Hidden Impacts of Peritoneal Dialysis on the Endocrine System
Abstract
1. Introduction
2. Fluid Retention
3. Autonomic Nervous System Abnormality
4. Impaired Glucose Tolerance
5. Bone and Mineral Disorders
6. Renal Anemia
7. Limitations and Future Perspectives
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Bonomini, V.; Orsoni, G.; Stefoni, S.; Vangelista, A. Hormonal Changes in Uremia. Clin. Nephrol. 1979, 11, 275–280. [Google Scholar] [PubMed]
- Maher, J.F. Endocrine Abnormalities in Patients Treated by Continuous Ambulatory Peritoneal Dialysis. Blood Purif. 1990, 8, 69–75. [Google Scholar] [CrossRef]
- Kang, S.H.; Choi, E.W.; Park, J.W.; Cho, K.H.; Do, J.Y. Clinical Significance of the Edema Index in Incident Peritoneal Dialysis Patients. PLoS ONE 2016, 11, e0147070. [Google Scholar] [CrossRef]
- Guo, Q.; Lin, J.; Li, J.; Yi, C.; Mao, H.; Yang, X.; Yu, X. The Effect of Fluid Overload on Clinical Outcome in Southern Chinese Patients Undergoing Continuous Ambulatory Peritoneal Dialysis. Perit. Dial. Int. 2015, 35, 691–702. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, M.; Miyazaki, M.; Honda, K.; Kasai, K.; Tomo, T.; Nakamoto, H.; Kawanishi, H. Encapsulating Peritoneal Sclerosis in the Era of a Multi-Disciplinary Approach Based on Biocompatible Solutions: The NEXT-PD Study. Perit. Dial. Int. 2014, 34, 766–774. [Google Scholar] [CrossRef]
- Williams, J.D.; Craig, K.J.; Topley, N.; Von Ruhland, C.; Fallon, M.; Newman, G.R.; Mackenzie, R.K.; Williams, G.T. Morphologic Changes in the Peritoneal Membrane of Patients with Renal Disease. J. Am. Soc. Nephrol. 2002, 13, 470–479. [Google Scholar] [CrossRef]
- Hashimoto, K.; Kamijo, Y. Current Progress in Peritoneal Dialysis: A Narrative Review of Progress in Peritoneal Dialysis Fluid. Life 2025, 15, 279. [Google Scholar] [CrossRef] [PubMed]
- Kooman, J.P.; Cnossen, N.; Konings, C.J.; van der Sande, F.M.; Leunissen, K.M. Is There a Competition between Urine Volume and Peritoneal Ultrafiltration in Peritoneal Dialysis Patients? Contrib. Nephrol. 2006, 150, 111–118. [Google Scholar] [CrossRef]
- Tian, N.; Guo, Q.; Zhou, Q.; Cao, P.; Hong, L.; Chen, M.; Yang, X.; Yu, X. The Impact of Fluid Overload and Variation on Residual Renal Function in Peritoneal Dialysis Patient. PLoS ONE 2016, 11, e0153115. [Google Scholar] [CrossRef]
- Stoiser, B.; Mörtl, D.; Hülsmann, M.; Berger, R.; Struck, J.; Morgenthaler, N.G.; Bergmann, A.; Pacher, R. Copeptin, a Fragment of the Vasopressin Precursor, as a Novel Predictor of Outcome in Heart Failure. Eur. J. Clin. Investig. 2006, 36, 771–778. [Google Scholar] [CrossRef]
