In Vitro and In Vivo Interventions Reveal the Health Benefits of Levan-Type Exopolysaccharide Produced by a Fish Gut Isolate Lactobacillus reuteri FW2
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strain and Biosynthesis of EPS
2.1.1. Bacterial Strain
2.1.2. Biosynthesis of EPS
2.2. In Vitro Experiments
2.2.1. Cell Culturing
2.2.2. Cell Viability and Cytotoxicity
2.2.3. Antioxidant Activity of FW2 Levan
2.3. In Vivo Experiments Using Mice Models
2.3.1. Preparation of Mice Feed
2.3.2. Animal Models and Dietary Strategy
2.3.3. Body Weight Analysis
2.3.4. Determination of Blood Glucose Analysis
2.3.5. Investigation of Serum Cholesterol Levels
2.4. Statistical Analyses
3. Results
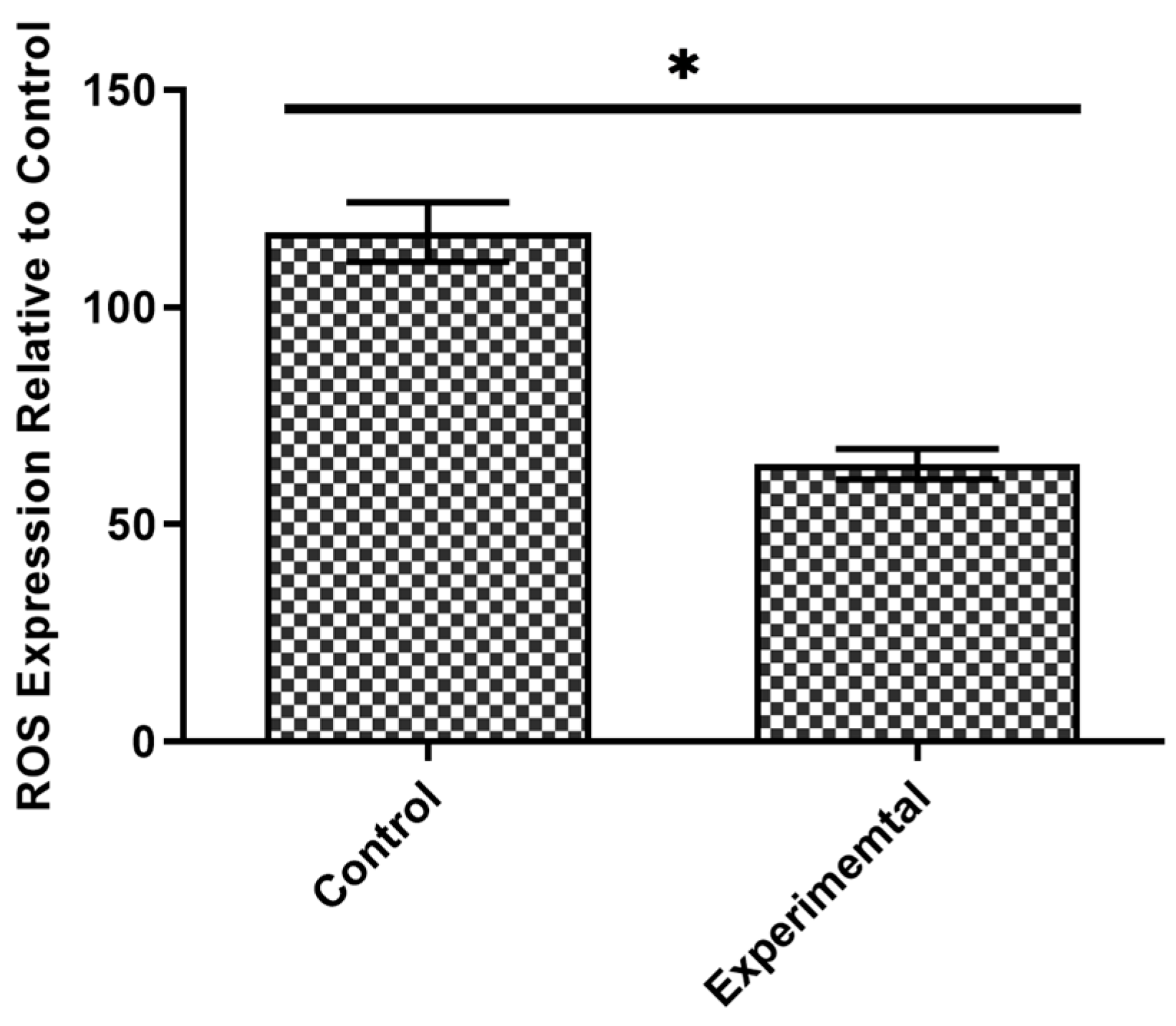
3.1. Intestinal Cell Viability and Antioxidant Potential in Response to FW2 Levan Exposure
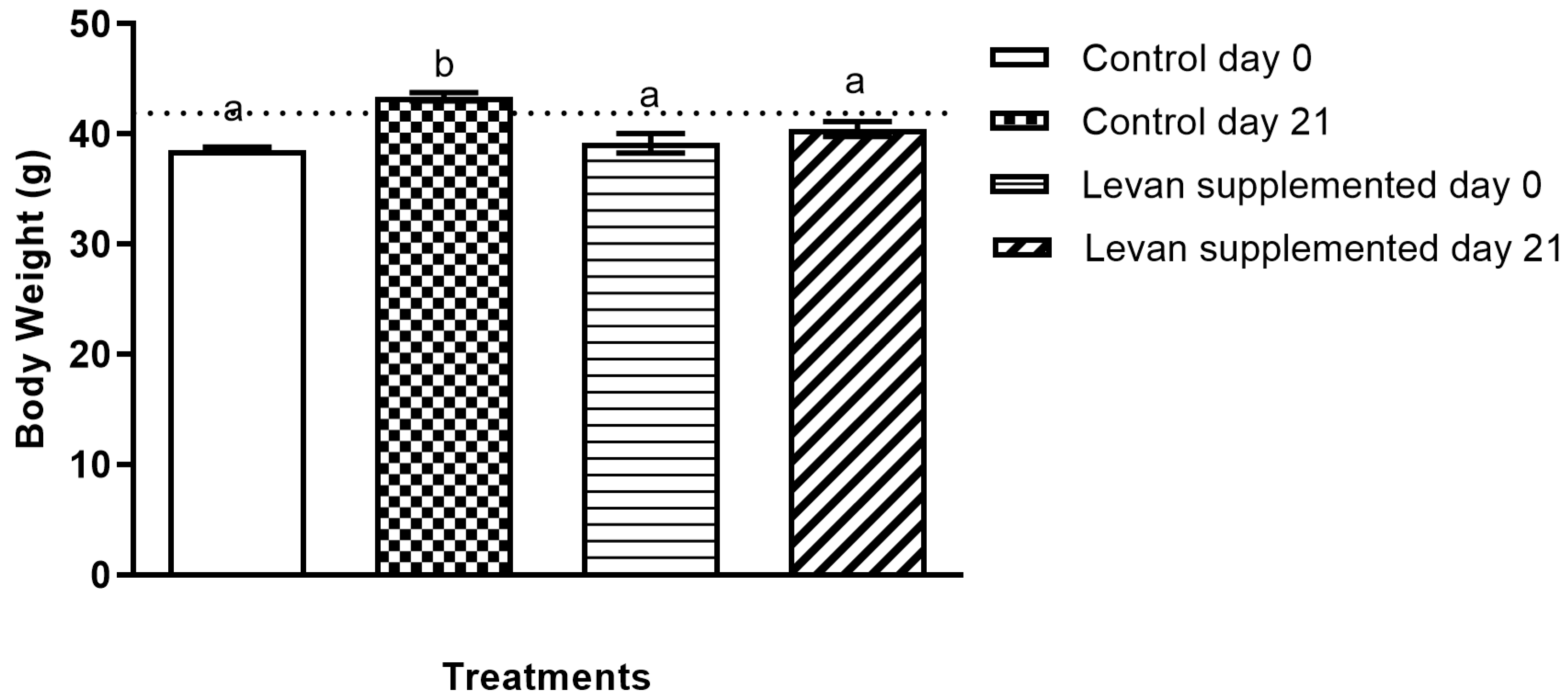
3.2. Determination of Levan’s Effect on Body Weight
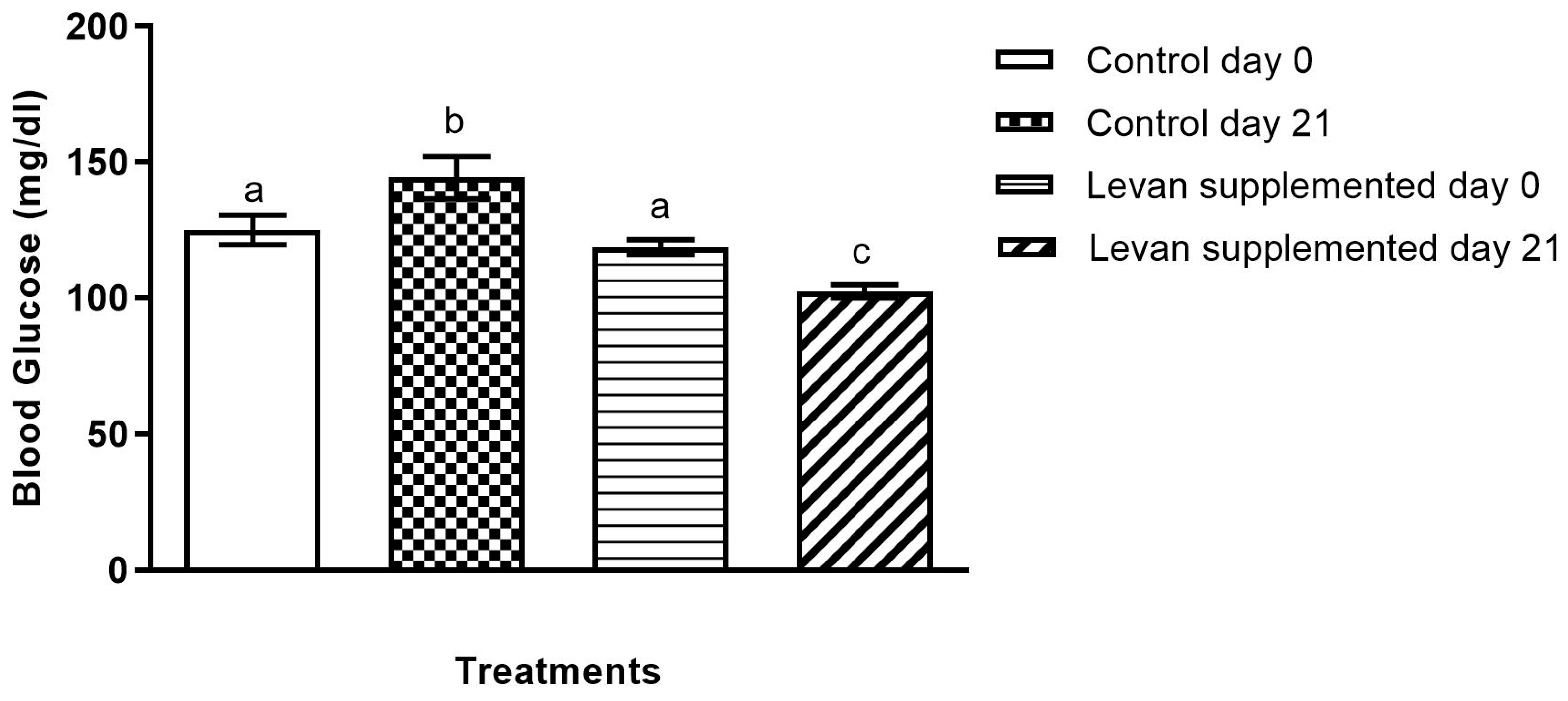
3.3. Analysis of Blood Glucose Levels in Mice Treated with Levan
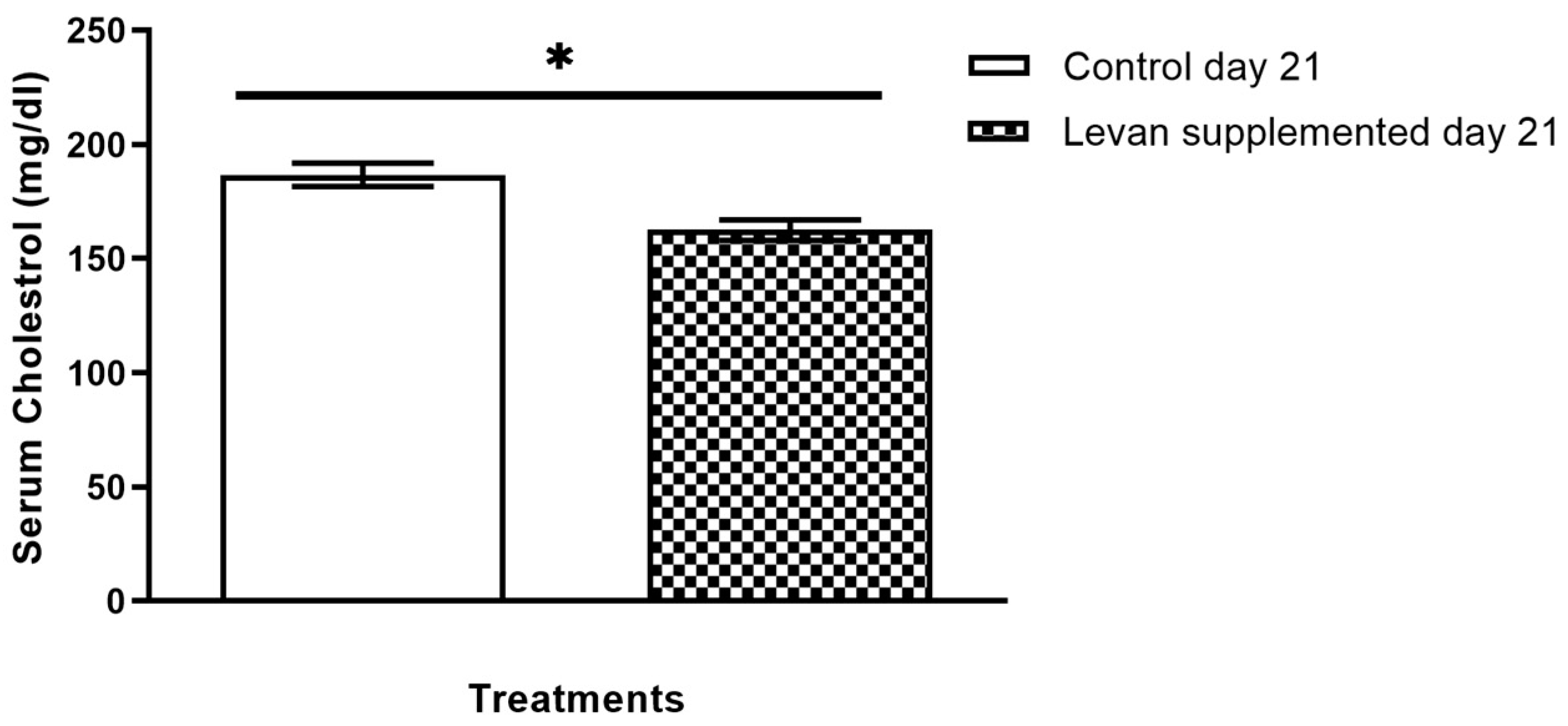
3.4. Investigation of Serum Cholesterol Levels in Mice Treated with Levan
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eckburg, P.B.; Bik, E.M.; Bernstein, C.N.; Purdom, E.; Dethlefsen, L.; Sargent, M.; Relman, D.A. Diversity of the human intestinal microbial flora. Science 2005, 308, 1635–1638. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W. Intestinal microbiota in various animals. Integr. Zool. 2022, 17, 331–332. [Google Scholar] [CrossRef] [PubMed]
- Flint, H.J.; Duncan, S.H.; Scott, K.P.; Louis, P. Interactions and competition within the microbial community of the human colon: Links between diet and health. Environ. Microb. 2007, 9, 1101–1111. [Google Scholar] [CrossRef] [PubMed]
- Meyer, D. Chapter Two—Health benefits of prebiotic fibers. Adv. Food Nutr. Res. 2015, 74, 47–91. [Google Scholar] [CrossRef]
