Heme Oxygenase-1 Expression as a Prognostic Marker in Early-Stage HCC Undergoing Resection or Liver Transplantation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Allocation
2.2. Study Groups
2.3. Immunohistochemistry
2.4. Immunohistochemical Assessment Method
2.5. Statistical Analysis
3. Results
3.1. Clinicopathological Parameters and HO-1 Expression in the Study Group
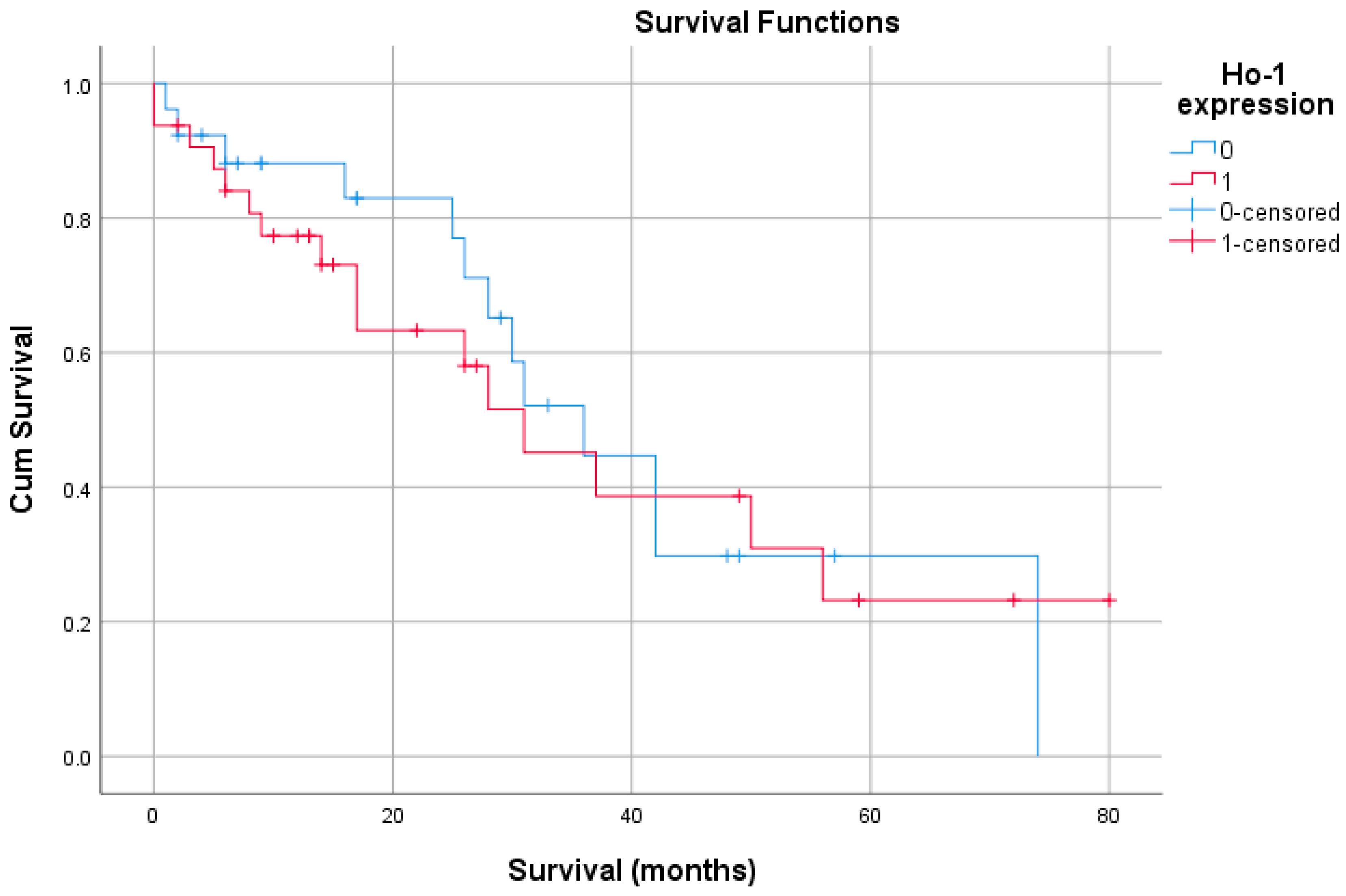
3.2. Survival and HO-1 Expression in the Study Group
4. Discussion
4.1. Clinical Implications
4.2. Strengths and Limitations of the Study
4.3. Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HCC | hepatocellular carcinoma |
| HO-1 | heme oxygenase-1 |
| HBV | hepatitis B virus |
| OS | overall survival |
| HCV | hepatitis C virus |
| NAFLD | nonalcoholic fatty liver disease |
| NASH | nonalcoholic steato-hepatitis |
| IAPs | inhibitors of apoptosis proteins |
| BCLC | Barcelona Clinic Liver Cancer |
| TACE | trans arterial chemoembolization |
| IRS | immunoreactivity score |
| ROC | receiver operating characteristic |
| AUC | area under the ROC curve |
| EMT | epithelial-to-mesenchymal transition. |
| VEGF | vascular endothelial growth factor |
Appendix A

References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Teng, M.L.; Ng, C.H.; Huang, D.Q.; Chan, K.E.; Tan, D.J.; Lim, W.H.; Yang, J.D.; Tan, E.; Muthiah, M.D. Global incidence and prevalence of nonalcoholic fatty liver disease. Clin. Mol. Hepatol. 2023, 29, S32–S42. [Google Scholar] [CrossRef]
- Petrick, J.L.; Florio, A.A.; Znaor, A.; Ruggieri, D.; Laversanne, M.; Alvarez, C.S.; Ferlay, J.; Valery, P.C.; Bray, F.; McGlynn, K.A. International trends in hepatocellular carcinoma incidence, 1978–2012. Int. J. Cancer 2020, 147, 317–330. [Google Scholar] [CrossRef]
- Nagtegaal, I.D.; Odze, R.D.; Klimstra, D.; Paradis, V.; Rugge, M.; Schirmacher, P.; Washington, K.M.; Carneiro, F.; Cree, I.A.; the WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020, 76, 182–188. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y. Changing etiology and epidemiology of hepatocellular carcinoma: Asia and worldwide. J. Liver Cancer 2024, 24, 622–670. [Google Scholar] [CrossRef]
- Schoemaker, M.H.; Ros, J.E.; Homan, M.; Trautwein, C.; Liston, P.; Poelstra, K.; van Goor, H.; Jansen, P.L.; Moshage, H. Cytokine regulation of pro- and anti-apoptotic genes in rat hepatocytes: NF-κB-regulated inhibitor of apoptosis protein 2 (cIAP2) prevents apoptosis. J. Hepatol. 2002, 36, 742–750. [Google Scholar] [CrossRef]
- Yeh, C.-N.; Wu, R.-C.; Cheng, C.-T.; Tsai, C.-Y.; Chang, Y.-R.; Yeh, T.-S.; Wu, T.-H.; Lee, W.-C.; Chiang, K.-C. HO-1 is a favorable prognostic factor for HBV-HCC patients who underwent hepatectomy. Cancer Manag. Res. 2018, 10, 6049–6059. [Google Scholar] [CrossRef] [PubMed]
- Maines, M.D. The heme oxygenase system: Past, present, and future. Antioxid. Redox Signal. 2004, 6, 797–801. [Google Scholar]