- Ueno, H.; Yoshimura, M.; Tanaka, K.; Nishimura, H.; Nishimura, K.; Sonoda, S.; Motojima, Y.; Saito, R.; Maruyama, T.; Miyamoto, T.; et al. Up-Regulation of Hypothalamic Arginine Vasopressin by Peripherally Administered Furosemide in Transgenic Rats Expressing Arginine Vasopressin-Enhanced Green Fluorescent Protein. J. Neuroendocrinol. 2018, 30, e12603. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, H.; Kawasaki, M.; Suzuki, H.; Matsuura, T.; Motojima, Y.; Ohnishi, H.; Yamanaka, Y.; Yoshimura, M.; Maruyama, T.; Saito, R.; et al. Neuropathic Pain Up-Regulates Hypothalamo-Neurohypophysial and Hypothalamo-Spinal Oxytocinergic Pathways in Oxytocin-Monomeric Red Fluorescent Protein 1 Transgenic Rat. Neuroscience 2019, 406, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Sanada, K.; Ueno, H.; Miyamoto, T.; Baba, K.; Tanaka, K.; Nishimura, H.; Nishimura, K.; Sonoda, S.; Yoshimura, M.; Maruyama, T.; et al. AVP-eGFP Was Significantly Upregulated by Hypovolemia in the Parvocellular Division of the Paraventricular Nucleus in the Transgenic Rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2022, 322, R161–R169. [Google Scholar] [CrossRef] [PubMed]
- Ueno, H.; Ueta, Y.; Nonaka, Y.; Shirouzu, T.; Ikeda, N.; Furuno, I.; Nakazono, K.; Hasegawa, E.; Shimizu, M.; Koga, J.; et al. Effects of Peritoneal Dialysis Fluids on Arginine Vasopressin Dynamics in Humans and Transgenic Rats. Perit. Dial. Int. 2025, 8968608251347093. [Google Scholar] [CrossRef]
- Iwahori, T.; Esaki, M.; Hinoue, H.; Esaki, S.; Esaki, Y. Tolvaptan Increases Urine and Ultrafiltration Volume for Patients with Oliguria Undergoing Peritoneal Dialysis. Clin. Exp. Nephrol. 2014, 18, 655–661. [Google Scholar] [CrossRef]
- Yu, Z.; Tan, B.K.; Dainty, S.; Mattey, D.L.; Davies, S.J. Hypoalbuminaemia, Systemic Albumin Leak and Endothelial Dysfunction in Peritoneal Dialysis Patients. Nephrol. Dial. Transplant. 2012, 27, 4437–4445. [Google Scholar] [CrossRef]
- Zager, P.G.; Frey, H.J.; Gerdes, B.G. Plasma 18-Hydroxycorticosterone during Continuous Ambulatory Peritoneal Dialysis. J. Lab. Clin. Med. 1983, 102, 604–612. [Google Scholar]
- Zabetakis, P.M.; Kumar, D.N.; Gleim, G.W.; Gardenswartz, M.H.; Agrawal, M.; Robinson, A.G.; Michelis, M.F. Increased Levels of Plasma Renin, Aldosterone, Catecholamines and Vasopressin in Chronic Ambulatory Peritoneal Dialysis (CAPD) Patients. Clin. Nephrol. 1987, 28, 147–151. [Google Scholar]
- Ito, Y.; Mizuno, M.; Suzuki, Y.; Tamai, H.; Hiramatsu, T.; Ohashi, H.; Ito, I.; Kasuga, H.; Horie, M.; Maruyama, S.; et al. Long-Term Effects of Spironolactone in Peritoneal Dialysis Patients. J. Am. Soc. Nephrol. 2014, 25, 1094–1102. [Google Scholar] [CrossRef]
- Li, P.K.-T.; Chow, K.-M.; Wong, T.Y.-H.; Leung, C.-B.; Szeto, C.-C. Effects of an Angiotensin-Converting Enzyme Inhibitor on Residual Renal Function in Patients Receiving Peritoneal Dialysis. A Randomized, Controlled Study. Ann. Intern. Med. 2003, 139, 105–112. [Google Scholar] [CrossRef]