- Roberfroid, M. Prebiotics: The concept revisited. J. Nutr. 2007, 137, 830S–837S. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, W.; Boyajian, J.L.; Abosalha, A.; Nasir, A.; Ashfaq, I.; Islam, P.; Schaly, S.; Thareja, R.; Hayat, A.; Rehman, M.u.; et al. High-Molecular-Weight Dextran-Type Exopolysaccharide Produced by the Novel Apilactobacillus waqarii Improves Metabolic Syndrome: In Vitro and In Vivo Analyses. Int. J. Mol. Sci. 2022, 23, 12692. [Google Scholar] [CrossRef] [PubMed]
- Chou, W.T.; Sheih, I.C.; Fang, T.J. The applications of polysaccharides from various mushroom wastes as prebiotics in different systems. J. Food Sci. 2013, 78, M1041–M1048. [Google Scholar] [CrossRef]
- Jawad, I.; Bin Tawseen, H.; Irfan, M.; Ahmad, W.; Hassan, M.; Sattar, F.; Akhtar, K.; Anwar, M.A. Dietary Supplementation of Microbial Dextran and Inulin Exerts Hypocholesterolemic Effects and Modulates Gut Microbiota in BALB/c Mice Models. Int. J. Mol. Sci. 2023, 24, 5314. [Google Scholar] [CrossRef]
- Teferra, T.F. Possible actions of inulin as prebiotic polysaccharide: A review. Food Front. 2021, 2, 407–416. [Google Scholar] [CrossRef]
- Domżał-Kędzia, M.; Ostrowska, M.; Lewińska, A.; Łukaszewicz, M. Recent Developments and Applications of Microbial Levan, A Versatile Polysaccharide-Based Biopolymer. Molecules 2023, 28, 5407. [Google Scholar] [CrossRef]
- Ashfaq, I.; Amjad, H.; Ahmad, W.; Nasir, A.; Ali, A.; Ali, W.R.; Khaliq, S.; Hayat, A.; Ali, H.; Sattar, F.; et al. Growth Inhibition of Common Enteric Pathogens in the Intestine of Broilers by Microbially Produced Dextran and Levan Exopolysaccharides. Curr. Microbiol. 2020, 77, 2128–2136. [Google Scholar] [CrossRef] [PubMed]
- Öner, E.T.; Hernández, L.; Combie, J. Review of levan polysaccharide: From a century of past experiences to future prospects. Biotechnol. Adv. 2016, 34, 827–844. [Google Scholar] [CrossRef]
