Continuous Glucose Monitoring in People at High Risk of Diabetes and Dysglycaemia: Transforming Early Risk Detection and Personalised Care
Abstract
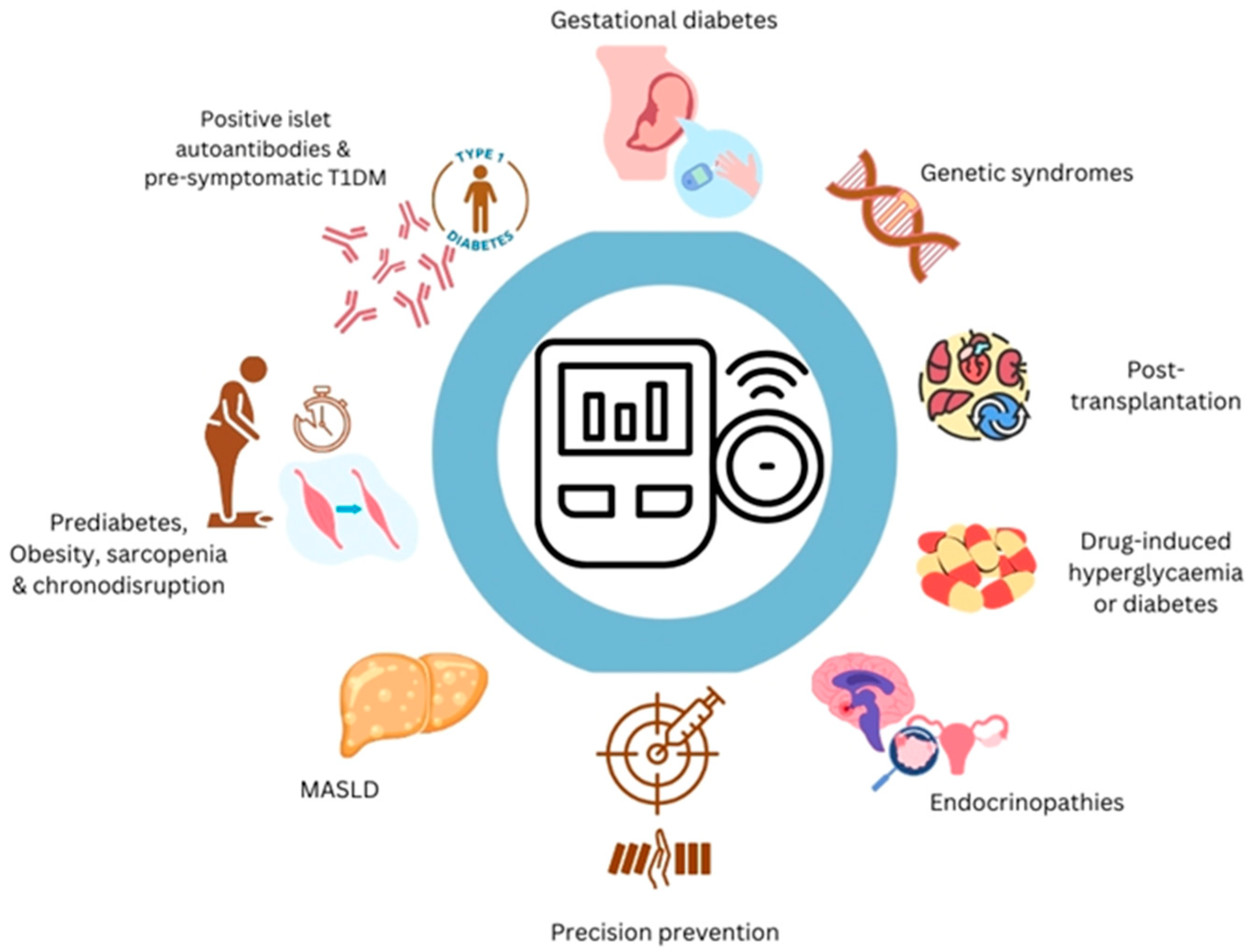
1. Introduction
2. Positive Islet Autoantibodies and Pre-Symptomatic Type 1 Diabetes
3. Metabolic Dysfunction
4. Solid Organ Transplantation
5. Medications
6. Genetic Syndromes
7. Precision Approaches
8. Future Directions and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABCC8 | ATP-binding cassette transporter sub-family C member 8 |
| CAH | congenital adrenal hyperplasia |
| CBG | capillary blood glucose |
| CFRD | cystic fibrosis-related diabetes |
| CGI | glucose time series index |
| CGM | continuous glucose monitoring |
| DKA | diabetic ketoacidosis |
| GCK | glucokinase |
| GDM | gestational diabetes mellitus |
| GV | glucose variability |
| GRADE | grading of recommendations, assessment, development, and evaluations |
| HbA1c | haemoglobin A1c |
| HCPs | healthcare professionals |
| ICIs | immune checkpoint inhibitors |
| IGT | impaired glucose tolerance |
| INSR | insulin receptor gene |
| KTRs | kidney transplant recipients |
| MASLD | metabolic dysfunction-associated steatotic liver disease |
| MIDD | maternally inherited diabetes and deafness |
| MODD | mean of daily differences |
| MODY | maturity-onset diabetes of the young |
| OGTT | oral glucose tolerance test |
| PCOS | polycystic ovarian syndrome |
| PTDM | post-transplant diabetes mellitus |
| RCTs | randomised controlled trials |
| ROC | receiver operating characteristic |
| RYGB | Roux-en-Y gastric bypass |
| SD | standard deviation |
| SIH | steroid-induced hyperglycaemia |
| T1DM | type 1 diabetes mellitus |
| T2DM | type 2 diabetes mellitus |
| TCF7L2 | transcription factor-7-like 2 gene |
| TIR | time-in-range |
References
- Leelarathna, L.; Evans, M.L.; Neupane, S.; Rayman, G.; Lumley, S.; Cranston, I.; Narendran, P.; Barnard-Kelly, K.; Sutton, C.J.; Elliot, R.A.; et al. Intermittently Scanned Continuous Glucose Monitoring for Type 1 Diabetes. N. Engl. J. Med. 2022, 387, 1477–1487. [Google Scholar] [CrossRef]
- Beck, R.W.; Riddlesworth, T.; Ruedy, K.; Ahmann, A.; Bergenstal, R.; Haller, S.; Kollman, C.; Kruger, D.; McGill, J.B.; Polonsky, W.; et al. Effect of Continuous Glucose Monitoring on Glycemic Control in Adults with Type 1 Diabetes Using Insulin Injections: The DIAMOND Randomized Clinical Trial. Jama 2017, 317, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, H.; Wilmot, E.G.; Gregory, R.; Barnes, D.; Narendran, P.; Saunders, S.; Furlong, N.; Kamaruddin, S.; Banatwall, R.; Herring, R.; et al. Effect of Flash Glucose Monitoring on Glycemic Control, Hypoglycemia, Diabetes-Related Distress, and Resource Utilization in the Association of British Clinical Diabetologists (ABCD) Nationwide Audit. Diabetes Care 2020, 43, 2153–2160. [Google Scholar] [CrossRef] [PubMed]
- Liarakos, A.L.; Lim, J.Z.M.; Leelarathna, L.; Wilmot, E.G. The use of technology in type 2 diabetes and prediabetes: A narrative review. Diabetologia 2024, 67, 2059–2074. [Google Scholar] [CrossRef]
- Jospe, M.R.; Richardson, K.M.; Saleh, A.A.; Bohlen, L.C.; Crawshaw, J.; Liao, Y.; Konnyu, K.; Schembre, S.M. Leveraging continuous glucose monitoring as a catalyst for behaviour change: A scoping review. Int. J. Behav. Nutr. Phys. Act. 2024, 21, 74. [Google Scholar] [CrossRef] [PubMed]
- Klonoff, D.C.; Nguyen, K.T.; Xu, N.Y.; Gutierrez, A.; Espinoza, J.C.; Vidmar, A.P. Use of Continuous Glucose Monitors by People Without Diabetes: An Idea Whose Time Has Come? J. Diabetes Sci. Technol. 2023, 17, 1686–1697. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 2. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes-2025. Diabetes Care 2025, 48 (Suppl. 1), S27–S49. [Google Scholar] [CrossRef]
- Steck, A.K.; Dong, F.; Geno Rasmussen, C.; Bautista, K.; Sepulveda, F.; Baxter, J.; Yu, L.; Frohnert, B.I.; Rewers, M.J.; ASK Study Group. CGM Metrics Predict Imminent Progression to Type 1 Diabetes: Autoimmunity Screening for Kids (ASK) Study. Diabetes Care 2022, 45, 365–371. [Google Scholar] [CrossRef]
- Steck, A.K.; Dong, F.; Taki, I.; Hoffman, M.; Simmons, K.; Frohnert, B.I.; Rewers, M.J. Continuous Glucose Monitoring Predicts Progression to Diabetes in Autoantibody Positive Children. J. Clin. Endocrinol. Metab. 2019, 104, 3337–3344. [Google Scholar] [CrossRef]
- Bravo, R.; Lee, K.H.; Nazeer, S.A.; Cornthwaite, J.A.; Fishel Bartal, M.; Pedroza, C. Glucose circadian rhythm assessment in pregnant women for gestational diabetes screening. Int. J. Obes. 2024, 49, 118–124. [Google Scholar] [CrossRef]
- Kang, M.; Zhu, C.; Lai, M.; Weng, J.; Zhuang, Y.; He, H.; Qiu, Y.; Wu, Y.; Qi, Z.; Zhang, W.; et al. Machine Learning-Based Prediction of Large-for-Gestational Age Infants in Mothers with Gestational Diabetes Mellitus. J. Clin. Endocrinol. Metab. 2024, 110, e1631–e1639. [Google Scholar] [CrossRef] [PubMed]