- Ueno, H.; Sanada, K.; Miyamoto, T.; Baba, K.; Tanaka, K.; Nishimura, H.; Nishimura, K.; Sonoda, S.; Yoshimura, M.; Maruyama, T.; et al. Oxytocin-Monomeric Red Fluorescent Protein 1 Synthesis in the Hypothalamus under Osmotic Challenge and Acute Hypovolemia in a Transgenic Rat Line. Physiol. Rep. 2020, 8, e14558. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, R.E.; Samson, W.K.; Fulton, R.J.; Stricker, E.M.; Verbalis, J.G. Central Oxytocin Inhibition of Salt Appetite in Rats: Evidence for Differential Sensing of Plasma Sodium and Osmolality. Proc. Natl. Acad. Sci. USA 1993, 90, 10380–10384. [Google Scholar] [CrossRef]
- Forsling, M.L.; Judah, J.M.; Windle, R.J. The Effect of Vasopressin and Oxytocin on Glomerular Filtration Rate in the Conscious Rat: Contribution to the Natriuretic Response. J. Endocrinol. 1994, 141, 59–67. [Google Scholar] [CrossRef]
- Gutkowska, J.; Jankowski, M.; Lambert, C.; Mukaddam-Daher, S.; Zingg, H.H.; McCann, S.M. Oxytocin Releases Atrial Natriuretic Peptide by Combining with Oxytocin Receptors in the Heart. Proc. Natl. Acad. Sci. USA 1997, 94, 11704–11709. [Google Scholar] [CrossRef]
- Walter, M.F.; Forsling, M.L.; Shirley, D.G. Contribution of Endogenous Oxytocin to Sodium Excretion in Anaesthetized, Surgically Operated Rats. J. Endocrinol. 2000, 165, 19–24. [Google Scholar] [CrossRef][Green Version]
- Elabd, C.; Basillais, A.; Beaupied, H.; Breuil, V.; Wagner, N.; Scheideler, M.; Zaragosi, L.-E.; Massiéra, F.; Lemichez, E.; Trajanoski, Z.; et al. Oxytocin Controls Differentiation of Human Mesenchymal Stem Cells and Reverses Osteoporosis. Stem Cells 2008, 26, 2399–2407. [Google Scholar] [CrossRef] [PubMed]
- Joo, K.W.; Jeon, U.S.; Kim, G.-H.; Park, J.; Oh, Y.K.; Kim, Y.S.; Ahn, C.; Kim, S.; Kim, S.Y.; Lee, J.S.; et al. Antidiuretic Action of Oxytocin Is Associated with Increased Urinary Excretion of Aquaporin-2. Nephrol. Dial. Transplant. 2004, 19, 2480–2486. [Google Scholar] [CrossRef]
- Kishi, T. Heart Failure as a Disruption of Dynamic Circulatory Homeostasis Mediated by the Brain. Int. Heart J. 2016, 57, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Denton, K.M.; Luff, S.E.; Shweta, A.; Anderson, W.P. Differential Neural Control of Glomerular Ultrafiltration. Clin. Exp. Pharma. Physio. 2004, 31, 380–386. [Google Scholar] [CrossRef]
- Grassi, G.; Seravalle, G.; Ghiadoni, L.; Tripepi, G.; Bruno, R.M.; Mancia, G.; Zoccali, C. Sympathetic Nerve Traffic and Asymmetric Dimethylarginine in Chronic Kidney Disease. Clin. J. Am. Soc. Nephrol. 2011, 6, 2620–2627. [Google Scholar] [CrossRef]
- Kaur, J.; Young, B.E.; Fadel, P.J. Sympathetic Overactivity in Chronic Kidney Disease: Consequences and Mechanisms. Int. J. Mol. Sci. 2017, 18, 1682. [Google Scholar] [CrossRef]
- Campese, V.M.; Kogosov, E.; Koss, M. Renal Afferent Denervation Prevents the Progression of Renal Disease in the Renal Ablation Model of Chronic Renal Failure in the Rat. Am. J. Kidney Dis. 1995, 26, 861–865. [Google Scholar] [CrossRef]
- Oshima, N.; Onimaru, H.; Matsubara, H.; Uchida, T.; Watanabe, A.; Takechi, H.; Nishida, Y.; Kumagai, H. Uric Acid, Indoxyl Sulfate, and Methylguanidine Activate Bulbospinal Neurons in the RVLM via Their Specific Transporters and by Producing Oxidative Stress. Neuroscience 2015, 304, 133–145. [Google Scholar] [CrossRef]