- Sacca, P.; Caballero, F.; Batlle, A.; Vazquez, E. Cell cycle arrest and modulation of HO-1 expression induced by acetyl salicylic acid in hepatocarcinogenesis. Int. J. Biochem. Cell Biol. 2004, 36, 1945–1953. [Google Scholar] [CrossRef]
- Jozkowicz, A.; Was, H.; Dulak, J. Heme oxygenase-1 in tumors: Is it a false friend? Antioxid. Redox Signal. 2007, 9, 2099–2118. [Google Scholar] [CrossRef]
- Liu, N.; Wang, X.; McCoubrey, W.K.; Maines, M.D. Developmentally regulated expression of two transcripts for heme oxygenase-2 with a first exon unique to rat testis: Control by corticosterone of the oxygenase protein expression. Gene 2000, 241, 175–183. [Google Scholar] [CrossRef]
- Waza, A.A.; Hamid, Z.; Ali, S.; Bhat, S.A.; Bhat, M.A. A review on heme oxygenase-1 induction: Is it a necessary evil. Inflamm. Res. 2018, 67, 579–588. [Google Scholar] [CrossRef]
- Ryter, S.W. Heme oxygenase-1/carbon monoxide as modulators of autophagy and inflammation. Arch. Biochem. Biophys. 2019, 678, 108186. [Google Scholar] [CrossRef] [PubMed]
- Sass, G.; Barikbin, R.; Tiegs, G. The multiple functions of heme oxygenase-1 in the liver. Z. Gastroenterol. 2012, 50, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Chiang, S.-K.; Chen, S.-E.; Chang, L.-C. A Dual Role of Heme Oxygenase-1 in Cancer Cells. Int. J. Mol. Sci. 2018, 20, 39. [Google Scholar] [CrossRef] [PubMed]
- Ghaziani, T.; Shan, Y.; Lambrecht, R.W.; Donohue, S.E.; Pietschmann, T.; Bartenschlager, R.; Bonkovsky, H.L. HCV proteins increase expression of heme oxygenase-1 (HO-1) and decrease expression of Bach1 in human hepatoma cells. J. Hepatol. 2006, 45, 5–12. [Google Scholar] [CrossRef]
- Abdalla, M.Y.; Britigan, B.E.; Wen, F.; Icardi, M.; McCormick, M.L.; LaBrecque, D.R.; Voigt, M.; Brown, K.E.; Schmidt, W.N. Down-regulation of heme oxygenase-1 by hepatitis C virus infection in vivo and by the in vitro expression of hepatitis C core protein. J. Infect. Dis. 2004, 190, 1109–1118. [Google Scholar] [CrossRef]
- Wen, F.; Brown, K.E.; Britigan, B.E.; Schmidt, W.N. Hepatitis C core protein inhibits induction of heme oxygenase-1 and sensitizes hepatocytes to cytotoxicity. Cell Biol. Toxicol. 2008, 24, 175–188. [Google Scholar] [CrossRef]
- Jopling, C.L.; Yi, M.; Lancaster, A.M.; Lemon, S.M.; Sarnow, P. Modulation of hepatitis C virus RNA abundance by a liver-specific MicroRNA. Science 2005, 309, 1577–1581. [Google Scholar] [CrossRef]
- Shan, Y.; Zheng, J.; Lambrecht, R.W.; Bonkovsky, H.L. Reciprocal effects of micro-RNA-122 on expression of heme oxygenase-1 and hepatitis C virus genes in human hepatocytes. Gastroenterology 2007, 133, 1166–1174. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Tian, Q.; Zheng, J.; Bonkovsky, H.L. MicroRNA-196 represses Bach1 protein and hepatitis C virus gene expression in human hepatoma cells expressing hepatitis C viral proteins. Hepatolgy 2010, 51, 1494–1504. [Google Scholar] [CrossRef] [PubMed]
- Antibodies, Proteins, Kits and Reagents for Life Science|Abcam. Available online: https://doc.abcam.com/datasheets/active/ab13248/en-cn/heme-oxygenase-1-antibody-ho-1-1-ab13248.pdf (accessed on 28 September 2025).
