infected with D. immitis (Table 3), but D. repens was only identified in Anopheles maculipennis pools (Table 4).

Table 3.
Statistical evaluation of the probability of D. immitis infection in mosquito species sampled in Tulcea area in 2024.

Table 4.
Statistical evaluation of the probability of D. repens infection in mosquito species sampled in Tulcea area in 2024.
Based on the MIR calculation formula, the total prevalence for Dirofilaria immitis is estimated to be 2.38%. The prevalence is 1.66% for Uranotaenia unguiculata, 14.28% for Aedes albopictus, 2.5% for Aedes vexans, 1.87% for Anopheles hyrcanus, 3.75% for Anopheles maculipennis, 2.06% for the Culex pipiens complex, and 1.42% for Aedes caspius. However, given the small and fixed number of mosquitoes per pool and the low CT values (ranging from 17.25 to 29.34), which indicate a high concentration of D. immitis DNA, we consider that this method does not accurately reflect the reality.
Climatically, Tulcea County is in a warm continental climate region (Dfa-Köppen climate classification), with warm oceanic climate influences (Cfa) in the coastal area of the Black Sea, without a dry season (ANM, 2008). Instead, our simulation using proposed climatic model highlights that average temperature will increase somewhere between 13.6 °C (low emissions—SSP126) and 16.1 °C (high emissions—SSP585) in 2100 (Figure 1).
The HDU over multiple decades from 1961 to 2020 was calculated to identify the periods favourable for development of Dirofilaria spp. from the microfilaria (L1) to the infectious stage (L3). On average, the temperature supports the start of microfilaria development on May 9 (1961–2020), with the first generation reaching the L3 stage by approximately June 6. However, the date after which temperatures drop below 14 °C has changed from October 30 in 1961–1990 decade to November 3 in 1991–2020 decade.
Climate-based forecasting systems typically use the concept of growing degree days (GDD), which represent the accumulation of heat units when the average daily temperature exceeds the threshold temperature by 1 °C. The seasonal transmission model of Dirofilaria spp. assumes that 130 HDUs must accumulate for the larvae to reach the infective stage, with a maximum lifespan of 30 days for the mosquito vector. Simulations of local thermal conditions using the proposed climatic model show that the favourable time window for mosquitoes developed will extend until the year 2100. The average annual number of Dirofilaria spp. generations will increase from 8.17 in 2020 to 13.75 by the end of the century (Figure 2).
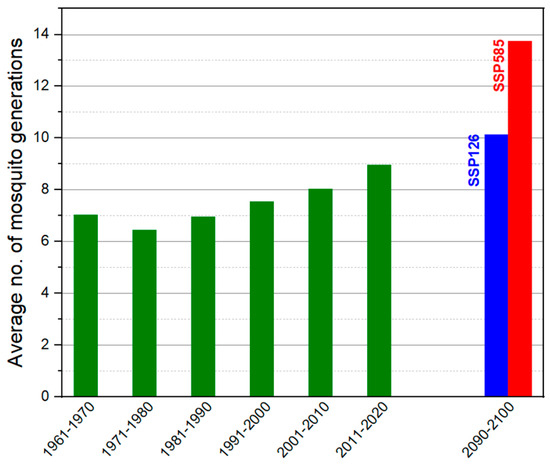
Figure 2.
The possible average number of mosquito generations that could develop under the temperature conditions in each decade from 1961 to 2020, and the estimates of the number of generations that will possibly develop in 2091–2100 decade. The estimations were calculated based on long-term temperature projections under the two extreme scenarios (SSP126 and SSP585) of CMIP6 climate model.
4. Discussion
A systematic review conducted in 2025 by Hattendorf and Lühken [20], which analyzed 3.847 publications, highlighted that Romania and Bulgaria have a large number of stray dogs, which serve as a significant reservoir for parasites. These countries also report a faster development of Dirofilaria spp. larvae within mosquitoes. The spread of Dirofilaria infestations had already been predicted in the early 2000s [2,68]. Moreover, rising temperatures in Europe have facilitated the widespread establishment of exotic, diurnal mosquito species such as Aedes albopictus, a major vector for D. immitis and D. repens. Consequently, the only tested pool in our study was found to be positive for D. immitis.