- Owyang, C.; Miller, L.J.; DiMagno, E.P.; Brennan, L.A.; Go, V.L. Gastrointestinal Hormone Profile in Renal Insufficiency. Mayo Clin. Proc. 1979, 54, 769–773. [Google Scholar]
- Swanson, L.W.; Sawchenko, P.E. Paraventricular Nucleus: A Site for the Integration of Neuroendocrine and Autonomic Mechanisms. Neuroendocrinology 1980, 31, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Bourque, C.W. Central Mechanisms of Osmosensation and Systemic Osmoregulation. Nat. Rev. Neurosci. 2008, 9, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Toney, G.M.; Stocker, S.D. Hyperosmotic Activation of CNS Sympathetic Drive: Implications for Cardiovascular Disease. J. Physiol. 2010, 588, 3375–3384. [Google Scholar] [CrossRef]
- Wijewickrama, P.; Williams, J.; Bain, S.; Dasgupta, I.; Chowdhury, T.A.; Wahba, M.; Frankel, A.H.; Lambie, M.; Karalliedde, J.; Bain, S.; et al. Narrative Review of Glycemic Management in People With Diabetes on Peritoneal Dialysis. Kidney Int. Rep. 2023, 8, 700–714. [Google Scholar] [CrossRef] [PubMed]
- Bailey, J.L.; Zheng, B.; Hu, Z.; Price, S.R.; Mitch, W.E. Chronic Kidney Disease Causes Defects in Signaling through the Insulin Receptor Substrate/Phosphatidylinositol 3-Kinase/Akt Pathway: Implications for Muscle Atrophy. J. Am. Soc. Nephrol. 2006, 17, 1388–1394. [Google Scholar] [CrossRef]
- Kezić, A.; Gajić, S.; Ostojić, A.R.; Bekić, I.; Bontić, A.; Pavlović, J.; Baralić, M.; Popović, L. Glycemic Control in Patients with Diabetes on Peritoneal Dialysis: From Glucose Sparing Approach to Glucose Monitoring. Life 2025, 15, 798. [Google Scholar] [CrossRef]
- Rivara, M.B.; Mehrotra, R. New-Onset Diabetes in Peritoneal Dialysis Patients—Which Predictors Really Matter? Perit. Dial. Int. 2016, 36, 243–246. [Google Scholar] [CrossRef]
- Szeto, C.-C.; Chow, K.-M.; Kwan, B.C.-H.; Chung, K.-Y.; Leung, C.-B.; Li, P.K.-T. New-Onset Hyperglycemia in Nondiabetic Chinese Patients Started on Peritoneal Dialysis. Am. J. Kidney Dis. 2007, 49, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Gu, Y.-Y.; Cui, C.-J.; Zhou, C.-C.; Wang, X.-D.; Ruan, M.-N.; Huang, L.-X.; Chen, S.-X.; Yang, B.; Chen, X.-J.; et al. New-Onset Glucose Disorders in Peritoneal Dialysis Patients: A Meta-Analysis and Systematic Review. Nephrol. Dial. Transplant. 2020, 35, 1412–1419. [Google Scholar] [CrossRef]
- Fortes, P.C.; de Moraes, T.P.; Mendes, J.G.; Stinghen, A.E.; Ribeiro, S.C.; Pecoits-Filho, R. Insulin Resistance and Glucose Homeostasis in Peritoneal Dialysis. Perit. Dial. Int. 2009, 29 (Suppl. S2), S145–S148. [Google Scholar] [CrossRef]
- Meuwese, C.L.; Snaedal, S.; Halbesma, N.; Stenvinkel, P.; Dekker, F.W.; Qureshi, A.R.; Barany, P.; Heimburger, O.; Lindholm, B.; Krediet, R.T.; et al. Trimestral Variations of C-Reactive Protein, Interleukin-6 and Tumour Necrosis Factor-α Are Similarly Associated with Survival in Haemodialysis Patients. Nephrol. Dial. Transplant. 2011, 26, 1313–1318. [Google Scholar] [CrossRef]