- Nasir, A.; Sattar, F.; Ashfaq, I.; Lindemann, S.R.; Chen, M.-H.; Van den Ende, W.; Toksoy, E.; Kirtel, O.; Khaliq, S.; Ghauri, M.A.; et al. Production and characterization of a high molecular weight levan and fructooligosaccharides from a rhizospheric isolate of Bacillus aryabhattai. LWT 2020, 123, 109093. [Google Scholar] [CrossRef]
- Vijn, I.; Smeekens, S. Fructan: More than a reserve carbohydrate? Plant Physiol. 1999, 120, 351–360. [Google Scholar] [CrossRef]
- Srikanth, R.; Reddy, C.H.S.; Siddartha, G.; Ramaiah, M.J.; Uppuluri, K.B. Review on production, characterization and applications of microbial levan. Carbohydr. Polym. 2015, 120, 102–114. [Google Scholar] [CrossRef]
- Hamdy, A.A.; Elattal, N.A.; Amin, M.A.; Ali, A.E.; Mansour, N.M.; Awad, G.E. Possible correlation between levansucrase production and probiotic activity of Bacillus sp. isolated from honey and honey bee. World J. Microbiol. Biotechnol. 2017, 33, 69. [Google Scholar] [CrossRef]
- Klaewkla, M.; Pichyangkura, R.; Charoenwongpaiboon, T.; Wangpaiboon, K.; Chunsrivirot, S. Computational design of oligosaccharide producing levansucrase from Bacillus licheniformis RN-01 to improve its thermostability for production of levan-type fructooligosaccharides from sucrose. Int. J. Biol. Macromol. 2020, 160, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Pan, L.; Wang, B.; Zou, X.; Zhang, A.; Zhou, Z.; Han, Y. Simulated Digestion and Fecal Fermentation Behaviors of Levan and Its Impacts on the Gut Microbiota. J. Agric. Food Chem. 2023, 71, 1531–1546. [Google Scholar] [CrossRef]
- Kovanda, L.; Zhang, W.; Wei, X.; Luo, J.; Wu, X.; Atwill, E.R.; Vaessen, S.; Li, X.; Liu, Y. In Vitro Antimicrobial Activities of Organic Acids and Their Derivatives on Several Species of Gram-Negative and Gram-Positive Bacteria. Molecules 2019, 24, 3770. [Google Scholar] [CrossRef]