To date, studies on the circulation of Dirofilaria species in Romania have focused mainly on monitoring dogs, with evidence of parasite circulation reported as early as 2007 [27], while studies focusing on vectors have received less attention. More recently, Tomazatos et al. (2018) [33] conducted a study in the Danube Delta and reported a prevalence of 4.53% for D. immitis in mosquitoes (the pools were large, with up to 250 mosquito specimens per pool) and 19.44% in dog blood samples. In contrast, our study highlighted a prevalence of 48% for D. immitis in mosquitoes, which aligns with data reported in dogs in the eastern part of Romania, where a prevalence of 60% was recorded in 2016 [70], and a recent study conducted in the same area as ours reported a prevalence of 50% for D. immitis [71].
Molecular detection of filarioid parasites in hematophagous vectors remains the most efficient method for assessing the prevalence of both vectors and pathogens in specific regions. This approach has been used in most studies reporting the prevalence of D. immitis and D. repens [72,73].
It is very difficult to determine the number of mosquitoes infected with Dirofilaria, as this requires significant human, material and financial resources. The binomial group testing statistical method used by us is accurate and efficient because it reduces testing time and costs compared to individual testing [74]. This method statistically analyses the probability of infection in a pool by calculating two parameters (point estimation and confidence interval) of the distribution function [75]. The ‘bgtCI()’ function used in the present study is based on the Wilson score, which provides accurate confidence intervals for small pool sizes (20 mosquitoes).
Previous studies in the Danube Delta and neighbouring areas confirm that Anopheles maculipennis and Aedes spp. have the highest Dirofilaria spp. infection rates [56,64], most likely due to specific local environmental conditions. Several studies have highlighted that the species of mosquito responsible for transmitting infection is influenced by the size of the mosquito population and the density of microfilariae in the blood of hosts [76], which is closely related to urbanisation, deforestation, pollution and organic matter [77,78], as well as climatic conditions [57].
Climatic conditions are important in this type of study because temperature and humidity are essential for mosquito colony development. Tulcea County is a special area because approximately 40% of its territory is covered by Danube Delta, which represents a favourable place for the development of mosquito populations. Our simulations, based on an adapted CMIP6 model and local conditions, highlight that thermal conditions will change locally, extending the mosquito development period to the end of autumn till the year 2100. This will increase the incidence of Dirofilaria spp. infection.
The results of this study highlight a high prevalence of D. immitis infection in mosquito populations collected from Tulcea County between April and October 2024, with a pool positivity rate of 48%. The species with the highest vectorial potential were Anopheles maculipennis (PTP = 75%) and Aedes vexans (PTP = 50%), both known for their adaptability to the region’s wet habitats. In contrast, D. repens was identified in only two pools belonging to the species Anopheles maculipennis, suggesting a lower circulation of this pathogen in the region compared to D. immitis. A study conducted for Europe shows that most reports identify species from the Culex pipiens complex and Anopheles maculipennis as the main vectors for D. immitis and D. repens, in agreement with our results [20].
The average estimated EIR value for all pools was 0.03217, with confidence intervals indicating a consistent probability of infection among the investigated species. Among them, Anopheles maculipennis showed the highest likelihood of being a vector (EIR = 0.1168), positioning it as a key species in the transmission of dirofilariosis in the area. Aedes albopictus was reported for the first time in Tulcea County, indicating the rapid spread tendency of this species in Romania. Only seven specimens were identified, and the single pool formed tested positive, suggesting a significant vector potential that requires further validation through subsequent collections.
From a climatic perspective, the analysis of air temperature evolution from 1961 to 2024 indicates a significant increase in the annual average, from 11.6 °C to forecasted values of up to 16.1 °C under the pessimistic SSP585 scenario. This trend is also confirmed by the analysis of the Heartworm Development Unit (HDU) index, which shows an extension of the favorable window for the development of Dirofilaria spp. larvae in vectors, both in terms of duration and number of generations. Our simulation suggests an increase in annual generations from an average of 8.17 at present to approximately 13.75 by the year 2100, implying a significant intensification of vector-borne transmission risk in the context of global warming.
The mosquito population in Tulcea/Dobrogea in 2024 had a very high transmission rate of Dirofilaria. D. immitis was predominant, with Anopheles maculipennis being the most important vector. The estimated infection rate of Anopheles hyrcanus is 10 times lower than that of Anopheles maculipennis.
The exceedance of the annual mean temperature by 2 °C—a threshold that can trigger the development of Dirofilaria immitis within mosquitoes—and the rise to an average of 16 °C in 2024 sound an alarm regarding the accelerated increase in the risk of transmission of this disease across the country. This highlights the urgent need to implement vector monitoring and control programs, as well as prophylactic measures among dogs, including those in shelters. If the current situation is already alarming due to the accelerated risk of parasite transmission—with a marked difference in the prevalence of Dirofilaria immitis in mosquitoes compared to the last study conducted in the region [33]—the outlook for the future is even more concerning, as a significant increase in the number of parasite generations is projected, from 8.17 to 13.75 by the year 2100. This study issues a clear warning, emphasizing the danger posed to both public health and veterinary medicine by the rapid spread of Dirofilaria spp.