- Lee, D.K. Alternatives to P value: Confidence interval and effect size. Korean J. Anesthesiol. 2016, 69, 555–562. [Google Scholar] [CrossRef]
- de Hond, A.A.H.; Steyerberg, E.W.; van Calster, B. Interpreting area under the receiver operating characteristic curve. Lancet Digit. Health 2022, 4, e853–e855. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wei, C. Advances in the early diagnosis of hepatocellular carcinoma. Genes Dis. 2020, 7, 308–319. [Google Scholar] [CrossRef]
- Chau, L.-Y. Heme oxygenase-1: Emerging target of cancer therapy. J. Biomed. Sci. 2015, 22, 22. [Google Scholar] [CrossRef]
- Mascaró, M.; Alonso, E.N.; Alonso, E.G.; Lacunza, E.; Curino, A.C.; Facchinetti, M.M. Nuclear Localization of Heme Oxygenase-1 in Pathophysiological Conditions: Does It Explain the Dual Role in Cancer? Antioxidants 2021, 10, 87. [Google Scholar] [CrossRef]
- Vanella, L.; Barbagallo, I.; Tibullo, D.; Forte, S.; Zappalà, A.; Volti, G.L. The non-canonical functions of the heme oxygenases. Oncotarget 2016, 7, 69075–69086. [Google Scholar] [CrossRef]
- Tsuji, M.H.; Yanagawa, T.; Iwasa, S.; Tabuchi, K.; Onizawa, K.; Bannai, S.; Toyooka, H.; Yoshida, H. Heme oxygenase-1 expression in oral squamous cell carcinoma as involved in lymph node metastasis. Cancer Lett. 1999, 138, 53–59. [Google Scholar] [CrossRef]
- Jun, S.-Y.; Hong, S.-M.; Bae, Y.K.; Kim, H.K.; Jang, K.Y.; Eom, D.W. Clinicopathological and prognostic significance of heme oxygenase-1 expression in small intestinal adenocarcinomas. Pathol. Int. 2018, 68, 294–300. [Google Scholar] [CrossRef]
- Xu, J.; Zhu, K.; Wang, Y.; Chen, J. The dual role and mutual dependence of heme/HO-1/Bach1 axis in the carcinogenic and anti-carcinogenic intersection. J. Cancer Res. Clin. Oncol. 2023, 149, 483–501. [Google Scholar] [CrossRef]
- Schmidt, W.N.; Mathahs, M.M.; Zhu, Z. Heme and HO-1 Inhibition of HCV, HBV, and HIV. Front. Pharmacol. 2012, 3, 129. [Google Scholar] [CrossRef]
- Bauer, I.; Rensing, H.; Florax, A.; Ulrich, C.; Pistorius, G.; Redl, H.; Bauer, M. Expression pattern and regulation of heme oxygenase-1/heat shock protein 32 in human liver cells. Shock 2003, 20, 116–122. [Google Scholar] [CrossRef]
- Zhu, Z.; Wilson, A.T.; Mathahs, M.M.; Wen, F.; Brown, K.E.; Luxon, B.A.; Schmidt, W.N. Heme oxygenase-1 suppresses hepatitis C virus replication and increases resistance of hepatocytes to oxidant injury. Hepatology 2008, 48, 1430–1439. [Google Scholar] [CrossRef] [PubMed]