- Fusco, W.; Lorenzo, M.B.; Cintoni, M.; Porcari, S.; Rinninella, E.; Kaitsas, F.; Lener, E.; Mele, M.C.; Gasbarrini, A.; Collado, M.C.; et al. Short-Chain Fatty-Acid-Producing Bacteria: Key Components of the Human Gut Microbiota. Nutrients 2023, 15, 2211. [Google Scholar] [CrossRef] [PubMed]
- Dahech, I.; Belghith, K.S.; Hamden, K.; Feki, A.; Belghith, H.; Mejdoub, H. Antidiabetic activity of levan polysaccharide in alloxan-induced diabetic rats. Int. J. Biol. Macromol. 2011, 49, 742–746. [Google Scholar] [CrossRef] [PubMed]
- Dahech, I.; Belghith, K.S.; Hamden, K.; Feki, A.; Belghith, H.; Mejdoub, H. Oral administration of levan polysaccharide reduces the alloxan-induced oxidative stress in rats. Int. J. Biol. Macromol. 2011, 49, 942–947. [Google Scholar] [CrossRef]
- Dal Bello, F.; Walter, J.; Hertel, C.; Hammes, W.P.J.S. In vitro study of prebiotic properties of levan-type exopolysaccharides from lactobacilli and non-digestible carbohydrates using denaturing gradient gel electrophoresis. Syst. Appl. Microbiol. 2001, 24, 232–237. [Google Scholar] [CrossRef]
- Marx, S.P.; Winkler, S.; Hartmeier, W. Metabolization of β-(2, 6)-linked fructose-oligosaccharides by different bifidobacteria. FEMS Microbiol. Lett. 2000, 182, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, W.; Nasir, A.; Sattar, F.; Ashfaq, I.; Chen, M.-H.; Hayat, A.; Rehman, M.U.; Zhao, S.; Khaliq, S.; Ghauri, M.A.; et al. Production of bimodal molecular weight levan by a Lactobacillus reuteri isolate from fish gut. Folia Microbiol. 2022, 67, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Nasir, A.; Ahmad, W.; Sattar, F.; Ashfaq, I.; Lindemann, S.R.; Chen, M.-H.; Van den Ende, W.; Toksoy, E.; Kirtel, O.; Khaliq, S.; et al. Production of a high molecular weight levan by Bacillus paralicheniformis, an industrially and agriculturally important isolate from the buffalo grass rhizosphere. Anton. van Leeuwen. 2022, 115, 1101–1112. [Google Scholar] [CrossRef] [PubMed]