- Lim, B.S.Y.; Yang, Q.; Choolani, M.; Gardner, D.S.L.; Chong, Y.S.; Zhang, C.; Chan, S.Y.; Li, L.J. Utilizing Continuous Glucose Monitoring for Early Detection of Gestational Diabetes Mellitus and Pregnancy Outcomes in an Asian Population. Diabetes Care 2024, 47, 1916–1921. [Google Scholar] [CrossRef] [PubMed]
- Shivaswamy, V.; Boerner, B.; Larsen, J. Post-Transplant Diabetes Mellitus: Causes, Treatment, and Impact on Outcomes. Endocr. Rev. 2016, 37, 37–61. [Google Scholar] [CrossRef]
- Sharif, A.; Chakkera, H.; de Vries, A.P.J.; Eller, K.; Guthoff, M.; Haller, M.C.; Hornum, M.; Nordheim, E.; Kautzky-Willer, A.; Krebs, M.; et al. International consensus on post-transplantation diabetes mellitus. Nephrol. Dial. Transplant. 2024, 39, 531–549. [Google Scholar] [CrossRef]
- Eide, I.A.; Halden, T.A.S.; Hartmann, A.; Dahle, D.O.; Åsberg, A.; Jenssen, T. Associations Between Posttransplantation Diabetes Mellitus and Renal Graft Survival. Transplantation 2017, 101, 1282–1289. [Google Scholar] [CrossRef]
- Eide, I.A.; Halden, T.A.; Hartmann, A.; Åsberg, A.; Dahle, D.O.; Reisaeter, A.V.; Jenssen, T. Mortality risk in post-transplantation diabetes mellitus based on glucose and HbA1c diagnostic criteria. Transpl. Int. 2016, 29, 568–578. [Google Scholar] [CrossRef]
- Topitz, D.; Schwaiger, E.; Frommlet, F.; Werzowa, J.; Hecking, M. Cardiovascular events associate with diabetes status rather than with early basal insulin treatment for the prevention of post-transplantation diabetes mellitus. Nephrol. Dial. Transplant. 2020, 35, 544–546. [Google Scholar] [CrossRef]
- Besser, R.E.J.; Bell, K.J.; Couper, J.J.; Ziegler, A.G.; Wherrett, D.K.; Knip, M.; Speake, C.; Casteels, K.; Discroll, K.A.; Jacobsen, L.; et al. ISPAD Clinical Practice Consensus Guidelines 2022: Stages of type 1 diabetes in children and adolescents. Pediatr. Diabetes 2022, 23, 1175–1187. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, A.G.; Rewers, M.; Simell, O.; Simell, T.; Lempainen, J.; Steck, A.; Winkler, C.; Ilonen, J.; Veijola, R.; Knip, M.; et al. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA 2013, 309, 2473–2479. [Google Scholar] [CrossRef] [PubMed]
- Wherrett, D.K.; Chiang, J.L.; Delamater, A.M.; DiMeglio, L.A.; Gitelman, S.E.; Gottlieb, P.A.; Herold, K.C.; Lovell, D.J.; Orchard, T.J.; Ryan, C.M.; et al. Defining pathways for development of disease-modifying therapies in children with type 1 diabetes: A consensus report. Diabetes Care 2015, 38, 1975–1985. [Google Scholar] [CrossRef] [PubMed]
- Goldman, J.D.; Choi, H. Teplizumab: The First Treatment to Delay the Progression of Type 1 Diabetes. Clin. Diabetes 2023, 41, 474–476. [Google Scholar] [CrossRef]
- Phillip, M.; Achenbach, P.; Addala, A.; Albanese-O’Neill, A.; Battelino, T.; Bell, K.J.; Besser, R.E.; Bonifacio, E.; Colhoun, H.M.; Couper, J.J.; et al. Consensus guidance for monitoring individuals with islet autoantibody-positive pre-stage 3 type 1 diabetes. Diabetologia 2024, 67, 1731–1759. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.M.; Pietropaolo, S.L.; Acevedo-Calado, M.; Huang, S.; Anyaiwe, D.; Scheinker, D.; Steck, A.K.; Vasudevan, M.M.; McKay, S.V.; Sherr, J.L.; et al. CGM Metrics Identify Dysglycemic States in Participants from the TrialNet Pathway to Prevention Study. Diabetes Care 2023, 46, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Desouter, A.K.; Keymeulen, B.; Van de Velde, U.; Van Dalem, A.; Lapauw, B.; De Block, C.; Gillard, P.; Seret, N.; Balti, E.V.; Van Vooren, E.R.; et al. Repeated OGTT Versus Continuous Glucose Monitoring for Predicting Development of Stage 3 Type 1 Diabetes: A Longitudinal Analysis. Diabetes Care 2025, 48, 528–536. [Google Scholar] [CrossRef]
- Ylescupidez, A.; Speake, C.; Pietropaolo, S.L.; Wilson, D.M.; Steck, A.K.; Sherr, J.L.; Gaglia, J.L.; Bender, C.; Lord, S.; Greenbaum, C.J. OGTT Metrics Surpass Continuous Glucose Monitoring Data for T1D Prediction in Multiple-Autoantibody-Positive Individuals. J. Clin. Endocrinol. Metab. 2023, 109, 57–67. [Google Scholar] [CrossRef]
- Sims, E.K.; Besser, R.E.J.; Dayan, C.; Geno Rasmussen, C.; Greenbaum, C.; Griffin, K.J.; Hagopian, W.; Knip, M.; Long, A.E.; Martin, F.; et al. Screening for Type 1 Diabetes in the General Population: A Status Report and Perspective. Diabetes 2022, 71, 610–623. [Google Scholar] [CrossRef]
- Lean, M.E.; Leslie, W.S.; Barnes, A.C.; Brosnahan, N.; Thom, G.; McCombie, L.; Peters, C.; Zhyzhneuskaya, S.; Al-Mrabeh, A.; Hollingsworth, K.G.; et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): An open-label, cluster-randomised trial. Lancet 2018, 391, 541–551. [Google Scholar] [CrossRef]