- Nitti, M.; Ivaldo, C.; Traverso, N.; Furfaro, A.L. Clinical Significance of Heme Oxygenase 1 in Tumor Progression. Antioxidants 2021, 10, 789. [Google Scholar] [CrossRef]
- Gong, G.; Waris, G.; Tanveer, R.; Siddiqui, A. Human hepatitis C virus NS5A protein alters intracellular calcium levels, induces oxidative stress, and activates STAT-3 and NF-κB. Proc. Natl. Acad. Sci. USA 2001, 98, 9599–9604. [Google Scholar] [CrossRef]
- Bai, Z.; Li, H.; Jiao, B. Potential Therapeutic Effect of Sinigrin on Diethylnitrosamine-Induced Liver Cancer in Mice: Exploring the Involvement of Nrf-2/HO-1, PI3K–Akt–mTOR Signaling Pathways, and Apoptosis. ACS Omega 2024, 9, 46064–46073. [Google Scholar] [CrossRef]
- Abdalla, M.Y.; Mathahs, M.M.; Ahmad, I.M. Protective role of heme oxygenase-1 in liver. Biologia 2012, 67, 623–628. [Google Scholar] [CrossRef]
- Gandini, N.A.; Alonso, E.N.; Fermento, M.E.; Mascaró, M.; Abba, M.C.; Coló, G.P.; Arévalo, J.; Ferronato, M.J.; Guevara, J.A.; Núñez, M.; et al. Heme Oxygenase-1 Has an Antitumor Role in Breast Cancer. Antioxid. Redox Signal. 2019, 30, 2030–2049. [Google Scholar] [CrossRef] [PubMed]
- Gandini, N.A.; Fermento, M.E.; Salomón, D.G.; Blasco, J.; Patel, V.; Gutkind, J.S.; Molinolo, A.A.; Facchinetti, M.M.; Curino, A.C. Nuclear localization of heme oxygenase-1 is associated with tumor progression of head and neck squamous cell carcinomas. Exp. Mol. Pathol. 2012, 93, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Fang, J.; Liao, L.; Maeda, H.; Su, Q. Upregulation of heme oxygenase-1 in colorectal cancer patients with increased circulation carbon monoxide levels, potentially affects chemotherapeutic sensitivity. BMC Cancer 2014, 14, 436. [Google Scholar] [CrossRef]
- Krukowska, K.; Magierowski, M. Carbon monoxide (CO)/heme oxygenase (HO)-1 in gastrointestinal tumors pathophysiology and pharmacology—Possible anti- and pro-cancer activities. Biochem. Pharmacol. 2022, 201, 115058. [Google Scholar] [CrossRef]
- Hofmann, A.; Hamann, B.; Klimova, A.; Müglich, M.; Wolk, S.; Busch, A.; Frank, F.; Sabarstinski, P.; Kapalla, M.; Nees, J.A.; et al. Pharmacotherapies and Aortic Heme Oxygenase-1 Expression in Patients with Abdominal Aortic Aneurysm. Antioxidants 2022, 11, 1753. [Google Scholar] [CrossRef]
- Loboda, A.; Jozkowicz, A.; Dulak, J. HO-1/CO system in tumor growth, angiogenesis and metabolism—Targeting HO-1 as an anti-tumor therapy. Vasc. Pharmacol. 2015, 74, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Nitti, M.; Piras, S.; Marinari, U.M.; Moretta, L.; Pronzato, M.A.; Furfaro, A.L. HO-1 Induction in Cancer Progression: A Matter of Cell Adaptation. Antioxidants 2017, 6, 29. [Google Scholar] [CrossRef] [PubMed]