D. repens infection was detected at a significantly lower rate. The discovery of the species Aedes albopictus in Tulcea County represents a noteworthy observation and suggests a possible expansion of the range of this invasive species. Climate models indicate a significant extension of the period favorable for the transmission of dirofilariosis by vectors until the year 2100, in correlation with the increase in average annual temperatures, which supports the need to implement proactive entomological and epidemiological monitoring measures in the region, as well as to develop integrated vector control strategies adapted to changing climatic conditions, while continuously taking into account the predictions made using accepted mathematical models.
5. Conclusions
The proportion of positive D. immitis pools was very high for all tested samples (PTP > 29%) but low for D. repens infection (PTP = 16.67%) in all the mosquito populations tested, with 75% PTP for Anopheles maculipennis and 50% PTP for Aedes vexans, emphasizing the important role of these two species as competent vectors. The species Aedes albopictus was also reported; however, due to the small number of specimens collected, we can only indicate the presence of Dirofilaria immitis and highlight the need for continued monitoring of this species in the future. The mathematical models used indicate a future increase in the number of Dirofilaria spp. generations developing annually within mosquito vectors, from 8.17 to 13.75, emphasizing the necessity of implementing control programs that are always correlated with ongoing climate changes.
The results of the study raise an alarm both in human and veterinary medicine. Considering that stray dogs represent a major problem in Romania, they remain the main natural reservoir for dirofilariosis. Therefore, the administration of prophylactic treatment becomes mandatory for both shelter dogs and dogs with owners. Moreover, the findings of our study should serve as an important criterion in the differential diagnosis of respiratory conditions involving pulmonary masses, prior to proposing any invasive procedure.
Author Contributions
Conceptualization, L.M., L.I. and D.A.; methodology, I.B., L.M. and L.I.; soft-ware, I.B. and S.H.; validation, L.M., L.I., D.A. and I.B.; formal analysis, R.M.; investigation, D.A., S.H., L.I., L.A. and R.M.; resources, G.-V.A., S.H., R.M., D.A. and L.A.; data curation, L.I. and I.B.; writing—original draft preparation, L.M. and L.I.; writing—review and editing, L.M., L.I., I.B. and D.A.; visualization, L.M. and I.B.; supervision, L.M. and D.A.; project administration, L.I.; funding acquisition, S.H., G.-V.A. and L.A. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the European Union (NextGenerationEU instrument) through the National Recovery and Resilience Plan, “PNRR-III-C9-2022—I5 Establishment and operationalization of Competence Centers” competition, “Competence Center for Climate Change Digital Twin for Earth forecasts and societal redressment: DTEClimate” project, contract no. 760008/30.12.2022, code 7/16.11.2022.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Genchi, C.; Kramer, L.H. The prevalence of Dirofilaria immitis and D. repens in the Old World. Vet. Parasitol. 2020, 280, 108995. [Google Scholar] [CrossRef]
- Genchi, C.; Mortarino, M.; Rinaldi, L.; Cringoli, G.; Traldi, G.; Genchi, M. Changing climate and changing vector-borne disease distribution: The example of Dirofilaria in Europe. Vet. Parasitol. 2011, 176, 295–299. [Google Scholar] [CrossRef]