- Voipio, H.-M.; Baneux, P.; De Segura, I.A.G.; Hau, J.; Wolfensohn, S. Guidelines for the veterinary care of laboratory animals: Report of the FELASA/ECLAM/ESLAV Joint Working Group on Veterinary Care. Lab. Anim. 2008, 42, 1–11. [Google Scholar] [CrossRef]
- Bhavani, A.L.; Nisha, J. Dextran—The polysaccharide with versatile uses. Int. J. Pharm. Bio Sci. 2010, 1, 569–573. [Google Scholar]
- Du, R.; Qiao, X.; Zhao, F.; Song, Q.; Zhou, Q.; Wang, Y.; Zhou, Z. Purification, characterization and antioxidant activity of dextran produced by Leuconostoc pseudomesenteroides from homemade wine. Carbohydr. Polym. 2018, 198, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Soeiro, V.C.; Melo, K.R.; Alves, M.G.; Medeiros, M.J.; Grilo, M.L.; Almeida-Lima, J.; Rocha, H.A. Dextran: Influence of molecular weight in antioxidant properties and immunomodulatory potential. Int. J. Mol. Sci. 2016, 17, 1340. [Google Scholar] [CrossRef]
- Thomas, J.; Roy, P.; Ghosh, A.; Mete, M.; Sil, S.K.; Das, D. Prebiotic levan type fructan from Bacillus subtilis PR-C18 as a potent antibiofilm agent: Structural elucidation and in silico analysis. Carbohydr. Res. 2024, 538, 109075. [Google Scholar] [CrossRef] [PubMed]
- Saadat, Y.R.; Khosroushahi, A.Y.; Gargari, B.P. A comprehensive review of anticancer, immunomodulatory and health beneficial effects of the lactic acid bacteria exopolysaccharides. Carbohydr. Polym. 2019, 217, 79–89. [Google Scholar] [CrossRef]
- Zhou, X.; Qi, W.; Hong, T.; Xiong, T.; Gong, D.; Xie, M.; Nie, S. Exopolysaccharides from Lactobacillus plantarum NCU116 regulate intestinal barrier function via STAT3 signaling pathway. J. Agric. Food Chem. 2018, 66, 9719–9727. [Google Scholar] [CrossRef] [PubMed]
- Andrew, M.; Jayaraman, G. Structural features of microbial exopolysaccharides in relation to their antioxidant activity. Carbohydr. Res. 2020, 487, 107881. [Google Scholar] [CrossRef]
- Pei, F.; Ma, Y.; Chen, X.; Liu, H. Purification and structural characterization and antioxidant activity of levan from Bacillus megaterium PFY-147. Int. J. Biol. Macromol. 2020, 161, 1181–1188. [Google Scholar] [CrossRef]