- Furfaro, A.L.; Traverso, N.; Domenicotti, C.; Piras, S.; Moretta, L.; Marinari, U.M.; Pronzato, M.A.; Nitti, M. The Nrf2/HO-1 Axis in Cancer Cell Growth and Chemoresistance. Oxidative Med. Cell. Longev. 2016, 2016, 1958174. [Google Scholar] [CrossRef]
- Xiong, Y.; Cao, P.; Lei, X.; Tang, W.; Ding, C.; Qi, S.; Chen, G. Accurate prediction of microvascular invasion occurrence and effective prognostic estimation for patients with hepatocellular carcinoma after radical surgical treatment. World J. Surg. Oncol. 2022, 20, 328. [Google Scholar] [CrossRef]
- Lu, J.-J.; Abudukeyoumu, A.; Zhang, X.; Liu, L.-B.; Li, M.-Q.; Xie, F. Heme oxygenase 1: A novel oncogene in multiple gynecological cancers. Int. J. Biol. Sci. 2021, 17, 2252–2261. [Google Scholar] [CrossRef]
- Giannelli, G.; Koudelkova, P.; Dituri, F.; Mikulits, W. Role of epithelial to mesenchymal transition in hepatocellular carcinoma. J. Hepatol. 2016, 65, 798–808. [Google Scholar] [CrossRef]
- Riquelme, S.A.; Carreño, L.J.; Espinoza, J.A.; Mackern-Oberti, J.P.; Alvarez-Lobos, M.M.; Riedel, C.A.; Bueno, S.M.; Kalergis, A.M. Modulation of antigen processing by haem-oxygenase 1. Implications on inflammation and tolerance. Immunology 2016, 149, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Vijayan, V.; Wagener, F.A.; Immenschuh, S. The macrophage heme-heme oxygenase-1 system and its role in inflammation. Biochem. Pharmacol. 2018, 153, 159–167. [Google Scholar] [CrossRef]
- Jie, X.X.; Zhang, X.Y.; Xu, C.J. Epithelial-to-mesenchymal transition, circulating tumor cells and cancer metastasis: Mechanisms and clinical applications. Oncotarget 2017, 8, 81558–81571. [Google Scholar] [CrossRef]
- Calay, D.; Mason, J.C. The multifunctional role and therapeutic potential of HO-1 in the vascular endothelium. Antioxid. Redox Signal. 2014, 20, 1789–1809. [Google Scholar] [CrossRef]
- Salloom, R.J.; Sahtout, D.Z.; Ahmad, I.M.; Abdalla, M.Y. Synergistic effects of HO-1 inhibition and chemotherapy on tumor proliferation and immune infiltration: An in vitro and in vivo approach to enhancing prostate cancer treatment. Transl. Oncol. 2025, 54, 102339. [Google Scholar] [CrossRef]
- Goodman, A.I.; Choudhury, M.; da Silva, J.-L.; Schwartzman, M.L.; Abraham, N.G. Overexpression of the heme oxygenase gene in renal cell carcinoma. Proc. Soc. Exp. Biol. Med. 1997, 214, 54–75. [Google Scholar] [CrossRef]