- Lean, M.E.; Leslie, W.S.; Barnes, A.C.; Brosnahan, N.; Thom, G.; McCombie, L.; Kelly, T.; Irvine, K.; Peters, C.; Zhyzhneuskaya, S.; et al. 5-year follow-up of the randomised Diabetes Remission Clinical Trial (DiRECT) of continued support for weight loss maintenance in the UK: An extension study. Lancet Diabetes Endocrinol. 2024, 12, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Anson, M.; Henney, A.E.; Broadwell, N.; Zhao, S.S.; Ibarburu, G.H.; Lip, G.Y.H.; Wilding, J.P.H.; Cuthberson, D.J.; Alam, U. Incidence of new onset type 2 diabetes in adults living with obesity treated with tirzepatide or semaglutide: Real world evidence from an international retrospective cohort study. EClinicalMedicine 2024, 75, 102777. [Google Scholar] [CrossRef]
- Battelino, T.; Lalic, N.; Hussain, S.; Ceriello, A.; Klobucar, S.; Davies, S.J.; Topsever, P.; Heverly, J.; Ulivi, F.; Brady, K.; et al. The use of continuous glucose monitoring in people living with obesity, intermediate hyperglycemia or type 2 diabetes. Diabetes Res. Clin. Pract. 2025, 223, 112111. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 2. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes-2024. Diabetes Care 2024, 47 (Suppl. 1), S20–S42. [Google Scholar] [CrossRef] [PubMed]
- Radin, M.S. Pitfalls in hemoglobin A1c measurement: When results may be misleading. J. Gen. Intern. Med. 2014, 29, 388–394. [Google Scholar] [CrossRef]
- Dimova, R.; Chakarova, N.; Daniele, G.; Bianchi, C.; Dardano, A.; Del Prato, S.; Tankova, T. Insulin secretion and action affect glucose variability in the early stages of glucose intolerance. Diabetes Metab. Res. Rev. 2022, 38, e3531. [Google Scholar] [CrossRef] [PubMed]
- Chakarova, N.; Dimova, R.; Grozeva, G.; Tankova, T. Assessment of glucose variability in subjects with prediabetes. Diabetes Res. Clin. Pract. 2019, 151, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Zahalka, S.J.; Galindo, R.J.; Shah, V.N.; Low Wang, C.C. Continuous Glucose Monitoring for Prediabetes: What Are the Best Metrics? J. Diabetes Sci. Technol. 2024, 18, 835–846. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Segade, S.; Rodriguez, J.; Camiña, F.; Fernández-Arean, M.; García-Ciudad, V.; Pazos-Couselo, M.; Garcia-Lopez, J.M.; Alonso-Sampedro, M.; Gonzalez-Quintela, A.; Gude, F. Continuous glucose monitoring is more sensitive than HbA1c and fasting glucose in detecting dysglycaemia in a Spanish population without diabetes. Diabetes Res. Clin. Pract. 2018, 142, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, J.M.; van Veen, L.; Fossat, Y. Screening for Impaired Glucose Homeostasis: A Novel Metric of Glycemic Control. Mayo Clin. Proc. Digit. Health 2023, 1, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.; Gastaldelli, A.; Yki-Järvinen, H.; Scherer, P.E. Why does obesity cause diabetes? Cell Metab. 2022, 34, 11–20. [Google Scholar] [CrossRef]
- Mansour, S.; Alkhaaldi, S.M.I.; Sammanasunathan, A.F.; Ibrahim, S.; Farhat, J.; Al-Omari, B. Precision Nutrition Unveiled: Gene-Nutrient Interactions, Microbiota Dynamics, and Lifestyle Factors in Obesity Management. Nutrients 2024, 16, 581. [Google Scholar] [CrossRef]
- Chakhtoura, M.; Haber, R.; Ghezzawi, M.; Rhayem, C.; Tcheroyan, R.; Mantzoros, C.S. Pharmacotherapy of obesity: An update on the available medications and drugs under investigation. EClinicalMedicine 2023, 58, 101882. [Google Scholar] [CrossRef] [PubMed]
- Hegedus, E.; Salvy, S.J.; Wee, C.P.; Naguib, M.; Raymond, J.K.; Fox, D.S.; Vidmar, A.P. Use of continuous glucose monitoring in obesity research: A scoping review. Obes. Res. Clin. Pract. 2021, 15, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Bailey, K.J.; Little, J.P.; Jung, M.E. Self-Monitoring Using Continuous Glucose Monitors with Real-Time Feedback Improves Exercise Adherence in Individuals with Impaired Blood Glucose: A Pilot Study. Diabetes Technol. Ther. 2016, 18, 185–193. [Google Scholar] [CrossRef]
- Richardson, K.M.; Schembre, S.M.; da Silva, V.; Blew, R.M.; Behrens, N.; Roe, D.J.; Marvasti, F.F.; Hingle, M. Adding a Brief Continuous Glucose Monitoring Intervention to the National Diabetes Prevention Program: A Multimethod Feasibility Study. J. Diabetes Res. 2024, 2024, 7687694. [Google Scholar] [CrossRef] [PubMed]
- Naguib, M.N.; Hegedus, E.; Raymond, J.K.; Goran, M.I.; Salvy, S.J.; Wee, C.P.; Durazo-Arvizu, R.; Moss, L.; Vidmar, A.P. Continuous Glucose Monitoring in Adolescents with Obesity: Monitoring of Glucose Profiles, Glycemic Excursions, and Adherence to Time Restricted Eating Programs. Front. Endocrinol. 2022, 13, 841838. [Google Scholar] [CrossRef] [PubMed]
- Beaudart, C.; Demonceau, C.; Reginster, J.Y.; Locquet, M.; Cesari, M.; Cruz Jentoft, A.J.; Bruyere, O. Sarcopenia and health-related quality of life: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2023, 14, 1228–1243. [Google Scholar] [CrossRef] [PubMed]
- Sayer, A.A.; Cooper, R.; Arai, H.; Cawthon, P.M.; Ntsama Essomba, M.J.; Fielding, R.A.; Grounds, M.R.; Witham, M.D.; Cruz-Jentoft, A.J. Sarcopenia. Nat. Rev. Dis. Primers 2024, 10, 68. [Google Scholar] [CrossRef]