- Kavitake, D.; Veerabhadrappa, B.; Sudharshan, S.; Kandasamy, S.; Devi, P.B.; Dyavaiah, M.; Shetty, P.H. Oxidative stress alleviating potential of galactan exopolysaccharide from Weissella confusa KR780676 in yeast model system. Sci. Rep. 2022, 12, 1089. [Google Scholar] [CrossRef] [PubMed]
- Şengül, N.; Işık, S.; Aslım, B.; Uçar, G.; Demirbağ, A.E. The effect of exopolysaccharide-producing probiotic strains on gut oxidative damage in experimental colitis. Digest. Dis. Sci. 2011, 56, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Peng, Q.; Zhang, Y.; Tian, D.; Zhang, P.; Huang, Y.; Shi, B. A novel exopolysaccharide produced by Lactobacillus coryniformis NA-3 exhibits antioxidant and biofilm-inhibiting properties in vitro. Food Nutr. Res. 2020, 64, 3744–3757. [Google Scholar] [CrossRef] [PubMed]
- Busserolles, J.r.m.; Gueux, E.; Rock, E.; Demigne, C.; Mazur, A.; Rayssiguier, Y. Oligofructose protects against the hypertriglyceridemic and pro-oxidative effects of a high fructose diet in rats. J. Nutr. 2003, 133, 1903–1908. [Google Scholar] [CrossRef] [PubMed]
- Cai, G.; Wu, D.; Li, X.; Lu, J. Levan from Bacillus amyloliquefaciens JN4 acts as a prebiotic for enhancing the intestinal adhesion capacity of Lactobacillus reuteri JN101. Int. J. Biol. Macromol. 2020, 146, 482–487. [Google Scholar] [CrossRef]
- Andersson, H.B.; Ellegård, L.H.; Bosaeus, I.G. Nondigestibility characteristics of inulin and oligofructose in humans. J. Nutr. 1999, 129, 1428S–1430S. [Google Scholar] [CrossRef]
- Chambers, E.S.; Byrne, C.S.; Morrison, D.J.; Murphy, K.G.; Preston, T.; Tedford, C.; Holmes, E. Dietary supplementation with inulin-propionate ester or inulin improves insulin sensitivity in adults with overweight and obesity with distinct effects on the gut microbiota, plasma metabolome and systemic inflammatory responses: A randomised cross-over trial. Gut 2019, 68, 1430–1438. [Google Scholar] [CrossRef] [PubMed]