- Moroni, B.; Rossi, L.; Meneguz, P.G.; Orusa, R.; Zoppi, S.; Robetto, S.; Marucco, F.; Tizzani, P. Dirofilaria immitis in wolves recolonizing northern Italy: Are wolves competent hosts? Parasites Vectors 2020, 13, 482. [Google Scholar] [CrossRef]
- Medkour, H.; Laidoudi, Y.; Marié, J.L.; Fenollar, F.; Davoust, B.; Mediannikov, O. Molecular investigation of vector-borne pathogens in red foxes (Vulpes vulpes) from southern France. J. Wildl. Dis. 2020, 56, 837–850. [Google Scholar] [CrossRef]
- Markakis, G.; Sioutas, G.; Bitchava, D.; Komnenou, A.; Ganoti, M.; Papadopoulos, E. Is the European badger a new host for Dirofilaria immitis? The first records in Greece. Parasitol. Res. 2024, 123, 118. [Google Scholar] [CrossRef] [PubMed]
- Alsarraf, M.; Dwużnik-Szarek, D.; Hildebrand, J.; Mierzejewska, E.J.; Kloch, A.; Kot, K.; Kurek, K.; Nowak, S.; Mysłajek, R.W.; Myśliwy, I.; et al. Occurrence of Dirofilaria repens in wild carnivores in Poland. Parasitol. Res. 2023, 122, 1229–1237. [Google Scholar] [CrossRef] [PubMed]
- Ionică, A.M.; Deak, G.; Boncea, R.; Gherman, C.M.; Mihalca, A.D. The European badger as a new host for Dirofilaria immitis and an update on the distribution of the heartworm in wild carnivores from Romania. Pathogens 2022, 11, 420. [Google Scholar] [CrossRef]
- Otranto, D.; Deplazes, P. Zoonotic nematodes of wild carnivores. Int. J. Parasitol. Parasites Wildl. 2019, 9, 370–383. [Google Scholar] [CrossRef]
- Alsarraf, M.; Levytska, V.; Mierzejewska, J.; Poliukhovych, V.; Rodo, A.; Alsarraf, M.; Kavalevich, D.; Dwużnik-Szarek, D.; Behnke, J.M.; Bajer, A. Emerging risk of Dirofilaria spp. infection in Northeastern Europe: High prevalence of Dirofilaria repens in sled dog kennels from the Baltic countries. Sci. Rep. 2021, 11, 1068. [Google Scholar] [CrossRef]
- Garrity, S.; Lee-Fowler, T.; Reinero, C. Feline asthma and heartworm disease: Clinical features, diagnostics and therapeutics. J. Feline Med. Surg. 2019, 21, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Kuna, A.; Poblocki, P.; Baranowicz, K.; Grzybek, M. Lesion on the right testicle of 21-year-old patient. One Health 2024, 19, 100863. [Google Scholar] [CrossRef]
- Procop, G.W.; Neafie, R.C. Human parasitic pulmonary infections. In Pulmonary Pathology, 2nd ed.; Zander, D.S., Farver, C.F., Eds.; Foundations in Diagnostic Pathology; Elsevier: Amsterdam, The Netherlands, 2018; pp. 289–314. [Google Scholar]
- Fontes-Sousa, A.P.; Silvestre-Ferreira, A.C.; Carretón, E.; Esteves-Guimarães, J.; Maia-Rocha, C.; Oliveira, P.; Lobo, L.; Morchón, R.; Araújo, F.; Simón, F.; et al. Exposure of humans to the zoonotic nematode Dirofilaria immitis in Northern Portugal. Epidemiol. Infect. 2019, 147, 282. [Google Scholar] [CrossRef]
- Capelli, G.; Genchi, C.; Baneth, G.; Bourdeau, P.; Brianti, E.; Cardoso, L.; Danesi, P.; Fuehrer, H.-P.; Giannelli, A.; Ionică, A.M.; et al. Recent advances on Dirofilaria repens in dogs and humans in Europe. Parasites Vectors 2018, 11, 663. [Google Scholar] [CrossRef] [PubMed]
- Momčilović, S.; Gabrielli, S.; Đenić, N.; Živković, N.; Stevanović, G.; Krstić, M.; Ranđelović, M.; Tasić-Otašević, S. New cases of human dirofilariosis on the Balkan Peninsula—“Masked intruders” uncovered by a surgeon. Parasitol. Int. 2021, 86, 102482. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.V. Human dirofilariasis: An emerging zoonosis. Trop. Parasitol. 2013, 3, 2–3. [Google Scholar] [PubMed]
- Perles, L.; Dantas-Torres, F.; Krücken, J.; Morchón, R.; Walochnik, J.; Otranto, D. Zoonotic dirofilariases: One, no one, or more than one parasite. Trends Parasitol. 2024, 40, 257–270. [Google Scholar] [CrossRef]