- Norton, L.; Shannon, C.; Gastaldelli, A.; DeFronzo, R.A. Insulin: The master regulator of glucose metabolism. Metabolism 2022, 129, 155142. [Google Scholar] [CrossRef]
- Park, H.; Metwally, A.A.; Delfarah, A.; Wu, Y.; Perelman, D.; Mayer, C.; McGinity, C.; Rodgar, M.; Celli, A.; McLaughlin, T.; et al. High-resolution lifestyle profiling and metabolic subphenotypes of type 2 diabetes. npj Digit. Med. 2025, 8, 352. [Google Scholar] [CrossRef]
- Metwally, A.A.; Perelman, D.; Park, H.; Wu, Y.; Jha, A.; Sharp, S.; Celli, A.; Ayhan, E.; Abbasi, F.; Gloyn, A.L.; et al. Prediction of metabolic subphenotypes of type 2 diabetes via continuous glucose monitoring and machine learning. Nat. Biomed. Eng. 2025, 9, 1222–1239. [Google Scholar] [CrossRef] [PubMed]
- Vetter, C.; Devore, E.E.; Ramin, C.A.; Speizer, F.E.; Willett, W.C.; Schernhammer, E.S. Mismatch of Sleep and Work Timing and Risk of Type 2 Diabetes. Diabetes Care 2015, 38, 1707–1713. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, A.K.; van den Donk, M.; Hilding, A.; Östenson, C.G. Work stress, sense of coherence, and risk of type 2 diabetes in a prospective study of middle-aged Swedish men and women. Diabetes Care 2013, 36, 2683–2689. [Google Scholar] [CrossRef]
- Marhefkova, N.; Sládek, M.; Sumová, A.; Dubsky, M. Circadian dysfunction and cardio-metabolic disorders in humans. Front. Endocrinol. 2024, 15, 1328139. [Google Scholar] [CrossRef] [PubMed]
- Fujino, Y.; Iso, H.; Tamakoshi, A.; Inaba, Y.; Koizumi, A.; Kubo, T.; Yoshimura, T.; Japanese Collaborative Cohort Study Group. A prospective cohort study of shift work and risk of ischemic heart disease in Japanese male workers. Am. J. Epidemiol. 2006, 164, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Gu, W.; Chen, Y.; Li, X.; Shi, J.; Lv, A.; Hu, J.; Zhang, R.; Liu, R.; Hong, J.; et al. The impact of shift work on glycemic characteristics assessed by CGM and its association with metabolic indices in non-diabetic subjects. Acta Diabetol. 2020, 57, 53–61. [Google Scholar] [CrossRef]
- Brandt, R.; Park, M.; Wroblewski, K.; Quinn, L.; Tasali, E.; Cinar, A. Sleep quality and glycaemic variability in a real-life setting in adults with type 1 diabetes. Diabetologia 2021, 64, 2159–2169. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, L.H.; Li, D.D.; Xu, F.; Wang, X.H.; Lu, C.F.; Wang, C.H.; Yu, C.; Zhang, X.L.; Ning, L.Y.; et al. Association of sleep quality with glycemic variability assessed by flash glucose monitoring in patients with type 2 diabetes. Diabetol. Metab. Syndr. 2021, 13, 102. [Google Scholar] [CrossRef] [PubMed]
- Inaishi, J.; Kashiwagi, K.; Kinoshita, S.; Wada, Y.; Hanashiro, S.; Shiga, K.; Kitazawa, M.; Tsutsumi, S.; Yamakawa, H.; Kishimoto, T. Associations between glycemic variability, sleep quality, and daily steps in subjects without diabetes using wearable devices. Metab. Open 2023, 20, 100263. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, N.; Chivese, T.; Werfalli, M.; Sun, H.; Yuen, L.; Hoegfeldt, C.A.; Powe, C.E.; Immanuel, J.; Karuranga, S.; et al. IDF Diabetes Atlas: Estimation of Global and Regional Gestational Diabetes Mellitus Prevalence for 2021 by International Association of Diabetes in Pregnancy Study Group’s Criteria. Diabetes Res. Clin. Pract. 2022, 183, 109050. [Google Scholar] [CrossRef]
- Sweeting, A.; Wong, J.; Murphy, H.R.; Ross, G.P. A Clinical Update on Gestational Diabetes Mellitus. Endocr. Rev. 2022, 43, 763–793. [Google Scholar] [CrossRef]
- McIntyre, H.D.; Catalano, P.; Zhang, C.; Desoye, G.; Mathiesen, E.R.; Damm, P. Gestational diabetes mellitus. Nat. Rev. Dis. Primers 2019, 5, 47. [Google Scholar] [CrossRef] [PubMed]
- Feig, D.S.; Donovan, L.E.; Corcoy, R.; Murphy, K.E.; Amiel, S.A.; Hunt, K.F.; Asztalos, E.; Barrett, J.F.R.; Sanchez, J.J.; de Leiva, A.; et al. Continuous glucose monitoring in pregnant women with type 1 diabetes (CONCEPTT): A multicentre international randomised controlled trial. Lancet 2017, 390, 2347–2359. [Google Scholar] [CrossRef]
- Lee, T.T.M.; Collett, C.; Bergford, S.; Hartnell, S.; Scott, E.M.; Lindsay, R.S.; Hunt, K.F.; McCance, D.R.; Barnard-Kelly, K.; Rankin, D.; et al. Automated Insulin Delivery in Women with Pregnancy Complicated by Type 1 Diabetes. N. Engl. J. Med. 2023, 389, 1566–1578. [Google Scholar] [CrossRef]
- Diabetes in Pregnancy: Management from Preconception to the Postnatal Period. NICE Guideline [NG3]. Available online: https://www.nice.org.uk/guidance/ng3/chapter/Recommendations#antenatal-care-for-women-with-diabetes (accessed on 4 November 2024).
- Continuous Glucose Monitoring Amongst Pregnant Women with Early-Onset Type 2 Diabetes. PROTECT Study. Available online: https://www.isrctn.com/ISRCTN12804317?q=ISRCTN12804317&filters=&sort=&offset=1&totalResults=1&page=1&pageSize=10 (accessed on 17 March 2025).