- Zhang, L.; Du, J.; Hu, Z.; Han, G.; Delafontaine, P.; Garcia, G.; Mitch, W.E. IL-6 and Serum Amyloid A Synergy Mediates Angiotensin II-Induced Muscle Wasting. J. Am. Soc. Nephrol. 2009, 20, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Koppe, L.; Pillon, N.J.; Vella, R.E.; Croze, M.L.; Pelletier, C.C.; Chambert, S.; Massy, Z.; Glorieux, G.; Vanholder, R.; Dugenet, Y.; et al. P-Cresyl Sulfate Promotes Insulin Resistance Associated with CKD. J. Am. Soc. Nephrol. 2013, 24, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, A.C.; Carrero, J.J.; Abensur, H.; Lindholm, B.; Stenvinkel, P. Systemic and Local Inflammation in Peritoneal Dialysis: Mechanisms, Biomarkers and Effects on Outcome. Contrib. Nephrol. 2009, 163, 132–139. [Google Scholar] [CrossRef]
- Nascimento, M.M.; Suliman, M.E.; Silva, M.; Chinaglia, T.; Marchioro, J.; Hayashi, S.Y.; Riella, M.C.; Lindholm, B.; Anderstam, B. Effect of Oral N-Acetylcysteine Treatment on Plasma Inflammatory and Oxidative Stress Markers in Peritoneal Dialysis Patients: A Placebo-Controlled Study. Perit. Dial. Int. 2010, 30, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Blaine, E.; Tumlinson, R.; Colvin, M.; Haynes, T.; Whitley, H.P. Systematic Literature Review of Insulin Dose Adjustments When Initiating Hemodialysis or Peritoneal Dialysis. Pharmacotherapy 2022, 42, 177–187. [Google Scholar] [CrossRef]
- Zager, P.G.; Spalding, C.T.; Frey, H.J.; Brittenham, M.C. Low Dose Adrenocorticotropin Infusion in Continuous Ambulatory Peritoneal Dialysis Patients. J. Clin. Endocrinol. Metab. 1985, 61, 1205–1210. [Google Scholar] [CrossRef]
- Iwasaki, Y.; Kumari, P.; Wang, L.; Hidema, S.; Nishimori, K.; Yada, T. Relay of Peripheral Oxytocin to Central Oxytocin Neurons via Vagal Afferents for Regulating Feeding. Biochem. Biophys. Res. Commun. 2019, 519, 553–558. [Google Scholar] [CrossRef]
- Heaton, A.; Johnston, D.G.; Haigh, J.W.; Ward, M.K.; Alberti, K.G.; Kerr, D.N. Twenty-Four Hour Hormonal and Metabolic Profiles in Uraemic Patients before and during Treatment with Continuous Ambulatory Peritoneal Dialysis. Clin. Sci. 1985, 69, 449–457. [Google Scholar] [CrossRef]
- Yamada, S.; Nakano, T. Role of Chronic Kidney Disease (CKD)-Mineral and Bone Disorder (MBD) in the Pathogenesis of Cardiovascular Disease in CKD. J. Atheroscler. Thromb. 2023, 30, 835–850. [Google Scholar] [CrossRef] [PubMed]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int. Suppl. 2017, 7, 1–59. [Google Scholar] [CrossRef] [PubMed]
- Noordzij, M.; Korevaar, J.C.; Bos, W.J.; Boeschoten, E.W.; Dekker, F.W.; Bossuyt, P.M.; Krediet, R.T. Mineral Metabolism and Cardiovascular Morbidity and Mortality Risk: Peritoneal Dialysis Patients Compared with Haemodialysis Patients. Nephrol. Dial. Transplant. 2006, 21, 2513–2520. [Google Scholar] [CrossRef]