- Yilmaz, E.; Fritzenwanker, M.; Pantchev, N.; Lendner, M.; Wongkamchai, S.; Otranto, D.; Kroidl, I.; Dennebaum, M.; Le, T.H.; Le, T.A.; et al. The mitochondrial genomes of the zoonotic canine filarial parasites Dirofilaria (Nochtiella) repens and Candidatus Dirofilaria (Nochtiella) hongkongensis provide evidence for presence of cryptic species. PLoS Neglected Trop. Dis. 2016, 10, e0005028. [Google Scholar] [CrossRef]
- Boldis, V.; Ondriska, F.; Bošák, V.; Hajdúk, O.; Antolová, D.; Miterpáková, M. Pseudotumor of the epididymis, a rare clinical presentation of human Dirofilaria repens infection: A report of autochthonous case of dirofilariasis in southwestern Slovakia. Acta Parasitol. 2020, 65, 550–553. [Google Scholar] [CrossRef]
- Hattendorf, C.; Lühken, R. Dirofilaria immitis and D. repens in Europe: A systematic literature review on vectors, host range, and the spatial distribution in the 20th and 21st century. bioRxiv 2025. [Google Scholar] [CrossRef]
- Masny, A.; Gołąb, E.; Cielecka, D.; Sałamatin, R. Vector-borne helminths of dogs and humans—Focus on central and eastern parts of Europe. Parasites Vectors 2013, 6, 38. [Google Scholar] [CrossRef]
- Széll, Z.; Bacsadi, Á.; Szeredi, L.; Nemes, C.; Fézer, B.; Bakcsa, E.; Kalla, H.; Tolnai, Z.; Sréter, T. Rapid spread and emergence of heartworm resulting from climate and climate-driven ecological changes in Hungary. Vet. Parasitol. 2020, 280, 109067. [Google Scholar] [CrossRef] [PubMed]
- Pampiglione, S.; Rivasi, F.; Angeli, G.; Boldorini, R.; Incensati, R.M.; Pastormerlo, M.; Pavesi, M.; Ramponi, A. Dirofilariasis due to Dirofilaria repens in Italy, an emergent zoonosis: Report of 60 new cases. Histopathology 2001, 38, 344–354. [Google Scholar] [CrossRef] [PubMed]
- Genchi, C.; Bowman, D.; Drake, J. Canine Heartworm Disease (Dirofilaria immitis) in Western Europe: Survey of Veterinary Awareness and Perceptions. Parasites Vectors 2014, 7, 206. [Google Scholar] [CrossRef]
- Herrin, B.H.; Peregrine, A.S.; Goring, J.; Beall, M.J.; Little, S.E. Canine Infection with Borrelia burgdorferi, Dirofilaria immitis, Anaplasma spp. and Ehrlichia spp. in Canada, 2013–2014. Parasites Vectors 2017, 10, 244. [Google Scholar] [CrossRef]
- Schäfer, I.; Volkmann, M.; Beelitz, P.; Merle, R.; Müller, E.; Kohn, B. Retrospective Analysis of Vector-Borne Infections in Dogs after Travelling to Endemic Areas (2007–2018). Vet. Parasitol. 2019, 276, 100015. [Google Scholar] [CrossRef]
- Coman, S.; Băcescu, B.; Coman, T. Epidemiological and paraclinical aspects of canine dirofilariosis. Lucr. Stiinł. Med. Vet. 2007, 40, 333–339. [Google Scholar]
- Ciocan, R.; Dărăbuș, G.; Igna, V. Morphometric study of microfilariae of Dirofilaria spp. on dogs. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca. Vet. Med. 2010, 67, 45–49. [Google Scholar]
- Ciocan, R.; Mederle, N.; Jacsó, O.; Tánczos, B.; Fok, É. Autochthonous cases of Dirofilaria in dogs from Timiș county (western part) Romania. Glob. J. Med. Res. 2013, 13, 29–34. [Google Scholar]
- Mircean, V.; Dumitrache, M.O.; Györke, A.; Pantchev, N.; Jodies, R.; Mihalca, A.D.; Cozma, V. Seroprevalence and geographic distribution of Dirofilaria immitis and tick-borne infections (Anaplasma phagocytophilum, Borrelia burgdorferi sensu lato, and Ehrlichia canis) in dogs from Romania. Vector-Borne Zoonotic Dis. 2012, 12, 595–604. [Google Scholar] [CrossRef]
- Ionică, A.M.; Matei, I.A.; Mircean, V.; Dumitrache, M.O.; D’aMico, G.; Győrke, A.; Pantchev, N.; Annoscia, G.; Albrechtová, K.; Otranto, D.; et al. Current surveys on the prevalence and distribution of Dirofilaria spp. and Acanthocheilonema reconditum infections in dogs in Romania. Parasitol. Res. 2015, 114, 975–982. [Google Scholar] [CrossRef]