- Castorino, K.; Durnwald, C.; Ehrenberg, S.; Ehrhardt, N.; Isaacs, D.; Levy, C.J.; Valent, A.M. Continuous Glucose Monitoring for Gestational Diabetes Management Working Group. Practical Considerations for Using Continuous Glucose Monitoring in Patients with Gestational Diabetes Mellitus. J. Womens Health 2024, 34, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Durnwald, C.; Beck, R.W.; Li, Z.; Norton, E.; Bergenstal, R.M.; Johnson, M.; Dunnigan, S.; Banfield, M.; Krumwiede, K.; Sibayan, J.; et al. Continuous Glucose Monitoring Profiles in Pregnancies with and Without Gestational Diabetes Mellitus. Diabetes Care 2024, 47, 1333–1341. [Google Scholar] [CrossRef] [PubMed]
- Quah, P.L.; Tan, L.K.; Lek, N.; Thain, S.; Tan, K.H. Glycemic Variability in Early Pregnancy May Predict a Subsequent Diagnosis of Gestational Diabetes. Diabetes Metab. Syndr. Obes. 2022, 15, 4065–4074. [Google Scholar] [CrossRef]
- Li, Z.; Beck, R.; Durnwald, C.; Carlson, A.; Norton, E.; Bergenstal, R.; Johnson, M.; Dunnigan, S.; Banfield, M.; Krumwiede, K.; et al. Continuous Glucose Monitoring Prediction of Gestational Diabetes Mellitus and Perinatal Complications. Diabetes Technol. Ther. 2024, 26, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Márquez-Pardo, R.; Baena-Nieto, M.G.; Córdoba-Doña, J.A.; Cruzado-Begines, C.; García-García-Doncel, L.; Aguilar-Diosdado, M.; Torres-Barea, I.M. Glycemic variability in diagnosis of gestational diabetes as predictor of pharmacological treatment. Endocrinol. Diabetes Nutr. 2024, 71, 96–102. [Google Scholar] [CrossRef]
- Liang, X.; Fu, Y.; Lu, S.; Shuai, M.; Miao, Z.; Gou, W.; Shen, L.; Liang, Y.; Xu, F.; Tian, Y.; et al. Continuous glucose monitoring-derived glycemic metrics and adverse pregnancy outcomes among women with gestational diabetes: A prospective cohort study. Lancet Reg. Health West. Pac. 2023, 39, 100823. [Google Scholar] [CrossRef] [PubMed]
- Kytö, M.; Koivusalo, S.; Tuomonen, H.; Strömberg, L.; Ruonala, A.; Marttinen, P.; Heinonen, S.; Jacucci, G. Supporting the Management of Gestational Diabetes Mellitus with Comprehensive Self-Tracking: Mixed Methods Study of Wearable Sensors. JMIR Diabetes 2023, 8, e43979. [Google Scholar] [CrossRef]
- Lai, M.; Weng, J.; Yang, J.; Gong, Y.; Fang, F.; Li, N.; Kang, M.; Xu, X.; Wang, X. Effect of continuous glucose monitoring compared with self-monitoring of blood glucose in gestational diabetes patients with HbA1c<6%: A randomized controlled trial. Front. Endocrinol. 2023, 14, 1174239. [Google Scholar]
- Murphy, H.R. Roadmap to the Effective Use of Continuous Glucose Monitoring in Pregnancy. Diabetes Spectr. 2023, 36, 315–319. [Google Scholar] [CrossRef]
- Harmon, K.A.; Gerard, L.; Jensen, D.R.; Kealey, E.H.; Hernandez, T.L.; Reece, M.S.; Barbour, L.A.; Bessesen, D.H. Continuous glucose profiles in obese and normal-weight pregnant women on a controlled diet: Metabolic determinants of fetal growth. Diabetes Care 2011, 34, 2198–2204. [Google Scholar] [CrossRef]
- Di Filippo, D.; Henry, A.; Bell, C.; Haynes, S.; Chang, M.H.Y.; Darling, J.; Welsh, A. A new continuous glucose monitor for the diagnosis of gestational diabetes mellitus: A pilot study. BMC Pregnancy Childbirth 2023, 23, 186. [Google Scholar] [CrossRef]
- Kusinski, L.C.; Brown, J.; Hughes, D.J.; Meek, C.L. Feasibility and acceptability of continuous glucose monitoring in pregnancy for the diagnosis of gestational diabetes: A single-centre prospective mixed methods study. PLoS ONE 2023, 18, e0292094. [Google Scholar] [CrossRef]
- Battelino, T.; Danne, T.; Bergenstal, R.M.; Amiel, S.A.; Beck, R.; Biester, T.; Bosi, E.; Buckingham, B.A.; Cefalu, W.T.; Close, K.L.; et al. Clinical Targets for Continuous Glucose Monitoring Data Interpretation: Recommendations from the International Consensus on Time in Range. Diabetes Care 2019, 42, 1593–1603. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Levi, A.M.; Rubio-Herrera, M.A.; Matía-Martín, P.; Pérez-Ferre, N.; Marcuello, C.; Sánchez-Pernaute, A.; Torres-Garcia, A.J.; Calle-Pascual, A.L. Mixed Meal Tolerance Test Versus Continuous Glucose Monitoring for an Effective Diagnosis of Persistent Post-Bariatric Hypoglycemia. J. Clin. Med. 2023, 12, 4295. [Google Scholar] [CrossRef]
- Yu, Y.; Groth, S.W. Use of Continuous Glucose Monitoring in Patients Following Bariatric Surgery: A Scoping Review. Obes. Surg. 2023, 33, 2573–2582. [Google Scholar] [CrossRef] [PubMed]
- Lupoli, R.; Lembo, E.; Rainone, C.; Schiavo, L.; Iannelli, A.; Di Minno, M.N.D.; Capaldo, B. Rate of post-bariatric hypoglycemia using continuous glucose monitoring: A meta-analysis of literature studies. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Quevedo, R.; de Melo, M.E.; Cercato, C.; Fernandes, A.E.; Dantas, A.C.B.; Santo, M.A.; Pajecki, D.; Mancini, M.C. Continuous Glucose Monitoring Captures Glycemic Variability After Roux-en-Y Gastric Bypass in Patients with and Without Type 2 Diabetes Mellitus: A Prospective Cohort Study. Obes. Surg. 2024, 34, 2789–2798. [Google Scholar] [CrossRef] [PubMed]
- Dorcely, B.; DeBermont, J.; Gujral, A.; Reid, M.; Vanegas, S.M.; Popp, C.J.; Verano, M.; Jay, M.; Schmidt, A.M.; Bergman, M.; et al. Continuous glucose monitoring captures glycemic variability in obesity after sleeve gastrectomy: A prospective cohort study. Obes. Sci. Pract. 2024, 10, e729. [Google Scholar] [CrossRef]
- Ostrovsky, V.; Knobler, H.; Lazar, L.O.; Pines, G.; Kuniavsky, T.; Cohen, L.; Schiller, T.; Kirzhner, A.; Zornitzki, T. Persistent post-bariatric-surgery hypoglycemia: A long-term follow-up reassessment. Nutr. Metab. Cardiovasc. Dis. 2023, 33, 1197–1205. [Google Scholar] [CrossRef]
- Cummings, C.; Jiang, A.; Sheehan, A.; Ferraz-Bannitz, R.; Puleio, A.; Simonson, D.C.; Dreyfuss, J.M.; Patti, M.E. Continuous glucose monitoring in patients with post-bariatric hypoglycaemia reduces hypoglycaemia and glycaemic variability. Diabetes Obes. Metab. 2023, 25, 2191–2202. [Google Scholar] [CrossRef]