- Was, H.; Dulak, J.; Jozkowicz, A. Heme oxygenase-1 in tumor biology and therapy. Curr. Drug Targets 2010, 11, 1551–1570. [Google Scholar] [CrossRef] [PubMed]
- Schacter, B.A.; Kurz, P. Alterations in microsomal drug metabolism and heme oxygenase activity in isolated hepatic parenchymal and sinusoidal cells in Murphy-Sturm lymphosarcoma-bearing rats. Clin. Investig. Med. Med. Clin. Exp. 1986, 9, 150–155. [Google Scholar]
- Wang, H.; Cheng, Q.; Bao, L.; Li, M.; Chang, K.; Yi, X. Cytoprotective Role of Heme Oxygenase-1 in Cancer Chemoresistance: Focus on Antioxidant, Antiapoptotic, and Pro-Autophagy Properties. Antioxidants 2023, 12, 1217. [Google Scholar] [CrossRef] [PubMed]
- Berberat, P.O.; Dambrauskas, Z.; Gulbinas, A.; Giese, T.; Giese, N.; KünZli, B.; Autschbach, F.; Meuer, S.; BücHler, M.W.; Friess, H. Inhibition of heme oxygenase-1 increases responsiveness of pancreatic cancer cells to anticancer treatment. Clin. Cancer Res. 2005, 11, 3790–3798. [Google Scholar] [CrossRef]
- Miyake, M.; Fujimoto, K.; Anai, S.; Ohnishi, S.; Nakai, Y.; Inoue, T.; Matsumura, Y.; Tomioka, A.; Ikeda, T.; Okajima, E.; et al. Inhibition of heme oxygenase-1 enhances the cytotoxic effect of gemcitabine in urothelial cancer cells. Anticancer Res. 2010, 30, 2145–2152. [Google Scholar]
- Woo, H.Y.; Heo, J. The role of c-MET inhibitors in advanced hepatocellular carcinoma: Now and future. Ann. Transl. Med. 2020, 8, 1617. [Google Scholar] [CrossRef] [PubMed]
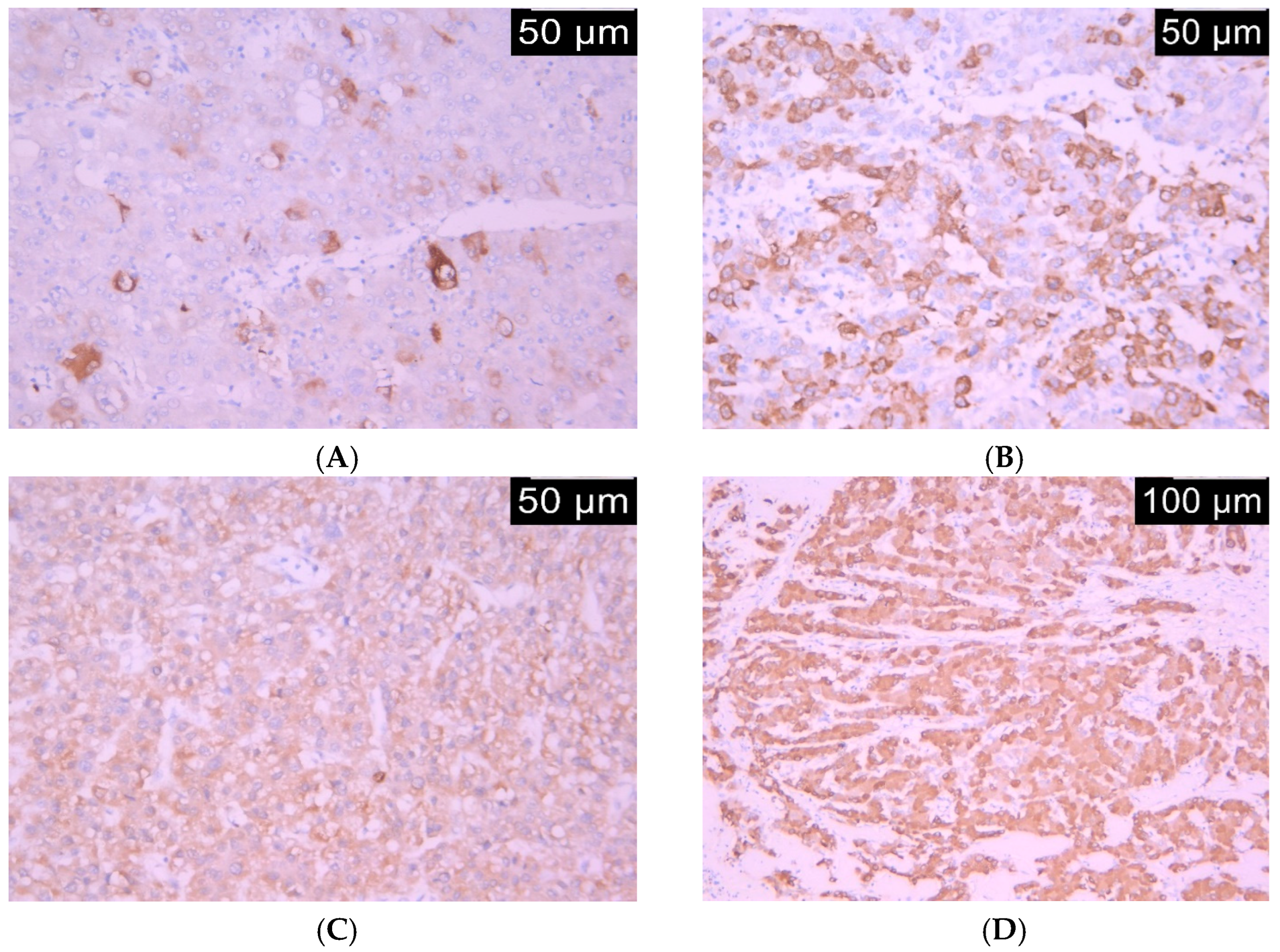
| Age Years, mean (SD) | 63.8 (11.13) |
| Male Genger, n, (%) | 35 (60.3) |
| Urban living environment, n, (%) | 36 (62.0) |
| ASA Score, n, (%) 0 1 2 | 12 (20.6) 16 (27.5) 30 (51.7) |
| Liver Cirhosis | 45 (77.5) |
| CHILD-PUGH SCORE A B C | 36 (62.0) 8 (13.7) 1 (1.72) |
| MILAN CRITERIA EXTRAMILAN | 34 (58.6) 24 (41.3) |
| Aetiology, n, (%) HBV HCV Toxic | 6 (10.3) 33 (56.9) 7 (12.1) |
| Clinicopathological Parameters | n | HO-1 Expression | p-Value | Cramer’s V | ||
|---|---|---|---|---|---|---|
| Low | High | |||||
| Gender | male | 49 | 36 (62.1%) | 13 (22.4%%) | 0.69 | 0.055 |
| female | 9 | 6 (10.3%) | 3 (5.2%) | |||
| Age (years) | <50 | 5 | 3 (5.2%) | 2 (3.4%) | 0.60 | 0.085 |
| >50 | 53 | 39 (67.2%) | 14 (24.1%) | |||
| HCV etiology | absent | 25 | 23 (39.7%) | 2 (3.4%) | 0.004 | 0.381 |
| present | 33 | 19 (32.8%) | 14 (24.1%) | |||
| HBV etiology | absent | 52 | 36 (62.1%) | 16 (27.8%) | 0.17 | 0.210 |