- Ionică, A.M.; Matei, I.A.; D’aMico, G.; Daskalaki, A.A.; Juránková, J.; Ionescu, D.T.; Mihalca, A.D.; Modrý, D.; Gherman, C.M. Role of golden jackals (Canis aureus) as natural reservoirs of Dirofilaria spp. in Romania. Parasites Vectors 2016, 9, 240. [Google Scholar] [CrossRef] [PubMed]
- Tomazatos, A.; Cadar, D.; Török, E.; Maranda, I.; Horváth, C.; Keresztes, L.; Spinu, M.; Jansen, S.; Jöst, H.; Schmidt-Chanasit, J.; et al. Circulation of Dirofilaria immitis and Dirofilaria repens in the Danube Delta Biosphere Reserve, Romania. Parasites Vectors 2018, 11, 392. [Google Scholar] [CrossRef]
- Morchón, R.; Montoya-Alonso, J.; Rodríguez-Escolar, I.; Carretón, E. What has happened to heartworm disease in Europe in the last 10 years? Pathogens 2022, 11, 1042. [Google Scholar] [CrossRef]
- Simón, F.; Diosdado, A.; Siles-Lucas, M.; Kartashev, V.; González-Miguel, J. Human dirofilariosis in the 21st century: A scoping review of clinical cases reported in the literature. Transbound. Emerg. Dis. 2022, 69, 2424–2439. [Google Scholar] [CrossRef]
- Rodríguez-Escolar, I.; Hernández-Lambraño, R.E.; Sánchez-Agudo, J.A.; Collado-Cuadrado, M.; Savić, S.; Stosic, Z.M.; Marcic, D.; Morchón, R. Prediction and validation of potential transmission risk of Dirofilaria spp. infection in Serbia and its projection to 2080. Front. Vet. Sci. 2024, 11, 1352236. [Google Scholar] [CrossRef]
- Yan, J.; Mackay, A.; Stone, C.M. Dynamics of invasive mosquitoes: Introduction pathways, limiting factors, and their potential role in vector-borne pathogen transmission. Front. Trop. Dis. 2024, 5, 1503120. [Google Scholar] [CrossRef]
- Ames, M.K.; Atkins, C.E. Treatment of dogs with severe heartworm disease. Vet. Parasitol. 2020, 283, 109131. [Google Scholar] [CrossRef] [PubMed]
- Pinto, C.J.; Labarthe, N.V.; Paiva, J.P.; Reifur, L.; Mendes-de-Almeida, F.; Merlo, A.; Juliani, P.J.; Ornelas de Almeida, M.A.; Alves, L.C. Updated canine infection rates for Dirofilaria immitis in areas of Brazil previously identified as having a high incidence of heartworm-infected dogs. Parasites Vectors 2014, 7, 493. [Google Scholar]
- Saha, B.K.; Bonnier, A.; Chong, W.H.; Chieng, H.; Austin, A.; Hu, K.; Shkolnik, B. Human pulmonary dirofilariasis: A review for the clinicians. Am. J. Med. Sci. 2022, 363, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Miterpáková, M.; Antolová, D.; Rampalová, J.; Undesser, M.; Krajčovič, T.; Víchová, B. Dirofilaria immitis pulmonary dirofilariasis, Slovakia. Emerg. Infect. Dis. 2022, 28, 482–485. [Google Scholar] [CrossRef]
- Silva, M.J.; Costa, A.R.; Calvinho, P. Human pulmonary dirofilariasis: A pitfall in solitary pulmonary nodule. Pulmonology 2022, 28, 413–414. [Google Scholar] [CrossRef]
- Diakou, A.; Prichard, R.K. Concern for Dirofilaria immitis and macrocyclic lactone loss of efficacy: Current situation in the USA and Europe, and future scenarios. Pathogens 2021, 10, 132. [Google Scholar] [CrossRef]
- Aykur, M.; Yağcı, A.; Simşek, S.; Palamar, M.; Yaman, B.; Korkmaz, M.; Dagci, H. First time identification of subconjunctival Dirofilaria immitis in Turkey: Giant episcleral granuloma mimicking scleritis. Parasitol. Res. 2021, 120, 3909–3914. [Google Scholar] [CrossRef] [PubMed]
- Cope, E.D.; Gupta, N.; Koehler, A.V.; Gasser, R.B.; Crowe, A. Ocular dirofilariasis in migrant from Sri Lanka, Australia. Emerg. Infect. Dis. 2024, 30, 829–830. [Google Scholar] [CrossRef] [PubMed]
- Mahesh, M.; Pauly, M.; Krishna, S.M.; Raman, M.; Biswas, J. Clinicopathological study of parasitic lesions of the eye and ocular adnexa in a tertiary care ophthalmic center in South India. Indian J. Ophthalmol. 2022, 70, 1713–1717. [Google Scholar] [CrossRef]