- Ma, J.; Huang, X.; Zhao, J.; Lu, J.; Lu, W.; Bao, Y.; Zhou, J.; Han, J. CGM for insulinoma screening: A prospective and observational case-control study. Endocr. Relat. Cancer 2021, 28, 291–300. [Google Scholar] [CrossRef]
- Magliozzo, M.; Tumminia, A.; Arpi, M.L.; Deiana, E.; Guglielmo, M.; Giannone, G.; Frasca, F.; Gullo, D. Intraoperative intermittently scanned continuous glucose monitoring in the management of patients with pancreatic insulinoma. J. Endocrinol. Investig. 2024, 48, 777–781. [Google Scholar] [CrossRef] [PubMed]
- Ciardullo, S.; Muraca, E.; Vergani, M.; Invernizzi, P.; Perseghin, G. Advancements in pharmacological treatment of NAFLD/MASLD: A focus on metabolic and liver-targeted interventions. Gastroenterol. Rep. 2024, 12, goae029. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD). J. Hepatol. 2024, 81, 492–542. [Google Scholar] [CrossRef] [PubMed]
- Bhat, N.; Mani, A. Dysregulation of Lipid and Glucose Metabolism in Nonalcoholic Fatty Liver Disease. Nutrients 2023, 15, 2323. [Google Scholar] [CrossRef]
- Gancheva, S.; Roden, M.; Castera, L. Diabetes as a risk factor for MASH progression. Diabetes Res. Clin. Pract. 2024, 217, 111846. [Google Scholar] [CrossRef] [PubMed]
- Keshet, A.; Shilo, S.; Godneva, A.; Talmor-Barkan, Y.; Aviv, Y.; Segal, E.; Rossman, H. CGMap: Characterizing continuous glucose monitor data in thousands of non-diabetic individuals. Cell Metab. 2023, 35, 758–769.e3. [Google Scholar] [CrossRef]
- Zhong, H.; Zhang, K.; Lin, L.; Yan, Y.; Shen, L.; Chen, H.; Liang, X.; Chen, J.; Miao, Z.; Zheng, J.S.; et al. Two-week continuous glucose monitoring-derived metrics and degree of hepatic steatosis: A cross-sectional study among Chinese middle-aged and elderly participants. Cardiovasc. Diabetol. 2024, 23, 322. [Google Scholar] [CrossRef] [PubMed]
- Schiaffini, R.; Liccardo, D.; Alisi, A.; Benevento, D.; Cappa, M.; Cianfarani, S.; Nobili, V. Early Glucose Derangement Detected by Continuous Glucose Monitoring and Progression of Liver Fibrosis in Nonalcoholic Fatty Liver Disease: An Independent Predictive Factor? Horm. Res. Paediatr. 2016, 85, 29–34. [Google Scholar] [CrossRef]
- Tao, M.; Zhou, J.; Zhu, J.; Lu, W.; Jia, W. Continuous glucose monitoring reveals abnormal features of postprandial glycemic excursions in women with polycystic ovarian syndrome. Postgrad. Med. 2011, 123, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Li, X.; Chen, P.; Chen, L.; Zhao, X. The Beta-Cell Function and Glucose Profile of Newly Diagnosed Acromegalic Patients with Normal Glucose Tolerance. Int. J. Endocrinol. 2021, 2021, 3666692. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Sun, Q.; Wang, Y.; Zhou, J.; Zhao, X. Correlation between insulin-like growth factor and complexity of glucose time series index in patients with newly diagnosed acromegaly: A PILOT study. Endocrine 2024, 87, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Ma, X.; Lu, J.; Tao, R.; Yu, X.; Mo, Y.; Lu, W.; Bao, Y.; Zhou, J.; Jia, W. Decreasing complexity of glucose time series derived from continuous glucose monitoring is correlated with deteriorating glucose regulation. Front. Med. 2023, 17, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Jo, E.A.; Woo, H.Y.; Cho, A.; Ko, M.; Kim, S.; Han, A.; Ha, J.; Min, S. Perioperative glucose monitoring with continuous glucose monitors identifies risk factors for post-transplant diabetes mellitus in kidney transplant recipients. Sci. Rep. 2024, 14, 21240. [Google Scholar] [CrossRef]
- Eleftheriadis, G.; Naik, M.G.; Osmanodja, B.; Liefeldt, L.; Choi, M.; Halleck, F.; Schrezenmeier, E.; Eckardt, K.U.; Pigorsch, M.; Tura, A.; et al. Continuous glucose monitoring for the prediction of posttransplant diabetes mellitus and impaired glucose tolerance on day 90 after kidney transplantation-A prospective proof-of-concept study. Am. J. Transplant. 2024, 24, 2225–22234. [Google Scholar] [CrossRef]
- Fathallah, N.; Slim, R.; Larif, S.; Hmouda, H.; Ben Salem, C. Drug-Induced Hyperglycaemia and Diabetes. Drug Saf. 2015, 38, 1153–1168. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.X.; Zhu, X.M.; Miao, Q.; Ye, H.Y.; Zhang, Z.Y.; Li, Y.M. Hyperglycemia induced by glucocorticoids in nondiabetic patients: A meta-analysis. Ann Nutr. Metab. 2014, 65, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Management of Hyperglycaemia and Steroid (Glucocorticoid) Therapy, 2023. Joint British Diabetes Societies (JBDS) for Inpatient Care Group. Available online: https://abcd.care/resource/current/jbds-08-management-hyperglycaemia-and-steroid-glucocorticoid-therapy (accessed on 4 November 2024).
- Mills, E.; Devendra, S. Steroid-induced hyperglycaemia in primary care. Lond. J. Prim. Care 2015, 7, 103–106. [Google Scholar] [CrossRef]
- Ulene, S.; Wang, S.; Cook, J.R.; McAuley, F.; Wooster, M.E.; Faheem, K.F.; Varoli, A.; McGuinness, J.E.; Vasan, N.; Trivedi, M.S.; et al. Continuous glucose monitoring and rates of hyperglycemia during chemotherapy for early-stage breast cancer. Breast Cancer Res. Treat. 2025, 212, 511–519. [Google Scholar] [CrossRef]
- Kleinhans, M.; Albrecht, L.J.; Benson, S.; Fuhrer, D.; Dissemond, J.; Tan, S. Continuous Glucose Monitoring of Steroid-Induced Hyperglycemia in Patients with Dermatologic Diseases. J. Diabetes Sci. Technol. 2024, 18, 904–910. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.S.; Barroso-Sousa, R.; Tolaney, S.M.; Hodi, F.S.; Kaiser, U.B.; Min, L. Endocrine Toxicity of Cancer Immunotherapy Targeting Immune Checkpoints. Endocr. Rev. 2019, 40, 17–65. [Google Scholar] [CrossRef] [PubMed]
- Stelmachowska-Banaś, M.; Czajka-Oraniec, I. Management of endocrine immune-related adverse events of immune checkpoint inhibitors: An updated review. Endocr. Connect. 2020, 9, R207–R228. [Google Scholar] [CrossRef]
- Kyriacou, A.; Melson, E.; Chen, W.; Kempegowda, P. Is immune checkpoint inhibitor-associated diabetes the same as fulminant type 1 diabetes mellitus? Clin. Med. 2020, 20, 417–423. [Google Scholar] [CrossRef]