| present | 6 | 6 (10.3%) | 0 (0%) | |||
| Alcohol-related etiology | absent | 51 | 36 (62.1%) | 15 (25.9%) | 0.66 | 0.110 |
| present | 7 | 6 (10.3%) | 1 (1.7%) | |||
| Serum AFP (ng/mL) | 1–9 | 9 | 6 (10.3%) | 3 (5.2%) | 0.91 | 0.061 |
| 10–99 | 35 | 26 (44.8%) | 9 (15.5%) | |||
| >100 | 14 | 10 (17.2%) | 4 (6.9%) | |||
| Tumor size (cm) | 0-3 | 50 | 36 (62.1%) | 14 (24.1%) | 0.30 | 0.237 |
| 4/5 | 7 | 6 (10.3%) | 1 (1.7%) | |||
| >5 | 1 | 0 (0%) | 1 (1.7%) | |||
| Tumor number | 0–3 | 16 | 12 (20.7%) | 4 (6.9%) | 0.86 | 0.076 |
| 4/5 | 15 | 10 (17.2%) | 5 (8.6%) | |||
| >5 | 27 | 20 (34.5%) | 7 (12.1%) | |||
| Milan criteria for liver transplantation | eligible | 31 | 26 (44.8%) | 5 (8.6%) | 0.03 | 0.275 |
| noneligible | 27 | 16 (27.6%) | 11 (19%) | |||
| BCLC grading | Stage A | 48 | 35 (60.3%) | 13 (22.4%) | 1.000 | 0.025 |
| Stage B | 10 | 7 (12.1%) | 3 (5.2%) | |||
| Perioperative anticoagulant therapy | absent | 31 | 27 (46.6%) | 4 (6.9%) | 0.007 | 0.352 |
| present | 27 | 15 (25.9%) | 12 (20.7%) | |||
| Antiviral therapy | absent | 52 | 38 (65.5%) | 14 (24.1%) | 0.66 | 0.044 |
| present | 6 | 4 (6.9%) | 2 (3.4%) | |||
| Perioperative TACE | absent | 54 | 38 (65.5%) | 16 (27.6%) | 0.56 | 0.168 |
| present | 4 | 4 (6.9%) | 0 (0%) | |||
| Postoperative TACE | absent | 55 | 40 (69%) | 15 (25.9%) | 1.000 | 0.030 |
| present | 3 | 2 (3.4%) | 1 (1.7%) | |||
| Chemotherapy | absent | 43 | 30 (51.7%) | 13 (22.4%) | 0.52 | 0.100 |
| present | 15 | 12 (20.7%) | 3 (5.2%) | |||
| Liver cirrhosis | Absent | 18 | 14 (24.1%) | 4 (6.9%) | 0.75 | 0.081 |
| Present | 40 | 28 (48.3%) | 12 (20.7%) | |||
| Pathological Parameters | n | HO-1 Expression | p-Value | Cramer’s V | ||
|---|---|---|---|---|---|---|
| Low | High | |||||
| Trabecular growth pattern | absent | 4 | 2 (3.4%) | 2 (3.4%) | 0.30 | 0.136 |
| present | 54 | 40 (69%) | 14 (24.1%) | |||
| Solid growth pattern | absent | 31 | 23 (39.7%) | 8 (13.8%) | 0.77 | 0.043 |
| present | 27 | 19 (32.8%) | 8 (13.8%) | |||
| Pseudoglandular growth pattern | absent | 17 | 9 (15.5%) | 8 (13.8%) | 0.05 | 0.281 |
| present | 41 | 33 (56.9%) | 8 (13.8%) | |||
| Histological grade | well differentiated | 8 | 7 (12.1%) | 1 (1.7%) | 0.71 | 0.136 |
| moderately differentiated | 43 | 30 (51.7%) | 13 (22.4%) | |||
| poorly differentiated | 7 | 5 (8.6%) | 2 (3.4%) | |||
| Tumor stage | 1 | 19 | 17 (29.3%) | 2 (3.4%) | 0.08 | 0.339 |
| 2 | 29 | 17 (29.3%) | 12 (20.7%) | |||