- Mistry, R.H.; Gu, F.; Schols, H.A.; Verkade, H.J.; Tietge, U.J. Effect of the prebiotic fiber inulin on cholesterol metabolism in wildtype mice. Sci. Rep. 2018, 8, 13238. [Google Scholar] [CrossRef]
- Rao, M.; Gao, C.; Xu, L.; Jiang, L.; Zhu, J.; Chen, G.; Xu, Y. Effect of inulin-type carbohydrates on insulin resistance in patients with type 2 diabetes and obesity: A systematic review and meta-analysis. J. Diabetes Res. 2019, 27, 5101423. [Google Scholar] [CrossRef] [PubMed]
- Jang, K.-H.; Kim, M.-J. A mixture of poly-γ-glutamic acid and levan ameliorates obesity in high fat diet-induced mice. Food Sci. Biotechnol. 2022, 31, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.-A.; Jang, K.-H.; Lee, J.-C.; Chang, B.-I.; Lim, Y.-A.; Song, B.-C. The effects of fructose polymer levan on the body fat accumulation and serum lipid profiles of Korean women. Korean J. Community Nutr. 2003, 8, 986–992. [Google Scholar]
- Jiao, A.; Yu, B.; He, J.; Yu, J.; Zheng, P.; Luo, Y.; Luo, J.; Yan, H.; Wang, Q.; Wang, H.; et al. Sodium acetate, propionate, and butyrate reduce fat accumulation in mice via modulating appetite and relevant genes. Nutrition 2021, 87, 111198. [Google Scholar] [CrossRef] [PubMed]
- Bahroudi, S.; Shabanpour, B.; Combie, J.; Shabani, A.; Salimi, M. Levan exerts health benefit effect through alteration in bifidobacteria population. Iranian Biomed. J. 2020, 24, 54. [Google Scholar] [CrossRef]
- Belghith, K.S.; Dahech, I.; Hamden, K.; Feki, A.; Mejdoub, H.; Belghith, H. Hypolipidemic effect of diet supplementation with bacterial levan in cholesterol-fed rats. Int. J. Biol. Macromol. 2012, 50, 1070–1074. [Google Scholar] [CrossRef] [PubMed]