- Schneider, B.J.; Naidoo, J.; Santomasso, B.D.; Lacchetti, C.; Adkins, S.; Anadkat, M.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; et al. Management of Immune-Related Adverse Events in Patients Treated with Immune Checkpoint Inhibitor Therapy: ASCO Guideline Update. J. Clin. Oncol. 2021, 39, 4073–4126. [Google Scholar] [CrossRef]
- Quandt, Z.; Young, A.; Perdigoto, A.L.; Herold, K.C.; Anderson, M.S. Autoimmune Endocrinopathies: An Emerging Complication of Immune Checkpoint Inhibitors. Annu. Rev. Med. 2021, 72, 313–330. [Google Scholar] [CrossRef]
- Moran, A.; Dunitz, J.; Nathan, B.; Saeed, A.; Holme, B.; Thomas, W. Cystic fibrosis-related diabetes: Current trends in prevalence, incidence, and mortality. Diabetes Care 2009, 32, 1626–1631. [Google Scholar] [CrossRef] [PubMed]
- Kirigin Biloš, L.S.; Altabas, V.; Vukić Dugac, A.; Baretić, M. The Role of Continuous Glucose Monitoring in Detecting Early Dysglycemia and Clinical Outcomes in Patients with Cystic Fibrosis. Medicina 2024, 60, 477. [Google Scholar] [CrossRef]
- Kumar, S.; Pallin, M.; Soldatos, G.; Teede, H. Comparison of continuous glucose monitoring to reference standard oral glucose tolerance test for the detection of dysglycemia in cystic Fibrosis: A systematic review. J. Clin. Transl. Endocrinol. 2022, 30, 100305. [Google Scholar] [CrossRef]
- Scully, K.J.; Sherwood, J.S.; Martin, K.; Ruazol, M.; Marchetti, P.; Larkin, M.; Zheng, H.; Sawicki, G.S.; Uluer, A.; Neuringer, I.; et al. Continuous Glucose Monitoring and HbA1c in Cystic Fibrosis: Clinical Correlations and Implications for CFRD Diagnosis. J. Clin. Endocrinol. Metab. 2022, 107, e1444–e1454. [Google Scholar] [CrossRef] [PubMed]
- Scully, K.J.; Brenner, L.; Martin, K.; Ruazol, M.; Sawicki, G.S.; Uluer, A.; Nueringer, I.; Yonker, L.M.; Sicilian, L.; Putman, M.S. Continuous glucose monitoring and advanced glycation endproducts for prediction of clinical outcomes and development of cystic fibrosis-related diabetes in adults with CF. Front. Endocrinol. 2024, 15, 1293709. [Google Scholar] [CrossRef]
- Identification of Dysglycemia with Continuous Glucose Monitoring to Assess Clinical Evolution in Cystic Fibrosis (ProspeC-F). Available online: https://clinicaltrials.gov/study/NCT05099939 (accessed on 4 November 2024).
- Whittaker, R.G.; Schaefer, A.M.; McFarland, R.; Taylor, R.W.; Walker, M.; Turnbull, D.M. Prevalence and progression of diabetes in mitochondrial disease. Diabetologia 2007, 50, 2085–2089. [Google Scholar] [CrossRef]
- Ren, Q.; Zhang, P.; Pang, W.; Ma, Y.; Gong, S.; Ba, T.; Liu, W.; Zhang, F.; Zhang, X.; Zhang, R.; et al. A Comparison of Daily Glucose Fluctuation Between GCK-MODY and Type 2 Diabetes Using Continuous Glucose Monitoring Technology. Diabetes 2023, 72, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Thuesen, A.C.B.; Jensen, R.T.; Maagensen, H.; Kristiansen, M.R.; Sørensen, H.T.; Vaag, A.; Beck-Nielsen, H.; Pedersen, O.B.; Grarup, N.; Nielsen, J.S.; et al. Identification of pathogenic GCK variants in patients with common type 2 diabetes can lead to discontinuation of pharmacological treatment. Mol. Genet. Metab. Rep. 2023, 35, 100972. [Google Scholar] [CrossRef]
- Williamson, A.; Norris, D.M.; Yin, X.; Broadaway, K.A.; Moxley, A.H.; Vadlamudi, S.; Wilson, E.P.; Jackson, A.U.; Ahuja, V.; Andersen, M.K.; et al. Genome-wide association study and functional characterization identifies candidate genes for insulin-stimulated glucose uptake. Nat. Genet. 2023, 55, 973–983. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.E.; Walker, M. Genetics of Insulin Resistance and the Metabolic Syndrome. Curr. Cardiol. Rep. 2016, 18, 75. [Google Scholar] [CrossRef] [PubMed]
- van der Kroef, S.; Noordam, R.; Deelen, J.; Akintola, A.A.; Jansen, S.W.; Postmus, I.; Wijsman, C.A.; Beekman, M.; Mooijaart, S.P.; Slagboom, P.E.; et al. Association between the rs7903146 Polymorphism in the TCF7L2 Gene and Parameters Derived with Continuous Glucose Monitoring in Individuals without Diabetes. PLoS ONE 2016, 11, e0149992. [Google Scholar] [CrossRef] [PubMed]
- Torekov, S.S.; Iepsen, E.; Christiansen, M.; Linneberg, A.; Pedersen, O.; Holst, J.J.; Kanters, J.K.; Hansen, T. KCNQ1 long QT syndrome patients have hyperinsulinemia and symptomatic hypoglycemia. Diabetes 2014, 63, 1315–1325. [Google Scholar] [CrossRef] [PubMed]
- Clemente, M.; Cobo, P.; Antolín, M.; Campos, A.; Yeste, D.; Tomasini, R.; Caimari, M.; Masas, M.; Garcia-Arumi, E.; Fernandez-Cancio, M.; et al. Genetics and Natural History of Non-pancreatectomized Patients With Congenital Hyperinsulinism Due to Variants in ABCC8. J. Clin. Endocrinol. Metab. 2023, 108, e1316–e1328. [Google Scholar] [CrossRef]
- Hanley, M.; Ryan, D.T.; Kyle, E.; Kavanagh, E.C. Radiographic appearances of a continuous glucose monitor in a patient with lipodystrophy. Radiol. Case Rep. 2023, 18, 3287–3290. [Google Scholar] [CrossRef]
- Arlt, W.; Willis, D.S.; Wild, S.H.; Krone, N.; Doherty, E.J.; Hahner, S.; Han, T.S.; Carroll, P.V.; Conway, G.S.; Rees, D.A.; et al. Health status of adults with congenital adrenal hyperplasia: A cohort study of 203 patients. J. Clin. Endocrinol. Metab. 2010, 95, 5110–5121. [Google Scholar] [CrossRef] [PubMed]
- Barbot, M.; Mazzeo, P.; Lazzara, M.; Ceccato, F.; Scaroni, C. Metabolic syndrome and cardiovascular morbidity in patients with congenital adrenal hyperplasia. Front. Endocrinol. 2022, 13, 934675. [Google Scholar] [CrossRef]
- Galderisi, A.; Kariyawasam, D.; Stoupa, A.; Quoc, A.N.; Pinto, G.; Viaud, M.; Brabant, S.; Beltrand, J.; Polak, M.; Samara-Boustani, D. Glucose pattern in children with classical congenital adrenal hyperplasia: Evidence from continuous glucose monitoring. Eur. J. Endocrinol. 2023, 189, K19–K24. [Google Scholar] [CrossRef] [PubMed]
- Merino, J.; Linenberg, I.; Bermingham, K.M.; Ganesh, S.; Bakker, E.; Delahanty, L.M.; Chan, A.T.; Pujol, J.C.; Wolf, J.; Khatib, H.A.; et al. Validity of continuous glucose monitoring for categorizing glycemic responses to diet: Implications for use in personalized nutrition. Am. J. Clin. Nutr. 2022, 115, 1569–1576. [Google Scholar] [CrossRef] [PubMed]