| 3 | 7 | 5 (8.6%) | 2 (3.4%) | |||
| 4 | 3 | 3 (5.2%) | 0 (0%) | |||
| Lymphatic invasion | absent | 51 | 38 (65.5%) | 13 (22.4%) | 0.38 | 0.127 |
| present | 7 | 4 (6.9%) | 3 (5.2%) | |||
| Vascular invasion | absent | 29 | 25 (43.1%) | 4 (6.9%) | 0.02 | 0.309 |
| present | 29 | 17 (29.3%) | 12 (20.7%) | |||
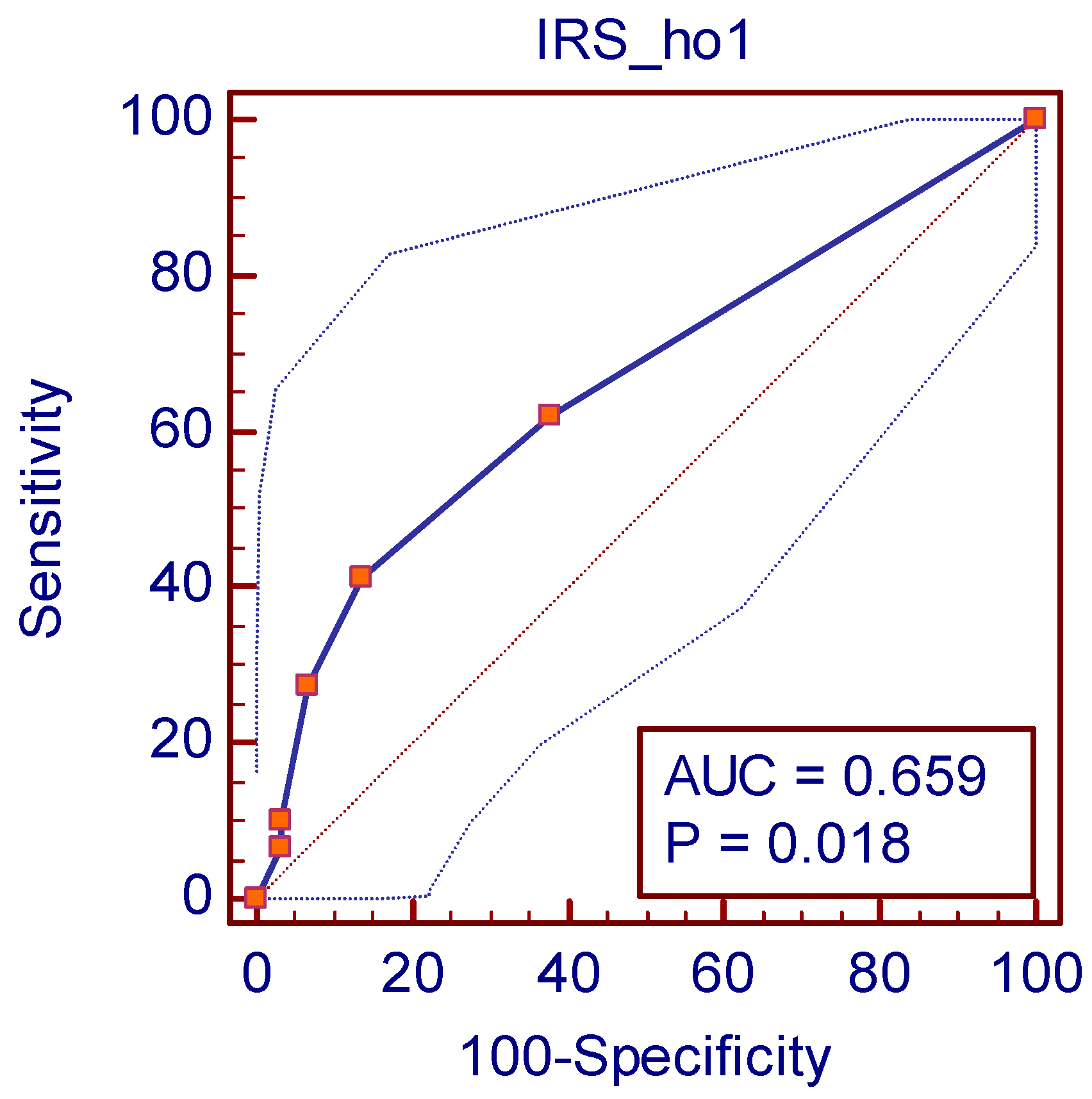
| Youden Index J | 0.2759 |
|---|---|
| Associated criterion | >1 |
| Sensitivity | 41.38 |
| Specificity | 86.21 |
| Estimate | Std. Error | 95% Confidence Interval | ||
|---|---|---|---|---|
| Lower Bound | Upper Bound | |||
| Negative | 40.829 | 5.996 | 29.077 | 52.581 |
| Positive | 37.607 | 6.147 | 25.560 | 49.655 |
| Overall | 38.606 | 4.259 | 30.258 | 46.953 |
| Ho-1 | Overall Survival | Disease Recurrence | ||
|---|---|---|---|---|
| Overall survival | Pearson Correlation | −0.061 | 1 | −0.047 |
| Sig. (2-tailed) | 0.650 | 0.728 | ||
| N | 58 | 58 | 58 | |
| Disease recurrence | Pearson Correlation | 0.014 | −0.047 | 1 |
| Sig. (2-tailed) | 0.915 | 0.728 | ||
| N | 58 | 58 | 58 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cadar, R.; Vasilescu, A.M.; Trofin, A.M.; Nastase, A.G.; Zabara, M.; Muzica, C.; Ursulescu, C.L.; Danciu, M.; Pascu, A.; Buzincu, I.; et al. Heme Oxygenase-1 Expression as a Prognostic Marker in Early-Stage HCC Undergoing Resection or Liver Transplantation. Life 2025, 15, 1589. https://doi.org/10.3390/life15101589
Cadar R, Vasilescu AM, Trofin AM, Nastase AG, Zabara M, Muzica C, Ursulescu CL, Danciu M, Pascu A, Buzincu I, et al. Heme Oxygenase-1 Expression as a Prognostic Marker in Early-Stage HCC Undergoing Resection or Liver Transplantation. Life. 2025; 15(10):1589. https://doi.org/10.3390/life15101589
Chicago/Turabian StyleCadar, Ramona, Alin Mihai Vasilescu, Ana Maria Trofin, Alexandru Grigorie Nastase, Mihai Zabara, Cristina Muzica, Corina Lupascu Ursulescu, Mihai Danciu, Andrei Pascu, Iulian Buzincu, and et al. 2025. "Heme Oxygenase-1 Expression as a Prognostic Marker in Early-Stage HCC Undergoing Resection or Liver Transplantation" Life 15, no. 10: 1589. https://doi.org/10.3390/life15101589
APA StyleCadar, R., Vasilescu, A. M., Trofin, A. M., Nastase, A. G., Zabara, M., Muzica, C., Ursulescu, C. L., Danciu, M., Pascu, A., Buzincu, I., Ciobanu, D., Victor, I., & Lupascu, C. D. (2025). Heme Oxygenase-1 Expression as a Prognostic Marker in Early-Stage HCC Undergoing Resection or Liver Transplantation. Life, 15(10), 1589. https://doi.org/10.3390/life15101589