- Adragao, T.; Branco, P.; Birne, R.; Curto, J.D.; de Almeida, E.; Prata, M.M.; Pais, M.J. Bone Mineral Density, Vascular Calcifications, and Arterial Stiffness in Peritoneal Dialysis Patients. Perit. Dial. Int. 2008, 28, 668–672. [Google Scholar] [CrossRef] [PubMed]
- Nitta, K.; Hanafusa, N.; Tsuchiya, K. Mineral Bone Disorders (MBD) in Patients on Peritoneal Dialysis. Ren. Replace. Ther. 2019, 5, 4. [Google Scholar] [CrossRef]
- Delmez, J.A.; Slatopolsky, E.; Martin, K.J.; Gearing, B.N.; Harter, H.R. Minerals, Vitamin D, and Parathyroid Hormone in Continuous Ambulatory Peritoneal Dialysis. Kidney Int. 1982, 21, 862–867. [Google Scholar] [CrossRef]
- Rahman, R.; Heaton, A.; Goodship, T.H.J.; Stuart, R.; Rodger, C.; Tapson Leslie, J.S.; Ellis, S.H.A.; Wilkinson, R.; Ward, M.K. Renal Osteodystrophy in Patients on Continuous Ambulatory Peritoneal Dialysis: A Five Year Study. Perit. Dial. Int. 1987, 7, 20–26. [Google Scholar] [CrossRef]
- Loschiavo, C.; Fabris, A.; Adami, S.; Tomelleri, L.; Tessitore, N.; Valvo, E.; Lupo, A.; Oldrizzi, L.; Rugiu, C.; Gammaro, L.; et al. Effects of Continuous Ambulatory Peritoneal Dialysis (CAPD) on Renal Osteodystrophy. Perit. Dial. Int. 1985, 5, 53–55. [Google Scholar] [CrossRef]
- Isakova, T.; Xie, H.; Barchi-Chung, A.; Vargas, G.; Sowden, N.; Houston, J.; Wahl, P.; Lundquist, A.; Epstein, M.; Smith, K.; et al. Fibroblast Growth Factor 23 in Patients Undergoing Peritoneal Dialysis. Clin. J. Am. Soc. Nephrol. 2011, 6, 2688–2695. [Google Scholar] [CrossRef]
- Bi, S.; Liang, Y.; Cheng, L.; Wang, Y.; Wang, T.; Han, Q.; Zhang, A. Hemodialysis Is Associated with Higher Serum FGF23 Level When Compared with Peritoneal Dialysis. Int. Urol. Nephrol. 2017, 49, 1653–1659. [Google Scholar] [CrossRef]
- Martínez, M.E.; Miguel, J.L.; Gómez, P.; Selgas, R.; Salinas, M.; Gentil, M.; Mateos, F.; Montero, J.L.; Sánchez Sicilia, L. Plasma Calcitonin Concentration in Patients Treated with Chronic Dialysis: Differences between Hemodialysis and CAPD. Clin. Nephrol. 1983, 19, 250–253. [Google Scholar]
- Elabd, C.; Cousin, W.; Upadhyayula, P.; Chen, R.Y.; Chooljian, M.S.; Li, J.; Kung, S.; Jiang, K.P.; Conboy, I.M. Oxytocin Is an Age-Specific Circulating Hormone That Is Necessary for Muscle Maintenance and Regeneration. Nat. Commun. 2014, 5, 4082. [Google Scholar] [CrossRef] [PubMed]
- Hofbauer, L.C.; Gori, F.; Riggs, B.L.; Lacey, D.L.; Dunstan, C.R.; Spelsberg, T.C.; Khosla, S. Stimulation of Osteoprotegerin Ligand and Inhibition of Osteoprotegerin Production by Glucocorticoids in Human Osteoblastic Lineage Cells: Potential Paracrine Mechanisms of Glucocorticoid-Induced Osteoporosis. Endocrinology 1999, 140, 4382–4389. [Google Scholar] [CrossRef] [PubMed]
- Shuto, T.; Kukita, T.; Hirata, M.; Jimi, E.; Koga, T. Dexamethasone Stimulates Osteoclast-like Cell Formation by Inhibiting Granulocyte-Macrophage Colony-Stimulating Factor Production in Mouse Bone Marrow Cultures. Endocrinology 1994, 134, 1121–1126. [Google Scholar] [CrossRef]