- Pai, R.; Barua, S.; Kim, B.S.; McDonald, M.; Wierzchowska-McNew, R.A.; Pai, A.; Deutz, N.E.P.; Kerr, D.; Sabharwal, A. Estimating Breakfast Characteristics Using Continuous Glucose Monitoring and Machine Learning in Adults With or at Risk of Type 2 Diabetes. J. Diabetes Sci. Technol. 2024, 19322968241274800. [Google Scholar] [CrossRef] [PubMed]
- Bowler, A.M.; Whitfield, J.; Marshall, L.; Coffey, V.G.; Burke, L.M.; Cox, G.R. The Use of Continuous Glucose Monitors in Sport: Possible Applications and Considerations. Int. J. Sport Nutr. Exerc. Metab. 2023, 33, 121–132. [Google Scholar] [CrossRef]
- Scheelbeek, P.; Green, R.; Papier, K.; Knuppel, A.; Alae-Carew, C.; Balkwill, A.; Key, T.J.; Beral, V.; Dangour, A.D. Health impacts and environmental footprints of diets that meet the Eatwell Guide recommendations: Analyses of multiple UK studies. BMJ Open 2020, 10, e037554. [Google Scholar] [CrossRef] [PubMed]
- Basiri, R.; Cheskin, L.J. Personalized Nutrition Therapy without Weight Loss Counseling Produces Weight Loss in Individuals with Prediabetes Who Are Overweight/Obese: A Randomized Controlled Trial. Nutrients 2024, 16, 2218. [Google Scholar] [CrossRef]
- Bermingham, K.M.; Linenberg, I.; Polidori, L.; Asnicar, F.; Arrè, A.; Wolf, J.; Badri, F.; Bernard, H.; Capdevila, J.; Bulsiewicz, W.J.; et al. Effects of a personalized nutrition program on cardiometabolic health: A randomized controlled trial. Nat. Med. 2024, 30, 1888–1897. [Google Scholar] [CrossRef]
- Ben-Yacov, O.; Godneva, A.; Rein, M.; Shilo, S.; Kolobkov, D.; Koren, N.; Dolev, N.C.; Shmul, T.T.; Wolf, B.C.; Kosower, N.; et al. Personalized Postprandial Glucose Response-Targeting Diet Versus Mediterranean Diet for Glycemic Control in Prediabetes. Diabetes Care 2021, 44, 1980–1991. [Google Scholar] [CrossRef]
- Hengist, A.; Ong, J.A.; McNeel, K.; Guo, J.; Hall, K.D. Imprecision nutrition? Intraindividual variability of glucose responses to duplicate presented meals in adults without diabetes. Am. J. Clin. Nutr. 2025, 121, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Wolever, T.M. Personalized nutrition by prediction of glycemic responses: Garbage in → garbage out. Am. J. Clin. Nutr. 2025, 121, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, C.; Irace, C.; Wilmot, E.G.; Akra, B.; Del Prato, S.; Cuesta, M.; Adolfsson, P.; Klupa, T.; Renard, E.; Battelino, T. Minimum expectations for market authorization of continuous glucose monitoring devices in Europe-‘eCGM’ compliance status. Diabetes Obes. Metab. 2025, 27, 1025–1031. [Google Scholar] [CrossRef] [PubMed]
| High-Risk Group | Rationale for CGM Use | Potential Future Applications | Limitations/Considerations |
|---|---|---|---|
| Pre-symptomatic type 1 diabetes (islet autoantibody positivity) |
|
| Lack of evidence for the following:
|
| Metabolic dysfunction (Prediabetes, early type 2 diabetes, and altered body composition) |
|
|
|
| Gestational diabetes mellitus |
|
|
|
| MASLD PCOS Acromegaly | Commonly associated with insulin resistance and dysglycaemia |
|
|
| Solid organ transplantation | High risk of post-transplant diabetes associated with reduced graft survival and mortality [13,14,15,16,17] |
|
|
| Steroid/Immune checkpoint inhibitors therapy | Therapy-induced hyperglycaemia is often unpredictable |
|
|
| Genetic syndromes (e.g., MODY, CFRD, etc.) | Unique pathophysiology of dysglycaemia Early identification of high glycaemic variability |
|
|
| High-Risk Group | CGM Metric(s) | Cut-Off Thresholds | Predictive Values |
|---|---|---|---|
| Pre-symptomatic type 1 diabetes (islet autoantibody positivity) [8] | Interstitial fluid glucose levels | Glucose ≥ 140 mg/dL (≥7.8 mmol/L) for >10% per day | 88% sensitivity and 91% specificity for diabetes prediction |
| Glycaemic variability | 20 mg/dL (1.1 mmol/L) standard deviation | 81% sensitivity and 81% specificity for diabetes prediction | |
| Mean amplitude of glucose excursion | 37 mg/dL (2.1 mmol/L) | 69% sensitivity and 91% specificity for diabetes prediction | |
| Prediabetes [37] | Functional assessment of glucose homeostasis (FLAG) | Prediabetes defined as per American Diabetes Association criteria | 86% sensitivity and 71–78% specificity for prediabetes |
| Gestational diabetes mellitus [66] | Second trimester and gestational week 13–14 percent time > 140 mg/dL (>7.8 mmol/L) | Percent time > 140 mg/dL (>7.8 mmol/L) |
|
| PTDM [99] | Percent time > 140 mg/dL (>7.8 mmol/L) | Exploratory screening thresholds of 31.8% on day 8 and 13.2% on day 30 | AUROC for days 8–90 post-transplant: 0.88–0.99 |
| CFRD [114] | Percent time > 140 mg/dL (>7.8 mmol/L) and/or 180 mg/dL (10 mmol/L) | 17.5% time > 140 mg/dL (>7.8 mmol/L); 3.4% time > 180 mg/dL (>10 mmol/L) |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liarakos, A.L.; Panagiotou, G.; Chondronikola, M.; Wilmot, E.G. Continuous Glucose Monitoring in People at High Risk of Diabetes and Dysglycaemia: Transforming Early Risk Detection and Personalised Care. Life 2025, 15, 1579. https://doi.org/10.3390/life15101579
Liarakos AL, Panagiotou G, Chondronikola M, Wilmot EG. Continuous Glucose Monitoring in People at High Risk of Diabetes and Dysglycaemia: Transforming Early Risk Detection and Personalised Care. Life. 2025; 15(10):1579. https://doi.org/10.3390/life15101579
Chicago/Turabian StyleLiarakos, Alexandros L., Grigorios Panagiotou, Maria Chondronikola, and Emma G. Wilmot. 2025. "Continuous Glucose Monitoring in People at High Risk of Diabetes and Dysglycaemia: Transforming Early Risk Detection and Personalised Care" Life 15, no. 10: 1579. https://doi.org/10.3390/life15101579
APA StyleLiarakos, A. L., Panagiotou, G., Chondronikola, M., & Wilmot, E. G. (2025). Continuous Glucose Monitoring in People at High Risk of Diabetes and Dysglycaemia: Transforming Early Risk Detection and Personalised Care. Life, 15(10), 1579. https://doi.org/10.3390/life15101579