- Camacho, M.; Antonietti, M.; Sayegh, Y.; Colson, J.D.; Kunkler, A.L.; Clauss, K.D.; Muniz-Castro, H.; Lee, W.W.; Yoo, S.H.; Johnson, T.E.; et al. Ocular dirofilariasis: A clinicopathologic case series and literature review. Ocul. Oncol. Pathol. 2024, 10, 43–52. [Google Scholar] [CrossRef]
- Redón-Soriano, M.; Blasco, A.; Gomila, B.; González-Sánchez, M.; Simón, F.; Esteban, J.G. Subconjunctival human dirofilariasis by Dirofilaria repens in the Mediterranean Basin. Am. J. Ophthalmol. Case Rep. 2022, 26, 101570. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, Z.; Kar, P.; Mohanty, S.; Dey, M.; Kumar Samal, D. Ocular dirofilariasis: A report from Odisha. Indian J. Med. Microbiol. 2023, 45, 100388. [Google Scholar] [CrossRef]
- Poliakova, S.I.; Karliuga, I.A.; Moloda, A.L.; Linchevska, O.G. Dirofilariasis of eyelid and orbit (clinic, diagnosis, treatment). J. Ophthalmol. 2023, 1, 27–33. [Google Scholar]
- Potters, I.; Vanfraechem, G.; Bottieau, E. Dirofilaria repens nematode infection with microfilaremia in traveler returning to Belgium from Senegal. Emerg. Infect. Dis. 2018, 24, 1761. [Google Scholar] [CrossRef]
- Pupić-Bakrač, A.; Pupić-Bakrač, J.; Beck, A.; Jurković, D.; Polkinghorne, A.; Beck, R. Dirofilaria repens microfilaremia in humans: Case description and literature review. One Health 2021, 13, 2352–7714. [Google Scholar] [CrossRef]
- Noack, S.; Harrington, J.; Carithers, D.S.; Kaminsky, R.; Selzer, P.M. Heartworm disease–Overview, intervention, and industry perspective. Int. J. Parasitol. Drugs Drug Resist. 2021, 16, 65–89. [Google Scholar] [CrossRef]
- Otranto, D.; Dantas-Torres, F.; Brianti, E.; Traversa, D.; Petrić, D.; Genchi, C.; Capelli, G. Vector-borne helminths of dogs and humans in Europe. Parasites Vectors 2013, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Cancrini, G.; Gabrielli, S. Dirofilariosis in humans: A review of world literature. Parassitologia 2007, 49, 121–125. [Google Scholar]
- Genchi, C.; Kramer, L.H. Subcutaneous dirofilariosis (Dirofilaria repens): An infection spreading throughout the old world. Parasites Vectors 2017, 10, 517. [Google Scholar] [CrossRef]
- Șuleșco, T.; von Thien, H.; Toderaș, L.; Toderaș, I.; Lühken, R.; Tannich, E. Circulation of Dirofilaria repens and Dirofilaria immitis in Moldova. Parasites Vectors 2016, 9, 627. [Google Scholar] [CrossRef]
- Rinaldi, L.; Genchi, C.; Cascone, C.; Oliva, G.; Musella, V. Canine and feline dirofilariosis in Italy: An update. Parasitol. Res. 2011, 109, 17–23. [Google Scholar]
- Morchón, R.; Carretón, E.; González-Miguel, J.; Mellado-Hernández, I. Heartworm disease (Dirofilaria immitis) and their vectors in Europe–new distribution trends. Front. Physiol. 2012, 3, 196. [Google Scholar] [CrossRef]
- Becker, N.; Petric, D.; Zgomba, M.; Boase, C.; Madon, M.B.; Dahl, C.; Kaiser, A. Mosquitoes and Their Control; Springer: Heidelberg, Germany, 2010. [Google Scholar] [CrossRef]
- Turcitu, M.A. Universal workflow for pathogen detection using real time (RT) PCR. Rom. J. Vet. Med. Pharmacol. 2024, 49, 276–287. [Google Scholar]
- Sandu, I.; Deak, G.; Turcitu, M.; O’Brien, P.J.; Mircean, V. A severe clinical case of Ehrlichia canis and Toxoplasma gondii in a dog (with the first morphological detection of tachyzoites in peripheral blood). Vet. Med. Sci. 2025, 11, e70380. [Google Scholar] [CrossRef] [PubMed]
- Pękacz, M.; Basałaj, K.; Kalinowska, A.; Klockiewicz, M.; Stopka, D.; Bąska, P.; Długosz, E.; Karabowicz, J.; Młocicki, D.; Wiśniewski, M.; et al. Selection of new diagnostic markers for Dirofilaria repens infections with the use of phage display technology. Sci. Rep. 2022, 12, 2288. [Google Scholar] [CrossRef]