- Li, P.K.T.; Choy, A.S.M.; Bavanandan, S.; Chen, W.; Foo, M.; Kanjanabuch, T.; Kim, Y.-L.; Nakayama, M.; Yu, X. Anemia Management in Peritoneal Dialysis: Perspectives From the Asia Pacific Region. Kidney Med. 2021, 3, 405–411. [Google Scholar] [CrossRef]
- De Paepe, M.B.J.; Schelstraete, K.H.G.; Ringoir, S.M.G.; Lameire, N.H. Influence of Continuous Ambulatory Peritoneal Dialysis on the Anemia of Endstage Renal Disease. Kidney Int. 1983, 23, 744–748. [Google Scholar] [CrossRef]
- Summerfield, G.P.; Gyde, O.H.; Forbes, A.M.; Goldsmith, H.J.; Bellingham, A.J. Haemoglobin Concentration and Serum Erythropoietin in Renal Dialysis and Transplant Patients. Scand. J. Haematol. 1983, 30, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, N.; Yokoyama, K.; Maruyama, Y.; Ueda, Y.; Yoshida, H.; Tanno, Y.; Yamamoto, R.; Terawaki, H.; Ikeda, M.; Hanaoka, K.; et al. Clinical Impact of a Combined Therapy of Peritoneal Dialysis and Hemodialysis. Clin. Nephrol. 2010, 74, 209–216. [Google Scholar] [CrossRef]
- Meytes, D.; Bogin, E.; Ma, A.; Dukes, P.P.; Massry, S.G. Effect of Parathyroid Hormone on Erythropoiesis. J. Clin. Investig. 1981, 67, 1263–1269. [Google Scholar] [CrossRef]
- Rao, D.S.; Shih, M.S.; Mohini, R. Effect of Serum Parathyroid Hormone and Bone Marrow Fibrosis on the Response to Erythropoietin in Uremia. N. Engl. J. Med. 1993, 328, 171–175. [Google Scholar] [CrossRef]
- Trunzo, J.A.; McHenry, C.R.; Schulak, J.A.; Wilhelm, S.M. Effect of Parathyroidectomy on Anemia and Erythropoietin Dosing in End-Stage Renal Disease Patients with Hyperparathyroidism. Surgery 2008, 144, 915–918; discussion 919. [Google Scholar] [CrossRef]
- David, V.; Martin, A.; Isakova, T.; Spaulding, C.; Qi, L.; Ramirez, V.; Zumbrennen-Bullough, K.B.; Sun, C.C.; Lin, H.Y.; Babitt, J.L.; et al. Inflammation and Functional Iron Deficiency Regulate Fibroblast Growth Factor 23 Production. Kidney Int. 2016, 89, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Coe, L.M.; Madathil, S.V.; Casu, C.; Lanske, B.; Rivella, S.; Sitara, D. FGF-23 Is a Negative Regulator of Prenatal and Postnatal Erythropoiesis. J. Biol. Chem. 2014, 289, 9795–9810. [Google Scholar] [CrossRef]
- Agoro, R.; Montagna, A.; Goetz, R.; Aligbe, O.; Singh, G.; Coe, L.M.; Mohammadi, M.; Rivella, S.; Sitara, D. Inhibition of Fibroblast Growth Factor 23 (FGF23) Signaling Rescues Renal Anemia. FASEB J. 2018, 32, 3752–3764. [Google Scholar] [CrossRef]
- Czaya, B.; Faul, C. The Role of Fibroblast Growth Factor 23 in Inflammation and Anemia. Int. J. Mol. Sci. 2019, 20, 4195. [Google Scholar] [CrossRef]
- Mayer, B.; Németh, K.; Krepuska, M.; Myneni, V.D.; Maric, D.; Tisdale, J.F.; Hsieh, M.M.; Uchida, N.; Lee, H.-J.; Nemeth, M.J.; et al. Vasopressin Stimulates the Proliferation and Differentiation of Red Blood Cell Precursors and Improves Recovery from Anemia. Sci. Transl. Med. 2017, 9, eaao1632. [Google Scholar] [CrossRef] [PubMed]