- Dahech, I.; Harrabi, B.; Hamden, K.; Feki, A.; Mejdoub, H.; Belghith, H.; Belghith, K.S. Antioxidant effect of nondigestible levan and its impact on cardiovascular disease and atherosclerosis. Int. J. Biol. Macromol. 2013, 58, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.-A.; Hong, K.-H.; Jang, K.-H.; Kim, S.-H.; Lee, K.-H.; Chang, B.-I.; Choue, R.-W. Anti-obesity and hypolipidemic effects of dietary levan in high fat diet-induced obese rats. J. Microbiol. Biotechnol. 2004, 14, 796–804. [Google Scholar]
- Burnett, L.C.; Lunn, G.; Coico, R. Biosafety: Guidelines for working with pathogenic and infectious microorganisms. Curr. Protoc. Microbiol. 2009, 13, 1A.1.1–1A.1.14. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, W.; Nasir, A.; Prakash, S.; Hayat, A.; Rehman, M.u.; Khaliq, S.; Akhtar, K.; Anwar, M.A.; Munawar, N. In Vitro and In Vivo Interventions Reveal the Health Benefits of Levan-Type Exopolysaccharide Produced by a Fish Gut Isolate Lactobacillus reuteri FW2. Life 2025, 15, 89. https://doi.org/10.3390/life15010089
Ahmad W, Nasir A, Prakash S, Hayat A, Rehman Mu, Khaliq S, Akhtar K, Anwar MA, Munawar N. In Vitro and In Vivo Interventions Reveal the Health Benefits of Levan-Type Exopolysaccharide Produced by a Fish Gut Isolate Lactobacillus reuteri FW2. Life. 2025; 15(1):89. https://doi.org/10.3390/life15010089
Chicago/Turabian StyleAhmad, Waqar, Anam Nasir, Satya Prakash, Azam Hayat, Mujaddad ur Rehman, Shazia Khaliq, Kalsoom Akhtar, Munir Ahmad Anwar, and Nayla Munawar. 2025. "In Vitro and In Vivo Interventions Reveal the Health Benefits of Levan-Type Exopolysaccharide Produced by a Fish Gut Isolate Lactobacillus reuteri FW2" Life 15, no. 1: 89. https://doi.org/10.3390/life15010089
APA StyleAhmad, W., Nasir, A., Prakash, S., Hayat, A., Rehman, M. u., Khaliq, S., Akhtar, K., Anwar, M. A., & Munawar, N. (2025). In Vitro and In Vivo Interventions Reveal the Health Benefits of Levan-Type Exopolysaccharide Produced by a Fish Gut Isolate Lactobacillus reuteri FW2. Life, 15(1), 89. https://doi.org/10.3390/life15010089