- Negron, V.; Saleh, M.N.; Sobotyk, C.; Luksovsky, J.L.; Harvey, T.V.; Verocai, G.G. Probe-based qPCR as an alternative to modified Knott’s test when screening dogs for heartworm (Dirofilaria immitis) infection in combination with antigen detection tests. Parasites Vectors 2022, 15, 306. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, K.; Peng, J. Some properties of the exact and score methods for binomial proportion and sample size calculation. Commun. Stat.—Simul. Comput. 2007, 36, 1171–1186. [Google Scholar] [CrossRef]
- Zhang, B.; Bilder, C.; Biggerstaff, B.; Schaarschmidt, F.; Hitt, B. binGroup: Evaluation and Experimental Design for Binomial Group Testing, Version 2.2-3; The R Foundation: Kaysville, UT, USA, 2025. [CrossRef]
- Boucher, O.; Denvil, S.; Levavasseur, G.; Cozic, A.; Caubel, A.; Foujols, M.A.; Meurdesoif, Y.; Devilliers, M.; Flavoni, S.; Gastineau, G.; et al. IPSL IPSL-CM6A-LR Model Output Prepared for CMIP6 DCPP dcppC-amv-pos, Version VLR; Earth System Grid Federation: Rockville, MD, USA, 2019.
- Genchi, C.; Rinaldi, L.; Mortarino, M.; Genchi, M.; Cringoli, G. Climate and Dirofilaria infection in Europe. Vet. Parasitol. 2009, 163, 286–292. [Google Scholar] [CrossRef]
- Ivănescu, L.M.; Bodale, I.; Grigore-Hristodorescu, S.; Martinescu, G.; Andronic, B.; Matiut, S.; Azoicai, D.; Miron, L. The risk of emerging of dengue fever in Romania, in the context of global warming. Trop. Med. Infect. Dis. 2023, 8, 65. [Google Scholar] [CrossRef]
- Ciucă, L.; Musella, V.; Miron, L.D.; Maurelli, M.P.; Cringoli, G.; Bosco, A.; Rinaldi, L. Geographic distribution of canine heartworm (Dirofilaria immitis) infection in stray dogs of eastern Romania. Geospat. Health 2016, 11, 499. [Google Scholar] [CrossRef]
- Matfei, A.; Ivănescu, L.; Mîndru, R.; Martinescu, G.; Lazăr, A.H.; Miron, L. Molecular detection of canine dirofilariosis (D. immitis, D. repens) in Danube Delta region. Rev. Rom. Med. Vet. 2025, 35, 83–88. [Google Scholar]
- Younes, L.; Davoust, B.; Varloud, M.; Niang, E.A.; Fenollar, F.; Medianniko, O. Development of a multiplex qPCR-based approach for the diagnosis of Dirofilaria immitis, D. repens and Acanthocheilonema reconditum. Parasites Vectors 2020, 13, 319. [Google Scholar] [CrossRef]
- Younes, L.; Barré-Cardi, H.; Bedjaoui, S.; Ayhan, N.; Varloud, M.; Mediannikov, O. Dirofilaria immitis and Dirofilaria repens in mosquitoes from Corsica Island, France. Parasites Vectors 2021, 14, 427. [Google Scholar] [CrossRef]
- Kariuki, F.M.; Wanyonyi, R.W.; Islam, A.S. Analysis of a two-stage negative binomial group testing model for estimating the prevalence of a rare trait. Open Access Libr. J. 2023, 10, 6. [Google Scholar] [CrossRef]
- Stoleriu, L.; Bodale, I.; Apetrei, A.; Stancu, A. Realistic reversible magnetization component in Preisach-type models. IEEE Trans. Magn. 2010, 46, 2341–2344. [Google Scholar] [CrossRef]
- Lai, C.H.; Tung, K.C.; Ooi, H.K.; Wang, J.S. Competence of Aedes albopictus and Culex quinquefasciatus as vector of Dirofilaria immitis after blood meal with different microfilarial density. Vet. Parasitol. 2000, 90, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Chandra, G. Nature limits filarial transmission. Parasites Vectors 2008, 1, 13. [Google Scholar] [CrossRef] [PubMed]
- Ledesma, N.; Harrington, L. Mosquito vectors of dog heartworm in the United States: Vector status and factors influencing transmission efficiency. Top. Companion Anim. Med. 2011, 26, 178–185. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).