- Schill, F.; Engström, G.; Melander, O.; Timpka, S.; Enhörning, S. The Possible Role of the Vasopressin System in Hematopoiesis. Sci. Rep. 2024, 14, 5085. [Google Scholar] [CrossRef] [PubMed]
- Cambien, G.; Dupuis, A.; Guihenneuc, J.; Bauwens, M.; Belmouaz, M.; Ayraud-Thevenot, S. Endocrine Disruptors in Dialysis Therapies: A Literature Review. Environ. Int. 2023, 178, 108100. [Google Scholar] [CrossRef] [PubMed]

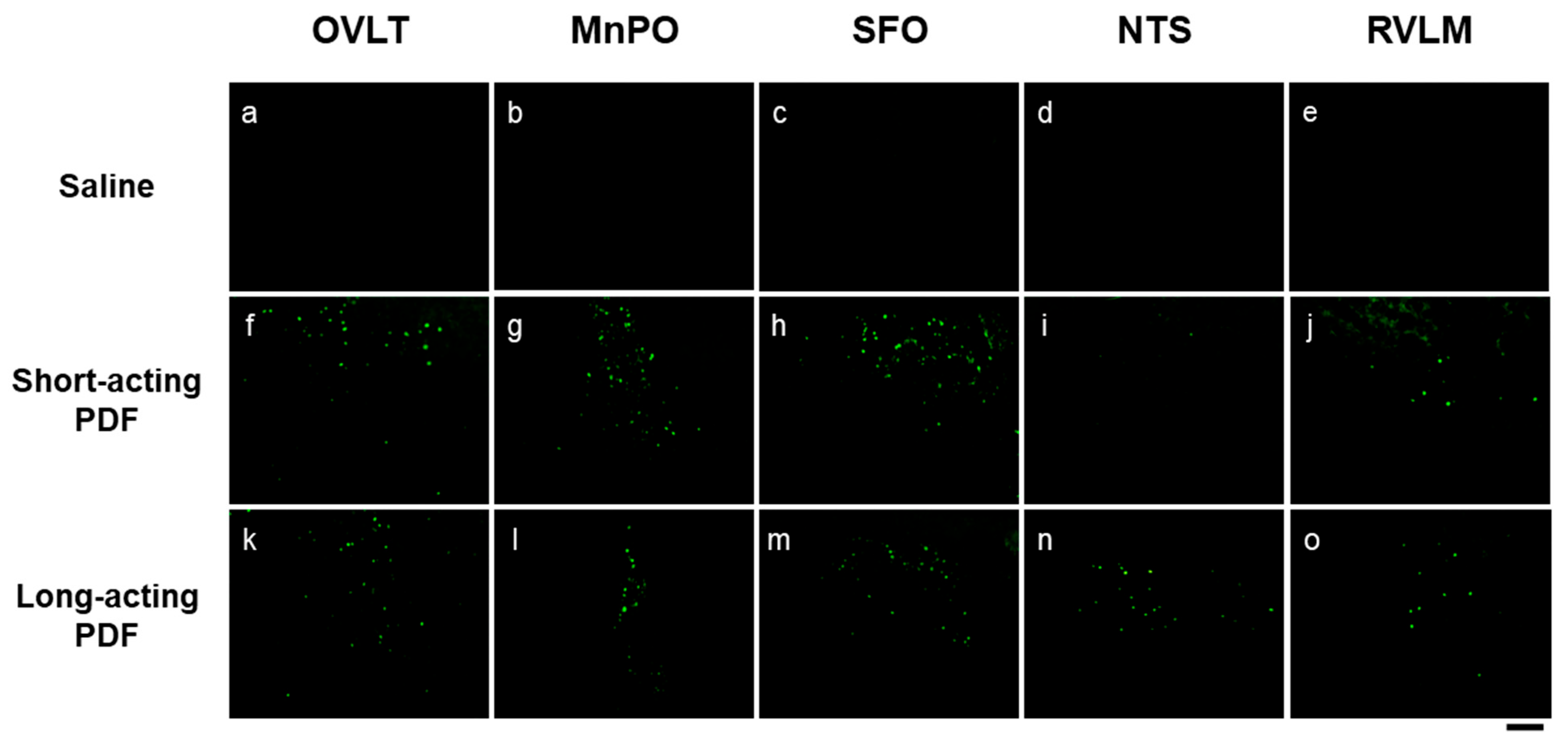
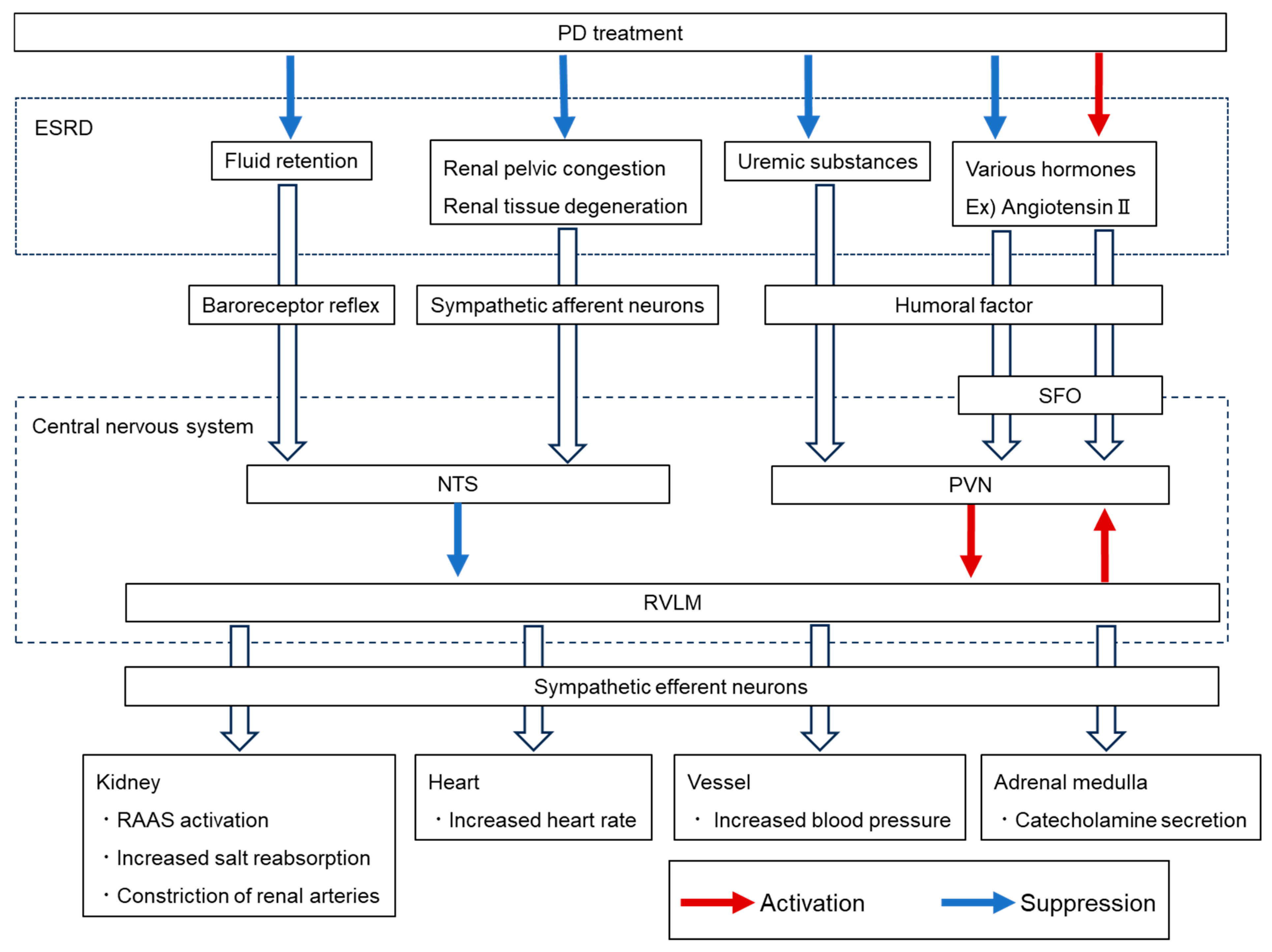
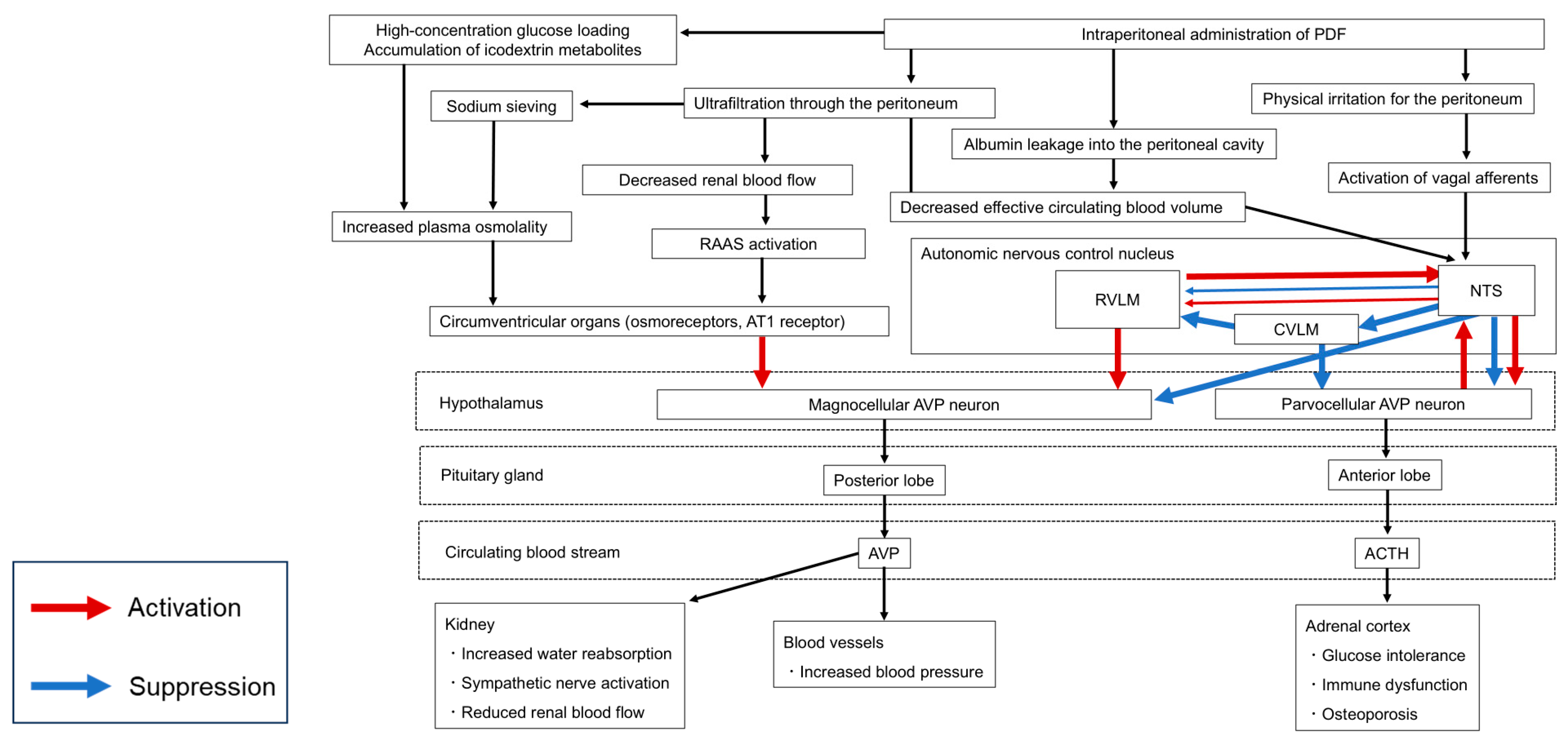
| Associated Complication | Hormone | Description | Key Reference |
|---|---|---|---|
| Impaired Glucose Tolerance | Insulin | · In CKD patients, insulin resistance develops early, irrespective of diabetes status, but tends to improve after initiation of PD. | [44] |
| Cortisol | · Clinical studies have shown elevated plasma cortisol levels in patients undergoing PD, and this increase appears to be driven primarily by central rather than peripheral mechanisms. | [17,53] | |
| · Intraperitoneal retention of PDF may activate parvocellular AVP neurons in the hypothalamus, a key site controlling corticosterone synthesis. | [13,14] | ||
| Glucagon | · Plasma glucagon levels are elevated in ESRD patients and are even higher in those on PD. | [53] | |
| CKD-MBD | PTH | · No clear consensus exists regarding changes in plasma PTH levels in PD patients. | [60,61] |
| · Plasma FGF-23 levels are higher in PD patients than in HD patients | [63] | ||
| Calcitonin | · Plasma calcitonin levels are elevated in PD patients, but remain lower than in HD patients. | [64] | |
| Oxytocin | PDF administration may stimulate hypothalamic OXT neuronal activity and promote its synthesis | [21] | |
| Renal Anemia | Erythropoietin | · Switching from HD to PD increases plasma erythropoietin levels, and switching from PD monotherapy to combined PD and HD improves erythropoietin-resistant anemia. | [70,71] |
| PTH, FGF-23 | · See “CKD-MBD” section | [57,58,63] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ueno, H.; Ueta, Y.; Koga, J.-i.; Maruyama, T.; Miyamoto, T.; Kataoka, M. Hidden Impacts of Peritoneal Dialysis on the Endocrine System. Life 2025, 15, 1588. https://doi.org/10.3390/life15101588
Ueno H, Ueta Y, Koga J-i, Maruyama T, Miyamoto T, Kataoka M. Hidden Impacts of Peritoneal Dialysis on the Endocrine System. Life. 2025; 15(10):1588. https://doi.org/10.3390/life15101588
Chicago/Turabian StyleUeno, Hiromichi, Yoichi Ueta, Jun-ichiro Koga, Takashi Maruyama, Tetsu Miyamoto, and Masaharu Kataoka. 2025. "Hidden Impacts of Peritoneal Dialysis on the Endocrine System" Life 15, no. 10: 1588. https://doi.org/10.3390/life15101588
APA StyleUeno, H., Ueta, Y., Koga, J.-i., Maruyama, T., Miyamoto, T., & Kataoka, M. (2025). Hidden Impacts of Peritoneal Dialysis on the Endocrine System. Life, 15(10), 1588. https://doi.org/10.3390/life15101588






